Abstract
Background. An explanation for the immunologic dysfunction that causes children to be prone to repeated episodes of acute otitis media (AOM) has long been sought. Poor antibody response has been associated with the otitis-prone condition; however, there is no precise mechanistic explanation for this condition.
Methods. Non–otitis-prone and otitis-prone children with AOM or nasopharyngeal (NP) colonization caused by either Streptococcus pneumoniae or Haemophilus influenzae were compared for pathogen-specific CD4+ T-helper memory responses by stimulating peripheral blood mononuclear cells using 6 vaccine candidate S. pneumoniae and 3 H. influenzae protein antigens. Samples were analyzed by multi-parameter flow cytometry.
Results. Significantly reduced percentages of functional CD45RALow memory CD4+ T cells producing specific cytokines (interferon γ, interleukin [IL]–2, IL-4 and IL-17a) were observed in otitis-prone children following AOM and NP colonization with either S. pneumoniae or H. influenzae. Immunoglobulin (Ig) G responses to the studied protein antigens were reduced, which suggests that antigen-specific B-cell function may be compromised as a result of poor T-cell help. Staphylococcal enterotoxin B stimulated similar cytokine patterns in memory CD4+T cells in both groups of children.
Conclusions. Otitis-prone children have suboptimal circulating functional T-helper memory and reduced IgG responses to S. pneumoniae or H. influenzae after colonization and after AOM; this immune dysfunction causes susceptibility to recurrent AOM infections.
Acute otitis media (AOM) is a common infectious disease in children worldwide and is associated with a substantial burden of temporary deafness and delayed speech development in developed countries and more-serious complications in developing countries [1, 2]. Although 60%–70% of children experience at least 1 episode during the first 3 years of life, a subpopulation of children, representing 30% of the total population of children, experience ≥3 episodes of AOM within 6 months or 4 infections within a year and are considered to be otitis-prone [3].
Streptococcus pneumoniae and nontypeable Haemophilus influenzae are the 2 most common pathogens causing AOM [4]. In animal models, CD4+ T lymphocytes have been shown to be critical for protective immunity against these prevalent bacterial respiratory pathogens [5–7]. More recently, Th-17 cells secreting interleukin (IL)–17, IL-21, and IL-22 have been described to impart antibody-independent protection in mouse model of pneumococcal infection [8]. In older children (median age, 5 years) and adults, antigen-specific CD4+ T cells have been shown to reduce S. pneumoniae nasopharyngeal colonization [9, 10]. An effective pathogen-specific T-cell response in adults has been associated with protection from invasive S. pneumoniae disease (IPD) and chronic obstructive pulmonary disease (COPD) caused by S. pneumoniae and H. influenzae, respectively [11, 12]. However, there are no data that correlate a protective role of CD4+ T-helper subsets among children who experience AOM.
Robust memory T- and B-cell responses are generated during onset of a natural infection as well as upon vaccination, with memory lymphocytes populating lymphoid and nonlymphoid sites [12–14]. Once generated, memory T cells and antibodies can be detected in the blood circulation over a period of time [12, 15]. In both humans and mice, CD4+ T cells comprise functionally distinct populations that are characterized by specific cytokine profiles produced in response to antigens [16, 17]. More recently, follicular helper T (Tfh) cells have been shown as a major subset to provide help to B cells for antibody responses [18–20].
To explain the immunological dysfunction that leads to recurrent AOM, earlier studies, including ours, have found lower levels of otopathogen-specific antibody concentrations in otitis-prone children, compared with non–otitis-prone children [21, 22]. In this work, we sought a better understanding of the immunologic dysfunction in otitis-prone children, focusing on the generation of different subsets (Th-1, Th-2, and Th-17) of memory CD4+ T-helper cells in correlation with B-cell antibody responses as a possible novel explanation. Using 6 pneumococcal and 3 H. influenzae protein antigens, we enumerated S. pneumoniae– and H. influenzae–specific functional CD4+ T-helper memory cell subsets in the peripheral blood of a cohort of non–otitis-prone and otitis-prone children. Serum immunoglobulin (Ig) G responses to the same antigens were also measured in these children.
METHODS
Subjects
Subjects were participants from our 5-year prospective longitudinal AOM study funded by the National Institutes of Health National Institute on Deafness and Other Communication Disorders [22]. Enrolled children were from a middle-class, suburban socio-demographic population in Rochester, New York. Healthy children 6 months of age without prior AOM were enrolled and had blood, nasopharyngeal (NP), and oropharyngeal (OP) cultures obtained 7 times, at the ages of 6, 9, 12, 15, 18, 24, and 30 months. Middle ear fluid (MEF) was obtained by tympanocentesis during AOM episodes. Colonization with Spn and/or NTHi in the NP, OP, and MEF samples was routinely determined by standard microbiologic culture. To identify the otitis-prone children in the study population, all of the children had tympanocentesis-confirmed infections, and all received antibiotic therapy directed to the otopathogen isolated from MEF for each AOM event. Peripheral blood mononuclear cells (PBMCs) were isolated from the collected blood and were frozen in liquid nitrogen until used. Children who had ≥ 3 episodes of AOM within 6 months or at least 4 episodes within 1 year were considered to be otitis-prone, whereas others who had fewer episodes were placed into the non–otitis-prone group. Written informed consent was obtained in association with a protocol approved by the Rochester General Hospital Investigational Review Board.
Antigens
Six different pneumococcal protein antigens were used in this study: pneumococcal histidine triad proteins D (PhtD) and E (PhtE), LytB, PcpA, PlyD1 (a detoxified derivative of pneumolysin that has 3 point mutations that do not interfere with anti-pneumolysin antibody responses), and PspA. H. influenzae protein antigens used were P6, OMP26, and Protein D. An optimal dosage for stimulation was determined by the absence of detectable cell toxicity, by the use of tryptan blue staining and/or flow cytometry analysis after propidium iodide staining (data not shown). Staphylococcal enterotoxin B (Sigma) was used as a positive control.
T-Cell Stimulation
T-cell stimulation and intracellular cytokine profiling were standardized in our laboratory using a procedure adapted from elsewhere [23]. Briefly, PBMCs were stimulated with 6-pneumococcal or 3–H. influenzae antigens individually depending on the NP-colonization or AOM-causative pathogen. Prior to stimulation, frozen PBMCs were quickly thawed in a 37°C water bath, followed by slowly adding complete culture medium (Roswell Park Memorial Institute [RPMI] 1640 supplemented with 10% of fetal bovine serum [FBS], 2 mM l-glutamine, 0.1 mM sodium pyruvate, nonessential amino acids, 100 U/mL penicillin, and 100 μg/mL streptomycin). Cells were then washed and rested overnight in complete culture media in 24-well plates. PBMCs were stimulated using a standardized protocol in our laboratory. Briefly, cells were counted, and 1 × 106 cells were placed in the each well of a 96-well flat-bottom culture plate for stimulation with either 1 μg/mL of various protein antigens individually or with 1 μg/mL of staphylococcal enterotoxin B (SEB). Cells that were left untreated served as negative controls. Cells were then incubated for 2 hours at 37°C in the presence of 5% CO2 for antigen processing. After 2 hours, Golgi transport inhibitors (Brefeldin A and Monensis; BD Biosciences) were added to preserve cytokines intracellularly, and incubation was then continued for an additional 4 hours. Then 1 μg/mL concentrations of anti-CD28 and anti-CD49d antibodies (clones L293 and L25, respectively; BD Biosciences) were added to the cells to provide costimulation and enhance the detection of antigen-specific responses. Anti-CD28 and -CD49d antibodies have been widely used for costimulation without affecting background levels [15].
Cell Surface Staining and Cytokine Profiling
An intracellular cytokine staining assay was used to evaluate antigen-specific CD4+ T-cell subsets (Th-1, Th-2, and Th-17). After stimulation, cells were transferred to 96-well V-bottom plates and washed once with fluorescence-activated cell-sorting (FACS) buffer (phosphate-buffered saline [PBS] with 5% FBS) and stained with the antibodies to various cell surface markers. Antibodies used were anti-CD4 APC Alexafluor 750 (clone RPA T4; eBiosciences), PE-Texas Red anti-CD45RA (clone MEM56; Invitrogen), and anti-CCR7 PerCP/Cy5.5 conjugate (clone TG8/CCR7; Biolegend). To identify Tfh cells in the PBMC samples, cells were surface stained with anti-CXCR5 perCP cy5.5 (Biolegend), anti-CD4 APC Alexafluor 750, PE-Texas Red anti- CD45RA, and anti-CD3 Qdot (clone UCHT1; Invitrogen) separately. Cells were then permeabilized with fixation and permeabilization solution (BD Biosciences) for 20 minutes and washed 3 times with 1 × permeabilization buffer (BD Biosciences). A cocktail of various cytokine-specific antibodies was used to stain intracellularly captured cytokines as a result of stimulation. Antibodies used were PE-Cy7–conjugated anti–interferon (IFN)-γ (clone B27; BD biosciences), Pacific blue– conjugated anti-IL17A (clone BL168; Biolegend), Alexa fluor 700 anti–IL-2 (clone MQ1-17H12; Biolegend), PE-conjugated anti–IL-4 (clone 8D4-8; BD Biosciences), AF 488–conjugated TNF-α, anti-CD3 Qdot 605 (clone UCHT1; Invitrogen), and PE-Cy5 anti-CD69 (clone FN50; BD biosciences). After intracellular staining, cells were further washed 3 times with 1 × permeabilization buffer and underwent a final wash with FACS buffer before they were resuspended into the FACS tubes. A custom made BD LSR II flow cytometer equipped for the detection of 12 fluorescent parameters was used to collect 2–5 × 105 events for each sample, and data were analyzed using FLOW JO (Tree Star) software. Gates for cytokine-positive cells were determined with the help of unstimulated and SEB-stimulated cells, and cytokine responders were confirmed by excessive back-gating.
Humoral Responses
For measuring IgG antibody levels in the samples, an enzyme-linked immunosorbent assay (ELISA) was performed as described previously [22]. Briefly, 96-well ELISA plates (Nunc-Immulon) were coated with 0.5 μg/mL of individual antigens (100 μl/well) in coating buffer (bicarbonate, pH 9.4) and incubated overnight at 4°C. After washing, the plates were blocked with 3% skimmed milk at 37°C for 1 hour (200 μl per well). After 5 washes, 100 μl of serum at a starting dilution of 1:100 (in PBS-3% skim milk) was added to the wells and diluted serially 2 fold. The mixture was incubated at room temperature for 1 hour followed by the addition of affinity-purified goat antihuman IgG, IgM, or IgA antibody conjugated to horseradish-peroxidase (Bethyl Laboratories) as a secondary antibody. The reaction products were developed with TMB Microwell Peroxidase Substrate System (KPL), stopped by the addition of 1.0 molar phosphoric acid and read by an automated ELISA reader using a 450-nm filter. To provide quantitative results on antibody concentrations, the level of the specific antibody present in the unknown sample was determined by comparison with an internal reference serum (pool of human serum with high antigen titers). The levels of IgG in the reference serum were quantitatively measured by using a human IgG ELISA quantitation kit (Bethyl Laboratories). A 4-parameter logistic-log function was used to form the reference and sample curves.
Statistics
All data were analyzed using Graph Pad Prism software. Two-tailed P values for the data were calculated using the Mann–Whitney U test. Percentages of atopy and nonatopy between all non–otitis-prone and otitis-prone children were compared using the χ2 test.
RESULTS
Study Population
From a total study population of 387 children, 19 otitis-prone children were identified. From the remainder of the children, those with 1 or 2 AOMs who were of a similar age as the non–otitis-prone children were randomly selected as comparators for the study. Clinical characteristics of the study children are shown in Table 1. No significant differences were found in atopic and nonatopic children between the 2 cohorts (P = .5).
Table 1.
Basic Characteristics of Study Subjects
| Pneumococcal infection |
H. influenzae infection |
|||
| Variable | Otitis-prone children (n = 13) |
Non–otitis-prone children (n = 14) |
Otitis-prone children (n = 6) |
Non–otitis-prone children (n = 6) |
| Sex | ||||
| Male | 9 | 10 | 3 | 2 |
| Female | 4 | 4 | 3 | 4 |
| Age, mean months | 13.5 | 10.1 | 14.3 | 8.2 |
| No. of AOM episodes | ||||
| ≥3 within 6 months | 6 | 0 | 5 | 0 |
| ≥4 within 12 months | 7 | 0 | 1 | 0 |
| No. of NP colonizations with respective pathogen | ||||
| 1–3 | 10 | 10 | 4 | 5 |
| 4–5 | 3 | 1 | 1 | 0 |
| ≥6 | 0 | 0 | 1 | 0 |
| Ventilation tube placement | 4 | 0 | 3 | 0 |
| Adenoidectomy | 0 | 0 | 0 | 0 |
| Breast feeding | 12 | 8 | 3 | 1 |
| Atopy | 7 | 4 | 4 | 1 |
| Nonatopic | 6 | 10 | 2 | 5 |
NOTE. Data are no. of subjects, unless otherwise indicated. AOM, acute otitis media; NP, nasopharyngeal.
Otitis-Prone Children Have Reduced Percentages of Antigen-Specific Functional T-helper Memory Responses to Spn and NTHi in Their Circulation
The circulating frequencies of various Spn and NTHi antigen-specific memory T-helper cell subsets were compared between non–otitis-prone and otitis-prone children by stimulating their PBMCs with specific antigens. For that, the percentages of T-helper memory cells producing IFN-γ, IL-4, IL-2, or IL-17 a were calculated by gating on activated CD69+ T cells (Figure 1). No difference was found in the naive and memory CD4+ T-cell counts among both the cohorts (Table 2). Antigen-specific responses were normalized with the control PBMCs left unstimulated or stimulated with a nonspecific antigen (Keyhole limpet hemocyanin).
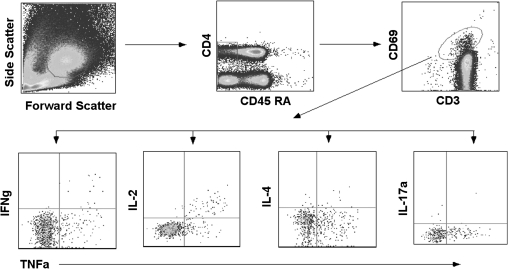
Figure 1.
Gating strategy for enumerating cytokine-specific memory CD4+ T cells among children. To exclude cell debris and clumps, cells were first gated on the basis of their forward- and side-scatter properties followed by sequential gating on CD4+ CD45RALow T cells and then to CD3+CD69+ cytokine positive cells before gating on to tumor necrosis factor (TNF)–α versus other cytokines. Low-frequency responders were confirmed by excessive back-gating. Preliminarily, the whole assay was standardized and compared with multiplex bead array (CBA; BD Biosciences) for the detection of CD4+ T-cell cytokine profiles.
Table 2.
Circulating CD4+ T-Cell Counts per Million Peripheral Blood Mononuclear Cells in Otitis-Prone and Non–Otitis-Prone Children
| Cell count, mean cells/μL ± standard deviation |
|||
| Cell type | Otitis-prone children (n = 19) | Non–otitis-prone children (n = 20) | P |
| CD3+CD4+ T cells | 2306±452 | 2099±380 | .17 |
| CD3+CD4+CD45RA+ (naive CD4+ T cells) | 1322±640 | 1240±590 | .10 |
| CD3+CD4+CD45RA− (memory CD4+ T cells) | 455±180 | 537±198 | .18 |
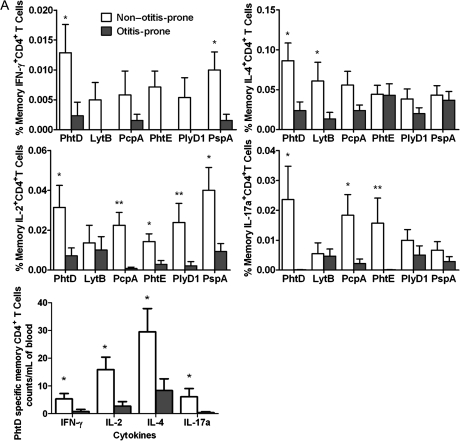
Figure 2A demonstrates frequencies of the various subsets of T-helper memory cells to all the Spn antigens used for stimulation in non–otitis-prone children (n = 15) following AOM (n = 6) or NP colonization (n = 9) with S. pneumoniae. In sharp contrast, otitis-prone children (n = 13) had a marked dysfunction of circulating S. pneumoniae–specific T-helper memory cells after AOM (n = 10) and NP colonization (n = 3). In particular, there was a complete lack of T-helper memory cells producing IFN-γ against LytB, PhtE, and PlyD1, whereas significantly lower levels of IFN-γ were produced in response to PhtD, PcpA, and PspA (P < .02). A significant decrease in IL-4–producing T-helper memory cells was observed against PhtD and LytB (P < .02) in the otitis-prone children. IL-2 responses to PhtD (P < .05), PcpA (P < .005), PhtE (P < .05), PlyD1 (P < .005), and PspA (0.02) were significantly lower in otitis-prone children, and a significant reduction in IL-17a–producing cells was found in otitis-prone children in response to PhtD, PcpA, and PhtE (P < .05).
Figure 2.
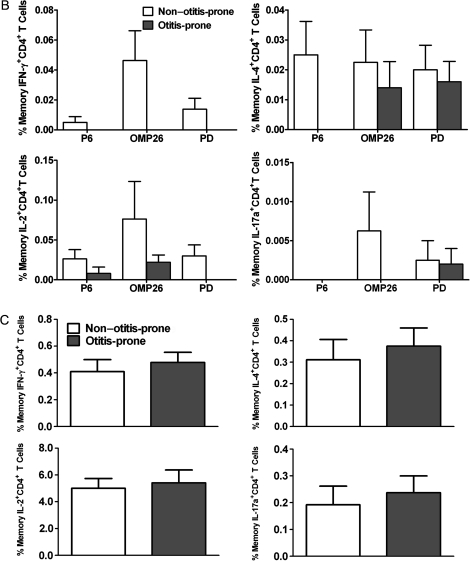
A, percentage frequencies of memory CD4+ T-cell subsets producing various cytokines (interferon [IFN]–γ, interleukin [IL]–4, IL-2, and IL-17a) against 6 pneumococcal antigens in the circulation of non–otitis-prone and otitis-prone children, whereas unstimulated cells serve as a negative control. Bar graphs represent normalized mean percentages of CD69+ CD4+ T cells gated on CD45RALow following stimulation. Absolute blood counts were calculated for the cytokine-producing cells in case of PhtD antigen. Error bars represent the standard error of the mean; P values were calculated using the Mann–Whitney U test. *P < .05; **P < .005. B, frequencies of memory CD4+ T-cell subsets producing various cytokines in response to 3 NTHi antigens (P6, OMP26, and Protein D) in the circulation of non–otitis-prone and otitis-prone children. Bar graphs represent mean percentage values of CD69+ CD4+ T cells that were CD45RALow following antigen stimulations. *P < .05. C, peripheral blood mononuclear cell samples from non–otitis-prone and otitis-prone children were stimulated with staphylococcal enterotoxin B, and cytokine production was observed in the CD45RALow CD4+ T-cell population (P > .5).
Figure 2B shows the results of a separate series of experiments involving 6 non–otitis-prone children (all NP colonized with H. influenzae) and 6otitis-prone children either NP colonized with H. influenzae (n = 2) or having an AOM episode caused by H. influenzae (n = 4). PBMCs were stimulated with H. influenzae protein antigens P6, OPM26 and protein D. Otitis-prone children were devoid of IFN-γ–producing T-helper memory cells against all 3 H. influenzae antigens used for stimulations. Otitis-prone children lacked an IL-4 response to P6 antigen (P < .05) but no significant differences were observed in the IL-4 response to OMP26 and protein D compared to non–otitis-prone children (P = .6). No T-helper memory cells were found in otitis-prone children who produced IL-2 upon stimulation with protein D, and the frequencies of cells responding to OMP26 and P6 were significantly reduced (P < .05).
Neither otitis-prone nor non–otitis-prone children showed IL-17a response upon stimulation with P6. Otitis-prone children were devoid of OMP26-specific memory Th-cells producing IL-17a, which is a significant difference from non–otitis-prone children (P = .05). The difference in the frequencies of IL-17a–producing memory T-helper cells, compared with protein D, was not significant (P = .7).
Otitis-Prone Children Are Not Deficient in Total Functional Memory T Cells
We tested whether the impaired T-helper memory cell responses to the S. pneumoniae and H. influenzae antigens among otitis-prone children were attributable to intrinsic T-cell defects among otitis-prone children. For that, PBMCs were stimulated with SEB (as described in Materials and Methods), which is an antigen that stimulates a T-cell response independent of antigen-presenting cell involvement [24]. Upon stimulation with SEB, the percentage of CD45RALow CD4+ T cells producing IFN-γ, IL-4, IL-2, or IL-17a was found to be the same for otitis-prone and non–otitis-prone children (P > .5; Figure 2C).
Antibody Responses to Spn and NTHi Protein Antigens Are Reduced in Otitis-Prone Children
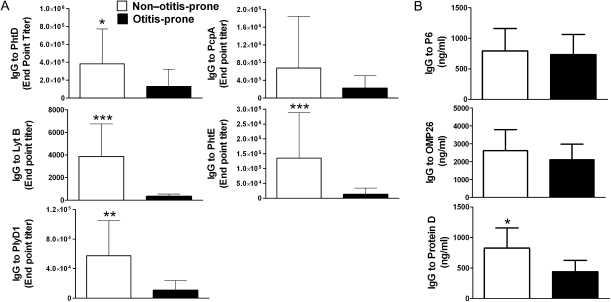
We evaluated antigen-specific IgG titers in the serum of non–otitis-prone and otitis-prone children. Serum IgG levels to the similar Spn and NTHi antigens in the respective groups are shown in Figure 3. As expected, with the increased T-helper memory cell frequencies, IgG titers to PhtD, LytB, PhtE, and PlyD1 were significantly higher in the non-otitis-prone group than in the otitis-prone group (P < .05; P < .001; P < .001; and P < .005, respectively), whereas PcpA levels were not significantly different between the groups (Figure 3A). Among NTHi antigens, significantly higher IgG levels were observed to Protein D in non–otitis-prone children, compared with the otitis-prone children (P < .05), whereas no significant differences in the levels of IgG antibody to P6 and OMP26 were measured between the groups (Figure 3B).
Figure 3.
A, comparison of immunoglobulin (Ig) G responses to 5 pneumococcal protein antigens (Phtd, LytB, PcpA, PhtE, and PlyD1) in the serum samples of 2 cohorts of non–otitis-prone and otitis-prone children. *P < .05; **P < .005; ***P < .001. Y-axis represents geometric mean titers, and error bars are upper 95% confidence intervals. B, IgG responses to NTHi protein antigens (P6, OMP26 and Protein D) were also observed in the serum samples of two cohorts of non-otitis-prone and otitis-prone children. *P < .05. Y-axis represents geometric mean titers, and error bars are upper 95% confidence intervals.
DISCUSSION
Children with repeated episodes of AOM experience the greatest morbidity from this infection, which sometimes results in permanent hearing loss [2, 25]. Previous studies have reported that otitis-prone children produce lower amounts of Spn- and NTHi-specific antibodies than do non–otitis-prone children and/or do not produce functional bactericidal antibodies [21, 22, 26]. These findings as well as our own suggested that a decreased concentration of circulating antibodies to the otopathogen antigens explained the otitis-prone condition. We sought a more precise immunological explanation for the observed lower antibody levels in the otitis-prone children to facilitate further research to circumvent the dysfunction. We postulated that the reduced antibody response observed in the otitis-prone children might be the result of impaired CD4+ T-helper cell responses to the pathogen. Hence, we compared generation of antigen-specific memory CD4+ T-helper cell subsets (Th-1, Th-2, and Th-17) between non–otitis-prone and otitis-prone populations of children. This becomes important because CD4+ T-helper cells have been shown to mediate help in fighting infections caused by Spn and NTHi [8, 11, 12]. However, there is no report demonstrating a protective role of pathogen-specific CD4+ T-helper-cells in AOM in children that is caused by these respiratory pathogens.
We found a clear reduction in the functional memory CD4+ T-cell frequencies producing various cytokines among children who are prone to AOM infection (Figure 2A and B). We speculate that otitis-prone children develop short-lived antibody responses because antibodies were detectable among these children after AOM and NP colonization with otopathogens (Figure 3A and B). However, in the absence of adequate pathogen-specific memory CD4+ T-cell frequencies and after the antibody levels wane, the child quickly becomes susceptible to additional AOM infections. Recent work on follicular T-helper cells (Tfh) has established their significance in generating B cell–mediated antibody responses. Hence, we expected that otitis-prone children may have reduced Tfh in their circulation. Surprisingly, staining of CD4+ T cells for CXCR5 expression did not identify a difference in the Tfh population in the circulation in otitis-prone or non–otitis-prone children (data not shown). First, only a low percentage of CXCR5-expressing CD4+ Tfh-cells can be detected in the peripheral blood, as demonstrated in adults [18]. Second, we have preliminary data to suggest that children of this age group lack overall CXCR5-expressing CD4+ T cells in their circulation. This makes it difficult to compare Tfh populations in the PBMCs of otitis-prone and non–otitis-prone children (unpublished data). We plan future studies to compare populations of Tfh cells in the lymphoidal tissues (tonsils and adenoids), which are the sites of germinal center formation and have higher Tfh populations than other tissues [18, 19]. Furthermore, as a result of SEB stimulation, similar percentages of functional memory CD4+ T cells were observed among both of the cohorts, which rules out an intrinsic defect in the CD4+ T cells of otitis-prone children (Figure 1C).
Previous work has demonstrated the role of Spn and NTHi antigens in CD4+ T-cell–proliferative responses (for 5–7 days) among children and adults [9, 10]. A prior study evaluated CD4+ T-cell proliferation in the cells collected from the adenoids and tonsils of otitis-prone children and found no proliferation in response to NTHi protein P6 [27]. Studies of this nature are imperative to evaluate antigen-specific T-cell proliferation but provide no information on the occurrence of antigen-specific memory CD4+ T cells. To the best of our knowledge, this is the first report that demonstrates increased frequencies of Spn- and NTHi-specific IL-17a–producing memory Th cells in the circulation of non–otitis-prone children, compared with otitis-prone children (Figure 1A and 1B). Although not directly demonstrated, the IL-17a–producing memory Th-cells may contribute to protection against the otitis-prone condition caused by Spn or NTHi by an antibody-independent mechanism, as demonstrated in a mouse model [8].
The cellular phenotyping of MEF during AOM as well as adenoids in similar individuals has indicated a large migration of CD45ROHigh/CD45RALow memory CD4+ T cells, as determined by the loss of homing receptors L-selectin [28, 29]. Local secondary lymphoid organs, such as adenoids, are the primary sites for T-cell priming during upper respiratory tract bacterial infections and nasopharyngeal colonization. Once an antigen-loaded APC migrates to local lymphoid organs (adenoids), the differentiation of lymphocytes (such as CD4+ T cells) takes place. After entering the blood circulation, the CD4+ T cells may eventually migrate to the middle ear mucosa (in the case of AOM) and/or the upper respiratory tract (during NP colonization) [12, 28]. Unlike in mice, it is practically impossible to track antigen-specific CD4+ T cells in human subjects. Nevertheless, the evaluation of MEF for the cellular phenotypes suggests that T-helper memory cells may play a key role in the elimination of AOM pathogens at the middle ear mucosa. Hence, we hypothesize that otopathogen-specific T-cell memory, if generated, would be helpful in the prevention of recurrent AOM.
A decreased antibody response has been reported previously after immunization with rubella vaccine in otitis-prone children [30]. A similar dysfunction in T-cell responses to vaccination has been observed among bone marrow or stem cell transplant recipients [31, 32]. Also, earlier studies have suggested a genetic polymorphism in the expression of various immunoresponsive genes, TNFa, IL-6, and IL-10 among otitis-prone children [33, 34]. Faulty function of APCs has been described as responsible for immature T-cell responses among infants and young children [35]. Furthermore, dendritic cells in infants have been shown to pose restrictions in generating vaccine-specific T-cell memory [36]. Collectively, based on the presented data as well as on prior reports, it is possible that APCs in otitis-prone children are unable to prime naive T cells for memory generation. Whether otitis-prone children possess an immature subset of APCs and therefore are unable to process and present antigens to the CD4+ T cells for effector/memory generation is now an area of investigation in our group.
Funding
This work was funded by the National Institute of Health (grant R01 DC008671) and Thrasher Research Fund to M. E. P.
Acknowledgments
We thank Dr Ravinder Kaur, Rochester General Hospital Research Institute, for performing the antibody measurements to the studied proteins; Dr Tim Murphy, University of Buffalo, and Dr Jennelle Kyd, University of Canberra, Australia, for providing P6 and OMP26 plasmids; Sanofi-Pasteur, for providing us pneumococcal protein antigens; and Sally Thomas, LPN, CCRC, for all of her clinical work with the study patients.
References
- 1.Pichichero ME, Casey JR. Otitis media. Expert Opin Pharmacother. 2002;3:1073–90. doi: 10.1517/14656566.3.8.1073. [DOI] [PubMed] [Google Scholar]
- 2.Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10:195–203. doi: 10.1016/S1473-3099(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 3.Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119:707–15. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 4.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005;102:4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCool TL, Weiser JN. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun. 2004;72:5807–13. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snapper CM, Shen Y, Khan AQ, et al. Distinct types of T-cell help for the induction of a humoral immune response to Streptococcus pneumoniae. Trends Immunol. 2001;22:308–11. doi: 10.1016/s1471-4906(01)01926-3. [DOI] [PubMed] [Google Scholar]
- 8.Malley R, Srivastava A, Lipsitch M, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74:2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mureithi MW, Finn A, Ota MO, et al. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J Infect Dis. 2009;200:783–93. doi: 10.1086/605023. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Bagrade L, Bernatoniene J, et al. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J Infect Dis. 2007;195:1194–202. doi: 10.1086/512617. [DOI] [PubMed] [Google Scholar]
- 11.King PT, Hutchinson PE, Johnson PD, Holmes PW, Freezer NJ, Holdsworth SR. Adaptive immunity to nontypeable Haemophilus influenzae. Am J Respir Crit Care Med. 2003;167:587–92. doi: 10.1164/rccm.200207-728OC. [DOI] [PubMed] [Google Scholar]
- 12.de Bree GJ, Daniels H, Schilfgaarde M, et al. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis. 2007;195:1718–25. doi: 10.1086/517612. [DOI] [PubMed] [Google Scholar]
- 13.Lanzavecchia A, Sallusto F. Human B cell memory. Curr Opin Immunol. 2009;21:298–304. doi: 10.1016/j.coi.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly DF, Pollard AJ, Moxon ER. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. JAMA. 2005;294:3019–23. doi: 10.1001/jama.294.23.3019. [DOI] [PubMed] [Google Scholar]
- 15.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–25. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 16.Fietta P, Delsante G. The effector T helper cell triade. Riv Biol. 2009;102:61–74. [PubMed] [Google Scholar]
- 17.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 18.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol. 2010;31:377–83. doi: 10.1016/j.it.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr. 2001;160:407–13. doi: 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 22.Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan N, Almudevar A. Antibody. response to Haemophilus influenzae outer membrane protein D, P6, and OMP26 after nasopharyngeal colonization and acute otitis media in children. Vaccine. 2010;28:7184–92. doi: 10.1016/j.vaccine.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507–16. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 24.Llewelyn M, Sriskandan S, Terrazzini N, Cohen J, Altmann DM. The TCR Vbeta signature of bacterial superantigens spreads with stimulus strength. Int Immunol. 2006;18:1433–41. doi: 10.1093/intimm/dxl076. [DOI] [PubMed] [Google Scholar]
- 25.Morris PS, Leach AJ. Acute and chronic otitis media. Pediatr Clin North Am. 2009;56:1383–99. doi: 10.1016/j.pcl.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy TF, Yi K. Mechanisms of recurrent otitis media: importance of the immune response to bacterial surface antigens. Ann N Y Acad Sci. 1997;830:353–60. doi: 10.1111/j.1749-6632.1997.tb51907.x. [DOI] [PubMed] [Google Scholar]
- 27.Kodama H, Faden H, Harabuchi Y, Kataura A, Bernstein JM, Brodsky L. Cellular immune response of adenoidal and tonsillar lymphocytes to the P6 outer membrane protein of non-typeable Haemophilus influenzae and its relation to otitis media. Acta Otolaryngol. 1999;119:377–83. doi: 10.1080/00016489950181422. [DOI] [PubMed] [Google Scholar]
- 28.Mattila PS, Nykanen A, Eloranta M, Tarkkanen J. Adenoids provide a microenvironment for the generation of CD4(+), CD45RO(+), L-selectin(-), CXCR4(+), CCR5(+) T lymphocytes, a lymphocyte phenotype found in the middle ear effusion. Int Immunol. 2000;12:1235–43. doi: 10.1093/intimm/12.9.1235. [DOI] [PubMed] [Google Scholar]
- 29.Skotnicka B, Stasiak-Barmuta A, Hassmann-Poznanska E, Kasprzycka E. Lymphocyte subpopulations in middle ear effusions: flow cytometry analysis. Otol Neurotol. 2005;26:567–71. doi: 10.1097/01.mao.0000169050.61630.da. [DOI] [PubMed] [Google Scholar]
- 30.Prellner K, Harsten G, Lofgren B, Christenson B, Heldrup J. Responses to rubella, tetanus, and diphtheria vaccines in otitis-prone and non-otitis-prone children. Ann Otol Rhinol Laryngol. 1990;99:628–32. doi: 10.1177/000348949009900808. [DOI] [PubMed] [Google Scholar]
- 31.Avetisyan G, Ragnavolgyi E, Toth GT, Hassan M, Ljungman P. Cell-mediated immune responses to influenza vaccination in healthy volunteers and allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2005;36:411–5. doi: 10.1038/sj.bmt.1705064. [DOI] [PubMed] [Google Scholar]
- 32.Avigan D, Pirofski LA, Lazarus HM. Vaccination against infectious disease following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:171–83. doi: 10.1053/bbmt.2001.v7.pm11302551. [DOI] [PubMed] [Google Scholar]
- 33.Emonts M, Veenhoven RH, Wiertsema SP, et al. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics. 2007;120:814–23. doi: 10.1542/peds.2007-0524. [DOI] [PubMed] [Google Scholar]
- 34.Revai K, Patel JA, Grady JJ, Nair S, Matalon R, Chonmaitree T. Association between cytokine gene polymorphisms and risk for upper respiratory tract infection and acute otitis media. Clin Infect Dis. 2009;49:257–61. doi: 10.1086/599833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–91. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–12. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]