Abstract
(See the editorial commentary by Clancy and Hong Nguyen, on pages 499–501.)
The identification of FKS1 mutations in Candida albicans associated with echinocandin resistance has raised concerns over the spread of drug-resistant strains. We studied the impact of fks1 mutations on C. albicans virulence and fitness. Compared with wild-type strains for FKS1, echinocandin-resistant C. albicans strains with homozygous fks1 hot-spot mutations had reduced maximum catalytic capacity of their glucan synthase complexes and thicker cell walls attributable to increased cell wall chitin content. The fks1 mutants with the highest chitin contents had reduced growth rates and impaired filamentation capacities. Fks1 mutants were hypovirulent in fly and mouse models of candidiasis, and this phenotype correlated with the cell wall chitin content. In addition, we observed reduced fitness of echinocandin-resistant C. albicans in competitive mixed infection models. We conclude that fks1 mutations that confer echinocandin resistance come at fitness and virulence costs, which may limit their epidemiological and clinical impact.
Candida species are the most common fungal pathogens in humans and are responsible for a spectrum of diseases ranging from mucosal infections to life-threatening invasive candidiasis [1]. Because of the increasing incidence of azole-resistant Candida isolates, the introduction to clinical practice of the echinocandins represented an important advance in the treatment of candidiasis. Echinocandins exert their antifungal activity through noncompetitive inhibition of 1,3-β-D-glucan synthase [2], the enzyme responsible for synthesis of the glucan polymer that comprises the bulk of C. albicans cell walls. Because of their excellent activity against Candida species, including azole-resistant strains, echinocandins are currently recommended as front-line therapy for many types of candidiasis [3].
As the clinical use of echinocandins expands, Candida species isolates with reduced susceptibility to echinocandins are increasingly encountered [4–9]. Although various adaptive mechanisms may result in elevated minimal inhibitory concentrations (MICs) of echinocandins [5, 10], resistance resulting in clinical failure is associated with point mutations in 2 highly conserved hot-spot regions of the echinocandin target Fks1p [6, 7]. These mutations result in significantly elevated echinocandin MICs, log-order increases in the enzymatic kinetic inhibition constant for echinocandins, and failure of echinocandin-based treatment in murine infection models [6].
The dissemination and persistence of drug-resistant organisms in nature depends on the relative fitness of resistant and susceptible genotypes [11]. Therefore, we sought to determine the effect of fks1 mutations that confer resistance to echinocandins on C. albicans fitness and virulence.
MATERIALS AND METHODS
C. albicans Strains, Culture Conditions, and Characterization
The laboratory and clinical C. albicans strains used in this study are shown in Table 1. Three spontaneous fks1 mutants (clonal group 3) were derived from C. albicans strain SC5314 with use of a 2-step method, as described elsewhere [12, 13]. Five additional C. albicans clinical isolates (groups 1 and 2) [6] were grouped using multilocus sequence typing [14] into 2 clonal groups, each isolated from a different patient and containing an echinocandin-susceptible FKS1 strain and related fks1 mutant or mutants. Echinocandin susceptibility testing was performed by broth microdilution in triplicate in accordance with Clinical and Laboratory Standards Institute document M27-A3 [15]. FKS1 gene sequence analysis for these strains is reported elsewhere [12]. Cell disruption, membrane protein extraction, partial 1,3-β-D-glucan synthase purification by product entrapment, and enzyme kinetic characterization were performed as described elsewhere [12].
Table 1.
In Vitro Susceptibility (MIC) and Inhibition Profiles (Half-Maximal Inhibitory Concentration) of Echinocandins and Kinetic Properties of the 1,3-β-Glucan Synthase Complexes Obtained From C. albicans Isolates
| Clonal group | Strain | Origin | FKS1 hot spot genotype | MIC (μg/mL)e |
IC50 (ng/mL)f |
Fks1p kineticsg |
|||||
| ANF | CSF | MCF | ANF | CSF | MCF | Km (mM of glucose) | Vmax (nmol.min-1) | ||||
| 1 | CLY16998a | Clinical | FKS1/FKS1 (WT) | 0.06 | 0.50 | 0.03 | 1.15 ± 0.11 | 0.56 ± 0.09 | 8.90 ± 0.12 | 0.101 ± 0.011 | 5.587 ± 0.150 |
| CLY16996a | Clinical | fks1/fks1 (S645F) | 2.00 | 4.00 | 2.67 | 787.0 ± 2.8 | 459.1 ± 3.4 | 449.8 ± 5.3 | 0.020 ± 0.008 | 1.980 ± 0.098 | |
| CLY16997a | Clinical | fks1/fks1 (S645P) | 4.00 | >8.00 | 4.00 | 695.1 ± 1.5 | 162.0 ± 1.6 | 995.0 ± 8.5 | 0.124 ± 0.015 | 4.998 ± 0.144 | |
| 2 | CLY19229b | Clinical | FKS1/FKS1 (WT) | 0.12 | 0.50 | 0.06 | 1.05 ± 0.03 | 0.40 ± 0.04 | 5.30 ± 0.11 | 0.100 ± 0.005 | 5.712 ± 0.241 |
| CLY19230b | Clinical | fks1/fks1 (S645F) | 2.00 | 4.00 | 2.00 | 754.50 ± 6.8 | 420.0 ± 4.7 | 469.7 ± 3.7 | 0.081 ± 0.007 | 1.995 ± 0.099 | |
| 3 | SC5314 c | Control | FKS1/FKS1 (WT) | 0.08 | 0.42 | 0.05 | 0.89 ± 0.02 | 3.88 ± 0.09 | 58.20 ± 2.81 | 0.125 ± 0.009 | 6.717 ± 0.246 |
| A15 c,d | Lab mutant | FKS1/fks1 (S645S/P) | 0.50 | 4.00 | 0.50 | 128.70 ± 1.4 (0.39) | 218.1 ± 2.9 (4.19) | 177.3 ± 0.9 (35.5) | Not done | Not done | |
NOTE. a and b Clinical clonal strain (6). c Homozygous laboratory isogenic spontaneous mutant (12, 13). d Heterozygous fks1 mutant. e Values presented as geometric means (three repetitions on three separate days). f Values presented as arithmetic means ± standard deviations (three repetitions). IC50 values shown in parentheses represent the IC50 of the susceptible allele of the heterozygous fks1 mutant A15 obtained using a two-site competition-fitting algorithm. g Values presented as arithmetic means ± standard deviations (three repetitions).
IC50, half-maximal inhibitory concentration; ANF, anidulafungin; CSF, caspofungin; MCF, micafungin; Km, Michaelis-Menten constant; WT, wild-type.
Transmission Electron Microscopy
We performed transmission electron microscopy to compare the cell wall morphologies of echinocandin-resistant fks1 mutant C. albicans strains with clonal wild-type strains in the absence of echinocandins. Samples were processed as described elsewhere [16] and examined under a JEM 1010 transmission electron microscope (JEOL USA). Cell wall thickness was determined using ImageJ (http://rsb.info.nih.gov/ij/). For each strain, ≥10 measurements were made from different cells at ×50,000 magnification.
Semiquantitative 1,3-β-Glucan and Chitin Measurement
The relative cellular 1,3-β-glucan content of each C. albicans strain was measured using aniline blue as described elsewhere [17]. The chitin content of C. albicans strains was determined using methods described by Lehmann et al [18].
Yeast Cell Growth Rate
Optical density (OD) curves for Candida blastospores in liquid medium allow for measurement of parameters that describe the growth phenotype of each strain and suggest alterations in fitness [19]. The OD at 600 nm (OD600) of blastospore suspensions (1 × 104 cells/mL in yeast extract peptone dextrose) was measured every 15 min for 24 h with use of a plate reader (PowerWave HT; BioTek) at 30°C. OD curves were fitted using a logistic growth-rate formula: Y = Ymax/(1+e−k(X−Xmax)), where Ymax is the maximal OD600, Xmax is the time point at which the growth rate is maximal (lag time), and k is a growth-rate constant.
Filamentation Assays
Inoculation of stationary C. albicans blastospores into fresh medium is a potent stimulus for hyphal growth [20]. We determined the efficiency of yeast-to-hyphal transition of matched echinocandin-resistant and -susceptible C. albicans isolates. Single colonies grown on yeast extract agar glucose (YAG) were selected and lightly inoculated in 10 mL of YPD broth. The resulting suspensions were incubated overnight at 30°C with shaking. Next, blastospores were transferred to YPD broth (OD600 adjusted to 0.5) and incubated at 37°C with shaking. Germ tube formation was determined at various time points in samples of at least 200 cells.
Virulence Testing in Toll-Deficient Drosophila melanogaster Flies
We used a high-throughput model of invasive candidiasis in Toll-deficient D. melanogaster flies to screen for altered virulence in fks1 mutants [21]. C. albicans inoculum suspensions were prepared at 1 × 108 cells/mL. Female flies aged 2–4 days were anesthetized using carbon dioxide in groups of 25 and inoculated by insertion of a 0.25-mm needle previously dipped in the inoculum suspension. After inoculation, the flies were housed at 29°C to maximize expression of the Tlr632 phenotype [22] and were transferred to fresh vials every 3 days. Fly survival was assessed daily for 7 days. All experiments were performed at least in triplicate on different days but at the same time of day (±2 hours) to prevent circadian variation in susceptibility to infection.
Murine Model of Hematogenously Disseminated Candidiasis
Based on survival rate differences in the fly model, we further tested 3 strains in a murine model of disseminated candidiasis: CLY16996 (fks1-S645F), CLY16997 (fks1-S645P), and the clonal FKS1 wild-type strain CLY16998. Procedures were performed in accordance with applicable laws and regulations and were approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee. C. albicans strains were grown in YPD broth as described above, and an inoculum suspension of each strain was prepared at 1 × 106 cells/mL. Eight-week-old female BALB/c mice weighing 20–22 g (National Cancer Institute) were inoculated in groups of 10 with 5 × 105 blastospores by lateral tail vein injection. Survival was assessed twice daily for 30 days. Mice that appeared moribund were killed using carbon dioxide–induced asphyxiation, and their death was recorded as having occurred 12 hours later.
In other experiments, mice were inoculated in groups of 5 as described above and were killed 48 hours later for assessment of fungal burden. One kidney and the spleen were removed aseptically from each animal, weighed, and homogenized in 1 mL of sterile normal saline. Serial 10-fold dilutions of the homogenates were spread on YAG plates, and colony-forming units (CFU)/g tissue was determined from the colony count after 48 hours incubation at 30°C. All mouse experiments were performed in triplicate at different times.
Macrophage Inflammatory Response
To test whether differences in mouse survival rates were related to differential host inflammatory responses, we used a modification of the methods of Wheeler et al [23]. In brief, blastospore suspension (5 × 107 cells/mL) was dispensed in flat-bottom microtiter wells (100 μL/well) and inactivated with UV radiation. Plates were centrifuged at 4000 g, and murine RAW264.7 macrophages with a secreted alkaline phosphatase (SEAP) reporter construct indicative of NF-kB/AP-1 transcription factor activity (Invivogen) were added at a ratio of 10:1 (target:effector). After 6 hours incubation at 37°C, supernatants were collected and assayed for SEAP reporter with use of Quanti-Blue assay (Invivogen). For specific experiments, 10 μg/mL anti–Dectin-1 or isotype control antibody (Invivogen) was added to macrophages 30 minutes before coculture with fungal cells. All experiments were performed in 3 replicates on at least 3 separate days.
Determination of C. albicans Strain Fitness in Competition Models
To determine the fitness cost of C. albicans fks1 mutations, we compared the relative fitness of CLY19229 (wild-type) with CLY19230 (fks1-S645F) strains with similar growth rates but modest differences in virulence in the Toll-deficient D. melanogaster model (Figure 1; online only). Relative fitness was measured in vitro using the methods of Cowen et al [24]. Competition experiments were performed in triplicate in RPMI 1640 at 35°C with an initial inoculum of 1 × 105 CFU/mL of each strain. Strains were allowed to grow together for one standard daily growth cycle, and the growth of each population was calculated from initial and final densities.
Furthermore, we measured the relative fitness of the same strain pair in vivo using murine models of single- and dual-strain infections. For single-strain infections, 6 mice were infected with equal inocula (1 × 106 CFU/mouse) of CLY19229 and CLY19230 via tail-vein injection. Similarly, for dual-strain infections, 6 mice were inoculated with a mixed inoculum of the wild-type and mutant strains (0.5 × 106 CFU/mouse of each strain for a final mixed inoculum of 1 × 106 CFU/mouse). On day 4 after injection, the mice were killed and DNA was extracted from their excised kidneys [25].
Molecular beacon probes and primers were designed on the basis of the C. albicans gene FKS1 sequence (GenBank accession number XM_716336) (Table 1; online only). Multiplex molecular beacon quantitative real-time polymerase chain reaction (PCR) experiments were performed to detect and quantify the different C. albicans strains in each mouse. Cycle threshold values obtained using kidney samples were extrapolated for C. albicans DNA quantification. The detection sensitivity and specificity of the multiplex real time PCR assay were assessed using DNA extracted from uninfected mouse kidneys spiked with log dilutions of C. albicans genomic DNA (mutant and wild-type). With these data, C. albicans genomic equivalents were obtained from the mixed challenge experiments.
Statistical Analysis
Continuous variables were compared using 1-sided analysis of variance or the nonparametric Kruskal-Wallis test, as appropriate. Survival curves were plotted using the Kaplan-Meier method, and the survival rates in the different infection groups were compared using the log-rank test. Linear correlation analyses were performed using Pearson’s correlation coefficient, and nonlinear correlations were calculated using the Spearman rank correlation coefficient. Two-tailed P values <.05 were considered to be statistically significant.
RESULTS
C. albicans Fks1p Hot-Spot Amino Acid Substitutions Alter Enzyme Kinetics
The geometric mean echinocandin MICs were 8–53-fold higher for C. albicans strains carrying homozygous fks1 hot-spot mutations (fks1/fks1), compared with the matched FKS1 wild-type strains (Table 1). Analysis of the kinetic properties of 1,3-β-D-glucan synthase complexes showed that fks1 mutations were associated with a 51% (range, 10%–65%) median decrease in the maximum catalytic velocity (Table 1). In contrast, we observed no statistically significant differences in the Michaelis-Menten constants of wild-type and mutant 1,3-β-D-glucan synthase complexes. These results are in accordance with previous reports by our group [12].
Fks1 Mutants Have Thickened Cell Walls and Increased Chitin Content
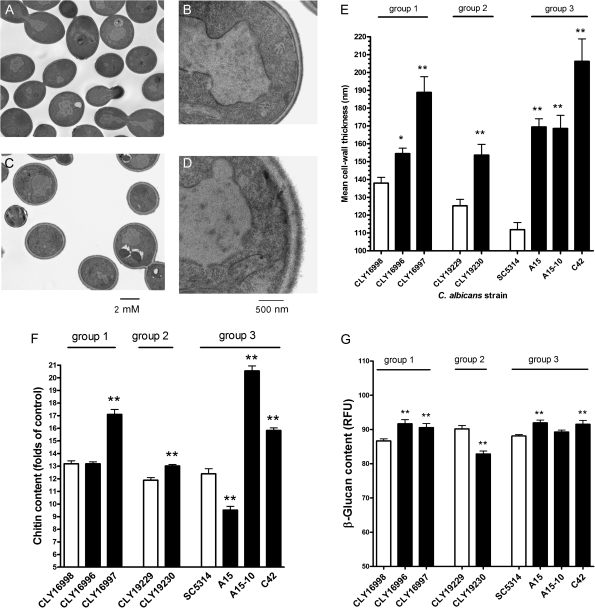
Cell wall thickness, as determined using transmission electron microscopy, was significantly greater in fks1 mutant C. albicans strains than in the clonal wild-type strains (median increase, 44%; range, 8%–84%; P < .0001) (Figure 1). Analysis of cell wall polysaccharides revealed that chitin content was significantly greater in 4 of the 5 homozygous fks1 mutants, compared with the clonal FKS1 wild-type strains (median increase, 28%; range, 9%–65%; P < .0001) (Figure 1). However, the relative chitin content of the fks1/FKS1 strain A-15 was 23% lower than in the clonal FKS1 wild-type (P < .0001). In comparison, the relative 1,3-β-glucan content differed only slightly in FKS1 wild-type and fks1 mutant strains: it was significantly elevated by 3%–7% in 4 of the 6 echinocandin-resistant strains and was significantly reduced by 8% in 1 strain (Figure 1).
Figure 1.
C. albicans fks1 mutants have thickened cell walls and increased chitin content. Transmission electron microscopic images of (A and B) FKS1 wild-type C. albicans strain SC5314 and the (C and D) clonal fks1 mutant C42 (F641S) showing the thickened cell walls of the mutant strains (A and C, ×5000 magnification; B and D, ×50,000 magnification). (E) Mean cell wall thicknesses in all of the wild-type and mutant strains. (F) The chitin content of the fks1 mutants was significantly higher than that of the clonal wild-type strains. (G) Minor differences in 1,3-β-glucan content among the different strains were noted. Group designation refers to clonal groups described in Table 1. Open bars: FKS1 wild-type strains; Black bars: fks1 mutants. Statistical significance determined using one-sided ANOVA with post hoc Dunn’s test (*P < .05; **P < .01). RFU, relative fluorescence units.
Growth Rate and Filamentation Are Impaired in fks1 Mutants
Three of 5 fks1/fks1 mutants had growth rates lower than those of the clonal wild-type strains, whereas the fks1/FKS1 strain A15 had a growth rate similar to that of the clonal FKS1 wild-type strain. (Figure 1A; online only; Table 2; online only). In addition, 2 fks1/fks1 mutants (C42 and A15-10), both of which had impaired growth, exhibited defects in filamentation in response to medium dilution (Figure 1B; online only). Of interest, the fks1 mutants with impaired growth and morphotype-transition characteristics were those with the highest relative cellular chitin content (Figure 1). Conversely, the fks1/FKS1 strain A15 formed filaments in excess of the clonal wild-type strain (Figure 1B; online only).
Homozygous fks1 Mutants Are Hypovirulent in Toll-Deficient D. melanogaster
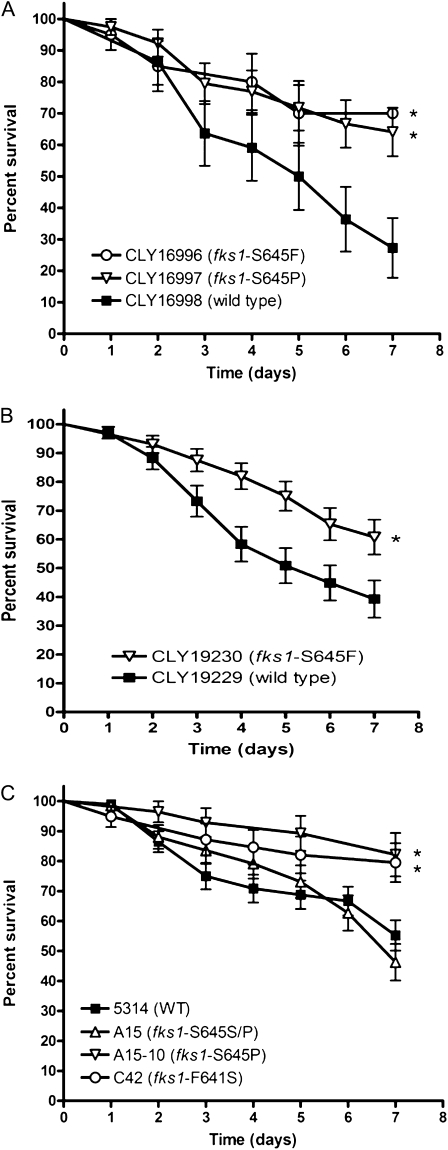
Homozygous fks1 mutants of C. albicans were significantly less lethal in Toll-deficient flies, compared with the matched FKS1 wild-type strains and the heterozygous fks1/FKS1 strain (Figure 2). Specifically, 7-day survival rates in Toll-deficient flies infected with fks1/fks1 strains were ≥60% in all cases (median, 70%; range, 60%–82%). In contrast, median 7-day survival rates (±standard deviation) were 27.2% ± 9.4%, 39.2% ± 6.4%, and 55.2% ± 5.0% for FKS1 wild-type strains CLY16998, CLY19230, and SC5314, respectively, and 46.2% ± 6.0% for the fks1/FKS1 strain A15 (P < .05, by log-rank test) (Figure 2). We found a significant inverse correlation between the echinocandin MICs and corresponding 7-day survival rates in Toll-deficient flies, because the fks1 mutants with the highest echinocandin MICs were those with the lowest virulence (Figure 2; online only).
Figure 2.
C. albicans fks1 mutants have attenuated virulence in Toll-deficient D. melanogaster flies. We used an invertebrate model of invasive candidiasis in Toll-deficient D. melanogaster flies to screen for variations in virulence among echinocandin-resistant and -susceptible Candida isolates. (A–C) Kaplan-Meier survival curves for three clonal groups of C. albicans strains. *P < .05; log-rank test.
Fks1 Mutants Are Hypovirulent in a Murine Model of Hematogenous Candidiasis
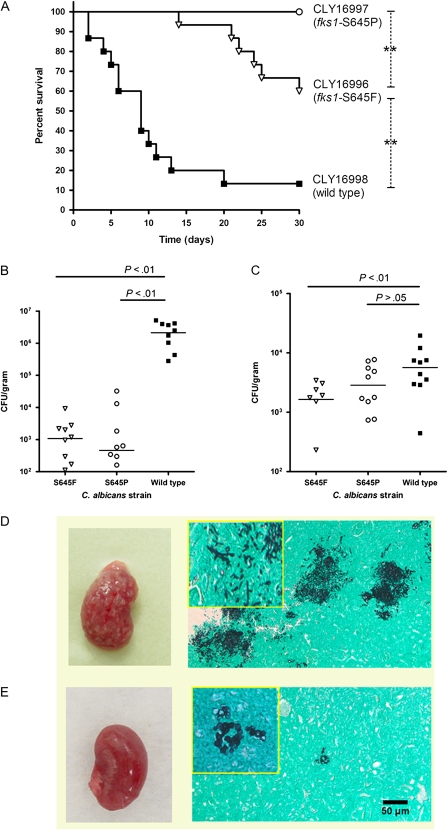
To confirm the attenuated virulence of fks1 mutants, we further tested the FKS1 wild-type isolate CLY16998 and 2 clonal fks1 mutants, CLY16996 and CLY16997, in a murine model of disseminated candidiasis. Consistent with our observations in the fly model, murine infection experiments revealed attenuated virulence of the fks1 mutant strains. Specifically, the mean 30-day survival rate among mice infected with CLY16998 was 13.3%, whereas those in mice infected with CLY16996 and CLY16997 were 60% and 100%, respectively (P < .0001; Figure 3). Mice infected with fks1 mutants had lower tissue fungal burdens in their kidneys (3 and 4 logs lower than the wild-type; P < .0001) and spleens (2-fold lower than the wild-type; P = .01) (Figure 3). Examination of Grocott-Gomori methenamine-silver nitrate stained murine tissue samples revealed sparse fungal elements in kidney samples obtained from fks1 mutant–infected mice. Moreover, whereas we observed extensive proliferation of hyphae in kidney samples from FKS1 wild-type–infected mice, only yeast forms were seen in kidney tissue samples from fks1 mutant–infected mice (Figure 3).
Figure 3.
C. albicans fks1 mutants have attenuated virulence in a murine model of hematogenously disseminated candidiasis. The virulence of a FKS1 wild-type C. albicans strain and two clonal fks1 mutant strains was assessed in nonimmunosuppressed BALB/c mice after intravenous injection of blastospores. (A) Kaplan Meier survival curves of 2 fks1 mutants and the clonal wild-type strain (log rank test P < .0001 for comparison among all 3 strains; dashed lines show intergroup comparisons; **P < .01). Tissue fungal burden in the (B) kidneys and (C) spleen 48 hours after inoculation (horizontal lines represent median values). One-sided ANOVA P values were <0.0001 for the kidney fungal burdens and 0.01 for the spleen fungal burdens. P values in the figure represent post hoc comparisons between wild-type and fks1 mutant fungal burdens. (D and E) Macroscopic appearance of kidneys (left) and findings of histopathologic examination of GMS-stained kidney tissue sections (right) are shown for (D) the wild-type strain and (E) an fks1 mutant (×100 magnification; inset, ×400 magnification). Whereas multiple fungal abscesses were observed on the surface of kidneys excised from wild-type–infected mice, those excised from fks1 mutant-infected mice appeared smooth. Moreover, in contrast with the extensive hyphal growth observed in wild-type–infected kidney tissue, fungal elements were rare in fks1 mutant-infected kidneys, and only yeast cells were observed.
Competitive Assays Reveal a Fitness Cost of fks1 Mutation
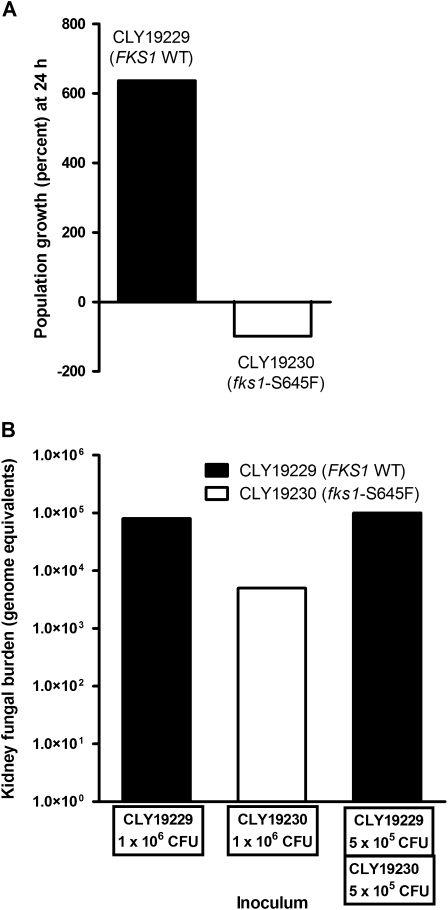
We assessed the relative fitness of CLY19229 (FKS1-WT) and the clonal fks1 mutant CLY19230 (fks1-S645F) in a competitive in vitro growth assay and in a murine model of mixed infection. After 24 hours of coculture in RPMI, the CLY19229 population increased by 636 ± 0.02% whereas the CLY19230 population decreased by 98 ± 0.6%, indicating lower relative fitness of the fks1 mutant. In single strain murine infection experiments, the median kidney fungal burden was >1 log lower in kidneys from fks1 mutant–infected mice than in kidneys from FKS1 wild-type–infected mice (P = .3) (Figure 4). In mixed challenge experiments, the kidney fungal burdens were similar to those produced by single strain infections (Figure 4). However, the C. albicans DNA present in infected kidneys belonged only to the FKS1 wild-type strain in 5 of the 6 mice studied. The only mouse in which DNA belonging to the mutant strain was detected had a kidney fks1 mutant DNA burden (90 genome equivalents) 3 logs lower than that of the FKS1 wild-type DNA (1 × 105 genome equivalents), confirming the attenuated fitness of the mutant strain (Figure 4).
Figure 4.
Competition experiments reveal reduced fitness of an fks1 mutant relative to the matched FKS1 wild-type strain. The relative fitness of 2 matched C. albicans strains, CLY19229 (FKS1 WT) and CLY19230 (fks1 S645F), was determined in competition experiments (A) in vitro and (B) in vivo. (A) Bars represent the growth of each strain in a mixed population during a single daily cycle. (B) Bars represent arithmetic means of six experiments performed using DNA extracted from 6 individual mouse kidneys infected with equal inoculums (106 CFU/mouse) of the fks1 mutant and the wild-type strain via tail injection. All mice were euthanized on day +4 post infection. Ct values and genome equivalents are beacon-specific. The value shown for the mixed infection experiment represents the signal for the FKS1 wild-type molecular beacon. Only one of the 6 dually infected mice showed a positive signal for the fks1 mutant molecular beacon.
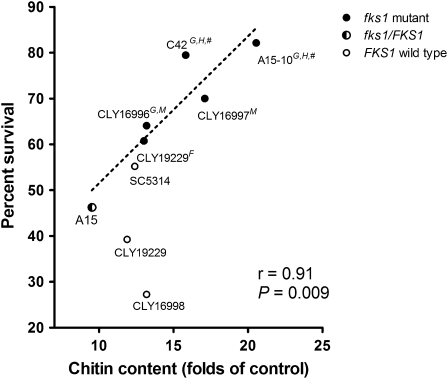
Cellular Chitin Content Is Inversely Correlated With Fks1 Mutant Virulence
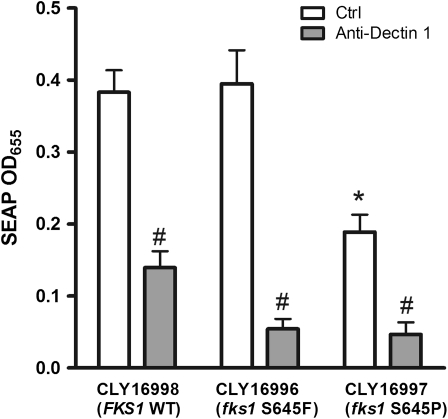
We hypothesized that formation of thickened, chitin-rich cell walls underlies the attenuated virulence of fks1 mutants. We therefore examined the correlation between the chitin content in fks1 mutant C. albicans strains and survival rates in Toll-deficient D. melanogaster flies inoculated with these isolates. We found a significant linear correlation between the fly survival rates and the fks1 mutant cellular chitin contents (correlation coefficient, r = 0.91; P = .009) (Figure 5). Cellular chitin content was also associated with attenuated virulence in the murine disseminated candidiasis model. Thus, strain CLY16997 (fks1-S645P), which had 37% greater cell wall thickness and 30% greater chitin content than those of the matched wild-type strain was avirulent in mice (Figures 1 and 3). In contrast, strain CLY16996 (fks1-S645F), which displayed a lesser degree of cell wall thickening (8% greater than the wild-type) and chitin content not significantly different from that of the wild-type strain, was hypovirulent relative to the wild-type strain (P = .0001) but more virulent than CLY16997 (P = .007). Of interest, CLY16997 was also associated with a significantly dampened inflammatory response in cocultured RAW264.7 macrophages (Figure 6). In contrast, CLY16996 induced a robust proinflammatory response similar to that observed with the FKS1 wild-type strain (Figure 6). All inflammatory responses were abrogated with anti–Dectin-1, indicating dependence on β-glucan ligation.
Figure 5.
Growth defects and attenuated virulence of C. albicans fks1 mutants correlate strongly with cell wall chitin content. A significant linear correlation was observed between the survival rates in Toll-deficient D. melanogaster flies infected with fks1 mutant C. albicans strains and the chitin content of those strains (r = 0.91; P = .009). In addition, defects in growth rate and morphotype-switching capacity were detected in the fks1 mutant strains with the highest chitin content. G, impaired growth rate; H, impaired transition to hyphal growth; F, reduced fitness in the mixed infection model; M, reduced virulence in the mouse model; #, virulence not assessed in the mouse model.
Figure 6.
Inflammatory response to FKS1 wild-type and mutant C. albicans strains. The inflammatory response to 3 C. albicans was assessed using the murine macrophage RAW264.7 cell line containing a secreted alkaline phosphatase (SEAP) reporter construct indicative of NF-kB/AP-1 transcription factor activity (see methods). Both CLY16998 (FKS1-WT) and CLY16996 (fks1-S645F not associated with increased cell-wall chitin content) induced a robust inflammatory response. In contrast, CLY16997 (fks1-S645P), a mutant with markedly increased cell-wall chitin content (Figure 1), induced less than 50% of SEAP levels of the wild-type strain, indicating a dampened inflammatory response. All responses were reversed by anti-Dectin-1 antibodies. Values represent the means of 3 separate experiments. *P < .05; one-sided ANOVA for comparison among controls. #P < .01; Students’s t-test for comparison between control and anti-Dectin-1 pretreated cells.
DISCUSSION
Echinocandins are currently the drugs of choice for treatment of invasive candidiasis in critically ill patients [3]. Therefore, reports of the emergence of echinocandin-resistant Candida isolates are of concern. Although researchers have elucidated the mechanism of echinocandin resistance in fks1 C. albicans mutants, the epidemiological and clinical consequences of these mutations are unknown. Because horizontal transfer of drug-resistance determinants does not readily occur in fungi, the spread of drug resistance in the population depends on the relative fitness of resistant strains [11]. We undertook the present study to characterize the impact of fks1 mutations on the fitness and virulence of C. albicans.
Homozygous fks1 C. albicans mutants had thicker cell walls and greater chitin content, compared with clonal FKS1 wild-type strains. All 5 homozygous fks1 mutants had attenuated virulence in Toll-deficient D. melanogaster flies. Moreover, we found an inverse linear correlation between the cell wall chitin contents of fks1 mutants and their lethality in Toll-deficient flies. Further assessment of 2 fks1 mutants revealed significantly higher survival rates and lower tissue fungal burdens than those for a clonal wild-type strain in a murine model of disseminated candidiasis. Finally, wild-type C. albicans strongly out-competed the clonal mutant fks1 strain in an in vitro competitive growth system and a murine mixed infection model despite similar growth rates of both strains, confirming the fitness cost of fks1 hot-spot mutations.
Exposure of C. albicans to echinocandins triggers cell wall salvage pathways, including the high-osmolarity glycerol mitogen-activated protein kinase, Ca+2/calcineurin, and protein kinase C pathways [26, 27]. Activation of these signaling pathways results in upregulation of chitin synthase gene expression, increased chitin synthase activity, and elevated cell wall chitin content [26, 27]. In addition, increased cell wall chitin content was shown to protect C. albicans against echinocandins [26]. Increased cellular chitin was detected in an echinocandin-resistant fks1 mutant C. albicans strain [26] and in echinocandin-resistant non-fks1 C. albicans mutants [10]. Moreover, chitin overproduction in C. albicans accounts for paradoxical growth in the presence of high echinocandin concentrations [28]. Thus, increased chitin synthesis is a recurring theme of echinocandin resistance in C. albicans.
Taken together, our results show that the presence of thick, chitin-rich cell walls in C. albicans fks1 mutants is associated with reduced virulence and fitness. The chitinous cell wall may be less amenable to remodeling, as evidenced in the impaired growth rates and morphotype switching capacities of the fks1 mutants with the highest chitin contents. Filamentation is a critical determinant of C. albicans resistance to phagocytosis [29]. Moreover, the cell wall is the interface through which Candida interacts with host immune effector cells. In the present study, increased cell wall chitin content was associated with an attenuated Dectin-1–mediated inflammatory response, suggesting that salvage chitin may act as an antiinflammatory signal. Indeed, chitin molecules have diverse immunoregulatory functions, depending on chitin fragment length [30] and interaction with fungal proteins [31]. Lastly, down-regulation of proinflammatory responses by salvage chitin may account for the attenuation of tissue damage and mortality from progressive sepsis in the murine model of disseminated candidiasis [32]. Of note, the FKS1/fks1 strain A15 (S645S/P) exhibited normal growth, formed hyphae in excess of the clonal wild-type strain, and had preserved virulence in Toll-deficient flies, suggesting that a single fks1 allele, which does not entail increased cellular chitin synthesis, is insufficient to compromise C. albicans virulence.
Our study has certain limitations. Previous studies of fluconazole resistance in C. albicans showed that, even when resistance was caused by a point mutation, it was accompanied by complex genome-wide changes in gene expression [33–35]. Thus, differential expression of undefined genes not related to cell wall structure may alter the fitness of fks1 mutants. Moreover, acquisition of additional mutations over successive generations can offset initial changes in the fitness of C. albicans [33]. However, we found a strong correlation between the fks1 genotype and reduced fitness and virulence in a wide range of matched clinical and laboratory C. albicans isolates of different genetic backgrounds. It is highly unlikely that the same or similar non-fks mutations were responsible for attenuated virulence in all of these strains. Moreover, the attenuated virulence of the clinical fks1 mutants suggests that these phenotypic changes remain stable in the host environment.
The attenuated fitness of echinocandin-resistant fks1 mutant C. albicans strains suggests that these isolates are at an evolutionary disadvantage in the absence of echinocandin exposure and are therefore predicted to have limited potential for spread in the population. This conclusion is consistent with data showing a low prevalence of fks1 hot-spot mutations in a large, geographically diverse collection of Candida species isolates, despite the increased use of echinocandins [36]. Moreover, our findings may provide a biological basis for the seemingly paradoxical results of studies showing better treatment outcomes in patients infected with Candida isolates having elevated caspofungin MICs (>2 μg/mL) than in those infected with caspofungin-susceptible isolates [37]. Finally, our results underscore the importance of cell wall integrity and plasticity for adaptation of C. albicans to its host environment and, thus, its capacity for dissemination and lethal infection.
Funding
This work was supported by the U. S. Investigator-Initiated Studies Program of Merck (to D. P. K.), the M. D. Anderson Cancer Center Faculty E. N. Cobb Scholar Award Research Endowment (to D. P. K.), the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant (CA016672), and National Institutes of Health (AI069397), and Pfizer (to D. S. P.).
Acknowledgments
We thank Nathan Albert, for excellent technical assistance, and Kenneth Dunner Jr, for assistance with transmission electron microscopy.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–51. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katiyar S, Pfaller M, Edlind T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2006;50:2892–4. doi: 10.1128/AAC.00349-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtz MB, Abruzzo G, Flattery A, et al. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect Immun. 1996;64:3244–51. doi: 10.1128/iai.64.8.3244-3251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49:3264–73. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat. 2007;10:121–30. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laverdiere M, Lalonde RG, Baril JG, Sheppard DC, Park S, Perlin DS. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother. 2006;57:705–8. doi: 10.1093/jac/dkl022. [DOI] [PubMed] [Google Scholar]
- 9.Miller CD, Lomaestro BW, Park S, Perlin DS. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy. 2006;26:877–80. doi: 10.1592/phco.26.6.877. [DOI] [PubMed] [Google Scholar]
- 10.Plaine A, Walker L, Da CG, et al. Functional analysis of Candida albicans GPI-anchored proteins: roles in cell wall integrity and caspofungin sensitivity. Fungal Genet Biol. 2008;45:1404–14. doi: 10.1016/j.fgb.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JB. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat Rev Microbiol. 2005;3:547–56. doi: 10.1038/nrmicro1179. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother. 2009;53:112–22. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balashov SV, Park S, Perlin DS. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother. 2006;50:2058–63. doi: 10.1128/AAC.01653-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robles JC, Koreen L, Park S, Perlin DS. Multilocus sequence typing is a reliable alternative method to DNA fingerprinting for discriminating among strains of Candida albicans. J Clin Microbiol. 2004;42:2480–8. doi: 10.1128/JCM.42.6.2480-2488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. Wayne, PA: National Committee for Clinical Laboratory Standards,; 2008. Reference method for broth dilution antifungal susceptibility testing of Yeasts. Approved Standard M27–A3. [Google Scholar]
- 16.Ben-Ami R, Lewis RE, Tarrand J, Leventakos K, Kontoyiannis DP. Antifungal activity of colistin against mucorales species in vitro and in a murine model of Rhizopus oryzae pulmonary infection. Antimicrob Agents Chemother. 2010;54:484–90. doi: 10.1128/AAC.00956-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekiya-Kawasaki M, Abe M, Saka A, et al. Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genetics. 2002;162:663–76. doi: 10.1093/genetics/162.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann PF, White LO. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect Immun. 1975;12:987–92. doi: 10.1128/iai.12.5.987-992.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toussaint M, Conconi A. High-throughput and sensitive assay to measure yeast cell growth: a bench protocol for testing genotoxic agents. Nat Protoc. 2006;1:1922–8. doi: 10.1038/nprot.2006.304. [DOI] [PubMed] [Google Scholar]
- 20.Enjalbert B, Whiteway M. Release from quorum-sensing molecules triggers hyphal formation during Candida albicans resumption of growth. Eukaryot Cell. 2005;4:1203–10. doi: 10.1128/EC.4.7.1203-1210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamilos G, Lionakis MS, Lewis RE, et al. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 2006;193:1014–22. doi: 10.1086/500950. [DOI] [PubMed] [Google Scholar]
- 22.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006;2:e35. doi: 10.1371/journal.ppat.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowen LE, Kohn LM, Anderson JB. Divergence in fitness and evolution of drug resistance in experimental populations of Candida albicans. J Bacteriol. 2001;183:2971–8. doi: 10.1128/JB.183.10.2971-2978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riggsby WS, Torres-Bauza LJ, Wills JW, Townes TM. DNA content, kinetic complexity, and the ploidy question in Candida albicans. Mol Cell Biol. 1982;2:853–62. doi: 10.1128/mcb.2.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008;4:e1000040. doi: 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munro CA, Selvaggini S, de Bruijn I, et al. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol Microbiol. 2007;63:1399–413. doi: 10.1111/j.1365-2958.2007.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. Escape of from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob Agents Chemother. 2006;50:3160–1. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie CG, Koser U, Lewis LE, et al. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun. 2010;78:1650–8. doi: 10.1128/IAI.00001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol. 2009;182:3573–82. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- 31.de Jong R, Thomma BP. Fungal LysM effectors: extinguishers of host immunity? Trends Microbiol. 2009;17:151–7. doi: 10.1016/j.tim.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Spellberg B, Ibrahim AS, Edwards JE, Jr., Filler SG. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis. 2005;192:336–43. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 33.Cowen LE, Nantel A, Whiteway MS, et al. Population genomics of drug resistance in Candida albicans. Proc Natl Acad Sci U S A. 2002;99:9284–9. doi: 10.1073/pnas.102291099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeRisi J, van den HB, Marc P, et al. Genome microarray analysis of transcriptional activation in multidrug resistance yeast mutants. FEBS Lett. 2000;470:156–60. doi: 10.1016/s0014-5793(00)01294-1. [DOI] [PubMed] [Google Scholar]
- 35.Rogers PD, Barker KS. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob Agents Chemother. 2003;47:1220–7. doi: 10.1128/AAC.47.4.1220-1227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castanheira M, Woosley LN, Diekema DJ, Messer SA, Jones RN, Pfaller MA. Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob Agents Chemother. 2010;54:2655–9. doi: 10.1128/AAC.01711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kartsonis N, Killar J, Mixson L, et al. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob Agents Chemother. 2005;49:3616–23. doi: 10.1128/AAC.49.9.3616-3623.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]