Abstract
The NMDA glutamate hypofunction model of schizophrenia is based in part upon acute effects of NMDA receptor blockade in humans and rodents. Several laboratories have reported glutamate system abnormalities following prenatal exposure to immune challenge, a known environmental risk factor for schizophrenia. Here we report indices of NMDA glutamate receptor hypofunction following prenatal immune activation, as well as the effects of treatment during periadolescence with the atypical antipsychotic medications risperidone and paliperidone. Pregnant Sprague-Dawley rats were injected with polyinosinic:polycytidylic acid (poly I:C) or saline on gestational day 14. Male offspring were treated orally via drinking water with vehicle, risperidone (0.01 mg/kg/day), or paliperidone (0.01 mg/kg/day) between postnatal days 35 and 56 (periadolescence) and extracellular glutamate levels in the prefrontal cortex were determined by microdialysis at PD 56. Consistent with decreased NMDA receptor function, MK-801 – induced increases in extracellular glutamate concentration were markedly blunted following prenatal immune activation. Further suggesting NMDA receptor hypofunction, prefrontal cortex basal extracellular glutamate was significantly elevated (P<0.05) in offspring of Poly I:C treated dams. Pretreatment with low dose paliperidone or risperidone (0.01 mg/kg/day postnatal days 35–56) normalized prefrontal cortical basal extracellular glutamate (P<0.05 vs. poly I:C vehicle-treatment). Pretreatment with paliperidone and risperidone also prevented the acute MK-801-induced increase in extracellular glutamate. These observations demonstrate decreased NMDA receptor function and elevated extracellular glutamate, two key features of the NMDA glutamate receptor hypofunction model of schizophrenia, during periadolescence following prenatal immune activation. Treatment with the atypical antipsychotic medications paliperidone and risperidone normalized basal extracellular glutamate. Demonstration of glutamatergic abnormalities consistent with the NMDA glutamate receptor hypofunction model of schizophrenia as an early developmental consequence of prenatal immune activation provides a model to identify novel early interventions targeting glutamatergic systems which play an important role in both positive and negative symptoms of schizophrenia.
Keywords: maternal, hippocampus, schizophrenia, animal model, prodrome, antipsychotic, NMDA receptor
Introduction
The NMDA glutamate receptor hypofunction model of schizophrenia has been a guiding paradigm in the schizophrenia research field for the past two decades. This model posits that lessened NMDA-subtype glutamate receptor activity in schizophrenia results in decreased stimulation of GABAergic inhibitory neurons in cortex and/or hippocampus. The loss of GABAergic activity thereby releases glutamatergic efferent neurons from inhibition, resulting in elevated glutamate release in the prefrontal cortex [13;15;19]. NMDA receptor hypofunction may account for both positive and negative symptoms of schizophrenia [4].
While several treatment trials targeting NMDA hypoactivity in schizophrenia have been promising [12;17], larger double-blind clinical trials, as well as meta-analyses of smaller studies, have not identified consistent beneficial effects in clinical trials of chronic patients with longstanding illness [39]. Earlier intervention with glutamatergic agents may hold greater therapeutic promise [36;42]. It is therefore of interest to identify NMDA receptor hypoactivity in an animal model of relevance to schizophrenia, and to determine the effect of early intervention on glutamatergic indices.
Prenatal immune activation provides one such developmental model of relevance to schizophrenia, based upon elevated schizophrenia risk following prenatal infection [3]. Prenatal infection stimulates maternal cytokines, soluble polypeptides mediating the innate inflammatory response. Consequences to the offspring of maternal cytokine elevation have been studied in prenatal immune activation animal models using immunogens including the synthetic nucleic acid poly I:C, which stimulates cytokine expression through Toll-like receptor TLR3 activation [37]. When combined with the appropriate host genetic background [6], prenatal immune activation is a suspected environmental risk factor for schizophrenia [28]. Several studies identified altered glutamate system function following early developmental immune activation [2;11;16], suggesting an opportunity to characterize the effects of early intervention on NMDA receptor hypofunction utilizing the prenatal immune activation model. Here we report the effects of periadolescent treatment with risperidone and paliperidone on prefrontal cortical glutamate following prenatal immune activation with poly I:C.
Materials and Methods
Eight-week old nulliparous female Sprague-Dawley rats used as breeders (total n = 52 dams) were obtained from Harlan Laboratories (Indianapolis, IN). Male breeders were generated within the facility. Following a minimum two week acclimation, males and females were co-housed overnight, and the following morning defined as gestational day 0 [38]. Pregnant rats were injected with poly I:C (Sigma P1530; 8 mg/kg i.p., n = 27) or saline (1 ml/kg i.p., n = 25) on gestational day 14, in order to stimulate a maternal inflammatory response. Poly I:C injection timing was based upon work of Zuckerman and colleagues describing outcomes following poly I:C injection on varying gestational dates in rats ([43]; Zuckerman gestational day 15 = [38] gestational day 14). Based on a previous study describing weight loss associated with maternal immune activation [9], pregnant dams were weighed on gestational day 15 [2]; offspring of poly I:C-injected dams without weight loss were excluded from study. Poly I:C-treated dams with offspring included in the study (n = 43) lost an average of 4 grams during the 24 hours following injection. Saline-treated dams with offspring included in the study (n = 63) gained an average of 6.8 grams during the same period.
Litters were culled to 8 on P1, weaned on postnatal day (PD) 21 and housed 2–3 per cage with same sex siblings in a temperature- and humidity-controlled room with 12-h light/dark cycle (0600 on:1800 off) and allowed food and water ad libitum. Male pups were randomly assigned among six treatment groups (minimum 8 rats/group) with variables of pretreatment (poly I:C vs. saline); and drug [risperidone (0.01 mg/kg/day), paliperidone (0.01 mg/kg/day) or vehicle]. Treatment groups were balanced across breeding cohorts, with no more than 2 rats/litter in any experimental group to avoid litter effect confounds. Animals undergoing surgery were single-housed postoperatively. Microdialysis was performed between PD 55–58.
Drugs and Drug Treatment
(+)-MK-801 hydrogen maleate and polyinosinic:polycytidylic acid (PolyI:C) were from Sigma-Aldrich (St. Louis, MO). Drugs were dissolved in 0.15 M NaCl. The NMDA receptor antagonist MK-801 dose was 0.3 mg/kg s.c. The atypical antipsychotic medications risperidone (oral solution) and paliperidone (powder) were from Janssen. Rats were treated with risperidone (0.01 mg/kg/day), paliperidone (0.01 mg/kg/day) or vehicle via drinking water from PD 34–35 until day of microdialysis (PD 55–58). Risperidone and paliperidone dosages were selected to be similar to commonly prescribed human oral dosages of 0.5 mg/day to a 50 kg adolescent.
All animal procedures were conducted in agreement with the Guide for the Care and Use of Laboratory Animals in accordance with NIH guidelines, and approved by University of Cincinnati and Cincinnati VA Medical Center Institutional Animal Care and Use Committees.
In vivo microdialysis
Rats were anesthetized with ketamine/xylazine (70:6 mg/kg, IM) 3 days prior to microdialysis for implantation of guide cannulae into the skull dorsal to prefrontal cortex. The afternoon of the day preceding dialysis, concentric style microdialysis probes (3 mm active membrane length) were inserted into medial prefrontal cortex (AP: 3.2 mm; L: 0.8 mm; V: −4.0 mm, according to Paxinos and Watson [29]). Probes were connected to a pump delivering Dulbecco’s PBS containing 1.2 mM CaCl2 and 0.5 mM glucose via infusion pump (rate 1.2 uL/min). On the morning of microdialysis, 3 baseline samples were collected after a 2 hr equilibration period (flow rate 2.0 uL/min). Following baseline sample collection, animals were administered saline or MK-801 (0.3 mg/kg). Dialysis samples were collected for an additional 3 hrs.
Analysis of glutamate dialysate concentrations
Extracellular glutamate concentrations were analyzed by HPLC-EC using published methods [7], as we have previously described [40].
Probe verification
Following microdialysis, probe placement in prefrontal cortex was verified visually on frontal sections of the brain. Only data from animals with correct probe placements were included for statistical analyses.
Statistical analysis
Values for glutamate were converted to percent of mean baseline and analyzed using two-way repeated-measures analysis of variance (ANOVA) (Sigma Stat, Jandel Scientific). ANOVAs were constructed using three baseline values and all values following drug administration. Subsequent multiple comparisons between treatment groups were conducted by post hoc analysis with Student-Newman-Keuls test. Student’s t test was used to compare baseline glutamate concentration absolute values between poly I:C and vehicle groups. Two-way ANOVA was used to analyze effects of pretreatment (risperidone and paliperidone) on baseline glutamate concentration absolute values between poly I:C and vehicle groups. Treatment differences were considered statistically significant at P<0.05.
Results
Prenatal immune activation blunts MK-801-induced increase in extracellular glutamate
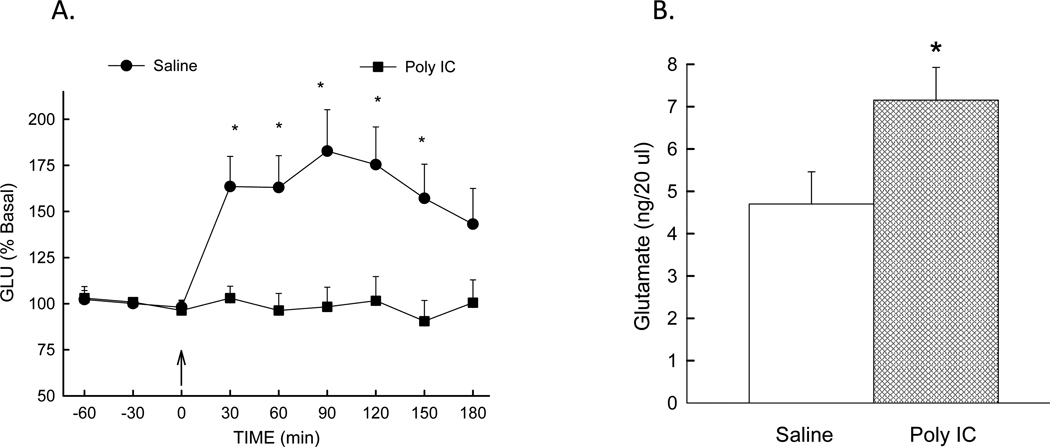
Since extracellular glutamate is modulated in part by NMDA subtype glutamate receptor stimulation [26;27], we determined extracellular glutamate in offspring of poly I:C and saline treated dams in response to NMDA glutamate receptor blockade using the antagonist MK-801 (0.3 mg/kg). As expected, extracellular glutamate is elevated following MK-801 injection in offspring of saline - treated dams (Figure 1A). In contrast, response to MK-801 is markedly blunted in offspring of poly I:C treated dams. ANOVA identified significant effects of treatment [F(1, 26) = 11.63, p=0.002], time [ F(8, 193) = 3.54, p<0.001], and treatment × drug × time interaction [ F(8, 193) = 3.67, p<0.001]. The blunted effect of MK-801 following prenatal immune activation supports the hypothesis that NMDA receptor function is decreased in offspring of poly I:C-treated dams.
Figure 1.
Left: MK-801-stimulated glutamate is blunted following prenatal immune activation: Glutamate levels determined in prefrontal cortex by microdialysis in offspring of saline and poly I:C treated dams. MK-801 (0.3 mg/kg) was injected at time 0. Extracellular glutamate values were converted to percent of mean baseline values ± S.E. N=18 (saline) and 12 (Poly I:C) rats/group. * (p<0.05) compared to poly I:C group. Right: Basal extracellular glutamate is elevated in offspring of poly I:C treated dams: Basal extracellular glutamate in prefrontal cortex of adult male offspring from saline and poly I:C treated dams. N=18 (saline) and 12 (Poly I:C) rats/group. * p<0.05 compared to saline group.
Prenatal immune activation increases basal extracellular glutamate
Because the NMDA hypofunction hypothesis of schizophrenia predicts elevated basal extracellular glutamate in schizophrenia, we determined basal prefrontal cortex extracellular glutamate at PD 55–58 in male offspring of poly I:C and saline -treated dams. As seen in Figure 1B, basal extracellular glutamate was significantly elevated in the poly I:C group (7.0 ± 0.8 ng/20µl) compared to the saline group (4.7 ± 0.7 ng/20µl) [p=0.049, poly I:C vs. saline group].
Effect of risperidone and paliperidone on basal extracellular glutamate
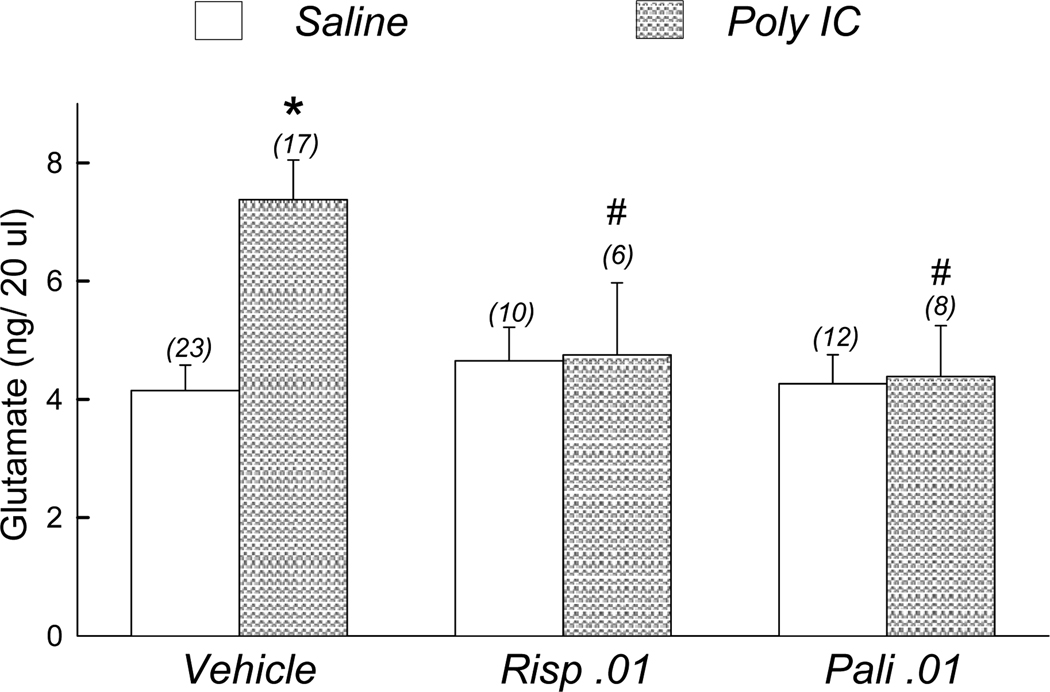
In order to determine the effect of antipsychotic medication on elevated basal glutamate, risperidone (0.01 mg/kg/day), paliperidone (0.01 mg/kg/day) or vehicle were delivered via drinking water between PD 34–58 to offspring of saline and poly I:C treated dams. Extracellular glutamate was determined in prefrontal cortex at PD 55–58. As seen in Figure 2, risperidone and paliperidone treatment lowered basal glutamate in offspring of poly I:C-treated dams to levels similar to offspring of saline -treated dams. Two-way ANOVA identified significant effects of pretreatment [F(1, 70) = 3.99, p=0.050] and pretreatment × treatment interaction [F(2,70) = 4.32, p=0.017]. There was no statistically significant effect of treatment alone [F(2,70) = 2.99, p=0.057]. Post-hoc tests demonstrated extracellular glutamate was significantly elevated in poly I:C offspring compared to saline offspring receiving vehicle post-treatment (p<0.05). Additionally, extracellular glutamate was significantly elevated in poly I:C offspring treated with vehicle compared to poly I:C offspring treated with either paliperidone or risperidone (p<0.05).
Figure 2.
Effect of risperidone and paliperidone on basal extracellular glutamate in prefrontal cortex of saline- and polyI:C-treated offspring: Rats received risperidone (0.01 mg/kg/day), paliperidone (0.01 mg/kg/day), or vehicle for 21 days prior to microdialysis. (x) number of rats per treatment group. * (p<0.05) compared to Saline offspring treated with vehicle. # (p<0.05) compared to poly I:C offspring treated with vehicle.
Risperidone and paliperidone suppress MK-801-induced increase in extracellular glutamate
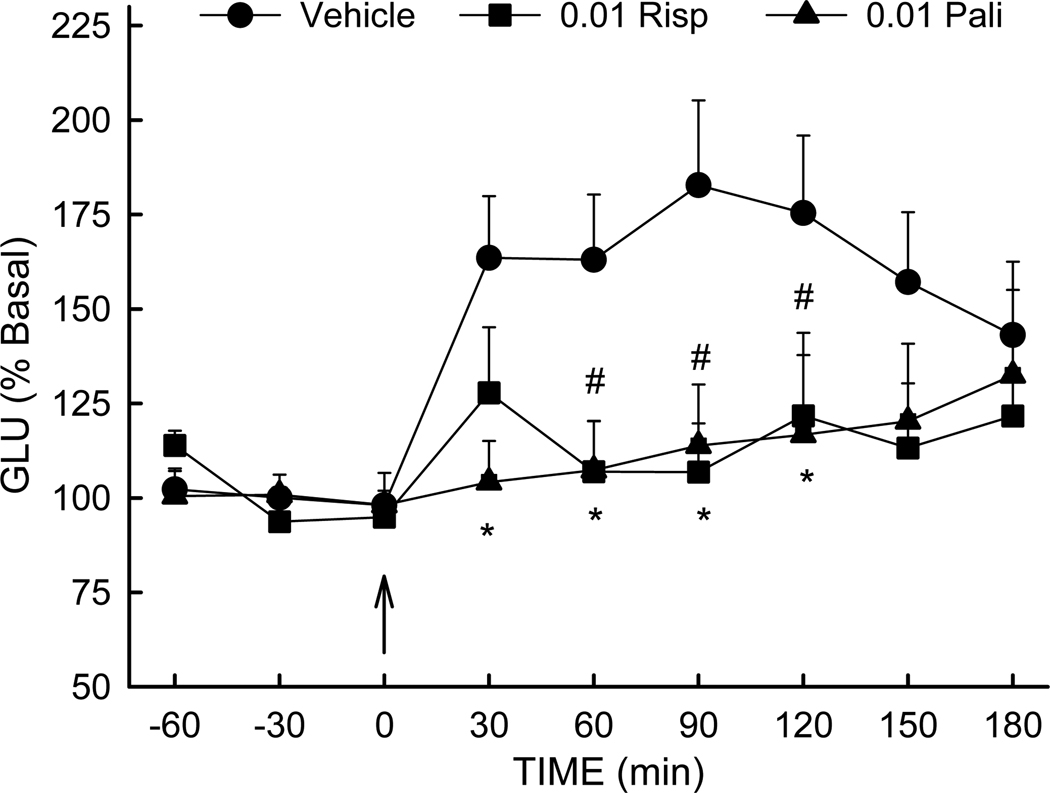
Previous studies have demonstrated suppression of NMDA blockade-mediated increase in extracellular glutamate by clozapine and other antipsychotic medications [1;20;21]. It was therefore of interest to determine if risperidone and paliperidone also share this ability to prevent NMDA blockade-mediated glutamate release. Following treatment with vehicle, risperidone or paliperidone between PD 34–58, MK-801-stimulated extracellular glutamate was determined in offspring of saline-treated dams. As seen in Figure 3, treatment with both risperidone and paliperidone prevented the increase in extracellular glutamate observed after MK-801 injection in vehicle-treated rats. ANOVA indicated significant effects of treatment [F(2,35) = 3.512, p=0.04], time [F(8,258) = 4.4, p<0.001], and treatment × time interaction [F(16,258) = 1.78, p=0.03]. Post-hoc tests demonstrated extracellular glutamate was significantly elevated in rats receiving vehicle post-treatment, compared to animals receiving risperidone (p<0.05) or paliperidone (p<0.05).
Figure 3.
Risperidone and paliperidone suppress MK-801-induced extracellular glutamate elevation: Rats were treated with vehicle, risperidone (0.01 mg/kg/day) or paliperidone (0.01 mg/kg/day) for 21 days prior to microdialysis. MK-801 (0.3 mg/kg) was injected at time 0. N=10–16 rats/group. *, # (p<0.05) compared to Vehicle rats.
Discussion
NMDA receptor hypofunction following prenatal immune activation
The findings presented above demonstrate decreased NMDA receptor function and elevated basal extracellular glutamate in prefrontal cortex, two key features of the NMDA glutamate receptor hypofunction model of schizophrenia, during late adolescence following prenatal immune activation. Prior studies have suggested elevated glutamate may result from removal of tonic NMDA receptor-mediated inhibition of glutamate release [26;27]. Previous research identified decreased NMDA receptor-dependent synaptic current and plasticity following prenatal lipopolysaccharide injection in rats [16], as well as elevated basal hippocampal extracellular glutamate following PD 2–6 poly I:C injection in mice (corresponding to early second trimester in humans) [11]. Additionally, we have previously observed decreased locomotor response to MK-801 treatment following prenatal immune activation [2], consistent with decreased NMDA receptor function observed in the current study following poly I:C treatment. Prenatal immune activation appears to be a common environmental exposure in schizophrenia, as evidence suggests as many as one-third of schizophrenia patients have had in utero exposure to conditions stimulating maternal immune activation [3]. Prenatal immune activation may therefore provide a useful model for studying the long-term effects of NMDA receptor hypofunction over the course of development, an advantage over acute NMDA receptor blockade models based upon effects of transient NMDA receptor inactivity.
In contrast, not all studies have observed glutamatergic abnormalities consistent with the NMDA hypofunction hypothesis following prenatal immune activation. Behavioral studies have identified elevated locomotor response to MK-801 in adult rats [44] and mice [22–24] following prenatal immune activation. Elevated, rather than decreased, response to NMDA receptor blockade has been attributed to a hyper-responsive dopamine system in adult rodents. Additionally, locomotor response to MK-801 was not affected by prenatal immune activation in pre-adolescent mice [23], perinatal immune activation in adult mice [11], or in adult mice following early/mid-gestational (gestational day 9) poly I:C exposure [24]. NMDA receptor expression assays following prenatal immune activation have also been variable. NR1 NMDA receptor subunit immunohistochemistry was decreased in dorsal hippocampus of adult mice following late gestational prenatal immune activation [24]. In contrast, a similar effect was absent following early/middle gestational prenatal immune activation [24]. Additionally, maternal IL-6 treatment during pregnancy increased adult hippocampal NR1 subunit mRNA expression [35].
Studies of glutamate levels following prenatal immune activation have also been variable. In addition to studies consistent with an NMDA hypofunction model described above, two studies observed basal tissue glutamate levels assayed in homogenized cerebellum [8] or dissected brain regions [41] were not altered by prenatal immune activation.
Differences in developmental stage of the offspring; gestational period of immune challenge; and immunogen dose between studies could account for the variable outcomes reported above. In combination these data suggest prenatal immune activation disrupts normal glutamatergic development, and that characterization of the developmental progression of NMDA receptor hypofunction following prenatal immune activation merits further study.
Normalization of elevated basal extracellular glutamate following prenatal immune activation
Treatment with risperidone and paliperidone prevented elevated extracellular glutamate in prefrontal cortex following prenatal immune activation. This finding adds to a growing literature suggesting that peri-adolescent antipsychotic medication administration following prenatal immune activation prevents emergence of behavioral and pharmacological abnormalities of relevance to schizophrenia. Treatment with clozapine, haloperidol, or fluoxetine prevented emergence of abnormalities in locomotor response to amphetamine, prepulse inhibition of startle and latent inhibition [25], while clozapine [31] and risperidone [30] protected against ventricular enlargement and reduced hippocampal volume. In combination, these studies demonstrate longstanding effects of early pharmacological intervention against measures of clinical relevance to schizophrenia.
The pharmacological target(s) responsible for the effect on basal extracellular glutamate is unknown. Both risperidone and paliperidone exhibit highest affinity binding to serotonin 5-HT2A receptors [34]. Functional interactions between serotonin 5-HT2A and glutamate mGluR2 receptors have been reported [10], providing a plausible mechanism linking 5-HT2A receptor binding to modulation of extracellular glutamate. We have previously suggested that serotonin 5-HT2A antagonism underlies the protective effects of risperidone on amphetamine-stimulated locomotion following neonatal hippocampal lesion [32;33], since protective effects were observed at low risperidone dosages with predominantly serotonin 5-HT2A antagonist effects. Further studies of prenatal immune activation may identify molecular targets underlying the observed effects upon NMDA receptor hypofunction.
Effect of risperidone and paliperidone on MK-801-mediated glutamate release
Peri-adolescent risperidone and paliperidone administration suppress the increase in extracellular glutamate evoked by NMDA blockade, a finding previously observed with clozapine and haloperidol [1;20;21;21]. Two independent mechanisms may combine to exert this effect upon MK-801-mediated glutamate release. It has been suggested that clozapine exerts this effect via serotonin 5HT2A receptor downregulation [1;20]. Because both risperidone and paliperidone exhibit high-affinity serotonin 5HT2A receptor binding, this provides one plausible molecular target for the observed effect. Additionally, because antipsychotic medications with prominent D2 receptor binding and more limited 5HT2A receptor affinity such as haloperidol also protect against MK-801-mediated glutamate elevation [21], an independent mechanism mediating this effect through dopamine D2 receptor blockade has also been suggested. This mechanism may result from the ability of D2 receptor antagonists such as haloperidol to facilitate NMDA receptor activity by an intracellular mechanism, through activation of second messenger signaling pathways in neurons also receiving NMDA receptor-mediated glutamatergic input [5;14;18]. Whether effects of prenatal immune activation and antipsychotic medications on extracellular glutamate result from direct actions on NMDA glutamate receptor subunit expression, or indirect effects mediated via other mechanisms, represents an important unanswered question. Further study will be necessary to elucidate the contribution of each of these independent mechanisms to the observed effect.
Conclusions
We present data suggesting prenatal immune activation provides a useful animal model to elucidate neurodevelopmental mechanisms underlying key features of the NMDA hypofunction model of schizophrenia. These findings add to a growing literature demonstrating prevention of behavioral and pharmacological abnormalities of relevance to schizophrenia through pharmacological intervention during the peri-adolescent period following prenatal immune activation. The presence of glutamatergic abnormalities consistent with NMDA hypofunction suggest the utility of determining if peri-adolescent treatment with glutamatergic interventions improves outcome measures of interest in schizophrenia.
Research Highlights.
Prenatal immune activation blunts MK-801–induced increase in extracellular glutamate
Prefrontal cortex basal glutamate is elevated by prenatal immune activation
Paliperidone treatment normalized PFC glutamate in poly I:C offspring
Palliperidone and risperidone prevent MK-801-induced glutamate increase
Acknowledgements
Supported by the Department of Veterans Affairs Medical Research Service, National Institute of Mental Health (R21MH083192-01), and Ortho McNeil Janssen Scientific Affairs L.L.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abekawa T, Ito K, Koyama T. Different effects of a single and repeated administration of clozapine on phencyclidine-induced hyperlocomotion and glutamate releases in the rat medial prefrontal cortex at s. Naunyn Schmiedebergs Arch. Pharmacol. 2007;375:261–271. doi: 10.1007/s00210-007-0154-x. [DOI] [PubMed] [Google Scholar]
- 2.Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: Contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson A, Waters N, Waters S, Carlsson ML. Network interactions in schizophrenia - therapeutic implications. Brain Res. Brain Res. Rev. 2000;31:342–349. doi: 10.1016/s0165-0173(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 5.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am. J Psychiatry. 2009;166:1025–1030. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- 7.Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- 8.Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, Smee DF, Pearce DA, Winter C, Sohr R, Juckel G. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr. Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am. J Physiol Regul. Integr. Comp Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, Takuma K, Kamei H, Noda Y, Nitta A, Nabeshima T, Yoneda Y, Yamada K. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64:297–305. doi: 10.1016/j.neures.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Javitt DC, Silipo G, Cienfuegos A, Shelley AM, Bark N, Park M, Lindenmayer JP, Suckow R, Zukin SR. Adjunctive high-dose glycine in the treatment of schizophrenia. Int. J Neuropsychopharmacol. 2001;4:385–391. doi: 10.1017/S1461145701002590. [DOI] [PubMed] [Google Scholar]
- 13.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 14.Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol. Ther. 2003;97:153–179. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krystal JH, D'Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS, Abi-Saab W, Madonick S. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv. Rev. Psychiatry. 1999;7:125–143. [PubMed] [Google Scholar]
- 16.Lante F, Meunier J, Guiramand J, Maurice T, Cavalier M, Jesus Ferreira MC, Aimar R, Cohen-Solal C, Vignes M, Barbanel G. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic. Biol. Med. 2007;42:1231–1245. doi: 10.1016/j.freeradbiomed.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Leiderman E, Zylberman I, Zukin SR, Cooper TB, Javitt DC. Preliminary investigation of high-dose oral glycine on serum levels and negative symptoms in schizophrenia: an open-label trial. Biol. Psychiatry. 1996;39:213–215. doi: 10.1016/0006-3223(95)00585-4. [DOI] [PubMed] [Google Scholar]
- 18.Leveque JC, Macias W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li XM, Coyle JT, Huganir RL, Heckers S, Konradi C. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J. Neurosci. 2000;20:4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Gil X, Artigas F, Adell A. Role of different monoamine receptors controlling MK-801-induced release of serotonin and glutamate in the medial prefrontal cortex: relevance for antipsychotic action. Int. J Neuropsychopharmacol. 2009;12:487–499. doi: 10.1017/S1461145708009267. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Gil X, Babot Z, Amargos-Bosch M, Sunol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- 22.Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry. 2008;13:208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- 23.Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- 24.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating Early Preventive Antipsychotic and Antidepressant Drug Treatment in an Infection-Based Neurodevelopmental Mouse Model of Schizophrenia. Schizophr. Bull. 2008 doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 28.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- 30.Piontkewitz Y, Arad M, Weiner I. Risperidone Administered During Asymptomatic Period of Adolescence Prevents the Emergence of Brain Structural Pathology and Behavioral Abnormalities in an Animal Model of Schizophrenia. Schizophr. Bull. 2010 doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol. Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Richtand NM, McNamara RK. Serotonin and dopamine interactions in psychosis prevention. Prog. Brain Res. 2008;172:141–153. doi: 10.1016/S0079-6123(08)00907-2. [DOI] [PubMed] [Google Scholar]
- 33.Richtand NM, Taylor B, Welge JA, Ahlbrand R, Ostrander MM, Burr J, Hayes S, Coolen LM, Pritchard LM, Logue A, Herman JP, McNamara RK. Risperidone Pretreatment Prevents Elevated Locomotor Activity Following Neonatal Hippocampal Lesions. Neuropsychopharmacology. 2006;31:77–89. doi: 10.1038/sj.npp.1300791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth BL, Lopez E, Beischel S, Westkaemper RB, Evans JM. Screening the receptorome to discover the molecular targets for plant-derived psychoactive compounds: a novel approach for CNS drug discovery. Pharmacol. Ther. 2004;102:99–110. doi: 10.1016/j.pharmthera.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am. J Physiol Regul. Integr. Comp Physiol. 2006;290:R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- 36.Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, O'Gorman RL, Barker GJ, McGuire PK. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol. Psychiatry. 2009;66:533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Suh HS, Brosnan CF, Lee SC. Toll-like receptors in CNS viral infections. Curr. Top. Microbiol. Immunol. 2009;336:63–81. doi: 10.1007/978-3-642-00549-7_4. 63–81. [DOI] [PubMed] [Google Scholar]
- 38.Taylor P. Practical Teratology. London: Academic Press; 1986. [Google Scholar]
- 39.Tsai GE, Lin PY. Strategies to Enhance N-Methyl-D-aspartate Receptor-Mediated Neurotransmission in Schizophrenia, a Critical Review and Meta-Analysis. Curr. Pharm. Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 40.Wallace TL, Vorhees CV, Gudelsky GA. Effects of lubeluzole on the methamphetamine-induced increase in extracellular glutamate and the long-term depletion of striatal dopamine. Synapse. 2001;40:95–101. doi: 10.1002/syn.1030. [DOI] [PubMed] [Google Scholar]
- 41.Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int. J Neuropsychopharmacol. 2009;12:513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- 42.Woods SW, Walsh B, Pearlson GD, McGlashan T, Simonsen E. Glycine treatment of prodromal symptoms. Schizophr. Res. 2006;86:S7. [Google Scholar]
- 43.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 44.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]