Abstract
The retrosplenial cortex (RSP) is highly interconnected with medial temporal lobe structures, yet relatively little is known about its specific contributions to learning and memory. One possibility is that RSP is involved in forming associations between multiple sensory stimuli. Indeed, damage to RSP disrupts learning about spatial or contextual cues and also impairs learning about co-occurring conditioned stimuli (CSs). Two experiments were conducted to test this notion more rigorously. In Experiment 1, rats were trained in a serial feature negative discrimination task consisting of reinforced presentations of a tone alone and non-reinforced serial presentations of a light followed by the tone. Thus, in contrast to prior studies, this paradigm involved serial presentation of conditioned stimuli (CS), rather than simultaneous presentation. Rats with damage to RSP failed to acquire the discrimination, indicating that RSP is required for forming associations between sensory stimuli regardless of whether they occur serially or simultaneously. In Experiment 2, a sensory preconditioning task was used to determine if RSP was necessary for forming associations between stimuli even in the absence of reinforcement. During the first phase of this procedure, one auditory stimulus was paired with a light while a second auditory stimulus was presented alone. In the next phase of training, the same light was paired with food. During the final phase of the procedure both auditory stimuli were presented alone during a single session. Control, but not RSP-lesioned rats, exhibited more food cup behavior following presentation of the auditory cue that was previously paired with light compared to the unpaired auditory stimulus, indicating that a stimulus-stimulus association was formed during the first phase of training. These results support the idea that RSP has a fundamental role in forming associations between environmental stimuli.
Keywords: medial temporal lobe, ambiguity, compound stimuli, conditioned inhibition, cingulate
Introduction
The retrosplenial cortex (RSP) provides a major source of polymodal sensory input to parahippocampal areas in both rodents and primates, including the postrhinal cortex (parahippocampal cortex in primates), the postsubiculum, and the medial entorhinal cortex (Burwell & Amaral, 1998; Suzuki & Amaral, 1994; van Groen & Wyss, 2003). Thus, RSP is well positioned to have a significant influence on learning and memory processes that are mediated by medial temporal lobe structures. Consistent with this notion, neuroimaging studies in humans demonstrate that RSP is active during tasks that require the medial temporal lobe, such as spatial navigation (Epstein, Higgins, Jablonski, & Feiler, 2007; Epstein, 2008). Likewise, damage to RSP commonly produces deficits in spatial learning and memory in rodents (Cain, Humpartzoomian, & Boon, 2006; Ennaceur, Neave, & Aggleton, 1997; Harker & Whishaw, 2002; Keene & Bucci, 2009; Lukoyanov et al., 2005; Parron & Save, 2004; Vann & Aggleton, 2002, 2004).
Nevertheless, the specific contribution of RSP to spatial learning, and to medial temporal lobe memory function in general, remains unclear and continues to be actively debated (Aggleton, 2009; Kobayashi & Amaral, 2007; Vann, Aggleton, & Maguire, 2009). Identifying the role of RSP in learning and memory is important for understanding the functional organization of cortico-hippocampal circuits and also has significant clinical implications. For example, RSP is one of the earliest regions to exhibit the neuropathology associated with Alzheimer’s disease (Buckner, Snyder, Shannon, LaRossa et al., 2005; Nestor, Fryer, Ikeda, & Hodges, 2003) and has been shown to have a central role in diencephalic amnesia (Vann & Albasser, 2009).
A growing body of evidence suggests that RSP may have a fundamental role in forming associations between multiple environmental stimuli, consistent with the involvement of related medial temporal lobe structures in configural learning (Sutherland & Hoesing, 1993; Rudy & Sutherland, 1989, 1995). This idea is supported by the results of a series of studies by Gabriel and colleagues showing that neurons in the posterior cingulate cortex of rabbits (thought to be comparable to RSP in rats) are sensitive to the formation of associations between a tone and different contexts in an approach/avoidance discrimination task (Freeman Jr., Cuppernell, Flannery, & Gabriel, 1996; Smith, Wakeman, Patel, & Gabriel, 2004) and exhibit task-relevant discriminative response to tones with different meanings (Gabriel, Sparenborg, & Stolar, 1987). More recent support has come from studies that implicate the RSP of rats in processing contextual information (Keene & Bucci, 2008a, 2008c; Bar & Aminoff, 2003; Fenske, Aminoff, Gronau, & Bar, 2006, Suzuki et al., 2005). In addition, we recently examined the effects of RSP lesions on a type of learning that involves the formation of associations between phasic conditioned stimuli (CS; Keene & Bucci, 2008b), as opposed to the static cues present during spatial and contextual learning. Rats were trained in a compound feature negative discrimination task that involved reinforced presentations of a tone on some trials, while on other trials a visual stimulus was simultaneously presented with the tone and not reinforced (necessitating an association between the light and tone). As with spatial and contextual learning, rats with RSP lesions indeed failed to learn the discrimination.
Although the aforementioned studies support a general role for RSP in forming associations between various environmental cues, several questions remain. First, it is unclear whether sensory stimuli must be presented simultaneously to engage RSP, which was the case in previous spatial learning, fear conditioning, and discrimination studies. In Experiment 1, we tested this by examining the involvement of RSP in forming associations between cues that are presented serially. Secondly, it is unknown if RSP is necessary for forming associations between stimuli even in the absence of reinforcement. In Experiment 2, we directly addressed this by testing the involvement of RSP in a sensory preconditioning task that requires the formation of a stimulus-stimulus association without reinforcement.
EXPERIMENT 1
Experiment 1 addressed whether the contribution of RSP to learning about multiple stimuli requires that the cues overlap in time. Control rats and RSP-lesioned rats were trained in a serial feature negative discrimination task, also referred to as negative occasion setting (Holland, Lamoureux, Han, & Gallgher, 1999; MacLeod & Bucci, 2010, MacLeod, Vucovich, & Bucci, 2010). Each session consisted of four reinforced trials in which a tone was presented by itself and followed by food reward, intermixed with twelve non-reinforced trials in which a light preceded presentation of the tone. According to contemporary theories of learning, the negative feature (light) enters into a relationship with the target cue (tone) in that it “gates” the inhibitory properties of the target stimulus rather than entering into a direct association with the outcome (Ross & Holland, 1981; Holland, 1985). If RSP is involved in forming stimulus-stimulus associations only when stimuli are present at the same time, then RSP lesions should not affect serial feature negative discrimination. Alternatively, a failure to learn the association between the light and tone would be expected to result in low levels of conditioning on both trial types since most trials were non-reinforced.
Materials and Methods
Subjects
Fifteen male Long Evans rats weighing ~225g were obtained from Harlan Laboratories (Indianapolis, IN). Rats were housed individually and allowed 7 days to acclimate to the vivarium with food available ad libitum (Purina standard rat chow; Nestle Purina, St. Louis, MO). Subsequently, rats were handled and weighed daily for three days to establish baseline body weights, which were then gradually reduced to 85% of baseline over a seven-day period. Throughout the study, rats were maintained on a 14:10 light-dark cycle and monitored and cared for in compliance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Surgery
Subjects were anesthetized with isoflurane gas (1.5–3% in oxygen) and placed in a Kopf stereotaxic apparatus. The skin was retracted and holes were drilled through each of the intended lesion sites using the rat brain atlas of Paxinos & Watson (1998). Seven rats received bilateral electrolytic lesions (2mA, 15sec at each site) of RSP prior to behavioral training using the stereotaxic coordinates outlined in Table 1. Electrolytic lesions were used to provide control over the extent of damage, which was an important factor in this study given the close proximity of RSP and posterior parietal cortex, which also provides visuo-spatial input to the medial temporal lobe (Burwell & Amaral, 1998). Control rats (n=8) received sham lesions consisting of a craniotomy and shallow, non-puncturing burr holes to minimize damage to underlying cortex. Rats were allowed to recover for 2 weeks before training began.
Table 1.
Stereotaxic coordinates for RSP lesions. All AP, ML and DV measurements are derived from bregma, midline, and skull surface, respectively (measurements are in mm). Electrolytic lesions were made by applying a 2mA current for 15sec at each site.
| AP | ML | DV |
|---|---|---|
| −2.0 | ±0.3 | −1.6 and −2.5 |
| −3.5 | ±0.4 | −1.8 and −2.5 |
| −5.0 | ±0.4 and ±1.0 | −1.6 and −2.2 (medial site), −1.6 (lateral site) |
| −6.5 | ±0.8 and ±1.4 | −1.6 and −2.2 (medial site), −2.7 (lateral site) |
| −8.0 | ±1.0 and ±2.0 | −2.0 (medial site), −2.4 (lateral site) |
Behavioral Apparatus
The behavioral apparatus was obtained from Med Associates Inc. (St. Albans, VT) and consisted of standard operant conditioning chambers (24cm X 30.5cm X 29cm) connected to a computer and enclosed in sound-attenuating chambers (62cm X 56cm X 56cm) outfitted with an exhaust fan to provide airflow and background noise (~68dB). The operant chambers consisted of aluminum front and back walls, clear acrylic sides and top, and grid floors. A dimly illuminated food cup was recessed in the center of the front wall, and a 6-W jeweled panel light serving as the visual stimulus was located 5cm above the opening to the recessed food cup. A house light providing background illumination was mounted 15cm above the food cup. A speaker was located 15cm above and to the right of the food cup and was used to present the auditory stimulus (1500Hz, 78dB). A pair of infrared photocells was mounted just inside the food cup to detect head entries into the cup. Surveillance cameras located inside the surrounding shell were used to videotape the rats’ behavior.
Behavioral Procedures
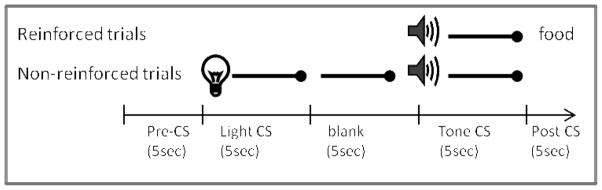
Rats learned to eat from the food cup during a single 32-min session during which two 45-mg food pellets (Noyes, New Brunswick, NJ) were randomly delivered eight times (variable intertrial interval (ITI) averaged 4 min). Training then took place over twelve daily sessions that lasted 64 min each and included sixteen trials of two types (see Figure 1). Rats received four trials per session consisting of a 5-second presentation of the tone followed immediately by delivery of two food pellets. For the other 12 trials, the light was presented for 5 sec, followed by a 5-sec period with no stimuli, and then the tone was presented by itself for 5 sec and not followed by food. The two trial types occurred randomly during the session and the order of trials differed on each day; the ITIs averaged 4 min.
Figure 1.
Schematic diagram of the serial feature negative discrimination task used in Experiment 1. The time line and epochs (pre-CS, CS, post-CS) noted on the bottom refer to the time periods used in the analyses described in the Materials and Methods in Experiment 1.
Behavioral Observations and Data Analysis
Breaks in the photobeam located across the entry of the food cup were monitored by the computer. The amount of time the beam was broken served as the measure of conditioned food cup behavior. Analysis of time spent in the food cup was conducted during the 5-sec period prior to CS onset (pre-CS responding), during presentation of the tone conditioned stimulus (CS), and during the 5 sec after the CS was turned off (post-CS responding). The data were subjected to a repeated measures ANOVA with Group (control, lesion) as the between-subjects variable and Session (2-session blocks) and Trial type (reinforced, non-reinforced) as the within-subjects variables. Significant main effects were followed up with appropriate pair-wise comparisons (two-tailed t-tests). An alpha level of 0.05 was used in all analyses.
Lesion Verification and Analysis
After the behavioral procedures were completed, rats were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 0.9% saline for 5 min, followed by 10% buffered formalin. Coronal brains sections (60μm) were collected using a freezing microtome and were Nissl-stained using thionin. Sections at 800μm intervals were used to assess the amount of tissue damage. Using StereoInvestigator software (version 6; Microbrightfield, Inc., Williston, VT) and a compound microscope (Axioskop I, Zeiss, Inc.), we identified gross tissue damage as necrosis, missing tissue, or marked thinning of the cortex. For each coronal section, areal measurements were obtained using the StereoInvestigator Cavalieri estimator probe with 100μm grid spacing. Measurements included the total area of the target region and the area of the target region that exhibited gross tissue damage. In addition, we recorded the number of coronal sections on which RSP damage was present along the entire rostro-caudal extent of the region (a total of ~30 sections for each rat). The number of sections containing damage to regions outside of the target area was also recorded.
Results
Histology
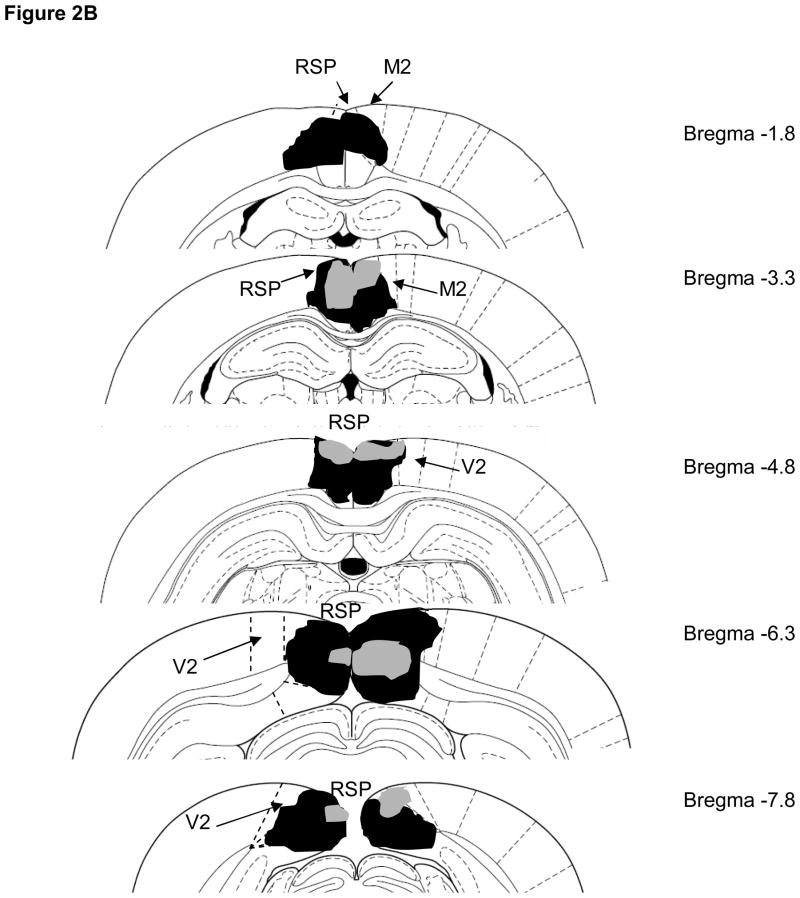
Bilateral damage of the RSP was observed in all rats and the average area of RSP damaged on each section analyzed was 74 ± 5%. A representative lesion is illustrated in Figure 2A. Damage to the RSP was present on 100% of the sections collected from each subject, indicating that damage extended throughout the rostro-caudal extent of RSP (Figure 2B). Minor damage to cortical regions outside of RSP (primarily unilateral anterior cingulate or motor cortex) was noted in some animals. In addition, 5 of 7 rats exhibited minor bilateral damage to the cingulum bundle.
Figure 2.

A) Photomicrograph of an RSP lesion illustrating typical damage to RSP on one side of the brain (approximately −3.0mm from bregma). The dotted line indicates the boundaries of RSP. B) Schematic diagram indicating the largest (black) and smallest (grey) lesions of RSP in Experiment 1 (adapted from Paxinos & Watson, 1998). Abbreviations: RSP, restrosplenial cortex; M2, secondary motor cortex; V2, secondary visual cortex.
Behavior
Conditioned responding during presentation of the tone on reinforced and non-reinforced trials is illustrated in Figure 3. Control and lesioned rats differed in their ability to discriminate between the two trials as evidenced by a significant Group X Trial type interaction [F(1,13)=5.7, P<0.03]. Subsequent analysis revealed that control rats responded more on reinforced trials than non-reinforced trials [t(7)=3.3, P<0.01] but that RSP-lesioned rats spent comparable amounts of time in the food cup on both types of trials [t(6)=.05, P>0.6]. These findings indicate that control rats successfully learned the conditional discrimination but that RSP-lesioned rats did not. The main effect of Group was not statistically significant (p>0.4).
Figure 3.
Damage to RSP impaired discrimination learning in Experiment 1, in which presentations of the tone alone were reinforced, but presentations of the light shortly before the tone signaled no reinforcement. Data are mean ± standard error.
There were no significant group differences in food cup behavior exhibited during any of the other epochs (Ps >0.5). Specifically, mean amount of time spent with the snout in the food cup during the pre-CS period was 0.4 ± 0.2 sec for the control group and 0.3 ± 0.1 sec for the lesion group. Responding during the 5-sec period after the food was delivered during reinforced trials was also comparable between groups: the mean time spent in the food cup during the post-CS period was 3.4 ± 0.4 sec and 3.1 ± 0.4 sec for control rats and RSP-lesioned rats, respectively.
Because we were concerned that potential damage to the cingulum bundle may have contributed to the inability of lesioned rats to learn the discrimination, we calculated a difference score for each lesioned rat by subtracting the amount of responding observed during the tone on non-reinforced trials from the amount of responding to the tone on reinforced trials. The mean difference scores for lesioned rats with or without cingulum damage were identical (0.07 ± 0.1 sec; sample sizes were 5 and 2, respectively), suggesting that cingulum damage was not responsible for the inability of lesioned rats to learn the discrimination.
EXPERIMENT 2
Together with our previous findings (Keene & Bucci, 2008b), the results of Experiment 1 suggest that RSP is necessary for forming associations between two phasic sensory stimuli regardless of whether they are presented simultaneously or serially, providing additional support for a fundamental role of RSP in forming associations between sensory stimuli. However, it remains unclear whether the involvement of RSP is limited to situations in which stimuli are reinforced. In other words, if RSP has a critical role in forming associations between multiple sensory stimuli, we would expect that RSP damage would disrupt the formation of association between sensory stimuli even in the absence of reinforcement. Indeed, in previous studies, the sensory cues of interest were either paired with an unconditioned stimulus (e.g., contextual cues and footshock) or had ambiguous meanings (e.g., paired with food or shock on some trials in a session, but not on others). Thus, the pairing of stimuli with specific outcomes from the onset of training makes it difficult to conclude that RSP is important for forming associations between sensory stimuli per se.
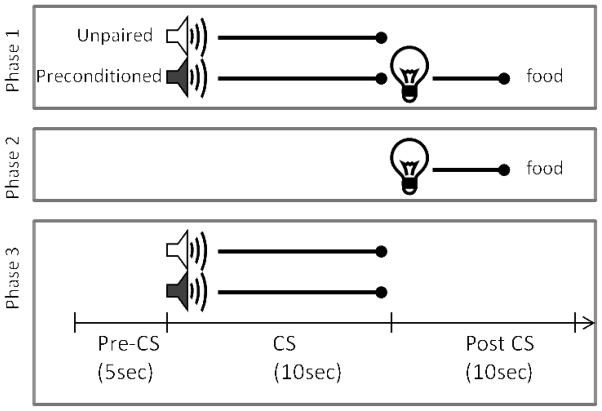
To address this, Experiment 2 tested control and RSP-lesioned rats in a sensory preconditioning task (Brogden, 1939; Blaisdell, Leising, Stahlman, & Waldman, 2009; Holland & Ross, 1983; Ward-Robinson, Coutureau, Good, Honey et al., 2001). The behavioral procedure is depicted in Figure 4. In the first phase of training, an auditory stimulus (e.g., a tone) was presented and followed immediately by a visual stimulus (a light) on half of the trials. During the other half of the trials in each session, another auditory stimulus (e.g., white noise) was presented alone. Importantly, no reinforcement was delivered during this phase of training. Thus, rats were given the opportunity to form a stimulus-stimulus association in the absence of reward. During the second phase of training, the same light was presented and followed by food reward (US). Finally, during a single test session, the auditory stimulus that was previously paired with light (referred to here on out as the “preconditioned cue”) and the unpaired auditory stimulus were presented alone on intermixed trials. In this paradigm, normal rats typically exhibit more conditioned responding to the preconditioned auditory cue compared to the unpaired cue during the test session (Blaisdell et al., 2009; Holland & Ross, 1983). This outcome is thought to reflect the formation of a stimulus-stimulus association between the two paired sensory stimuli during the initial training session, a phenomena referred to as sensory preconditioning. We predicted that if RSP was critically involved in forming associations between sensory stimuli, control but not RSP-lesioned rats would exhibit more conditioned responding to the preconditioned auditory cue during the test session.
Figure 4.
Schematic diagram of the sensory preconditioning task used in Experiment 2. The time line and epochs (pre-CS, CS, post-CS) noted on the bottom refer to the time periods used in the analyses described in the Materials and Methods in Experiment 2.
Materials and Methods
Subjects and Surgery
Forty-six rats were obtained from Harlan Laboratories and maintained as described in Experiment 1. Twenty-seven rats received electrolytic RSP lesions and 19 received sham lesions as described in the first study.
Behavioral Apparatus and Training Procedures
Training took place using the apparatus and stimuli described in Experiment 1, and also included a 78 dB white noise auditory stimulus. A schematic diagram of the training procedure is shown in Figure 4. During the first phase of training, rats received four daily 64-min training sessions each consisting of 12 trials. On half of the trials, one of the auditory stimuli (the “preconditioned cue”) was presented for 10 sec and followed immediately by a 5-sec presentation of the light. During the other six trials the other auditory stimulus (the “unpaired cue”) was presented alone for 10 sec. The trials types were randomly intermixed with an average ITI of 4.5 min and the use of the tone and white noise as the preconditioned and unpaired auditory stimuli was counterbalanced in each group of rats. During the second phase of training, five daily 64-min conditioning sessions each consisted of 8 presentations of the light (5 sec in duration, 7 min ITI) followed immediately by delivery of two 45-mg food pellets. Note that neither auditory stimulus was presented during the phase 2 sessions. Phase 3 consisted of a single test session during which each of the two auditory stimuli was presented alone 6 times in separate intermixed trials (64 min session).
Behavioral Observations and Data Analysis
As in Experiment 1, breaks in the photobeam located across the entry of the food cup were monitored by the computer and the amount of time the beam was broken served as the measure of conditioned food cup behavior. During the phase 2 sessions, the time spent in the food cup during the 5-sec period just prior to onset of the light (“pre-CS” responding) was subtracted from the amount of time spent in the food cup during presentation of the light, resulting in an ‘elevation score’ that served as the primary indicator of light-food conditioning. The elevation score was adopted because of the variability observed in the amount of food cup behavior exhibited by individual rats in both treatment groups. These data were subjected to a repeated measures ANOVA with Group (control, lesion) as the between-subjects variable and Session as the within subjects variable.
During the phase 3 test session, pre-CS responding was similarly subtracted from the amount of responding during presentation of each auditory stimulus (CS period) and also subtracted from the responding observed during the 10 sec period following termination of the stimulus (“post-CS period”; see Figure 4). Data from this post-CS epoch in the phase 3 session were particularly important to analyze because this period corresponded temporally to the time that the light was presented after the auditory stimulus in phase 1 and also the time that food would have been presented during light → food conditioning in phase 2. Thus, if rats formed an association between the auditory stimulus and light in phase 1, food cup behavior was expected to be particularly high during the post-CS epoch, as observed previously (Blaisdell et al., 2009). The elevations scores were analyzed using a repeated measures ANOVA with Group as the between subjects variable and Trial type (preconditioned or unpaired auditory stimulus) as the within-subjects variable. Significant main effects were followed up with appropriate pair-wise comparisons (two-tailed t-tests).
An additional comparison of the strength of sensory preconditioning between the control and lesion groups was carried out by calculating a difference score, defined as the amount of responding observed during the post-CS period following presentation of the preconditioned auditory stimulus divided by the sum of the post-CS responding observed following each of the auditory stimuli. Using one-sample t-tests, the resulting values for each group were compared to an expected value of 0.5 (i.e., chance), which would indicate no sensory preconditioning. An alpha level of 0.05 was used in all analyses.
Lesion Verification and Analysis
Histological preparation and lesion analysis were conducted as described in Experiment 1. The extent of the RSP damage was visualized in all lesioned rats, and tissue from 11 brains were used to make areas measurements of the lesion size. The tissue from the remaining lesioned rats was used for other purposes unrelated to this study.
Results
Histology
Damage to the RSP in lesioned rats was very similar to that observed in Experiment 1 (Figures 1A and 1B) and to previous studies from our lab (Keene & Bucci, 2008a, 2008b). Bilateral RSP damage was observed in all rats and the average area of RSP damaged on each section analyzed was 81 ± 5%. Damage to the RSP was present on 100% of the sections collected from each subject, indicating that damage extended throughout the rostro-caudal extent of RSP. Minor damage to cortical regions outside of RSP (primarily secondary visual cortex or motor cortex) was present on 71 ± 7% of the sections analyzed. In addition, 9 of 11 rats exhibited minor bilateral damage to the cingulum bundle.
Behavior
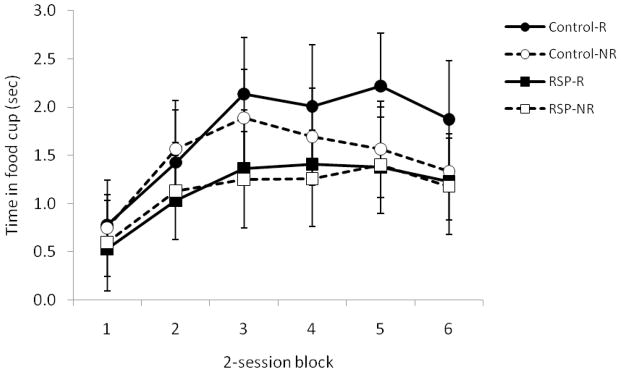
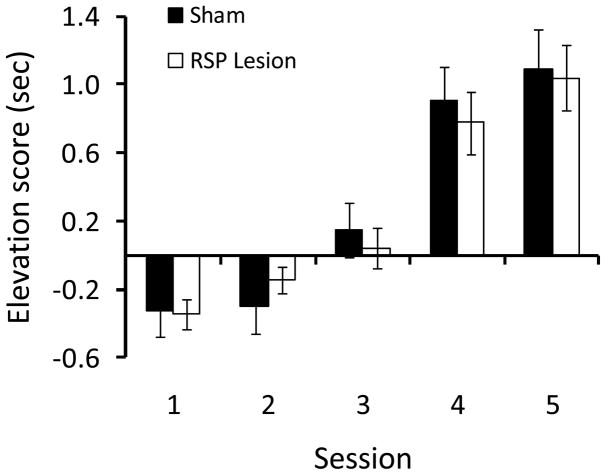
As training progressed during phase 2, rats in both groups exhibited increased food cup behavior during presentation of the light (Figure 5). This was confirmed by a repeated measures ANOVA that revealed a significant main effect of Session [F(4, 41)=20.3, P>0.001]. The main effect of Group and the Group X Session interaction were not statistically significant (Ps > 0.8), indicating that control rats and RSP-lesioned rats comparably learned the association between the light and food.
Figure 5.
Damage to RSP did not affect light-food conditioning during phase 2 in Experiment 2. Data are average food cup behavior during the 5-sec light presentation minus food cup behavior during the 5-sec pre-CS period. Data are mean ± standard error.
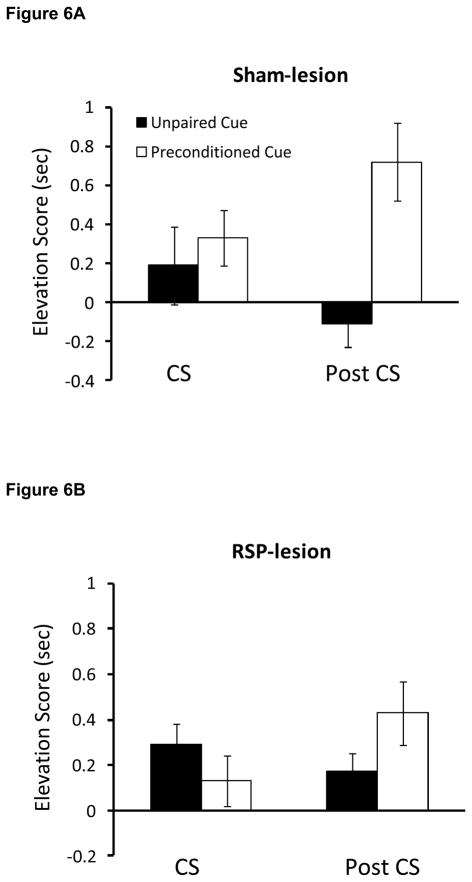
The critical test session data collected during phase 3 are illustrated in Figures 6 and 7. A repeated measures ANOVA that compared the food cup behavior of control and RSP-lesioned rats during the post-CS period revealed a significant main effect of Trial type [F(1,44)=20.6, P<0.001] and a significant Trial type X Group interaction [F(1,44)=5.7, P<0.02]. As shown in Figure 6A, control rats spent more time in the food cup (i.e., a higher elevation score) during the post-CS period on trials in which the preconditioned auditory stimulus was presented compared to trials in which the unpaired auditory stimulus was presented [t(18)=4.2, P<0.001]. In contrast, control rats did not spend more time in the food cup during the CS period on paired versus unpaired trials [t(18) = −0.8, p>0.05]. These data indicate that, as predicted, control animals formed a stimulus-stimulus association during phase 1. Unlike control rats, animals with RSP damage exhibited similar food cup responding during the post-CS period regardless of whether the preconditioned or unpaired auditory cue was presented (Figure 6B), suggesting that lesioned rats failed to form a stimulus-stimulus association between the auditory cue and the light during phase 1. No other pair-wise comparisons reached statistical significance (Ps>0.09). Importantly, there was no main effect of Group (P>0.9), indicating that control and RSP-lesioned rats exhibited overall levels of food cup responding that were comparable to those of control rats.
Figure 6.
Food cup responding during presentation of the two auditory stimuli during the third phase of Experiment 2. Control rats but not RSP-lesioned rats exhibited sensory preconditioning, evidenced by greater food cup responding during presentation of the auditory stimulus that was previously paired with the light compared to the unpaired auditory stimulus. Data are averaged food cup behavior (5 sec) minus 5-sec preCS baseline responding and mean ± standard error.
Figure 7.
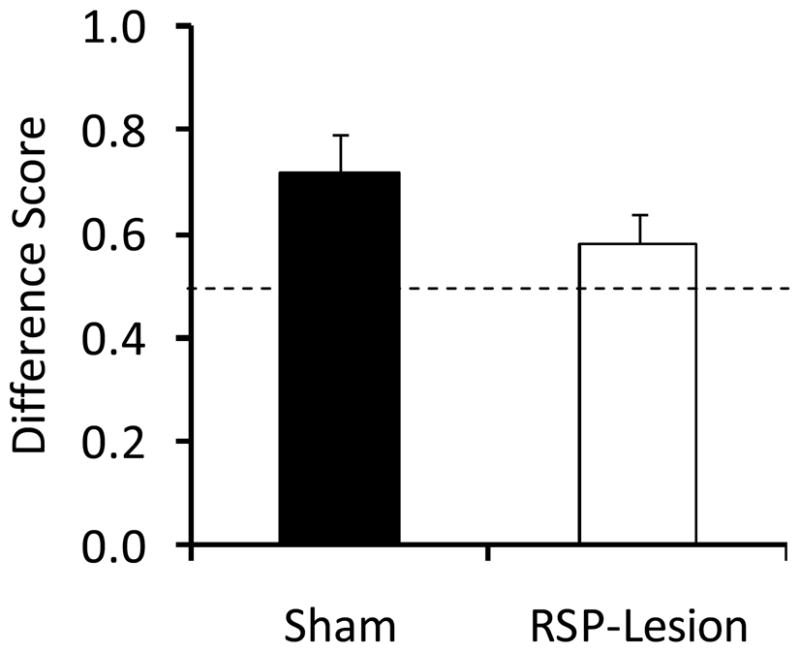
Difference scores during phase 3 of Experiment 2. Control, but not RSP-lesioned rats exhibited difference scores significantly different from 0.5, indicating that more time was spent with the nose in the food cup during presentation of the previously-paired auditory stimulus versus the unpaired auditory stimulus. Data are mean ± standard error.
The difference scores (calculated by dividing the time spent in the food cup during the post-CS period following presentation of the preconditioned cue by the sum of the post-CS responding observed following each of the auditory stimuli during phase 3) are presented in Figure 6. Consistent with the analyses above, control rats had a difference score that was significantly higher than 0.5 [t(18)=4.4, P<0.001] but RSP-lesioned rats did not (P>0.2), supporting the conclusion that RSP damage impaired sensory preconditioning.
Lastly, we were concerned that potential damage to the cingulum bundle may have contributed to the behavioral deficit and therefore compared the mean difference scores for lesioned rats with and without cingulum damage were 0.63 ± 0.1 (n=9) and 0.33 ± 0.04 (n=2), respectively. It is important to note that the mean difference score observed in the group with cingulum damage was actually higher than that observed in the no-cingulum damage group, which argues against the possibility that cingulum damage was responsible for the sensory preconditioning impairment observed in RSP-lesioned rats.
Discussion
The results of prior studies suggest that a contribution of RSP to learning and memory may involve forming associations between co-occurring stimuli in the environment. The present study included two experiments to test the boundaries of this theory. In Experiment 1, RSP damage impaired the ability of rats to learn a serial feature negative discrimination in which presentations of a tone alone were paired with food, whereas the occurrence of a light just before the tone signaled non-reinforcement. A similar outcome was obtained in our previous study (Keene & Bucci, 2008b) in that RSP-lesioned rats were impaired when the light and tone occurred simultaneously and were not reinforced (compound feature negative discrimination). These findings indicate that RSP is necessary for learning that involves forming associations between two sensory stimuli regardless of whether the cues are presented as simultaneous or serial compounds. In Experiment 2, rats with RSP lesions were impaired in a sensory preconditioning task that similarly involved forming an association between two sensory stimuli. However, in this procedure, the two sensory cues were paired prior to the delivery of any reinforcement, unlike the tasks used in Experiment 1 or previous studies (Keene & Bucci, 2008a, 2008b, 2008c). The finding that RSP damage impaired sensory preconditioning suggests that the involvement of RSP in learning associations between sensory stimuli is not restricted to conditions in which cues are paired with reinforcement.
Several observations from the current studies allow us to rule out potential confounding factors and alternative explanations. For instance, in Experiment 2, control and RSP-lesioned rats exhibited comparable levels of food cup behavior during presentation of the light in phase 2. Thus, any impairment observed during the sensory preconditioning test session cannot be attributed to an inability to learn the association between the light and food. This is consistent with prior studies that have shown that single-cue learning is intact following RSP damage in both aversive and appetitive conditioning paradigms (Keene & Bucci, 2008a, 2008b). Similarly, studies by Gabriel and colleagues have demonstrated that damage to posterior cingulate cortex in rabbits did not affect acquisition of a two-tone discrimination (Gabriel, Lambert, Foster, Orona et al., 1983). Interestingly, lesion effects were only observed later in training when performance depended on associations between the tones and contextual cues, consistent with involvement of this region in forming associations between sensory cues. In addition, it is important to note that there was no main effect of Group in Experiment 1, or during the phase 3 test session in Experiment 2. Indeed, in the prior study by Keene & Bucci (2008b), low levels of conditioned responding were observed on both reinforced and non-reinforced trials, raising the possibility that RSP damage altered performance factors such as motivation or mobility. The lack of significant main effect of Group indicates that the overall levels of conditioned responding in control and RSP-lesioned rats were comparable and argues against a lesion-induced change in motivation or locomotion to the food cup.
Together with our prior findings using a simultaneous compound feature negative paradigm (Keene & Bucci, 2008b), the results of these experiments support the view that RSP makes a fundamental contribution to forming associations between multiple sensory stimuli. Indeed, it is noteworthy that there are distinct procedural differences between the sensory preconditioning and feature negative discrimination tasks regarding the order of timing of the sensory cues. However, an important commonality is that they each involve the formation of stimulus-stimulus associations. The fact that RSP damage produces impairments regardless of the different procedural aspects of these tasks strongly supports a basic role for RSP in forming relationships between sensory cues. In fact, comparing the effects of manipulations across multiple higher-order conditioning preparations has recently been touted as a useful means to distinguish between different components of stimulus processing and learning (Gewirtz & Davis, 2011).
This formulation also accounts for many of the previously published observations following damage to RSP. Indeed, both spatial and contextual learning paradigms require forming associations between static sensory cues in the environment and numerous studies have shown that spatial and contextual learning are sensitive to RSP damage (Harker & Whishaw, 2002; Lukoyanov et al., 2005; Parron & Save, 2004; Vann & Aggleton, 2002, 2004; Keene & Bucci, 2008a, 2008c). Similarly, an extensive series of studies has shown that neurons in the posterior cingulate cortex of rabbits (comparable to RSP in rats) are engaged in tasks that required the processing of multi-modal stimuli and formation of sensory associations. For example, when neuronal responses in posterior cingulate cortex are blocked following fornix lesions, approach/avoidance behavior in rabbits was impaired when subjects were required to use background contextual cues to guide behavior (Smith et al., 2004). Likewise, other studies by Gabriel and colleagues demonstrate that posterior cingulate cortex neurons encode context specificity (Talk, Stoll, & Gabriel, 2005) and that damage to the posterior cingulate cortex eliminated discriminative responses in anterior cingulate cortex (Gabriel & Sparenborg, 1987). Interestingly, posterior cingulate cortex also exhibits theta rhythmicity, which is independent of hippocampal theta (Talk, Kang, & Gabriel, 2004).
This formulation of RSP function also fits well with its prominent position in the so called “where” processing circuit, which is thought to contribute aspects of contextual, spatial, and episodic information to memory (Burwell & Amaral, 1998; Eichenbaum, 2007; Murray, Bussey, & Saksida, 2007). At the same time, the present data clearly indicate that the role of RSP in forming associations between stimuli may not be relegated to spatial and contextual domains. Indeed, in the study by Keene & Bucci (2008b), rats were presented with phasic conditioned stimuli presented simultaneously. Likewise, forming associations between the sensory cues in Experiments 1 and 2 did not involve using background contextual or spatial information. It is interesting to note, however, that tasks such as the serial feature negative discrimination procedure used in Experiment 1 share important similarities to contextual and spatial learning. For instance, the paradigm used in Experiment 1 is often referred to as ‘negative occasion setting’ (Holland, 1984; Ross & Holland, 1981) in that the presence of the light sets the occasion for the meaning of the tone on those trials. In this regard, contextual fear conditioning is similar in that the training context sets the stage for the meaning of phasic auditory stimulus (Bouton, 2007). Thus, one function of the RSP may be to facilitate associations between cues that provide contextual meaning for subsequent stimuli.
The neuroanatomical features of RSP suggest that is well positioned to mediate the processing of sensory stimuli and to integrate hippocampal, thalamic and cortical sensory information (van Groen & Wyss, 1990, 1992). For example, RSP provides a major source of polymodal sensory input to parahippocampal areas in both rodents and primates, including the postrhinal cortex, the postsubiculum, and the medial entorhinal cortex (Burwell & Amaral, 1998; Suzuki & Amaral, 1994; van Groen & Wyss, 2003). Moreover, a recent study of the intrinsic connectivity of subregions of RSP in the rat indicates that there are dense interconnections along the transverse axis of RSP, providing a basis for the local integration of information and subsequent integration within the entire structure (Shibata, Honda, Sadaki, & Naito, 2009). Supportive of this notion, our laboratory has recently found evidence for learning-related plasticity in RSP as measured by the expression of the immediate-early gene arc after fear conditioning (Robinson, Iaccarino, NDong, DeLeo, & Bucci, 2010). However, additional insight into the mechanisms underlying the involvement of RSP in forming associations between sensory stimuli awaits future study.
Evidence that RSP contributes to mnemonic function by facilitating the formation of stimulus-stimulus associations informs the overarching and heavily researched question of how different cortico-hippocampal circuits contribute to medial temporal lobe dependent learning and memory. In that vein, it is useful to compare the effects of RSP and hippocampal damage across various paradigms. Interestingly, hippocampal damage has been shown to impair performance in a serial feature negative discrimination task (Holland et al., 1999) but spares learning a compound feature negative discrimination (Solomon, 1977; Chan, Jarrard, & Davidson, 2003). In contrast, RSP lesions impair both serial and compound feature negative discrimination (Experiment 1, and Keene & Bucci, 2008b). In addition, hippocampal damage has been shown to have an equivocal effect on sensory preconditioning, with some studies reporting deficits (Talk, Gandhi, & Matzel, 2002) and others observing no effects (Ward-Robinson et al., 2001). Yet even in the study by Talk et al. (2002), there was evidence that the hippocampus may not have been critically involved in the stimulus-stimulus association per se. These findings support the notion that RSP may have a more fundamental role than the hippocampus in forming associations between sensory stimuli. This is consistent with recent theories delineating functional distinctions of a medial temporal lobe system believed to support episodic memory (Davachi, 2006, Eichenbaum, Yonelinas & Ranganath, 2007, Diana et al., 2007). The present findings suggest these models should be expanded to account for the contribution of RSP in forming stimulus associations within the “where” processing circuit, which may ultimately contribute to the integration of object information into the contextual representation within the hippocampus (Komorowski, Manns & Eichenbaum, 2009; Manns & Eichenbaum, 2009).
In a similar fashion, it is noteworthy that damage to perirhinal cortex (PER), a key region in the complementary “what” processing stream, impairs sensory preconditioning (Nicholson & Freeman, 2000), compound feature negative discrimination (similar to Keene & Bucci, 2008b) and compound feature positive discrimination while having no effect on learning a serial feature positive discrimination (Campolattaro & Freeman, 2006a, 2006b). Based on these findings, the authors hypothesize that PER may play a role in resolving ambiguity in discriminations with overlapping stimulus elements (Campolattaro & Freeman, 2006b). Thus, it is possible that PER and RSP make unique contributions to complex learning paradigms by resolving stimulus ambiguity for overlapping stimulus elements and by forming or mediating associations between multiple stimuli, respectively. These proposed functions of PER and RSP are consistent with another recent study that found unique contributions of CA1 and dorsocaudal medial entorhinal (dcMEC) cortex to the disambiguation of overlapping experiences (Lipton, White, & Eichenbaum, 2007). In that study, rats were trained to run on a modified T-maze, continuously alternating between left turns and right turns. Activity from neurons in CA1 more selectively encoded places visited on each route, while neurons in dcMEC activity more strongly distinguished left-turn from right-turn trials. Critical to the present discussion, this study establishes the notion that nearby cortical structures make important and distinct contributions to hippocampal function in resolving ambiguity for closely related or overlapping experiences. This idea, along with the present findings, provides an intriguing avenue for future research regarding the unique contributions of closely related brain areas such as RSP, PER, and the hippocampus.
The present findings are largely consistent with other current theories about RSP function in rats and primates. One prominent theory suggests that RSP plays a key role in spatial navigation by translating between viewpoint-independent allocentric representations provided by hippocampal inputs into viewpoint-dependent egocentric representations that can be utilized by the posterior parietal cortex to guide navigation and motor behaviors (Bird & Burgess, 2008; Burgess, 2008). In two recent electrophysiological studies by Sato and colleagues (2006, 2009), recordings were made from medial parietal neurons (potentially including RSP) while monkeys navigated well-learned paths in a virtual reality environment. They found a large proportion of neurons whose responses were associated with specific behaviors (turn left, turn right, move forward) at specific locations. Epstein (2008) suggested that these results are consistent with the notion that RSP can translate “you are here” information into “your goal is to the left” information. Thus, RSP may aid in navigation by integrating and translating spatial information about the environment into a plan for goal-related actions. This role is consistent with other recent findings about RSP function. Epstein and colleagues (2007) found greater activity in RSP in response to familiar scenes relative to unfamiliar scenes, and Park and colleagues (2009) suggested that RSP may serve to integrate scenes or viewpoints in one visual context. Another study in rodents also demonstrated that RSP was involved in a spatial task when room and arena stimuli were in conflict, leading the authors to hypothesize that RSP is involved in navigation when segregation of relevant and irrelevant stimuli is required (Wesierska, Adamska, & Malinowska, 2009). The present results also suggest that associational significance of stimuli might be a critical part of this representation.
In summary, the present study expands upon the growing evidence implicating RSP in spatial and contextual learning as well as conditional discriminations, demonstrating an underlying role for RSP in associating multiple stimuli. The findings presented here offer potential insights both to the unique aspects of RSP function as well as a mechanism for its contributions to medial temporal lobe function. Specifically, the data support the notion that RSP has a fundamental role in mediating associations between sensory stimuli, consistent with previously ascribed functions in spatial navigation and contextual learning and memory. Future studies will further delineate the breadth of RSP involvement in these processes and identify limitations of this involvement.
Acknowledgments
Research supported by NSF Grants 0441934 and 0922075 (DJB), NIMH Grant F32MH092991 (SR), and a Howard Hughes Medical Institute Life Science Internship (DD). The authors wish to thank Dr. Howard Eichenbaum for valuable comments on previous versions of the manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Aggleton JP. Understanding retrosplenial amnesia: Insights from animal studies. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.09.030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Reviews Neuroscience. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Leising KJ, Stahlman WD, Waldmann MS. Rats distinguish between absence of events and lack of information in sensory preconditioning. International Journal of Comparative Psychology. 2009;22:1–18. [Google Scholar]
- Brogden WJ. Sensory preconditioning. Journal of Experimental Psychology. 1939;25:323– 332. [Google Scholar]
- Bouton ME. Learning and Behavior: A Contemporary Synthesis. Sunderland, MA: Sinauer; 2007. [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N. Spatial cognition and the brain. Annals of the New York Academy of Sciences. 2008;1124:77–97. doi: 10.1196/annals.1440.002. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Journal of Comparative Neurology. 1998;398:1–27. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Cain DP, Humpartzoomian R, Boon F. Retrosplenial cortex lesions impair water maze strategies learning or spatial place learning depending on prior experience of the rat. Behavioural Brain Research. 2006;170:316–325. doi: 10.1016/j.bbr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Perirhinal cortex lesions impair feature-negative discrimination. Neurobiology of Learning and Memory. 2006a;86:205–213. doi: 10.1016/j.nlm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Perirhinal cortex lesions impair simultaneous but not serial feature-positive discrimination learning. Behavioral Neuroscience. 2006b;120:970–975. doi: 10.1037/0735-7044.120.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Jarrard LE, Davidson TL. The effects of selective ibotenate lesions of the hippocampus on conditioned inhibition and excitation. Cognitive, Affective and Behavioral Neuroscience. 2003;3:111–119. doi: 10.3758/cabn.3.2.111. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum HB. Comparative cognition, hippocampal function, and recollection. Comparative Cognition & Behavior Reviews. 2007;2:47–66. [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;20:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Jablonski K, Feiler AM. Visual scene processing in familiar and unfamiliar environments. Journal of Neurophysiology. 2007;97:3670–3683. doi: 10.1152/jn.00003.2007. [DOI] [PubMed] [Google Scholar]
- Fenske MJ, Aminoff E, Gronau N, Bar M. Chapter 1 top-down facilitation of visual object recognition: object-based and context-based contributions. Progress in Brain Research. 2006;155:3–21. doi: 10.1016/S0079-6123(06)55001-0. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Cuppernell C, Flannery K, Gabriel M. Limbic thalamic, cingulate cortical and hippocampal neuronal correlates of discriminative approach learning in rabbits. Behavioural Brain Research. 1996;80:123–36. doi: 10.1016/0166-4328(96)00027-7. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Lambert RW, Foster K, Orona E, Sparenborg S, Maiorca RR. Anterior thalamic lesions and neuronal activity in the cingulate and retrosplenial cortices during discriminative avoidance behavior in rabbits. Behavioral Neuroscience. 1983;97:675–96. doi: 10.1037//0735-7044.97.5.675. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Sparenborg SP. Posterior cingulated cortical lesions eliminate learning- related unit activity in the anterior cingulate cortex. Brain Research. 1987;409:151–7. doi: 10.1016/0006-8993(87)90752-9. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Sparenborg SP, Stolar N. Hippocampal control of cingulate cortical and anterior thalamic information processing during learning in rabbits. Experimental Brain Research. 1987;67:131–52. doi: 10.1007/BF00269462. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Using Pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learning & Memory. 2011;7:257–266. doi: 10.1101/lm.35200. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Impaired spatial performance in rats with retrosplenial lesions: importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. Journal of Neuroscience. 2002;22:1155–1164. doi: 10.1523/JNEUROSCI.22-03-01155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. The Psychology of Learning and Motivation. Academic Press; 1984. Origins of behavior in Pavlovian conditioning; pp. 129–174. [Google Scholar]
- Holland PC. The nature of conditioned inhibition in serial and simultaneous feature negative discrimination. In: Miller RR, Spear NE, editors. Information processing in animals: Conditioned inhibition. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1985. pp. 267–298. [Google Scholar]
- Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Holland PC, Ross RT. The savings test for associations between neutral stimuli. Animal Learning & Behavior. 1983;11:83–90. [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience. 2008a;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behavioral Neuroscience. 2008b;122:651–658. doi: 10.1037/0735-7044.122.3.651. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignalled contextual fear conditioning. Behavioral Neuroscience. 2008c;122:1070–1077. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiology of Learning and Memory. 2009;91:408–414. doi: 10.1016/j.nlm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens. Journal of Neuroscience. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. Journal of Comparative Neurology. 2007;502:10–33. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Lipton PA, White JA, Eichenbaum H. Disambiguation of overlapping experiences by neurons in the medial entorhinal cortex. Journal of Neuroscience. 2007;27:5787–5795. doi: 10.1523/JNEUROSCI.1063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoyanov NV, Lukoyanova EA, Andrade JP, Paula-Barbosa MM. Impaired water maze navigation of Wistar rats with retrosplenial cortex lesions: effect of nonspatial pretraining. Behavioral Brain Research. 2005;158:175–182. doi: 10.1016/j.bbr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- MacLeod JE, Bucci DJ. Contributions of the subregions of medial prefrontal cortex to negative occasion setting. Behavioral Neuroscience. 2010;124:321–328. doi: 10.1037/a0019344. [DOI] [PubMed] [Google Scholar]
- MacLeod JE, Vucovich MM, Bucci DJ. Differential effects of ncotinic acetylcholine receptor stimulation on negative occasion setting. Behavioral Neuroscience. 2010;124:656–661. doi: 10.1037/a0020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learning & Memory. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Annual Review of Neuroscience. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease) European Journal of Neuroscience. 2003;18:2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Lesions of the perirhinal cortex impair sensory preconditioning in rats. Behavioral Neuroscience. 2000;112:69–75. doi: 10.1016/s0166-4328(00)00168-6. [DOI] [PubMed] [Google Scholar]
- Park S, Chun MM, Johnson MK. Refreshing and integrating visual scenes in scene-selective cortex. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21406. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parron C, Save E. Comparison of the effects of entorhinal and retrosplenial cortical lesions on habituation, reaction to spatial and non-spatial changes during object exploration in the rat. Neurobiology of Learning and Memory. 2004;82:1–11. doi: 10.1016/j.nlm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego, CA: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Robinson SN, Dong C, DeLeo JA, Bucci DJ. Fear conditioning is associated with increased Arc mRNA expression in the retrosplenial cortex. Society for Neuroscience Abstracts 2010 [Google Scholar]
- Ross RT, Holland PC. Conditioning of simultaneous and serial feature-positive discriminations. Animal Learning & Behavior. 1981;9:293–303. [Google Scholar]
- Rudy JW, Sutherland RJ. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behavioural Brain Research. 1989;34:97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: an appraisal and reconfiguration. Hippocampus. 1995;5:375–89. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Sato N, Sakata H, Tanaka YL, Taira M. Navigation-associated medial parietal neurons in monkeys. Proceedings of the National Academy of Sciences USA. 2006;103:17001–17006. doi: 10.1073/pnas.0604277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Sakata H, Tanaka YL, Taira M. Context-dependent place-selective responses of the neurons in the medial parietal region of macaque monkeys. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp147. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Shibata H, Honda Y, Sasaki H, Naito J. Organization of intrinsic connections of the retropslenial cortex in the rat. Anatomical Science International. 2009;84:280–292. doi: 10.1007/s12565-009-0035-0. [DOI] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, Gabriel M. Fornix lesions impair context-related cingulothalamic neuronal patterns and concurrent discrimination learning in rabbits (Oryctolagus cuniculus) Behavioral Neuroscience. 2004;118:1225–1239. doi: 10.1037/0735-7044.118.6.1225. [DOI] [PubMed] [Google Scholar]
- Solomon PR. Role of the hippocampus in blocking and conditioned inhibition of the rabbit’s nictitating membrane response. Journal of Comparative Physiology and Psychology. 1977;91:407–17. doi: 10.1037/h0077330. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Hoesing JM. Posterior cingulate cortex and spatial memory: A microlimnology analysis. In: Vogt BA, Gabriel M, editors. Neurobiology of the cingulate cortex and limbic thalamus: A comprehensive handbook. Boston: Birkhauser; 1993. pp. 461–477. [Google Scholar]
- Suzuki WA, Amaral DG. The perirhinal and parahippocampal cortices of the Macaque monkey: Cortical afferents. Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Tsukiura T, Matsue Y, Yamadori A, Fujii T. Dissociable brain activations during the retrieval of different kinds of spatial context memory. Neuroimage. 2005;25:993–1001. doi: 10.1016/j.neuroimage.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Talk AC, Kang E, Gabriel M. Independent generation of theta rhythm in the hippocampus and posterior cingulate cortex. Brain Research. 2004;1015:15–24. doi: 10.1016/j.brainres.2004.04.051. [DOI] [PubMed] [Google Scholar]
- Talk AC, Stoll E, Gabriel M. Cingulate cortical coding of context-dependent latent inhibition. Behavioral Neuroscience. 2005;119:15224–32. doi: 10.1037/0735-7044.119.6.1524. [DOI] [PubMed] [Google Scholar]
- Talk AC, Gandhi CC, Matzel LD. Hippocampal function during behaviorally silent associative learning: dissociation of memory storage and expression. Hippocampus. 2002;12:648–56. doi: 10.1002/hipo.10098. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. Journal of Comparative Neurology. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. Journal of Comparative Neurolog. 1992;315:200–16. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. Journal of Comparative Neurology. 1990;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behavioral Neuroscience. 2002;116:85–94. [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Testing the importance of the retrosplenial guidance system: effects of different sized retrosplenial cortex lesions on heading direction and spatial working memory. Behavioural Brain Research. 2004;155:97–108. doi: 10.1016/j.bbr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vann SD, Albasser MM. Hippocampal, retrosplenial, and prefrontal hypoactivity in a model of diencephalic amnesia: Evidence towards an interdependent subcortical-cortical memory network. Hippocampus. 2009;19:1090–1102. doi: 10.1002/hipo.20574. [DOI] [PubMed] [Google Scholar]
- Ward-Robinson J, Coutureau E, Good M, Honey RC, Killcross AS, Oswald CJ. Excitotoxic lesions of the hippocampus leave sensory preconditioning intact: implications for models of hippocampal function. Behavioral Neuroscience. 2001;115:1357–62. doi: 10.1037//0735-7044.115.6.1357. [DOI] [PubMed] [Google Scholar]
- Wesierska M, Adamska I, Malinowska M. Retrosplenial cortex lesion affected segregation of spatial information in place avoidance task in the rat. Neurobiology of Learning and Memory. 2009;91:41–49. doi: 10.1016/j.nlm.2008.09.005. [DOI] [PubMed] [Google Scholar]