Abstract
Background and Purpose
The risk of stroke shortly after TIA with infarction on DWI (also known as “transient symptoms with infarction” or “TSI”) is substantially higher than the risk after imaging-normal TIA. We sought to assess the utility of a web-based recurrence risk estimator (http://www.nmr.mgh.harvard.edu/RRE/) originally developed for use in patients with ischemic stroke for predicting 7-day risk of stroke in patients with TSI.
Methods
We calculated RRE and ABCD2 scores in a retrospective series of 257 consecutive patients with TSI diagnosed by DWI within 24 hours of symptom onset. We defined subsequent stroke as a clinical deterioration associated with new infarction spatially distinct from the index lesion. We assessed the predictive performance of each model by computing the area under receiver operating characteristics curve.
Results
Over 7-day follow-up, 16 patients developed a recurrent stroke (6.2%). The sensitivity and specificity of an RRE score of 2 or greater for predicting 7-day stroke risk were 87% and 73% respectively. The area under the ROC curve was 0.85 (95% CI, 0.78-0.92) for RRE and 0.57 (95% CI, 0.45-0.69) for the ABCD2 score (z-test, p<0.001).
Conclusion
The RRE score seems to predict the 7-day risk of stroke after a TSI. If further validated in larger datasets, the RRE score could be useful in identifying high-risk patients with TSI who may benefit from early intervention with targeted stroke prevention strategies.
Keywords: brain infarction, diffusion-weighted imaging, MRI, transient ischemic attack, stroke risk
Introduction
The new tissue-based definition endorsed by the AHA/ASA proposes to classify TIA as a transient episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia, without evidence of acute infarction1. All remaining neurological events, regardless of whether symptoms are transient or permanent, are called “ischemic stroke,” as long as they are associated with brain infarction. The tissue-based definition is an important step in the right direction because it is no longer based on the arbitrary 24-hour duration criterion and more importantly it is compatible with the pathophysiology (i.e. based on objective evidence of ischemia)1. Categorizations based on pathophysiology are crucial for accurate diagnosis and treatment of diseases with certain level of complexity. Categorizations are likely to serve even more ideally when they are also consonant with prognosis. Prognostic information allows individualization of timing and optimal method of diagnostic evaluation and treatment. While appealing from a pathophysiological point of view, the tissue-based definition might carry some negative impact from a prognostic point of view. Specifically, it is now known that the early risk (7-day) of stroke after imaging positive TIA (transient symptoms with infarction or TSI)2 is substantially higher – as much as fifteen times higher -- than the risk after ischemic stroke3-7; the 7-day risk of stroke ranges from 4% and 16% after TSI5-9 whereas the corresponding risk after an ischemic stroke changes between 1% and 3%2-4,6,10. Classifying “TSI” and “stroke” in the same category may unintentionally obscure the clinical importance of identifying TSI as the warning sign of an impending stroke within the lower risk pool of strokes. We sought to develop a means to retain the prognostic information in the familiar TIA terminology while using the new definition. For this, we assessed the utility of a prognostic score (recurrence risk estimator or RRE) for predicting 7-day risk of stroke in TSI10. The RRE tool was originally developed to predict 14- and 90-day risk of recurrence in patients with ischemic stroke (not TSI) per the conventional definition. If validated in TSI, the score could be used to identify patients with imminent risk of developing another stroke among the overall population of strokes whether the new definition or earlier definitions are used, thus keeping a way for practitioners to identify the highest risk subjects whether or not they have become familiar with current terminology.
Methods
Study population and data acquisition
This was a retrospective study of consecutive patients with TIA defined according to time-based traditional definition (symptoms lasting <24 hours)11 who were admitted during a 6-year period between 2003 and 2009. Patients were identified from a prospectively maintained database that included all admissions to the emergency department with possible diagnosis of TIA. The current study included patients with TIA who had a clinically relevant acute infarction on diffusion-weighted imaging (DWI) obtained within 24 hours of symptom onset. DWI was performed as a routine piece of diagnostic evaluation in all TIA patients who did not have contraindication to MRI. As previously stated, we used the term “TSI” (transient symptoms with infarction) to designate TIA with DWI evidence of acute infarction2.
Data on TSI characteristics, patient demographics, vascular risk factors, ABCD2 score, etiologic stroke subtype, and treatment for secondary prevention were collected from the database. The stroke etiology was classified using the automated Causative Classification of Stroke (CCS) system based on information available from clinical history, ECG, brain imaging, and brain vascular imaging within the first 24 hours of symptom onset12. The study protocol was approved by the local institutional review board.
The RRE score was computed for each patient by a stroke neurologist blinded to the patient’s recurrence status. RRE is a web-based (http://www.nmr.mgh.harvard.edu/RRE/), 7-point score composed of 2 clinical and 4 imaging predictors10. These predictors are prior TIA or stroke within the preceding month of TSI (1 point), etiologic CCS subtype (1 point for large artery atherosclerosis and other uncommon causes and zero point for all other remaining subtypes), the presence of multiple acute infarcts (1 point), simultaneous acute infarcts in both hemispheres or in both anterior and posterior circulations (1 point), multiple infarcts of different ages (combination of acute and subacute infarcts, 1 point), and isolated cortical location (1 point). The RRE provides estimates for 14-day and 90-day risk of recurrence after an ischemic stroke based on information available to physician immediately after initial stroke evaluation in typical clinical practice (based on clinical history, EKG, and baseline brain and vascular imaging). The RRE score has demonstrated adequate calibration and good discrimination in the prior derivation and validation datasets10. The current study aims to validate RRE in a different patient population (TSI instead of stroke) and outcome window (7 days instead of 14 or 90 days).
Follow-up assessment
The outcome parameter was recurrent ischemic stroke within 7 days of the index TSI. Follow-up information was collected retrospectively by an investigator blinded to RRE scores through inspection of inpatient medical record notes as well as from routine one to three month outpatient assessment notes by the treating neurologist. These notes included a detailed description and timing of the follow-up event. Recurrent stroke was defined as a clinical incident that was clearly attributable to a new area of brain infarction visualized by imaging as spatially distinct from the index lesion. Each clinically suspected recurrent event was adjudicated by a separate investigator using pertinent brain images without the knowledge of clinical and imaging characteristics of the index TSI.
Statistics
All numerical variables were expressed as mean ± standard deviation (SD) or median (inter-quartile range, IQR). Fisher‘s Exact Test, Mann-Whitney U and Kruskall-Wallis tests were used to explore the relationship between baseline categorical variables and recurrent stroke status as of day-7. We quantified the predictive validity of RRE and ABCD2 scores by computing the receiver operating characteristic (ROC) curve and calculated the area under the ROC curve (AUC). We compared the AUC for different scores using the z-test13. Statistical analyses were performed using SPSS 16.0.
Results
A total of 302 consecutive patients with TSI were admitted during the study period. Seven-day follow-up information was available in 257 patients. Patients with incomplete follow-up (n=45) had lower RRE scores as compared to patients with complete follow-up [n=257, median (IQR) RRE score 1 (0-2) vs. 2 (1-3), p=0.001]. Other baseline clinical predictors of stroke risk (etiologic stroke mechanism, recent history of stroke or TIA, and ABCD2 score) were similar between patients with and without complete follow-up.
Overall, 24 patients developed a recurrent clinical event within 7 days of TSI (9.3%). In 8 of these 24 patients, recurrent events were not associated with a new infarction on DWI. All 8 patients with clinical recurrence unconfirmed by imaging had lacunar infarcts at baseline; the recurrent event was stereotypical for the index TSI in all 8 (stuttering lacunar syndrome). The remaining 16 patients (6.2%) who developed a subsequent event associated with imaging evidence of a new infarct fulfilled the predefined criteria for recurrence.
The median age of the study population was 67 years (IQR, 55-76 years), the median time to MRI was 9.4 hours (IQR, 5.5-15.2 hours), the median ABCD2 score was 4 (IQR, 3-5), and the median RRE score was 2 (IQR, 1-3). Table 1 summarizes the baseline characteristics and clinical and imaging features with respect to subsequent stroke status. Patients with recurrence were more likely to have a prior TIA or stroke within the month preceding the index TSI (p=0.004) and “large artery atherosclerosis” and “other uncommon causes” (dissection, vasculitis, etc.) as the underlying TSI mechanism (p<0.001) (Table 1). There was no difference in mean and median ABCD2 scores between patients with and without subsequent stroke. There were 8 patients with and 28 patients without subsequent stroke who were eligible for carotid intervention. Five of the 8 (62.5%) with and 16 of the 28 (57.1%) without subsequent stroke eventually underwent carotid endarterectomy or stenting (fisher’s exact test p=1.000).
Table 1.
Baseline characteristics according to recurrence status
| Recurrence (n=16) |
No Recurrence (n=241) |
p | |
|---|---|---|---|
| Age | 70 (61-82) | 66 (55-76) | 0.255 |
| Female | 9 (56.2%) | 115 (47.7%) | 0.508 |
| Hypertension | 13 (81.2%) | 158 (65.6%) | 0.276 |
| Diabetes Mellitus | 3 (18.8%) | 44 (18.3%) | 1.000 |
| Atrial fibrillation | 1 (6.2%) | 39 (16.2%) | 0.479 |
| Prior TIA or stroke | 11 (68.8%) | 80 (33.2%) | 0.004 |
| Time to MRI (hours) | 9.6 (5.2-15.0) | 9.4 (5.5-15.5) | 0.907 |
| CCS stroke subtype | |||
| Large artery atherosclerosis | 11 (68.8%) | 53 (22.0%) | |
| Cardio-aortic embolism | 1 (6.2%) | 35 (14.5%) | <0.001 |
| Small artery occlusion | 0 (0.0%) | 21 (8.7%) | |
| Other rare causes | 4 (25.0%) | 15 (6.2%) | |
| Undetermined | 0 (0.0%) | 117 (48.5%) | |
| Treatment | |||
| Antiplatelet treatment | 12 (75.0%) | 196 (81.3%) | 0.533 |
| Anticoagulation | 11 (68.8%) | 116 (48.1%) | 0.110 |
| Mean ABCD2 Score ± SD | 4.5 ± 1.0 | 4.1 ± 1.6 | 0.292 |
| Median ABCD2 Score (IQR) | 5 (3-5) | 4 (3-5) | 0.321 |
| Mean RRE Score ± SD | 3.5 ± 1.0 | 1.7 ± 1.3 | <0.001 |
| Median RRE Score (IQR) | 3 (3-4) | 2 (1-3) | <0.001 |
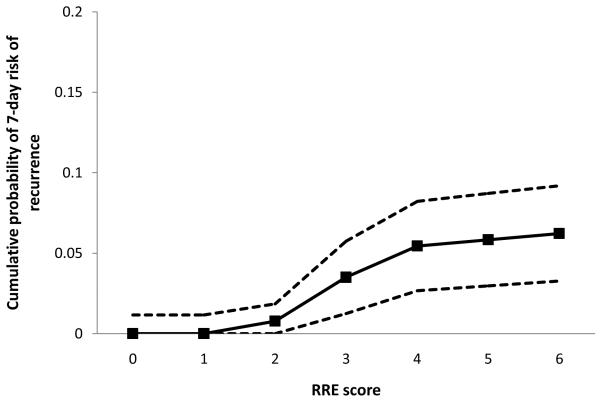
Table 2 shows the distribution of RRE scores in patients with and without recurrent stroke. The risk of subsequent stroke at 7 days continuously rose with increasing RRE scores (p<0.001, log rank test) (Figure 1); the risk was 0% (95% CI, 0-3%) with no or one predictor, 3% (95% CI, 0-3%) with two predictors, 15% (95% CI, 5-25%) with 3 predictors, and 22% (95% CI, 7-35%) with 4 or more predictors. Both mean and median RRE score was higher in patients with subsequent stroke. For scores 3 or greater, the sensitivity and specificity for predicting 7-day risk of subsequent stroke was 87% and 73% respectively.
Table 2.
Distribution of RRE score in patients with and without recurrence
| RRE score | Recurrence (n=16) |
No Recurrence (n=241) |
|---|---|---|
| 0 | 0 (0.0%) | 48 (19.9%) |
| 1 | 0 (0.0%) | 68 (28.2%) |
| 2 | 2 (12.5%) | 61 (25.3%) |
| 3 | 7 (43.8%) | 39 (16.2%) |
| 4 | 5 (31.2%) | 20 (8.3%) |
| 5 | 1 (6.2%) | 4 (1.7%) |
| 6 | 1 (6.2%) | 1 (0.4%) |
Figure 1.
Cumulative probability of 7-day risk of recurrence across the RRE scores. The dotted lines represent 95% confidence intervals.
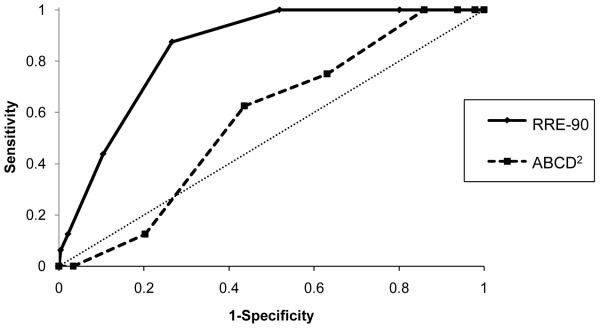
Figure 2 shows the receiver operator characteristics curve for predicting 7-day risk of stroke in patient with TSI. The AUC was 0.85 (95% CI, 0.78-0.92, p<0.001). The AUC for the ABCD2 score was 0.57 (95% CI, 0.45-0.69, p=0.331). The improvement in diagnostic performance as measured by AUC by the RRE score over the ABCD2 score was both clinically meaningful (from 0.57 to 0.85) and statistically significant (p<0.001, z test). The AUC remained essentially unchanged (0.86, 95% CI, 0.80-0.93) when analyses included the 45 patients with incomplete follow-up by carrying forward the available follow-up information.
Figure 2.
Receiver operating characteristics curves for 7-day risk of recurrent stroke
Discussion
TIA, when using the tissue-based definition, should be thought of as an extremely low-risk condition. According to a recent pooled analysis of 4574 patients with conventionally-defined TIA from 12 centers9, the 7-day risk of stroke after a “TIA with no infarction” is 0.4%. In contrast, TSI (that is, TIA with infarction) is a high-risk condition; the 7-day risk of stroke ranges from 4% and 16%5-8. In settings where brain imaging is available and feasible, the fundamental question is, therefore, no longer about whether a patient with a traditionally-defined TIA will develop a subsequent stroke, but rather, the question is which patient with TSI is at imminent risk of developing a stroke. Our results indicate that the RRE criteria segregate the TSI population into different risk groups with a high degree of predictive power (AUC=0.85). Using RRE, approximately two thirds of patients with TSI can be classified as low risk (risk ≤ 1%, score ≤ 2) and one third as high risk (risk > 18%, score >2). The implication of these findings for the practitioner is that the RRE tool can allow determination of who is at high short-term risk and therefore may need very urgent intervention.
Recent studies have shown that the risk of early recurrence after minor stroke is relatively comparable to the risk after TSI14, 15. One could, thus, retain the prognostic information in TSI by adjusting the current tissue-based definition to accommodate for prognostically different categories (such as defining a separate minor stroke category). Nevertheless, we suspect that such an approach would not be practical and may even cause confusion because prognostic categories in stroke are many (minor vs. moderate vs. major stroke, small vs. large infarct, single vs. multiple infarcts, etc.) and most are definition dependent; at least 4 different definitions have been used in the literature to describe minor stroke (NIHSS score ≤1, ≤3, ≤5, and ≤9)16, 17. Here, we offer an alternative approach: a simple score that can be universally applied to anyone with “ischemic stroke” as defined by the tissue-based definition to identify prognostically similar subsets. After one internal and one independent validation10, this is another validation of RRE for a new application in a different population. The score has been previously shown to reliably predict short-term risk (14-day risk) of recurrence in patients with ischemic stroke per the conventional definition. The present study shows that RRE can also predict short-term risk (7-day risk) of stroke after a TSI (which is now called stroke per the tissue-based definition). Hence, if further validated for 7 and 14 day risk prediction, RRE could be applied to any patient with clinically relevant brain infarction, regardless of whether neurological symptoms are transient or persistent, to identify individuals at risk of early recurrence. By this way, RRE may complement the tissue-based definition by providing a prognostic component to it.
In contrast to RRE, the ABCD2 score reveals little predictive value in patients with TSI; the point estimate of AUC (0.57) was not different from predictions based on chance alone although a modest predictive value could not be excluded (95% CI, 0.45–0.69). The ABCD2 score appears to predict stroke risk partly because of its ability to discriminate between a true TIA and a suspected TIA with eventual diagnosis of a non-vascular TIA-mimic18-20. The predictive value of the ABCD2 score shows marked variation among studies with different methods of patient selection and case-mix (true and suspected TIA)9,21. In contrast to the general TIA population, TSI represents a fairly homogenous subset consisting of definite cerebral infarctions. The prognostic value of a score in this homogenous group in large part relies on its ability to account for the underlying stroke mechanism3. All individual components of RRE directly relate to the underlying stroke mechanism. Etiologic stroke subtype itself is a predictor in RRE. Imaging predictors such as location, age, and number of infarcts and their spatial relationship with respect to each other provide information on whether the underlying stroke mechanism is unstable with potential to cause another stroke10. Other predictors such as “recent history of TIA/stroke” and “multiple infarcts of different ages” further add to the system’s ability to mark an unstable etiology by providing the continuity information.
The complexity of stroke and current nomenclature require one additional clarification. Pragmatic definition of recurrent stroke includes any clinical incident that is associated with a new ischemic lesion spatially distinct from the initial infarct. While in general this is straightforward, the special case of “stuttering lacunar infarction” causes some pause. In stuttering lacunar infarction, patients present with a cluster of repetitive, stereotypic, and short lasting events -- which sometimes might be thought of as TIAs -- and then subsequently develop a permanent deficit with no imaging evidence of a spatially distinct new lesion beyond the initial index lacunar infarct. This special case, then, consists of a TSI patient developing a subsequent clinical stroke without a new, spatially distinct infarct. The dissociation between the changing clinical symptoms and the stable imaging findings is important, and we propose the terminology, “lacunar infarct paradox,” to clarify this dissociation. Also, we point out that the RRE criteria should not be applied in this special case.
This study has several limitations. First, 7-day follow-up information was not available in approximately 15% of patients. Missing follow-up data, however, would not be expected to significantly alter the predictive performance of RRE unless patients with incomplete follow-up more often developed a recurrent stroke (hence they had lower RRE scores). It is unlikely that the stroke rate was higher in the cohort with missing follow-up information because, in our practice, the primary neurologist taking care of TIA is often notified in an event of stroke occurring within days of TIA and the data collection methods would have captured this. Second, the number of outcome events was small for reliable validation of a prognostic score and therefore limited the power of the predictive model22. The 95% confidence intervals around the point estimate for AUC were large. Nevertheless, the lower confidence limit was still well above the threshold set for random prediction. Moreover, the predictive performance of RRE in this dataset was comparable to those in prior validation and derivation datasets, suggesting that the current estimate for AUC reflects the true predictive capacity of the RRE score. Third, although it is recommended that MRI as the method of imaging in TIA1, approximately 5-10% of patients cannot be scanned because of contraindications and this may limits widespread applicability of RRE. While the RRE requires an imaging study but the ABCD2 score does not, the improved performance and widespread use of MRI in the evaluation of stroke reduce this concern for most practice settings. We note that current guidelines for the management for TIA already recognize the helpful role of imaging, and specifically state, “MRI…can help to determine which TIA patients to admit to hospital and it may help in identifying patients to treat with more aggressive therapies”1. Our current data support this recommendation and demonstrate the added value of imaging beyond the ABCD2 score in identifying patients for the most aggressive treatment. Finally, the predictive performance of RRE may be different in clinical settings where patient profile, and type and timing of preventive stroke treatments substantially differ from that is reported in this study. Therefore, further validation is critical for the generalizability of our results.
Our findings offer utility in clinical management of TSI. The major concept behind using prognostic tools in medicine is to identify individuals who are at risk of developing a potentially avoidable adverse event. Prognostic information becomes more critical if future events are prevalent and occur soon after the index disease so that acute care at specialized centers can be organized on an individual basis. TSI is an ideal condition for prognostic risk evaluation because the risk of subsequent stroke is high and imminent. The RRE score may allow physicians to identify high risk patients who benefit most from timely identification of the underlying etiology, early institution of specific preventive treatment such as carotid endarterectomy, and care at specialized centers where timely administration of acute treatments in the event of a subsequent stroke is possible23.
Acknowledgments
Funding Sources: EMA: None, KLF: NIH grant P50-NS051343, LHS: NIH grant P50-NS051343, AGS: NIH grant R01-NS038477, R01-NS063925, HA: NIH grant R01-NS059710
Footnotes
Disclosures: EMA, KLF, LHS: None, AGS: http://www.biomarkers.org/NewFiles/disclosures.html lists full disclosures, HA: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 2.Ay H, Koroshetz WJ, Benner T, Vangel MG, Wu O, Schwamm LH, et al. Transient ischemic attack with infarction: a unique syndrome? Ann Neurol. 2005;57:679–686. doi: 10.1002/ana.20465. [DOI] [PubMed] [Google Scholar]
- 3.Lowett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 4.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50:208–216. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 5.Ay H, Arsava EM, Johnston SC, Vangel M, Schwamm LH, Furie KL, et al. Clinical- and imaging-based prediction of stroke risk after transient ischemic attack: the CIP model. Stroke. 2009;40:181–186. doi: 10.1161/STROKEAHA.108.521476. [DOI] [PubMed] [Google Scholar]
- 6.Prabhakaran S, Chong JY, Sacco RL. Impact of abnormal diffusion-weighted imaging results on short-term outcome following transient ischemic attack. Arch Neurol. 2007;64:1105–1109. doi: 10.1001/archneur.64.8.1105. [DOI] [PubMed] [Google Scholar]
- 7.Calvet D, Touzé E, Oppenheim C, Turc G, Meder JF, Mas JL. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke. 2009;40:187–192. doi: 10.1161/STROKEAHA.108.515817. [DOI] [PubMed] [Google Scholar]
- 8.Asimos AW, Rosamond WD, Johnson AM, Price MF, Rose KM, Murphy CV, et al. Early diffusion weighted MRI as a negative predictor for disabling stroke after ABCD2 score risk categorization in transient ischemic attack patients. Stroke. 2009;40:3252–3257. doi: 10.1161/STROKEAHA.109.555425. [DOI] [PubMed] [Google Scholar]
- 9.Giles MF, Albers GW, Amarenco P, Arsava MM, Asimos A, Ay H, et al. Addition of Brain Infarction to the ABCD2 Score (ABCD2I). A Collaborative Analysis of Unpublished Data on 4574 Patients. Stroke. 2010;41:1907–1913. doi: 10.1161/STROKEAHA.110.578971. [DOI] [PubMed] [Google Scholar]
- 10.Ay H, Gungor L, Arsava EM, Rosand J, Vangel M, Benner T, et al. A score to predict early risk of recurrence after ischemic stroke. Neurology. 2010;74:128–135. doi: 10.1212/WNL.0b013e3181ca9cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute of Neurological Disorders and Stroke Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21:637–676. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 12.Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38:2979–2984. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 13.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 14.Coull AJ, Lovett JK, Rothwell PM. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328:326. doi: 10.1136/bmj.37991.635266.44. Oxford Vascular Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ois A, Gomis M, Rodríguez-Campello A, Cuadrado-Godia E, Jiménez-Conde J, Pont-Sunyer C, et al. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke. 2008;39:1717–1721. doi: 10.1161/STROKEAHA.107.505438. [DOI] [PubMed] [Google Scholar]
- 16.Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, De Marchis GM, et al. What is minor stroke? Stroke. 2010;41:661–666. doi: 10.1161/STROKEAHA.109.572883. [DOI] [PubMed] [Google Scholar]
- 17.Coutts SB, Hill MD, Simon JE, Sohn CH, Scott JN, Demchuk AM, VISION Study Group Silent ischemia in minor stroke and TIA patients identified on MR imaging. Neurology. 2005;65:513–517. doi: 10.1212/01.wnl.0000169031.39264.ff. [DOI] [PubMed] [Google Scholar]
- 18.Quinn TJ, Cameron AC, Dawson J, Lees KR, Walters MR. ABCD2 scores and prediction of noncerebrovascular diagnoses in an outpatient population: a case-control study. Stroke. 2009;40:749–753. doi: 10.1161/STROKEAHA.108.530444. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan OC, Merwick A, Kelly LA, Hannon N, Marnane M, Kyne L, et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke. 2009;40:3449–3454. doi: 10.1161/STROKEAHA.109.557074. [DOI] [PubMed] [Google Scholar]
- 20.Josephson SA, Sidney S, Pham TN, Bernstein AL, Johnston SC. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096–3098. doi: 10.1161/STROKEAHA.108.514562. [DOI] [PubMed] [Google Scholar]
- 21.Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 22.Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol. 2005;58:475–483. doi: 10.1016/j.jclinepi.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, et al. Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]