Abstract
The use of food products designed to mimic the sensory properties of sweet and fat while providing fewer calories has been promoted as a method for reducing food intake and body weight. However, such products may interfere with one mechanism that animals use to regulate energy balance, a learned relationship between the sensory properites of food and the caloric consequences of consuming those foods. Consistent with this hypothesis, previous data have shown that providing rats with sweet tastes that are not associated with the delivery of calories using high-intensity sweeteners results in increased food intake, body weight and adiposity, but only if the diet on which they are maintained also tastes sweet. In the present experiment, we examined whether use of the fat substitute, olestra, would have similar consequences by comparing the effects of consuming high-fat, high-calorie potato chips to the effects of consuming potato chips that sometimes signalled high calories (using high-fat potato chips) and that sometimes signalled lower calories (using non-fat potato chips manufactured with the fat substitute olestra). The results demonstrated that food intake, body weight gain and adiposity were greater for rats that consumed both the high-calorie chips and the low-calorie chips with olestra compared to rats that consumed consuming only the high-calorie chips, but only if animals were also consuming a chow diet that was high in fat and calories. When animals were maintained on a low-fat chow diet, intake, weight gain, and adiposity did not differ significantly based on chip type. However, rats previously exposed to both the low-calorie chips with olestra and the high-calorie chips exhibited increased body weight gain, food intake and adiposity when they were provided with a high fat, high calorie chow diet, even though the potato chips were no longer available. This suggests that the experience with the chips containing olestra affected the ability to predict high calories based on the sensory properties associated with fat. These results extend the generality of previous findings that interfering with a predictive relationship between sensory properties of foods and calories may contribute to dysregulation of energy balance, overweight and obesity.
Keywords: Learning, Appetitive behavior, Food intake, Obesity
Introduction
One method that has been suggested to combat the spread of obesity is to reduce the caloric density of foods, with the hope that reducing the number of calories per unit of food will result in lower total caloric intake. While caloric density can be reduced by simple dilution (e.g. the addition of water or other bulking agents) a reduction in caloric density of foods is also accomplished by replacement of high calorie ingredients (such as sugar or fat) with substitutes designed to mimic the sensory properties of sweet and fat, but with fewer or no calories. Such substitutions are assumed to contribute to reductions in energy intake, as common sense dictates that decreasing the number of calories in foods will result in decreased energy intake.
However, a variety of evidence challenges this common sense view. For example, several prospective studies in people have reported a link between use of high-intensity, non-caloric sweeteners and increased risk for overweight, obesity and related health concerns such as diabetes and the metabolic syndrome (Dhingra et al., 2007; Fowler et al., 2008; Lutsey, Steffen, & Stevens, 2008). In addition, previous work in our lab and others has demonstrated that in rats consumption of foods or fluids sweetened with high-intensity sweeteners such as saccharin, acesulfame potassium and stevia can result in increased food intake, body weight, adiposity, and decreased thermic effects of food (Davidson & Swithers, 2004; Swithers, Baker, & Davidson, 2009; Swithers & Davidson, 2008; Swithers, Martin, Clark, Laboy, & Davidson, 2010; Swithers, Martin, & Davidson, 2010).
Previous research established that orosensory cues can enter into excitatory associations with caloric or nutritive outcomes (e.g., Bolles, Hayward, & Crandall, 1981; also see Sclafani, 1997). We have proposed that dysregulation of energy balance may result from a weakening of such learned associations. (Davidson & Swithers, 2004; Swithers et al., 2009; Swithers & Davidson, 2008; Swithers, Martin, Clark et al., 2010; Swithers, Martin, & Davidson, 2010). Within our view, sweet taste typically provides a signal for an animal that a caloric outcome is imminent. Animals use this predictive relationship to mobilize physiological responses that prepare the body for the arrival of calories. Specifically, orosensory and other pre-absorptive stimuli are known to evoke a variety of physiological responses (e.g., salivation, hormonal secretions, metabolic and thermic responses) that anticipate the nutritive or energetic consequences of intake. These “cephalic phase” responses (e.g., Zafra, Molina, & Puerto, 2006) are thought to prepare for the arrival of calories in the gut, thereby promoting efficient energy utilization (e.g., Woods & Ramsey, 2000; Smeets, Erkner, & de Graaf, 2010). Consumption of high-intensity sweeteners which provide a strong signal for calories, but without the delivery of calories, reduces the validity of this signalling relationship. A reduction in the validity of this sweet taste-calorie relation may promote positive energy balance by weakening the ability of sweet taste to evoke physiological responses that underlie energy regulation.
A similar disruption in energy balance may be produced when other food-related cues, such as the sensory properties of fat, do not consistently predict the delivery of calories. For example, foods manufactured with non-caloric fat substitutes such as olestra are designed to mimic the sensory qualities of fat but with significantly fewer calories than the same foods produced with regular fats. In fact, we have demonstrated that rats exposed to potato chips made with olestra were impaired in their short-term ability to compensate for the calories provided in a novel high-fat, high-calorie snack (Swithers, Doerflinger, & Davidson, 2006), suggesting an impaired ability to use cues associated with fat to predict caloric outcomes. However, longer-term energy balance (e.g. body weight gain) was not affected, perhaps because the animals were maintained on a standard rat chow that was low in fat, and therefore low in sensory cues associated with fat. In other words, outside of the caloric compensation test with the novel high-fat snack food, animals had little need to use sensory cues associated with fat to modulate food intake, and therefore long-term energy balance was maintained. Increasing the fat content of the maintenance diet would increase the role of fat cues in modulating intake, and perhaps increase the impact of exposure to the low-calorie chips. Consistent with this hypothesis, we have recently demonstrated that when sweet cues in the maintenance diet are minimized, saccharin-sweetened dietary supplements do not result in increased body weight gain (Davidson, Martin, Clark, & Swithers, 2011).
The goal of the present experiment was to test whether consumption of a maintenance diet that was high in fat and calories resulted in increased food intake, body weight gain and/or adiposity when animals received high-calorie, high-fat and low-calorie fat-substituted potato chips compared to animals receiving only high-fat potato chips. Half of the animals received potato chip dietary supplements in which the sensory properties of fat always predicted high calories (using regular, high-fat, high-calorie Pringles potato chips; 5.4 kcal/g) while the other half received potato chip supplements in which the sensory properties of fat sometimes predicted high calories (using high-fat, high-calorie Pringles potato chips) and sometimes predicted low calories (using Pringles Light potato chips manfactured with the fat substitute olestra; 2.5 kcal/g). Potato chips were provided for 28 days to animals maintained on either a standard low-fat chow diet or a high-fat chow maintenance diet. Following this chip exposure, animals in the low-fat chow group were then switched to the high-fat maintenance chow for 16 days, with no potato chips provided, to examine whether altered responses to the sensory cues of fat may have been acquired during the exposure to chips, but not expressed due to the lack of fat cues in the maintenance chow. The results indicated that when the strength of the relationship between the sensory cues of fat and calories was weakened by consumption of both low-calorie, fat-substituted chips and high-calorie, high-fat chips, consuming the high-fat diet resulted in increased body weight gain, regardless of whether exposure to the low-calorie, fat-substituted chips occurred before or during exposure to the high-fat maintenance diet.
Methods
Subjects were 31 male Sprague-Dawley rats (Harlan, Indianapolis) weighing 325–350 g at the time of arrival. Rats were maintained on a 14:10 light:dark cycle with lights on at 0400 h and off at 1800 h and were fed standard, pelleted lab chow diet (LabDiets 5001) for 5 days prior to being assigned to one of four groups matched on body weight (group means = 378 – 384 g). Body composition was assessed prior to the start of testing using NMR (EchoMRI-900).
During testing, two groups of rats were maintained on a powdered, low-fat chow diet (LabDiets 5001) while the other two groups were switched to a powdered, high fat (HF) diet previously used in our lab (TestDiets modified diet 5012 with 16% peanut oil and 4% starch; ~5.48 kcal/g, with ~ 41% of calories provided by fat, 41% provided carbohydrate, and 18% provided by protein;). On the same day as animals were placed on powdered diets, animals began receiving 5 g of crushed potato chips as a supplement to their assigned diet and water (which were provided ad lib). Chips used were Pringles Original and Pringles Sour Cream and Onion Flavored (~5.3 kcal/g), along with Pringles Light Original and Pringles Light Sour Cream and Onion Flavored (~ 2.65 kcal/g). Two flavors of chips were used to minimize potential differences in sensory properties of the high-fat, high-calorie versus fat-substituted chips, low-calorie chips. Two groups of animals (one on the HF maintenance diet and on the Standard Chow diet) received 5 g of high-fat, high-calorie chips daily. Thus, for this group (HF Chips), the sensory attributes of fat always predicted high calories. Two additional groups of animals (one on the HF Chow and one on the Standard Chow) received HF, high-calorie chips on half of the days and low-calorie, fat-substituted (Olestra) chips on the remaining days. Thus, for this group (Olestra + HF chips), the sensory attributes of fat sometimes predicted high calories and sometimes predicted low calories. During the first four days of chip presentation, animals in the Olestra + HF chips groups received one exposure to each of the four chip types in an order counterbalanced across the animals, with flavor of chips offered to animals in the HF chips groups matched to the Olestra + HF chips across animals. For the remaining 24 days, the order of presentation of the chip flavors (original versus sour cream and onion) and chip energy content was pseudo-randomized such that animals did not receive the same flavor or chip type more than 3 days in a row and animals received equal exposure to both flavors (and both chip types in the Olestra + HF group).
Animals received chip supplements for 28 days, with one day of maintenance diet and water alone after 14 days of chip exposure. At the end of the 28 days of chip consumption, body composition was again assessed by NMR, and chip supplements were discontinued. All animals then consumed the powdered HF diet for 16 days; NMR was used to assess body composition at the end of those 16 days. Body weight, chip intake and maintenance diet intake were measured daily by weighing (corrected for spillage) throughout testing. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Purdue University Animal Care and Use Committee.
Body weight gain during the chip exposure was analyzed using a 3-Way (Base diet × Chip group × Day) Repeated Measures ANOVA, with Day as a within-subjects factor and Base diet and Chip group as between-subjects factors. The quantity of chips consumed and total weekly caloric intake (chip calories plus base diet calories) were assessed using separate 3-Way (Base diet × Chip group × Week) Repeated Measures ANOVA, with Week as a within-subjects factor and Base diet and Chip group as between-subjects factors. Body weight gain after discontinuation of chips was assessed with a 3-Way (Starting Base diet × Chip group × Day) Repeated Measures ANOVA, with Day as a within-subjects factor and Starting Base diet and Chip group as between-subjects factors. Chow intake after discontinuation of chips was assessed using separate One-Way (Chip group) ANOVAs. Fat mass and lean mass at the beginning of the study were assessed with separate Two-Way (Base diet × Chip) ANOVAs. Fat mass and lean mass after chip exposure and following chip discontinuation were assessed using separate Two-Way (Base diet × Chip) ANCOVAs with starting fat mass or starting lean mass used as the covariate as appropriate. Where indicated, post-hoc analyses were done using subsequent 1-Way or 2-Way ANOVAs, followed by Newman-Keuls tests as indicated. Alpha was set at 0.05 for all analyses.
Results
Body Weight Gain
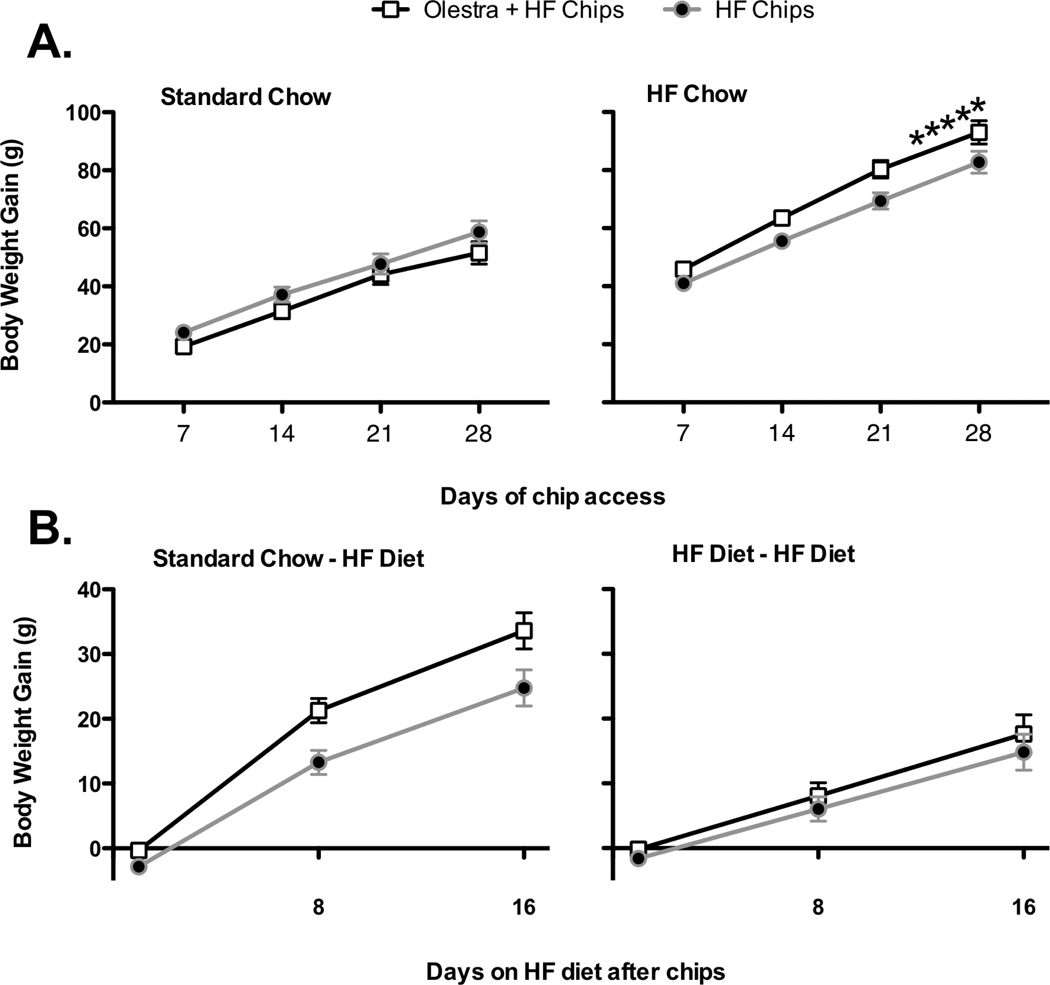
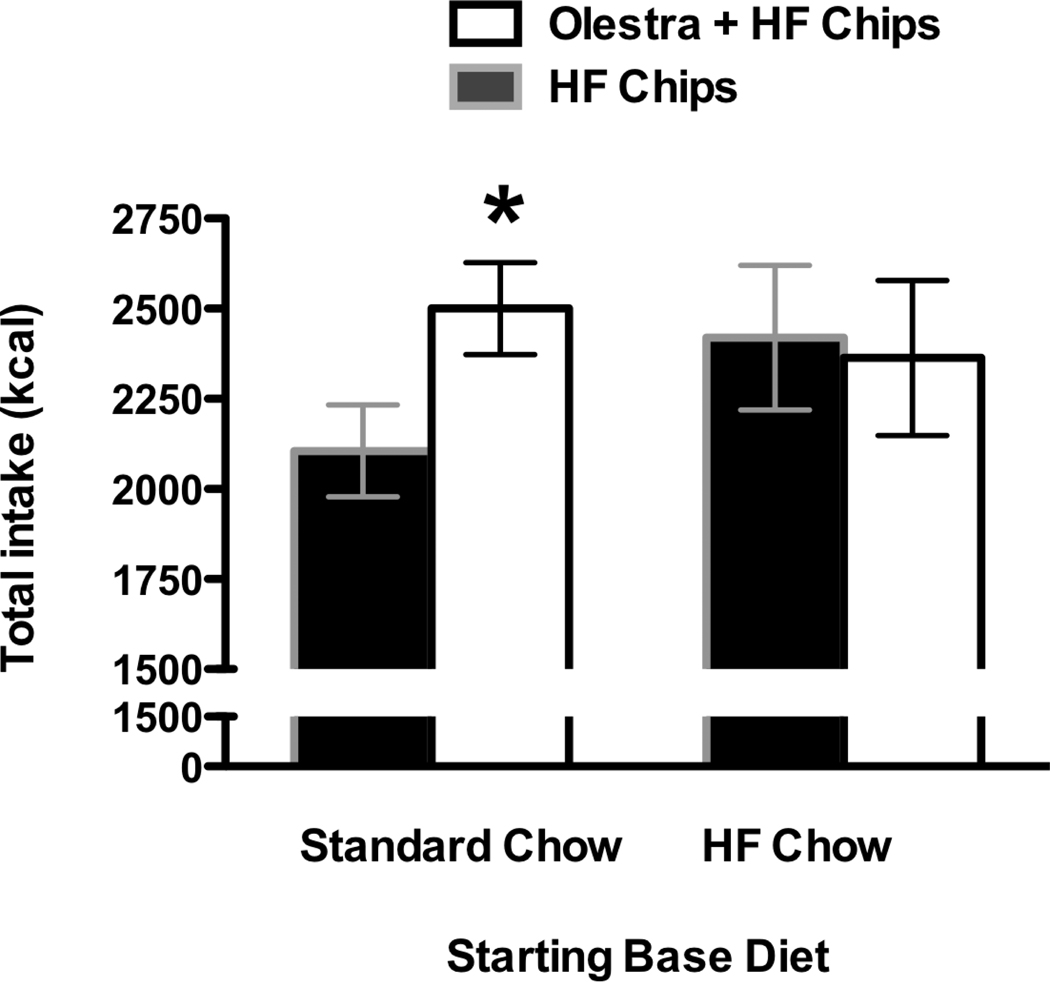
During the 28 days when the chips were presented, body weight gain was affected by both the diet on which the animals were maintained, and the type of chips offered (Main effect of Base diet, F 1, 27 = 122.9, p < 0.000001; Base Diet × Chip group, F 1, 27 = 8.7, p = 0.007; Day × Base diet, F 28, 756 = 20.9, p < 0.000001; Day × Base diet × Chip group, F 28, 756 = 1.9, p = 0.002). Subsequent analysis indicated that in animals maintained on the Standard low-fat Chow diet, body weight gain was not affected by the type of chips provided (No Main effect of Chip group or interaction of Chip group with day; Fs = 2.6 and 1.0; p’s = 0.13 and 0.42 respectively; Figure 1A). In contrast, animals maintained on the high fat diet showed significantly greater body weight gain on days 23 – 28 in the Olestra + HF Chips group compared to animals given only the HF chips (Main effect of Chip group, F 1, 13 = 6.9, p =0.02; Day × Chip type, F 28,364 = 2.1, p = 0.0008; Figure 1A).
Figure 1.
A. Body weight gain in rats maintained on a standard, low fat laboratory chow diet (left) was not differentially affected by consumption of non-predictive potato chips compared to predictive potato chips. In contrast, body weight gain was significantly greater in animals given Olestra + HF chips (right) compared to HF chips alone when animals were maintained on a high fat chow diet. B. When switched to a high fat diet, body weight gain was significantly greater in animals previously maintained on a standard chow diet and given Olestra + HF chips compared to animals maintained on a standard chow diet and given only HF potato chips (left). After discontinuation of chips, body weight gain did not differ in animals continued on the high fat chow diet (right). Body weight was measured daily – some points omitted for clarity.
* p< 0.05 compared to HF only group maintained on HF chow on days 23–28
Following discontinuation of the chips, the body weight gain was significantly affected by the type of Chip to which animals had previously been exposed, as well as by the Base chow diet on which the animals had begun, (Main effect of Starting Base diet, F 1, 27 = 24.7, p = 0.0003, Main effect of Chip group, F 1, 27 = 6.1, p =0.02; Day × Starting Base diet, F 14, 378 = 12.5, p < 0.000001). Post-hoc ANOVAs indicated that in animals previously maintained on the HF diet, there were no differences in body weight gain once the chips were discontinued (No main effect or interaction of Chip group, F’s = 1.3 and 0.4, respectively; Figure 1B). In contrast, when animals previously maintained on the low-fat Standard chow diet were placed onto a High Fat chow diet, previous experience with Olestra + HF chips resulted in increased body weight gain relative to animals that had previous experience with the HF chips alone (Main effect of Chip group, F 1, 14 = 5.1, p = 0.04; Day × Chip group interaction, F 14, 196 = 1.8, p =0.036; post-hoc analysis indicated no significant differences on any individual day; Figure 1B).
Body Composition
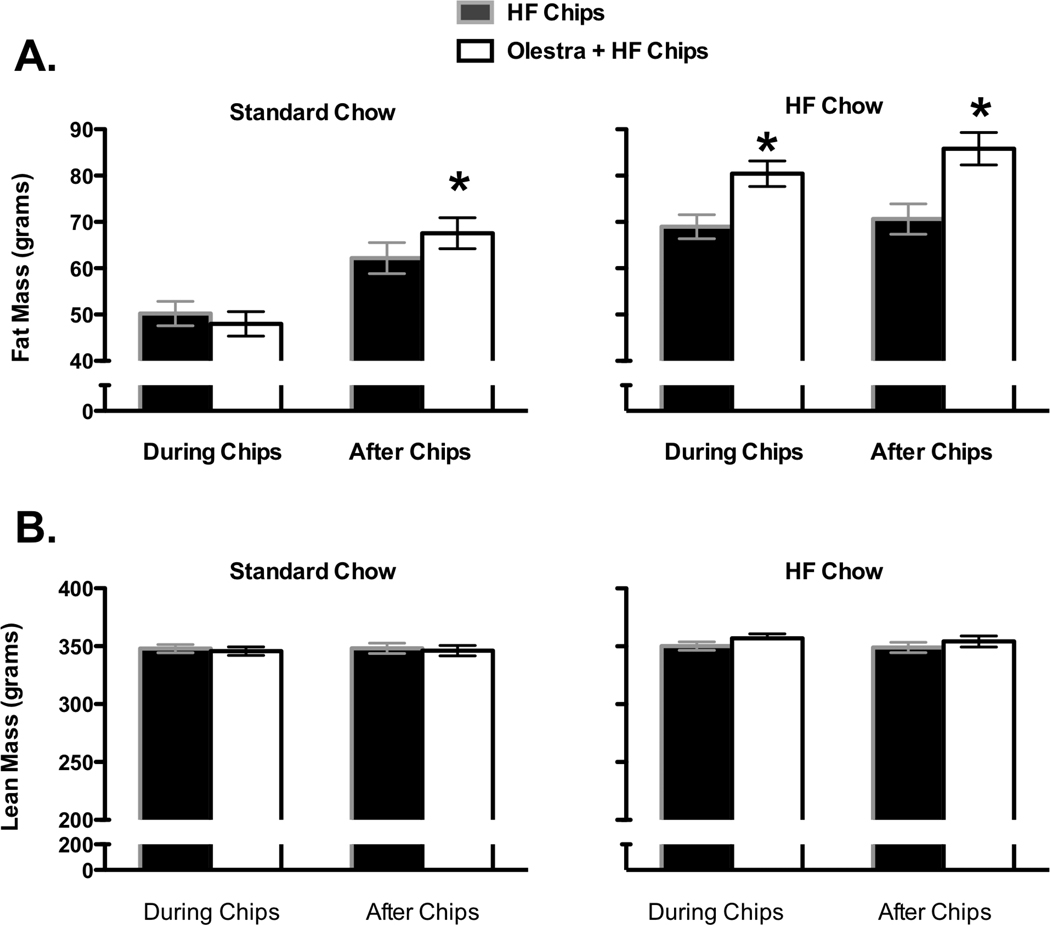
There were no differences in body fat or lean body mass at the start of the study (Mean + SEM fat ranged from 26.2 ± 1.00 g to 28.3 ± 1.1 g ; Mean + SEM lean ranged from 303.8 + 3.13 g to 304.2 + 3.3 g). Analysis of the effects of chip consumption on body composition using initial fat mass as a covariate indicated that fat mass was significantly affected by the base diet and the type of chip consumed (Main effect of Base diet, F 1, 27 = 93.93, p < 0.0000001, Base diet × Chips interaction, F 1, 27 = 6.52, p =0.017; Figure 2A). Post-hoc analysis indicated that animals on the HF Chow diet had significantly greater amounts of fat than animals on the Standard low-fat Chow diet. In addition, animals on the HF diet given the Olestra and HF chips had significantly more fat than animals given only the HF chips. There were no differences in fat between chip groups in animals maintained on the Standard low-fat Chow diet immediately following chip exposure (Figure 2A). In addition, there were no differences in lean mass based on chip type or base chow diet immediately following chip exposure (Figure 2B).
Figure 2.
A. In animals maintained on a Standard Chow diet during chip exposure (left), fat mass was not significantly affected by the type of chips consumed, but when animals were switched to the High Fat chow following discontinuation of the chips, fat mass was significantly greater in animals previously exposed to the Olestra + HF chips. In animals maintained on the HF chow (right), fat mass during and after exposure to chips was significantly greater in animals exposed to Olestra + HF chips. B. Lean mass was not affected by chip type or base diet during or after chip exposure.
* p < 0.05 compared to HF chips
When chips were discontinued and animals from the Standard low-fat Chow group were switched to the HF chow maintenance diet, body fat assessed using initial fat mass as a covariate was significantly affected by the Starting base diet and the type of chips to which the animals had previously been exposed (Main effect of Starting base, F 1, 27 = 16.06, p = 0.00046; Main effect of Chips, F 1, 27 = 9.05, p = 0.0058; Figure 2A). Post-hoc analysis indicated that animals that had been on the Standard low-fat Chow diet had less fat than animals maintained on the HF chow diet throughout testing and animals previously given the Olestra + HF chips had more fat than animals previously given only the HF chips. There were no differences in lean mass based on chip type or base chow diet after the chips were discontinued (Figure 2B).
Intake of Chips
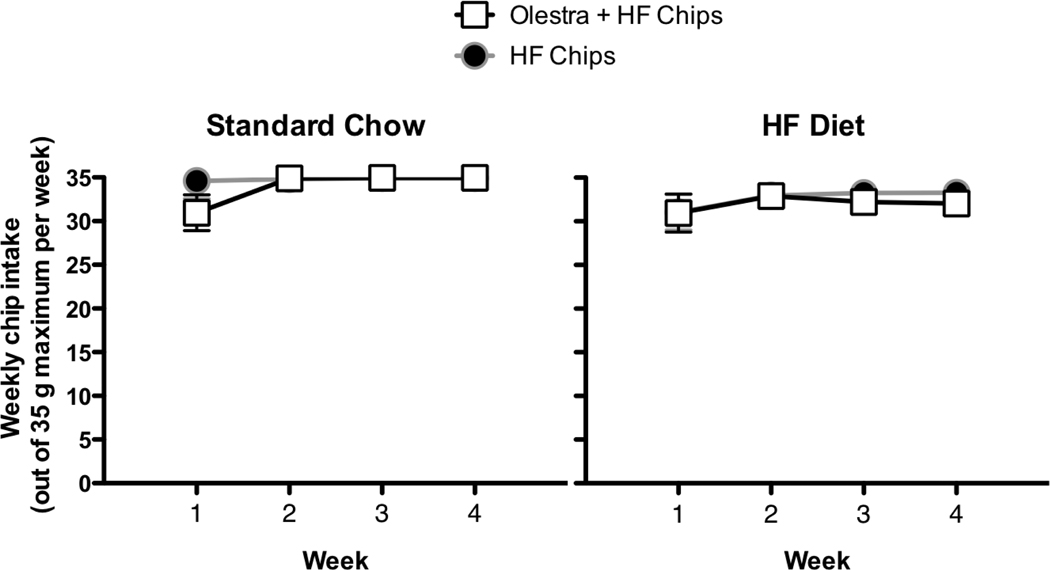
With the exception of the first week of testing, animals generally consumed the entire fixed portion (5 g daily) of chips provided. The amount of chips consumed was significantly affected by the week of testing (Main effect of Week, F 3, 81 = 3.32, p =0.02) and by the Base chow diet (Main effect of Base chow diet, F 1, 27 = 7.38, p = 0.01; Figure 3), but not by the type of chip offered. Animals consumed significantly fewer total grams of chips during the first week compared to the remaining weeks. In addition, animals on the HF chow diet consumed significantly fewer grams of chips compared to animals on the Standard low-fat Chow diet, but there were no differences in intake among groups given the Olestra + HF chips compared to groups given the HF chips alone.
Figure 3.
Weekly intake was significantly lower during the first week compared to the other weeks, and higher in animals maintained on the Standard Chow diet (left) compared to the HF diet (right), but there were no significant differences in chip consumption between animals given only HF chips versus Olestra + HF chips.
Total Energy Intake
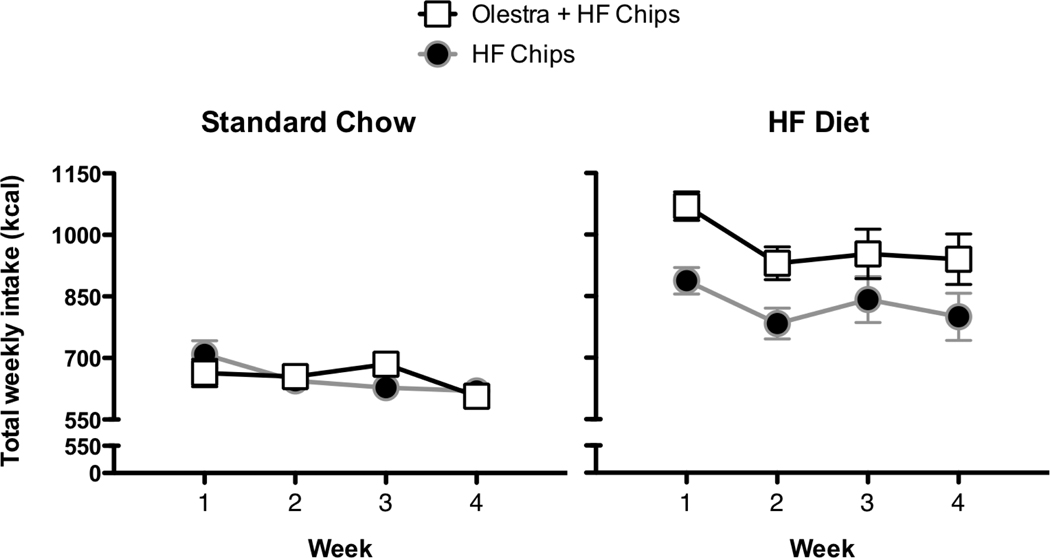
During the period when chips were consumed, weekly total caloric intake was affected by the Base chow diet, and the Chip group (Main effect of Base chow diet, F 1, 27 = 51.4, p < 0.0000001; Main effect of Chip group, F 1, 27 = 4.5, p =0.043, Base chow diet × Chip group, F 1, 27 = 4.3, p = 0.049, Week × Base chow diet × Chip group, F 3, 81 = 2.74, p = 0.049). Post-hoc ANOVAs indicated that in animals maintained on the standard chow diet, food intake was affected by the week of testing with intake being lower during the 4th week than the 1st week, but there were no main effects of Chip group or interactions (Main effect of Week, F 3, 42 = 4.72, p = 0.006; Figure 4). In contrast, in animals maintained on the high fat diet, food intake was affected by both the week of testing and the Chip group offered (Main effect of Chip group, F 1, 13 = 5.37, p = 0.037; Main effect of Week, F 3, 39 = 10.68, p = 0.000029). Post-hoc analyses indicated that food intake was significantly higher during the first week of testing compared to the other 3 weeks. In addition, animals consuming the Olestra + HF chips consumed significantly higher numbers of total calories than animals consuming the HF chips only (Figure 4).
Figure 4.
In animals maintained on the Standard Chow diet (left), total caloric intake was significantly higher during the first week compared to the fourth week, but there were no effects of chip type. In animals maintained on the High Fat diet (right), total caloric intake was significantly higher during the first week compared to the remaining 3 weeks, and significantly higher in animals fed the Olestra + HF chips compared to animals fed only the HF chips.
Following discontinuation of the chips, total caloric intake was significantly higher in animals previously offered the Olestra + HF chips and switched from the Standard low-fat Chow diet to the HF chow diet compared to animals offered the HF chips only and switched from Standard low-fat Chow diet to the HF chow diet (Main effect of chips, F 1, 14 = 4.29, p = 0.046; Figure 5). There were no differences in total caloric intake following discontinuation of the chips in animals that were maintained on the HF chow diet throughout the experiment.
Figure 5.
Total caloric intake across 16 days of HF diet was significantly greater in animals that previously received Olestra + HF chips while maintained on a Standard Chow diet compared to animals that previously received only HF chips while maintained on a Standard Chow diet (left). No differences in total caloric intake were observed following discontinuation of the chips in animals maintained on HF Chow diet throughout the experiment (right).
* p < 0.05 compared to HF only Chips
Discussion
The results of this experiment demonstrate that animals consuming snack chips where the sensory properties were not reliably associated with high calories and ate more, gained more weight, and were fatter than animals that consumed only high-fat, high-calorie chips when the maintenance chow diet was also high in fat. These data are consistent with the hypothesis that animals may use the sensory properties of food to predict the consequences of consuming food, and that reducing the validity of this predictive relation can lead to positive energy balance. If such cues are also present in the maintenance diet at the same time, food intake, body weight gain, and adiposity are increased.
An alternative hypothesis could be that rats in the Olestra + HF chips group were able to discriminate the Olestra chips from the HF chips, and were therefore exposed to increased variety relative to the animals given the HF chips alone. Differences in intake would therefore result from the increased sensory variety, rather than the lack of predictive relation between the sensory cues and calories. If so, then the effects of such variety would be expected to occur in both groups given the Olestra +HF chips. However, this hypothesis is challenged by the finding that animals maintained on a standard chow diet that was low in sensory cues associated with fat did not show impaired energy balance as a result of consuming the fat-substituted + HF chips. Thus, the greater sensory variety does not appear to account for our findings. Further, animals did appear to learn about the sensory properties of the chips even when maintained on the standard chow diets, since animals given Olestra + HF chips showed significantly greater body weight gain and increased adiposity when given a maintenance diet high in fat, even though they were no longer consuming the chips. The previous experience with chips in which the sensory properties of fat were not consistently related to calories appeared to impair their ability to regulate intake of the high-fat diet compared to animals given a relationship in which fat consistently predicted high calories. These effects of consuming the Olestra + HF chips that occur only after the chips are no longer available are also difficult to reconcile with an explanation based on increased sensory variety during chip exposure.
Our results are consistent with our previous data demonstrating that weakening the predictive relation between sweet taste and calories can also produce positive energy balance, depending on the sweetness of the maintenance diet. The present data show that this effect is not specific to the use of high intensity sweeteners, but extends to reduced calorie fat substitutes as well. Furthermore, we found that even after the opportunity to consume the fat substitutes had been suspended, differences in weight gain and adiposity were maintained. Animals did not lose the extra weight or fat when they were no longer consuming the chips. This outcome is also similar to that obtained with high intensity sweeteners (Swithers et al., 2009). Thus, these results enhance the generality of the principle that degrading the association between the orosensory properties of food or fluids and their caloric outcomes interferes with the learned control of energy regulation. This interference does not require that animals have no expectations about the caloric outcomes that are associated with the orosensory cues provided by sweet or high-fat foods. Rather, energy and body weight dysregulation are a consequence of a reduced ability to anticipate the actual caloric US that is produced when normal high-fat and high-energy foods are encountered. Although experience with reduced-calorie or no-calorie sweet or fatty foods might also involve other types of learning and memory processes, the present results seem wholly consistent with the relatively simple associative or Pavlovian framework that provided the initial interpretive foundation for our work (see Swithers, Martin, & Davidson, 2010 for review).
Further, consistent with other reports, consumption of the high fat maintenance diet appeared to promote positive energy balance as animals in both chip groups showed increased caloric intake, body weight gain and adiposity when maintained on the high-fat chow diet compared to the standard low-fat chow diet. In addition, animals switched from the standard low-fat chow diet to the high-fat chow diet after chip exposure showed more pronounced weight gain than animals maintained on the high-fat chow diet. Thus, exposure to high-fat chow diet alone promoted positive energy balance, and this effect was amplified by either current or previous experience that interfered with the relationship between fat cues and calories.
These findings call into question the conventional wisdom that reductions in intake, weight gain, and/or adiposity will necessarily follow from altering the energy density of foods by introducing low-calorie or no-calorie substitutes which mimic the sensory properties of sweet or fat (Bellisle & Drewnowski, 2007). The variety, availability, and consumption of such sensory substitutes, such as the fat substitute olestra and the sugar substitutes saccharin, aspartame, sucralose, and most recently extracts from the Stevia rebaudiana plant (stevia) have increased dramatically over the past 30 years, keeping pace with the increased prevalence of overweight and obesity (e.g. Yang 2010). While the typical interpretation of such a correlation is that people begin to consume such products after they have begun to gain weight, the alternative is that consumption of such products contributes to increases in body weight. Data from the present and previous experiments along with prospective correlational studies in humans (e.g. Dhingra et al., 2007; Fowler et al., 2008; Lutsey, Steffen & Stevens, 2008) are instead consistent with the idea that consumption of sweet and fat subsitutes may in fact contribute to overweight and obesity.
Acknowledgments
We thank Kiely Clark and Melissa McCurley for technical assistance. Supported by NIH grants R01DK076078 and P01 HD052112.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61(6):691–700. doi: 10.1038/sj.ejcn.1602649. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Hayward L, Crandall C. Conditioned Taste Preferences Based on Caloric Density. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7(1):59–69. doi: 10.1037//0097-7403.7.1.59. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Martin AA, Clark KM, Swithers SE. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control energy and body weight regulation. Quarterly Journal of Experimental Psychology. 2011 doi: 10.1080/17470218.2011.552729. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Swithers SE. A Pavlovian approach to the problem of obesity. Int J Obes Relat Metab Disord. 2004;28(7):933–935. doi: 10.1038/sj.ijo.0802660. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16(8):1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Learned controls of ingestive behavior. Appetite. 1997;29:153–158. doi: 10.1006/appe.1997.0120. [DOI] [PubMed] [Google Scholar]
- Smeets PAM, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutrition Reviews. 2010;68(11):643–655. doi: 10.1111/j.1753-4887.2010.00334.x. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Baker CR, Davidson TL. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav Neurosci. 2009;123(4):772–780. doi: 10.1037/a0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122(1):161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Doerflinger A, Davidson TL. Consistent relationships between sensory properties of savory snack foods and calories influence food intake in rats. Int J Obes (Lond) 2006;30(11):1685–1692. doi: 10.1038/sj.ijo.0803329. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Martin AA, Clark KM, Laboy AF, Davidson TL. Body weight gain in rats consuming sweetened liquids. Effects of caffeine and diet composition. Appetite. 2010;55(3):528–533. doi: 10.1016/j.appet.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100(1):55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behavioural Brain Research. 2000;110:175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- Yang Q. Gain weight by "going diet?" Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J Biol Med. 2010;83(2):101–108. [PMC free article] [PubMed] [Google Scholar]
- Zafra MA, Molina F, Puerto A. The neural/cephalic phase reflexes in the physiology of nutrition. Neuroscience and Biobehavioral Reviews. 2006;30:1032–1044. doi: 10.1016/j.neubiorev.2006.03.005. [DOI] [PubMed] [Google Scholar]