Abstract
In a previous paper we demonstrated in a large twin study that disordered gambling (DG) as defined by the DSM-IV symptom set runs in families, that about half of the variation in liability for DG was due to familial factors, and that all of this was explained by shared genetic rather than shared environmental influences (Slutske et al., 2010). The purpose of the present study was to extend this work to also include an alternative conceptualization of DG that is provided by the South Oaks Gambling Screen (SOGS) item set in order to (a) compare the magnitude of the familial resemblance obtained when using the two definitions of DG (based on the DSM-IV and the SOGS), (b) examine the extent to which the two definitions tap the same underlying sources of genetic and environmental variation, and (c) examine whether the same results will be obtained among men and women. The results of bivariate twin model-fitting analyses suggested that DG as defined by the DSM-IV and SOGS substantially overlapped at the etiologic level among both men and women, which supports the construct validity of both the DSM and SOGS conceptualizations of DG. This study highlights the utility of twin studies for appraising the validity of the diagnostic nomenclature.
Keywords: disordered gambling, pathological gambling, genetic influences, twin study, construct validity, South Oaks Gambling Screen
Pathological gambling was first introduced into the official diagnostic nomenclature with the publication of the 9th Edition of the International Classification of Diseases in 1977 (World Health Organization, 1977), followed by its inclusion into the DSM-III in 1980 (APA, 1980). A diagnosis of pathological gambling in the DSM-III required experiencing 3 of 7 symptoms that were primarily focused on legal and financial consequences of gambling. The diagnostic criteria for pathological gambling underwent a major overhaul for the DSM-III-R in 1987 when they were substantially rewritten to more closely resemble the diagnostic criteria for substance dependence. A diagnosis of pathological gambling in the DSM-III-R required experiencing 4 of 9 symptoms, with none of the diagnostic criteria from the DSM-III retained. The diagnostic criteria for pathological gambling were again revised with the DSM-IV. A diagnosis of pathological gambling in the DSM-IV requires experiencing 5 of 10 symptoms; six of these symptoms were carried forward from DSM-III-R, three were brought back from the DSM-III, and one symptom was completely new to the DSM. The DSM-IV diagnostic criteria for pathological gambling are now primarily composed of symptoms modeled on the substance dependence criteria with only two items referring to either legal or financial consequences of gambling.
There was not a widely-accepted standardized assessment of pathological gambling until the South Oaks Gambling Screen (SOGS; Lesieur & Blume, 1987) was introduced in 1987. (The Diagnostic Interview Schedule, a structured diagnostic interview developed in 1978 for use in the Epidemiologic Catchment Area study [Robins et al., 1981] and widely used in psychiatric research, included only an abbreviated 4-item assessment of DSM-III pathological gambling [Cunningham-Williams et al., 1998].) For many years, the SOGS was the primary instrument used to assess pathological gambling in research and was often the gold standard against which new measures of pathological gambling were compared. The SOGS was partly based on the DSM-III diagnostic criteria for pathological gambling, and also contains several items that were not a part of either of the previous versions, nor the current version of the DSM. Only three of the 10 current DSM-IV pathological gambling symptoms (chasing losses, lying about gambling, borrowing because of gambling) are approximated by similar diagnostic items in the SOGS. There are five pathological gambling symptoms that are unique to the DSM-IV (preoccupation with gambling, efforts to stop or reduce gambling, needing to increase the size or frequency of bets, restlessness or irritability if unable to gamble, and gambling to escape problems or dysphoria), and another two symptoms that are more narrowly covered by the SOGS than the DSM-IV (affected relationship, job, education or career, and committed illegal acts to finance gambling). There are seven pathological gambling symptoms that are unique to the SOGS (gambled more than intended, hid signs of gambling, defaulted on debts, people criticized or felt had a problem, argued with close family about gambling, felt guilty about gambling, and felt had a problem with gambling), and one symptom that is much more extensively covered by the SOGS than the DSM-IV (borrowing because of gambling).
A number of studies have directly compared the DSM-IV and SOGS operationalizations of pathological gambling. Research comparing the DSM-IV and SOGS serves as an important bridge from the earlier pathological gambling research based primarily on the SOGS to the more recent work that is based on the DSM-IV. More importantly, research comparing the DSM-IV and SOGS can provide valuable insights into the construct of pathological gambling. Like all psychological constructs, pathological gambling is still an “open concept” (Meehl, 1977, 1978, 1986). Although a DSM diagnosis is often interpreted as the “true” definition of a disorder (Blashfield & Burgess, 2007; Meehl, 1986), it is more appropriately regarded as a hypothetical construct that is formulated by clinical scientists (Widiger et al., 1987), operationalized with a standardized diagnostic interview based on the DSM criteria (Robins, 2004), and revised based on the results of DSM field trials and the corpus of existing empirical research (Widiger et al., 1991). Compared to other DSM disorders, the validity of the diagnosis of pathological gambling has undergone only limited testing in field trials (Lesieur, 1988; Bradford et al., 1996), and the results of empirical research have not had much influence on the changes made to the diagnostic criteria.
The majority of studies directly comparing the DSM-IV and SOGS operationalizations of pathological gambling have compared the prevalences of pathological gambling that are obtained when using the two. A consistent finding is that the SOGS classifies more individuals as affected with pathological gambling than does the DSM-IV symptom set (Shaffer et al, 1997). This has led to criticisms of the use of the SOGS in community-based studies because it tends to “over-diagnose” pathological gambling (Gambino et al., 2006; Gerstein et al., 1999), yielding rates that are about double those obtained with the DSM-IV symptom set. This is not an especially damning criticism, because it is primarily a function of the cut-offs used to make a diagnosis (Widiger et al., 1996), and this has been changed with every revision of the DSM, often without empirical justification. Whether the DSM-IV or SOGS lead to an over- or under-diagnosis of pathological gambling is of more concern for estimating the prevalence of and the public health significance of PG disorder than for an etiologic study. For many purposes it is of greater concern whether the different operationalizations of pathological gambling are tapping the same underlying construct.
A number of studies have examined the correlations between dimensional DSM-IV and SOGS measures obtained by summing the 10 DSM-IV pathological gambling criteria and the 20 SOGS items; these typically correlate with each other in excess of 0.70 (Cox et al., 2004; Hodgins et al., 2004; Stinchfield, 2002; Stinchfield, 2003; Welte et al, 2001; Wickwire et al., 2008). Although the prevalence estimates of pathological gambling diagnoses based on the DSM-IV and SOGS differ, dimensional measures based on these suggest a substantial overlap in the way that the two measures operationalize pathological gambling, which provides strong evidence that the DSM-IV symptom set and SOGS items tap overlapping pathological gambling constructs. The use of dimensional measures of pathological gambling are likely to become more popular because of an increasing appreciation for the idea of disordered gambling (DG), along with many other psychiatric disorders (e.g. Helzer et al., 2008; Widiger, 2005; Widiger & Samuel, 2005) as a continuum of pathology (e.g. Shaffer et al., 2004). The term “disordered gambling” was coined to describe the full continuum of gambling-related problems, including pathological gambling as well as subclinical problems (Shaffer et al, 1999), although this term is now being considered as a replacement for the diagnostic label “pathological gambling” in the DSM-V (APA, 2010). (For the remainder of this paper we use the term DG to refer to all manifestations of disordered gambling, including pathological gambling, in order to minimize confusion.)
Few studies have moved beyond simple cross-tabulations and correlations in comparing the DSM-IV-based and SOGS assessments of DG. A novel and informative approach used latent trait modeling (Strong et al, 2004). Analyses of past-year DSM-IV and SOGS item-level data obtained from clinical and community samples were conducted to determine the amount of information provided by the DSM-IV and SOGS at different levels of a latent dimensional DG construct. At all levels of the latent DG construct, the SOGS provided diagnostic resolution that was as good as or better than the DSM-IV symptom set. In particular, the SOGS appeared to be superior to the DSM-IV at differentiating low (sub-threshold) levels of DG, as well as very high (supra-threshold) levels of DG.
Evidence from family studies has always been an important step in the validation of a diagnosis because a valid diagnostic entity is expected to “run in families” (Robins & Guze, 1970). A family study can also determine whether some definitions of a disorder result in greater resemblance between relatives than others (Robins, 2004; Widiger et al., 1991). A twin study that includes assessments of DG according to both the DSM-IV and SOGS can also provide an incisive test of whether the DSM-IV and SOGS tap the same underlying sources of genetic and environmental variation in DG, that is, the extent to which they overlap at the etiologic level.
Previously, we demonstrated that DG as defined by the DSM-IV symptom set runs in families. In a large twin study, we estimated that 49% of the variation in liability for DG was due to familial factors, and all of this was explained by shared genetic rather than shared environmental influences (Slutske et al., 2010). The purpose of the present study was to extend this work to include DG as defined by both the DSM-IV symptoms and the SOGS items in order to: (a) compare the magnitude of the familial resemblance obtained when using the two definitions, and (b) examine the extent to which the two definitions tap the same underlying sources of genetic and environmental variation. Evidence that DG as defined by the DSM-IV and SOGS overlap at the etiologic level not only would contribute to the construct validity of the DSM conceptualization of DG, but also to the construct validity of the SOGS conceptualization of DG.
Women are underrepresented in etiologic research on DG. The causes of DG among women and potential sex differences in the causes of DG are largely uncharted territory. In the previous study, the only large-scale twin study of DG that included both men and women, we found no evidence for sex differences in the familial aggregation or the heritability of DG as defined by the DSM-IV PG symptom set (Slutske et al, 2010). The present study will extend this work by: (a) establishing that these results are not specific to the DSM, but will also be observed using the alternative conceptualization of DG provided by the SOGS item set, and (b) examining the extent to which DG as defined by the DSM-IV and SOGS overlap at the etiologic level among both men and women.
Method
Participants
Participants for this study were 4,764 members of the Australian Twin Registry (ATR) Cohort II (for more details about the study participants and the zygosity determination, see Slutske et al, 2009). In 2004–2007, a telephone interview containing a thorough assessment of gambling behaviors was conducted with the ATR Cohort II members (individual response rate of 80.4%). The mean age was 37.7 years (range = 32–43) and 57.2% of the sample was female. There were 1,875 complete twin pairs (867 MZ [520 female, 347 male], 1008 DZ [367 female-female, 227 male-male, and 414 female-male]), and 1,014 individual twins from incomplete pairs (304 MZ [151 female, 153 male], 710 DZ [181 female-female, 216 male-male, and 313 female-male]).
Procedure
Twins were assessed by structured telephone interview. Interviews were administered by trained lay-interviewers who were blind to the status of the cotwin. Interviewers were supervised by a project coordinator, a clinical psychologist with over ten years of experience. All interview protocols were reviewed either by the project coordinator or by research editors (veteran skilled interviewers from previous studies who had maintained consistently low error rates in coding). All interviews were tape-recorded and a random sample of 5% of the interview tapes was reviewed for quality control and coding inconsistencies. A small sub-sample of the participants (N = 166) were re-interviewed several months after their initial interview (mean interval = 3.4 months, SD = 1.4 months, range = 1.2 – 9.5 months) to establish the test-retest reliability of the interview measures. Individuals with a history of DG symptoms were over-sampled for the test-retest reliability study.
This study was approved by the Institutional Review Boards at the University of Missouri and the Queensland Institute of Medical Research. All of the participants provided informed consent.
Measures
Two different measures of DG were used in this study, the National Opinion Research Center DSM-IV Screen for Gambling Problems(NODS; Gerstein et al, 1999) and the South Oaks Gambling Screen (Lesieur & Blume, 1987). The NODS DSM-IV and SOGS diagnostic criteria were assessed for all participants who reported that they had ever gambled at least five times within a single 12 month period; the majority of participants, 77.5%, surpassed this gambling threshold.
The NODS is a structured interview that was developed for a national United States gambling prevalence survey conducted in 1999(Gerstein et al, 1999). The NODS assesses the 10 DSM-IV diagnostic criteria for DG. The test-retest reliability of the lifetime diagnosis of DG from the NODS was high (kappa = 0.67; Yule’s Y = 0.79). The test-retest and internal consistency reliabilities of the NODS lifetime symptom count were also high (test-retest r = 0.86; coefficient alpha = 0.85). Exploratory factor analyses provided strong and convincing evidence consistent with a single-factor model of DG for the DSM-IV symptom set: there was only a single large eigenvalue greater than one, and the root mean square error of approximation (RMSEA) and root mean square residual (RMSR) were 0.021 and 0.03, respectively. The factor loadings were all high and ranged from 0.87 to 0.95. Typically, a single eigenvalue greater than one, an RMSEA of less than 0.06, an RMSR of less than 0.05, and all of the indicators having high loadings on a single factor are evidence supporting the hypothesis that a single factor is sufficient for explaining the inter-item correlations. (This measure is referred to as “DSM-IV DG” throughout this paper.)
The SOGS(Lesieur & Blume, 1987) was originally developed as a screening instrument to assess gambling problems among individuals seeking treatment. The development of the SOGS pre-dated the DSM-IV. The SOGS was originally developed as a paper-and-pencil questionnaire to be filled out by the patient or study participant, but in the present study the SOGS was administered as a structured interview. The test-retest reliability of the lifetime diagnosis of DG from the SOGS was high (kappa = 0.78; Yule’s Y = 0.82). The test-retest and internal consistency reliabilities of the SOGS lifetime symptom count were also high (test-retest r = 0.86; coefficient alpha = 0.81). In exploratory factor analyses of the SOGS items, there was also only a single large eigenvalue greater than one, and the RMSEA and RMSR were 0.015 and 0.045, respectively. The factor loadings were all high and ranged from 0.65 to 0.95.
Data Analysis
Prior to conducting biometric modeling we tested for cross-sex measurement invariance of DG as measured by the 10 DSM-IV DG symptoms and by the 20 SOGS items. This was done to ensure that any sex differences that emerged in the biometric analyses could be interpreted as actual sex differences in DG, rather than sex differences in our chosen measurements of DG. The analyses were conducted using Mplus software (Muthen & Muthen, 2004) with a mean- and variance-adjusted weighted least squares estimator. The tests for measurement invariance proceeded in two steps(Byrne et al, 1989). In the first step, a baseline single-factor confirmatory factor analytic model of DG (either based on the DSM-IV or the SOGS) was fit to the data and all measurement parameters (i.e., item factor loadings and item thresholds) were freely estimated for males and females. In the second step, item factor loadings and item thresholds were constrained to be equal across males and females. The fit of the constrained model was compared to the fit of the unconstrained model via a Δχ2 test. A non-significant Δχ2 test indicates that the assumption of measurement invariance cannot be rejected (i.e., the data are consistent with measurement invariance). Measurement invariance analyses were conducted separately for the DSM-IV and SOGS measures.
Biometric models were fit by the method of maximum likelihood directly to the raw twin data using the Mx program(Neale et al, 2003). The data from incomplete as well as complete twin pairs were included in the analyses, which reduces potential biases in parameter estimates due to nonparticipation if the data are missing at random(Little & Rubin, 1987). Liability-threshold models were fit to the twin data(Neale et al., 1992). This model assumes that there are latent liability continua underlying the categorical diagnoses based on the DSM-IV and the SOGS. The decision to use this model was based on the following two considerations: (a) maintaining consistency with the previous twin studies of DG (Eisen et al, 1998; Slutske et al, 2000; Slutske et al, 2010), and (b) the use of continuous symptom count measures was intractable because the distributions for the DSM-IV and the SOGS (especially the DSM-IV) were highly skewed even after a data transformation.
In fitting a liability-threshold model, a decision must be made about the appropriate threshold to use. Typically, this is an easy decision because the threshold will correspond to whether or not an individual is affected versus unaffected with a disorder. However, with dimensional diagnoses such as DG, this diagnostic cut-point also represents a count on a continuous symptom scale (i.e. 5 out of 10 symptoms for DSM-IV DG and 5 out of 20 items for the SOGS). When the symptoms making up the scale are all indicators of the same unidimensional construct, as has been demonstrated for both the DSM-IV(Strong & Kahler, 2007) and the SOGS(Strong et al, 2004), the cut-point used for the threshold in the liability threshold model does not necessarily have to correspond to the cut-point used for a clinical diagnosis. The liability threshold model assumes that the causes of variation in risk will be the same at any point along the liability distribution and for any threshold imposed(Reich et al, 1975) -- an assumption that could not be rejected for DSM-III-R DG in a previous paper(Slutske et al, 2000). Therefore, to maximize the statistical power in testing sex differences and bivariate biometric models, we have dichotomized the DSM-IV and SOGS symptom counts at one or more symptoms. Although this threshold conforms most closely to the idea of problem gambling, the assumption of the underlying model that we are imposing suggests that the results will apply equally to all levels of disordered gambling behavior, including pathological gambling disorder. The results of the exploratory factor analyses support the proposition that all of the DSM-IV symptoms and all of the SOGS items are measuring the same underlying dimensions and that endorsing even a single symptom or item is informative about an individual’s DG liability.
Univariate model-fitting was conducted to partition the variation in DSM-IV and SOGS DG, each considered in isolation, into additive genetic, shared environmental or nonadditive genetic, and nonshared environmental influences. Bivariate model-fitting was conducted to similarly partition the covariation between the DSM-IV and SOGS, and to determine the extent to which the different sources of variation were common or specific to the DSM-IV and the SOGS operationalizations of DG. In the bivariate model-fitting, the genetic and environmental variation was partitioned into a common factor shared by the DSM-IV and SOGS and two sets of specific factors that were unique to each measure. In a factor model with only two indicators it is necessary to constrain the path coefficients for the common factor to be the same for each measure.
The evidence for two different types of sex difference was evaluated. Quantitative (also known as scalar) sex differences refers to differences in the magnitude of genetic or environmental effects in men and women and is detected from within-zygosity differences in the twin correlations obtained from same-sex male versus female twin pairs. Qualitative (also known as nonscalar) sex differences refers to differences in the actual genetic or environmental risk factors that contribute to variation in a trait, and is detected from smaller twin correlations obtained from unlike-sex than from same-sex dizygotic twin pairs.
Ideally, one would combine the measurement model used in the measurement invariance analyses with the univariate and bivariate biometric models(Neale et al, 2005). Unfortunately, the sparseness of some of the DSM-IV DG and SOGS symptom data among some of the five sex/zygosity subgroupings precluded the implementation of this analytic approach.
Results
The overall lifetime prevalence of pathological gambling disorder according to the DSM-IV was 2.2% (3.4% among men, 1.2% among women), and the overall lifetime prevalence of pathological gambling according to the SOGS was 3.5% (5.3% among men, 2.2% among women). The overall lifetime prevalence of ever experiencing one or more DG symptoms was 12.5% (18.2% among men, 8.3% among women) according to the DSM-IV and was 40.1 (49.2% among men, 33.3% among women) according to the SOGS.
Tests of Measurement Invariance
Of the 10 DSM-IV DG symptoms, one had to be excluded from the cross-sex measurement invariance analyses due to low rates of endorsement (committing illegal acts to finance gambling). Measurement invariance analyses suggested that the symptoms of DSM-IV DG functioned similarly for men and women, except for the symptom “gambles to escape personal problems or to relieve a dysphoric mood” which was endorsed by women at a lower level of the latent DG severity continuum than by men, suggesting partial measurement invariance of the DSM-IV PG symptoms across sex (Slutske et al., 2010). Notably, the single symptom that was not invariant across sex was the only symptom included in the DSM-IV DG diagnostic criteria set that was completely new to the DSM. Because partial measurement invariance was established, the full symptom set was used in both men and women for the biometric analyses.
Of the 20 SOGS items, 5 were excluded from the cross-sex measurement invariance analyses due to low rates of endorsement (defaulting on debts, borrowing from loan sharks, cashing in stocks, bonds, other securities, selling personal property, or passing bad checks to gamble or to pay gambling debts). The remaining 15 items of the SOGS were factor analyzed, and the fit of the baseline single-factor model, allowing all measurement parameters to differ across males and females was excellent (CFI:.99, TLI:.99, RMSEA, .02). The fit of the model constraining measurement parameters for all items to be the same for males and females was not significantly poorer than the fit of the unconstrained model (Δχ2 (9, N=4,764)=5.86, p=.75), suggesting measurement invariance across sex for the 15 SOGS items. Because measurement invariance was established, the full symptom set was used in both men and women for the biometric analyses.
Tests of Sex Differences
Prior to fitting biometric models, tests of the differences between the within-trait twin correlations for the different zygosity groups were conducted using Mx (Table 1). In these and all subsequent biometric models, thresholds (prevalences) for men and women were allowed to vary because they could not be constrained to be equal for either the DSM-IV (Δχ2 (1, N=4,758) = 92.4, p < .001) or the SOGS (Δχ2 (1, N=4,758)= 112.1, p< .001). In the previous paper (Slutske et al., 2010) we reported that for the DSM-IV, the following sets of twin correlations from (1) the two MZ groups (male and female), (2) the two same-sex DZ groups (male-male and female-female), and (3) the two same-sex DZ groups and the unlike-sex DZ group (male-male, female-female, and male-female) could each be constrained to be equal.
Table 1.
Univariate and bivariate twin correlations in liability for disordered gambling as defined by the DSM-IV (DSM-IV DG) and South Oaks Gambling Screen (SOGS).
| twin correlations | |||
|---|---|---|---|
| within-trait | cross-trait | ||
| Zygosity group | DSM-IV DGa | SOGS | DSM-IV and SOGS |
| MZ male | 0.49 (0.30 – 0.65) | 0.64 (0.52 – 0.74) | 0.48 (0.35 – 0.59) |
| DZ male-male | 0.21 (0.00 – 0.45) | 0.19 (0.00 – 0.38) | 0.18 (0.00 – 0.35) |
| MZ female | 0.55 (0.34 – 0.72) | 0.49 (0.37 – 0.60) | 0.46 (0.32 – 0.58) |
| DZ female-female | 0.21 (0.00 – 0.51) | 0.28 (0.12 – 0.43) | 0.23 (0.04 – 0.40) |
| DZ male-female | 0.22 (0.01 – 0.41) | 0.16 (0.01 – 0.30) | 0.12 (0.00 – 0.25) |
Note: DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Version 4. Cell entries are tetrachoric correlations; 95% confidence intervals are in parentheses.
Similarly for the SOGS, the following sets of twin correlations from (1) the two MZ groups (male and female), (2) the two same-sex DZ groups (male-male and female-female) (Δχ2 (2, N=4,759)= 3.76, p=.15), and (3) the two same-sex DZ groups and the unlike-sex DZ group (male-male, female-female, and male-female) could each be constrained to be equal (Δχ2 (1, N=4,760)= 0.01, p=.91).
These results indicate that there is no evidence of quantitative sex differences or qualitative sex differences. Based on these findings, it would be appropriate to proceed with biometric modeling without allowing for sex differences in parameter estimates, but because there are so little data on the etiology of DG among women we present results of fitting models to the male and female data separately in addition to fitting models to the pooled data from men and women.
Univariate Biometric Model Fitting
For both the DSM-IV and the SOGS the best fitting model was one that included additive genetic and nonshared environmental sources of variation -- shared environmental or nonadditive genetic factors did not account for significant portions of variation in liability. The results of fitting a full univariate biometric model that included additive genetic, shared environmental, and nonshared environmental sources of variation are presented in Table 2 for the purpose of delineating the confidence bounds around the parameter estimates (of the nonsignificant as well as the significant parameters). For example, shared environmental factors were estimated at zero for both the DSM-IV and the SOGS, but the narrow confidence intervals around these estimates suggest that shared environmental factors could have accounted for only 4% and 13% of the variation in liability at best. Parameter estimates for men and women did not significantly differ from each other for either the DSM-IV (Δχ2 (2, N = 4,760)= 0.1, p = 0.97) or the SOGS (Δχ2 (2, N = 4,760)= 2.3, p = 0.32).
Table 2.
Parameter estimates of additive genetic (A), shared environmental (C), and non-shared environmental (E) influences from univariate biometric model-fitting of liability for disordered gambling as defined by DSM-IV (DSM-IV DG) and South Oaks Gambling Screen (SOGS).
| Parameters | ||||||
|---|---|---|---|---|---|---|
| A | (95% CI) | C | (95% CI) | E | (95% CI) | |
| DSM-IV DGa | ||||||
| Full sample | 49.2 | (26.7 – 60.9) | 0.00 | (0.0 – 4.1) | 50.7 | (39.0 – 64.3) |
| Men | 48.5 | (10.3 – 64.1) | 0.01 | (0.0 – 46.1) | 51.4 | (35.8 – 70.6) |
| Women | 51.8 | (26.4 – 69.0) | 0.00 | (0.0 – 9.0) | 48.2 | (30.8 – 69.6) |
| SOGS | ||||||
| Full sample | 54.4 | (36.9 – 61.9) | 0.00 | (0.0 – 13.5) | 45.5 | (38.0 – 53.6) |
| Men | 62.2 | (37.1 – 72.6) | 0.00 | (0.0 – 20.7) | 37.7 | (27.3 – 50.0) |
| Women | 43.4 | (4.3 – 60.0) | 5.7 | (0.0 – 38.4) | 50.0 | (39.9 – 63.3) |
Note: DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Version 4. 95% confidence intervals are in parentheses.
As a check on the validity of the underlying assumption of the liability threshold model, we compared the heritability estimates obtained from fitting models to the data from the full sample when the diagnostic cut-offs were set at one or more, three or more, and five or more symptoms. For the DSM, this yielded heritability estimates of 49% (95% CI = 28–61%: see Table 2), 58% (95% CI = 35–78%), and 40% (95% CI = 9–74%), respectively. For the SOGS, this yielded heritability estimates of 55% (95% CI = 37–62%: see Table 2), 57% (95% CI = 32–70%), and 48% (95% CI = 17–77%), respectively. These results are consistent with the hypothesis that the causes of variation in risk are similar at any point along the liability distributions and for any diagnostic cut-off imposed.
Bivariate Biometric Model Fitting
DSM-IV DG and SOGS liabilities were substantially correlated with each other in the full sample (tetrachoric r = 0.72) and among men (tetrachoric r = 0.73) and women (tetrachoric r = 0.68). These are similar to the Pearson correlations that were obtained by using continuous DSM-IV DG and SOGS symptom scales (full sample: r = 0.83, men: r = 0.85, women: r = 0.82) and especially with log-transformed symptom scales (full sample: r = 0.68, men r = 0.71, women: r = 0.62). The similarity of these correlations supports the liability threshold model and suggests that the same level of covariation is being captured using the correlation in liability (which assumes an underlying continuous dimension of liability) as would be observed using a dimensional DG indicator.
The phenotypic correlation in liability of 0.72 represents a theoretical “upper-bound” with which to compare the cross-twin cross-trait correlations between DSM-IV DG and SOGS presented in Table 1. It is noteworthy that the cross-twin cross-trait correlation between DSM-IV DG and SOGS of 0.48 (the correlation between DSM-IV DG in one twin and SOGS in the cotwin) among MZ male twins is as large as the cross-twin within-trait correlation of 0.49 for DSM-IV DG (the correlation between DSM-IV DG in one twin and DSM-IV DG in the cotwin). Similar results were obtained for female twins and for comparisons with the cross-twin within-trait correlations for the SOGS (see Table 1).
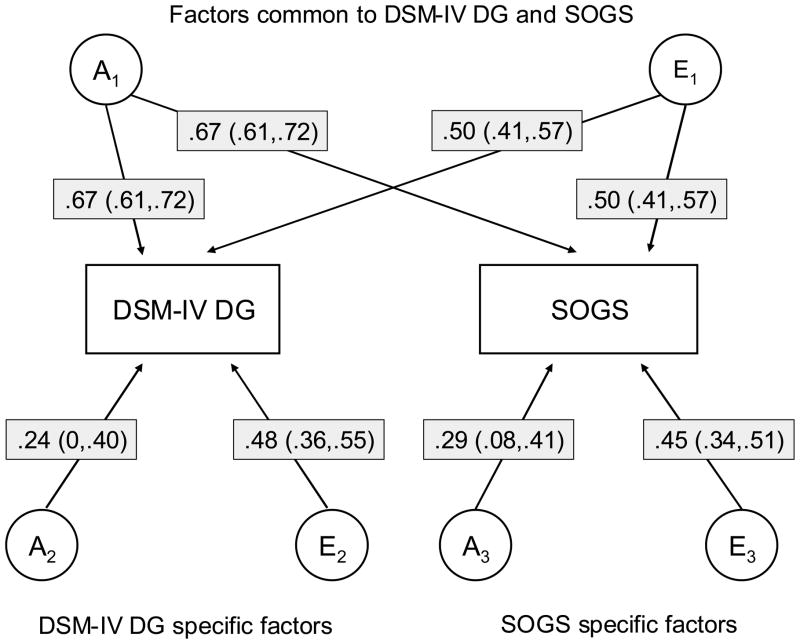
Bivariate models of DSM-IV DG and SOGS included additive genetic and nonshared environmental factors (Figure 1). Most of the genetic variation in DSM-IV DG (89%) was common with genetic variation in the SOGS, and the remaining genetic variation specific to DSM-IV DG did not significantly differ from zero. Similarly, most of the genetic variation in the SOGS (84%) was common with genetic variation in DSM-IV DG, but in this case there was significant remaining genetic variation that was specific to the SOGS. The correlation between the total genetic variation for the DSM-IV and the SOGS was 0.86. There were substantial contributions of nonshared environmental factors that were in common with and specific to DSM-IV and SOGS, and the correlation between the total nonshared environmental variation for the DSM-IV and the SOGS was 0.54.
Figure 1.
Parameter estimates (path coefficients) of additive genetic (A) and non-shared environmental (E) influences from bivariate biometric model-fitting of liability for disordered gambling as defined by the DSM-IV (DSM-IV DG) and South Oaks Gambling Screen (SOGS) in which the estimates for men and women were constrained to be equal. (Proportions of variation in risk for DSM-IV DG and SOGS attributable to each factor can be obtained by squaring the path coefficient). 95% confidence intervals are in parentheses
The overall pattern of results obtained from fitting models to the data from men and women separately was quite similar. In addition, the overlap between the genetic and environmental risk factors for DSM-IV and SOGS liability were similar in men and women. The correlation between the total genetic variation for the DSM-IV and the SOGS were 0.88 and 0.88, and the correlation between the total nonshared environmental variation in risk for the DSM-IV and the SOGS were 0.53 and 0.47 among men and women, respectively.
In sum, the results of the bivariate biometric model-fitting analyses suggest there may be a slightly but significantly greater familial resemblance and heritability in DG liability as defined by the SOGS than the DSM-IV. The DSM-IV and SOGS largely tap the same underlying sources of genetic and environmental variation in DG in both men and women.
Discussion
Many previous studies have established that dimensional measures of DG based on the DSM and SOGS are highly correlated. The present study suggests that the main explanation for this is that they share common etiologic structures. Despite differences between the DSM-IV DG diagnostic criteria set and the SOGS item set in their methods of construction and content coverage, from a behavioral genetic perspective they appear to measure the same underlying core disordered gambling construct.
Twin studies are a powerful tool for addressing nosologic questions in psychopathology (Kendler, 1993). For example, Kendler et al (1992a) compared the heritabilities obtained using nine different definitions of major depression (e.g., DSM-III, DSM-III-R) and Prescott and colleagues (Prescott & Kendler, 1999, Prescott, Aggen, & Kendler, 1999) compared the heritabilities obtained using six different definitions of alcohol use disorder (e.g., DSM-III-R alcohol abuse and dependence, DSM-IV alcohol abuse and dependence). One of the questions of interest in these investigations was the impact on the heritability of using broader versus narrower definitions of disorder. Such research can bridge findings based on older versus newer revised diagnostic criteria (ideally conducted prior to making revisions). Other common nosologic questions that can be addressed by twin studies are whether two different disorders merely represent different points along the same liability continuum (e.g., alcohol abuse versus alcohol dependence [Prescott & Kendler, 1999, Prescott, et al, 1999] and unipolar versus bipolar depression [McGuffin et al., 2003]), and whether and to what extent two different disorders share common etiologic structures (e.g., generalized anxiety disorder/major depression [Kendler et al., 1992b] and unipolar/bipolar depression [McGuffin et al., 2003]).
Despite differences in their breadth (the DSM-IV provides a narrower and the SOGS a broader definition of DG), the heritabilities of DG as defined by the DSM-IV and SOGS were nearly equivalent. Interestingly, the presence of DG as defined by the SOGS in a twin was as highly correlated with DG as defined by the DSM-IV in the cotwin as it was with the SOGS definition of DG, and conversely, the presence of DG as defined by the DSM-IV in a twin was as highly correlated with DG as defined by the SOGS in the cotwin as it was with the DSM-IV definition of DG. In other words, if one wanted to predict a twin’s risk for DSM-IV DG, one would do just as well by knowing the cotwin’s history of SOGS DG as one would by knowing the cotwin’s history of DSM-IV DG.
The present study also extended the previous study by demonstrating that the inability to detect any differences between men and women in the causes of liability for DG was not measure dependent. In the previous study, there was little evidence for sex differences, either quantitative or qualitative, in the causes of liability to DG as defined by the DSM-IV DG symptom set. The contribution of genetic, shared, and nonshared environmental factors to variation in DG liability did not significantly differ between men and women, and the estimated parameters of these effects were very similar (Slutske et al., 2010). The same results were obtained in the present study for DG as defined by the SOGS. DG as defined by the DSM-IV and SOGS are as heritable among women as among men.
Deeper insights into the construct of DG might be obtained by further exploring the relation between the DSM and SOGS definitions at the item level. Previous studies have conducted factor analyses (e.g. Orford et al., 2003) and latent trait modeling analyses (e.g. Strong et al., 2004) of the DSM-IV DG symptoms and SOGS items, but they have never included both item sets in the same analysis and have never incorporated measures of non-diagnostic gambling involvement (for an example from the alcoholism literature, see Krueger et al., 2004). The latent trait modeling of a comprehensive item set would allow for a comparison of the informativeness of each individual symptom at different levels of a latent DG continuum, as well as the combined informativeness of the two diagnostic criteria sets. The inclusion of non-diagnostic indicators of gambling involvement (such as daily or weekly gambling, or involvement in certain types of or numbers of different gambling activities) would allow for a better characterization of the full DG continuum (i.e. non-diagnostic items might provide information about lower levels of the latent DG continuum not well differentiated by the diagnostic items).
The SOGS might play an important role in describing the DG continuum. For the same reasons that it is said to “overdiagnose” pathological gambling, the SOGS may be especially useful in providing a broader measure than the DSM-IV for characterizing a DG continuum. The SOGS provides valuable information about clinically-significant variation in DG both above and below the DSM-IV diagnostic threshold (Strong et al., 2004). This characteristic of the SOGS will become especially valuable as the DSM moves forward in offering a dimensional diagnostic option because it is unlikely that the ten items of the DSM-IV will be sufficient to adequately describe the full spectrum of DG. This characteristic of the SOGS is often harnessed in treatment studies of clinical populations that might use the DSM-IV for screening participants, but then uses the SOGS for describing the variation in gambling pathology among those above the DSM-IV diagnostic threshold. For studies of the general population, the SOGS is also especially useful for describing continuous variation in gambling pathology below the DSM-IV threshold. This could be harnessed for the mapping of quantitative trait loci for DG because it will enable researchers to informatively phenotype more individuals.
This study suffers from a number of limitations. Australia was specifically chosen as the site for this study because it has a heavy-gambling culture and higher prevalence of DG (Slutske, et al., 2009), but the results of this study may not be generalizable beyond the heavy-gambling Australian context and culture. Also, the participants were all 32–43 years of age, and it is not clear the extent to which the same result would be obtained among older or younger adults or adolescents. Although the sample was relatively large, the sparseness of the data at the level of individual DSM-IV symptoms or SOGS items precluded the use of more sophisticated biometric models that allow one to estimate the heritability of and correlations between latent DG factors derived from inter-item correlations. The biometric modeling required the assumption that the underlying liability for DG is continuous, that is, that the risk factors for DG are the same throughout the continuum and that differences in severity are due to having more rather than different risk factors. Although the twin method provides an elegant test of this assumption (Neale & Cardon, 1992) it is usually underpowered to reject the continuity hypothesis. Thus, this assumption could not be rigorously tested.
Despite these limitations, this study represents an important step forward. The findings of a previous twin study of DSM-IV DG were extended to DG as defined by the most-used measure of gambling pathology, the SOGS. More importantly, the results of this study: (1) suggest that DG as defined by the DSM-IV and SOGS largely tap the same underlying sources of familial and genetic variation, and (2) support the construct validity of both the DSM-IV and the SOGS operationalizations and conceptualizations of DG.
Acknowledgments
Supported by National Institutes of Health Grant MH66206. We thank Dixie Statham, Bronwyn Morris, and Megan Fergusson for coordinating the data collection for the twins, and David Smyth, Olivia Zheng, and Harry Beeby for data management of the Australian Twin Registry. We thank the Australian Twin Registry twins for their continued participation.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn
Contributor Information
Wendy S. Slutske, Department of Psychological Sciences, University of Missouri
Gu Zhu, Genetic Epidemiology Unit, Queensland Institute of Medical Research.
Madeline H. Meier, Department of Psychological Sciences, University of Missouri
Nicholas G. Martin, Genetic Epidemiology Unit, Queensland Institute of Medical Research
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3. Washington, D.C: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. 3. Washington, D.C: American Psychiatric Association; 1987. rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. DSM-5 Development. 2010 (webpage). url: www.dsm5.org.
- Blashfield RK, Burgess DR. Classification provides an essential basis for organizing mental disorders. In: Lilienfeld SO, O’Donohue WT, editors. The great ideas of clinical science: 17 principles that every mental health professional should understand. New York, NY: Routledge/Taylor & Francis Group; 2007. pp. 93–117. [Google Scholar]
- Bradford J, Geller J, Lesieur HR, Rosenthal R, Wise M. Impulse control disorders. In: Widiger TA, Pincus AJ, Pincus HA, Ross R, First MB, Wakefield Davis W, editors. DSM-IV sourcebook. Vol. 2. Washington, DC: American Psychiatric Association; 1996. [Google Scholar]
- Byrne BM, Shavelson RJ, Muthén B. Testing for the equivalence of factor covariance and mean structures: The issue of partial measurement invariance. Psychological Bulletin. 1989;105:456–466. [Google Scholar]
- Cox BJ, Enns MW, Michaud V. Comparisons between the South Oaks Gambling Screen and a DSM-IV-based interview in a community survey of problem gambling. Canadian Journal of Psychiatry. 2004;49:258–264. doi: 10.1177/070674370404900406. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychological Bulletin. 1955;52:281–302. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- Cunningham-Williams RM, Cottler LB, Compton WM, III, Spitznagel EL. Taking chances: problem gamblers and mental health disorders--results from the St. Louis Epidemiologic Catchment Area Study. American Journal of Public Health. 1998;88:1093–1096. doi: 10.2105/ajph.88.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen SA, Lin N, Lyons MJ, Scherrer J, Griffith K, True WR, Goldberg J, Tsuang MT. Familial influences on gambling behavior: an analysis of 3,359 twin pairs. Addiction. 1998;93:1375–1384. doi: 10.1046/j.1360-0443.1998.93913758.x. [DOI] [PubMed] [Google Scholar]
- Gambino B, Lesieur H. The South Oaks Gambling Screen: A rebuttal to critics. Journal of Gambling Issues. 2006:17. on-line e-journal. [Google Scholar]
- Gerstein D, Hoffmann JP, Larison C, Engelman L, Murphy S, Palmer A, et al. Gambling Impact and Behavior Study: Report to the National Gambling Impact Study Commission. New York: Christiansen/Cummings Associates; 1999. [Google Scholar]
- Helzer JE, Kraemer HC, Krueger RF, Wittchen H, Sirovatka PJ, Regier DA. Dimensional approaches in diagnostic classification: Refining the research agenda for DSM-V. Arlington, VA: American Psychiatric Association; 2008. [Google Scholar]
- Hodgins D. Using the NORC DSM Screen for Gambling Problems as an outcome measure for pathological gambling: Psychometric evaluation. Addictive Behaviors. 2004;29:1685–1690. doi: 10.1016/j.addbeh.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A population-based twin study of major depression in women: The impact of varying definitions of illness. Archives of General Psychiatry. 1992a;49:257–266. doi: 10.1001/archpsyc.1992.01820040009001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder: Same genes, (partly) different environments? Archives of General Psychiatry. 1992b;49:716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: current status and future directions. Archives of General Psychiatry. 1993;50:905–915. doi: 10.1001/archpsyc.1993.01820230075007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Hwang I, LaBrie R, Petukhova M, Sampson NA, Winters KC, et al. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychological Medicine. 2008;38:1351–1360. doi: 10.1017/S0033291708002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Nichol PE, Hicks BM, Markon KE, Patrick CJ, Iacono WG, McGue M. Using latent trait modeling to conceptualize an alcohol problems continuum. Psychological Assessment. 2004;16:107–119. doi: 10.1037/1040-3590.16.2.107. [DOI] [PubMed] [Google Scholar]
- Lesieur HR. Altering the DSM-III criteria for pathological gambling. Journal of Gambling Behavior. 1988;4:38–47. [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. American Journal of Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York: John Wiley & Sons; 1987. [Google Scholar]
- McGuffin P, Rijsdijk F, Martin A, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Archives of General Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Specific etiology and other forms of strong influence: Some quantitative meanings. The Journal of Medicine and Philosophy. 1977;2:33–53. [Google Scholar]
- Meehl PE. Theoretical risks and tabular asterisks: Sir Karl, Sir Ronald, and the slow progress of soft psychology. Journal of Consulting and Clinical Psychology. 1978;46:806–834. [Google Scholar]
- Meehl PE. Diagnostic taxa as open concepts: Metatheoretical and statistical questions about reliability and construct validity in the grand strategy of nosological revision. In: Millon T, Klerman GL, editors. Contemporary directions in psychopathology. NY: Guilford; 1986. pp. 215–231. [Google Scholar]
- Muthén LK, Muthén B. Mplus User’s Guide. Los Angeles, CA: Author; 2004. [Google Scholar]
- National Research Council. Pathological gambling: A critical review. Washington DC: National Academy Press; 1999. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Lubke G, Aggen SH, Dolan CV. Problems with using sum scores for estimating variance components: contamination and measurement noninvariance. Twin Research and Human Genetics. 2005;8:553–568. doi: 10.1375/183242705774860231. [DOI] [PubMed] [Google Scholar]
- Orford J, Sproston K, Erens B. SOGS and DSM-IV in the British gambling prevalence survey: Reliability and factor structure. International Gambling Studies. 2003;3:53–65. [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005;66:564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcoholism: Clinical and Experimental Research. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Reich T, Cloninger CR, Guze SB. The multifactorial model of disease transmission: I. description of the model and its use in psychiatry. British Journal of Psychiatry. 1975;127:1–10. doi: 10.1192/bjp.127.1.1. [DOI] [PubMed] [Google Scholar]
- Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: Its application to schizophrenia. American Journal of Psychiatry. 1970;126:983–987. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- Robins LN. Using survey results to improve the validity of the standard diagnostic nomenclature. Archives of General Psychiatry. 2004;61:1188–1194. doi: 10.1001/archpsyc.61.12.1188. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, et al. The NIMH Diagnostic Interview Schedule: Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, Hall MN, Vander Bilt J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: A meta-analysis. Harvard Medical School Division on Addictions; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer HJ, Hall MN, Vander Bilt J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: A research synthesis. American Journal of Public Health. 1999;89:1369–1376. doi: 10.2105/ajph.89.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer HJ, LaBrie RA, LaPlante DA, Nelson SE, Stanton MV. The road less traveled: Moving from distribution to determinants in the study of gambling epidemiology. Canadian Journal of Psychiatry. 2004;49:504–516. doi: 10.1177/070674370404900802. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Eisen SA, True WR, Lyons MJ, Goldberg J, Tsuang MT. Common genetic vulnerability for pathological gambling and alcohol dependence in men. Archives of General Psychiatry. 2000;57:666–673. doi: 10.1001/archpsyc.57.7.666. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG. The Australian twin study of gambling (OZ-GAM): Rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Research and Human Genetics. 2009;12:63–78. doi: 10.1375/twin.12.1.63. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Zhu G, Meier MH, Martin NG. Genetic and environmental influences on disordered gambling in men and women. Archives of General Psychiatry. 2010;67:624–630. doi: 10.1001/archgenpsychiatry.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchfield R. Reliability, validity, and classification accuracy of the South Oaks Gambling Screen (SOGS) Addictive Behaviors. 2002;27:1–19. doi: 10.1016/s0306-4603(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Stinchfield R. Reliability, validity, and classification accuracy of a measure of DSM-IV diagnostic criteria for pathological gambling. American Journal of Psychiatry. 2003;160:180–182. doi: 10.1176/appi.ajp.160.1.180. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW. Evaluation of the continuum of gambling problems using the DSM-IV. Addiction. 2007;102:713–721. doi: 10.1111/j.1360-0443.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- Strong DR, Lesieur HR, Breen RB, Stinchfield R, Lejeuz CW. Using a Rasch model to examine the utility of the South Oaks Gambling Screen across clinical and community samples. Addictive Behaviors. 2004;29:465–481. doi: 10.1016/j.addbeh.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Welte J, Barnes G, Wieczorek W, Tidwell M, Parker J. Alcohol and gambling pathology among U.S. adults: Prevalence, demographic patterns, and comorbidity. Journal of Studies on Alcohol. 2001;62:706–712. doi: 10.15288/jsa.2001.62.706. [DOI] [PubMed] [Google Scholar]
- Wickwire EM, Burke RS, Brown SA, Parker JD, Ryan KM. Psychometric evaluation of the National Opinion Research Center DSM-IV Screen for Gambling Problems (NODS) The American Journal on Addictions. 2008;17:392–395. doi: 10.1080/10550490802268934. [DOI] [PubMed] [Google Scholar]
- Widiger TA. A dimensional model of psychopathology. Psychopathology. 2005;38:211–214. doi: 10.1159/000086094. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Frances A. Definitions and diagnoses: A brief response to Morey and McNamara. Journal of Abnormal Psychology. 1987;96:286–287. doi: 10.1037//0021-843x.96.3.286. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Frances AJ, Pincus HA, David WW, First MB. Toward an empirical classification for the DSM-IV. Journal of Abnormal Psychology. 1991;100:280–288. doi: 10.1037//0021-843x.100.3.280. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Cadoret R, Hare R, Robins L, Rutherford M, Zanarini M, et al. DSM-IV antisocial personality disorder field trial. Journal of Abnormal Psychology. 1996;105:3–16. doi: 10.1037//0021-843x.105.1.3. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Samuel DB. Diagnostic categories or dimensions? A question for the Diagnostic and Statistical Manual of Mental Disorders–Fifth edition. Journal of Abnormal Psychology. 2005;114:494–504. doi: 10.1037/0021-843X.114.4.494. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Manual of the international statistical classification of diseases, injuries, and causes of death: ICD-9. Geneva: World Health Organization; 1977. [PMC free article] [PubMed] [Google Scholar]