Abstract
Background
Obesity is a known risk factor for surgical site infection (SSI). Our hypothesis is that morphometric measures of midline subcutaneous fat will be associated with increased risk of SSI, and will predict SSI better than conventional measures of obesity.
Study Design
We identified 655 patients who underwent midline laparotomy (2006 - 2009) using the Michigan Surgical Quality Collaborative database. Using novel, semi-automated analytic morphometric techniques, the thickness of subcutaneous fat along the linea alba was measured between T12 and L4. To adjust for variations in patient size, subcutaneous fat was normalized to the distance between the vertebrae and anterior skin. Logistic regression analyses were used to identify factors independently associated with the incidence of SSI.
Results
Overall, SSIs were observed in 12.5% (n = 82) of the population. Logistic regression revealed that patients with increased subcutaneous fat had significantly greater odds of developing a superficial incisional SSI (OR = 1.76 per 10% increase, 95% CI: 1.10 – 2.83, p = 0.019). Smoking, steroid use, ASA classification, and incision-to-close operative time were also significant independent risk factors for superficial incisional SSI. When comparing subcutaneous fat and body mass index (BMI) as the only model variables, subcutaneous fat significantly improved model predictions of superficial incisional SSI (AUC: 0.60, p = 0.023) while BMI did not (AUC = 0.52, p = 0.73).
Conclusions
Abdominal subcutaneous fat is an independent predictor of superficial incisional SSI following midline laparotomy. Novel morphometric measures may improve risk stratification and help elucidate the pathophysiology of surgical complications.
Introduction
A novel concept in preoperative risk stratification is analytic morphomics. This approach involves using preoperative imaging to obtain morphometric measures such as core muscle size, bone mineral density, arterial calcification, and body composition. These novel techniques may help to objectively capture the subjective components of a surgeon's preoperative assessment, referred to as the “eyeball test” by some clinicians. Similar to this “eyeball test,” these metrics may potentially be used to assess a patient's ability to tolerate major surgery. Moreover, such measures may significantly improve preoperative risk stratification.1,2
One potential application of analytic morphomics involves the relationship between obesity and surgical site infection. Obesity is a known risk factor for surgical site infection (SSI).3-5 Although body mass index (BMI) is typically used to define obesity, there is clearly wide variation in how precisely it describes body composition.6-9 More specifically, although BMI is useful as a clinical tool, it is a relatively non-specific assessment of body composition that does not directly measure adiposity. In comparison, morphometric measures of body composition can directly quantify the amount of subcutaneous fat at the site of the surgical wound. Such targeted measures of body composition may improve assessments for SSI risk compared to less specific metrics such as BMI. Within this context, applying these morphometric measures of body composition to surgical patients may further inform preoperative risk stratification and clinical decision-making.
In this report, we evaluate a novel morphometric measure of abdominal subcutaneous fat as a predictor for SSI in patients undergoing midline laparotomy. Our hypothesis is that increased subcutaneous fat will be a strong risk factor for superficial incisional SSI, and that morphometric measures of subcutaneous fat will improve predictions of superficial incisional SSI compared to BMI. To address this hypothesis, we describe the incidence of SSI in 655 patients who underwent midline laparotomy at the University of Michigan. This work represents a pilot study in our efforts to develop clinically relevant analytic morphometric techniques. With further study, these measures may allow surgeons to use cross-sectional images not only to assess pathology, but also to develop an overall clinical impression of burden of disease and patient fitness for surgical intervention.
Methods
Study Population
This study included all patients in the Michigan Surgical Quality Collaborative (MSQC) database who underwent elective midline laparotomy at the University of Michigan from 2006 – 2009 and had a suitable preoperative abdominal CT scan. Eligible preoperative scans were completed within 90 days prior to the operation. Patients with midline ventral hernias were excluded from analysis, as it was not possible to obtain accurate morphometric measures for these patients. The MSQC database uses the data collection platform of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) and is well described in our previous work.3,10-13 In brief, this database includes information regarding patient demographics, preoperative comorbidities and laboratory values, operative events, and clinical outcomes. Variables of particular interest for this study included age, gender, race, height, weight, history of smoking, diabetes, steroid use, and incision-to-close operative time. In the MSQC database, SSI is defined as any occurrence of a superficial incisional, deep incisional, or organ space surgical site infection within 30 days of operation (as defined by ACS NSQIP). 14-16 The primary outcome for all models was superficial incisional SSI within 30 days post-operation.
Analytic Morphomics
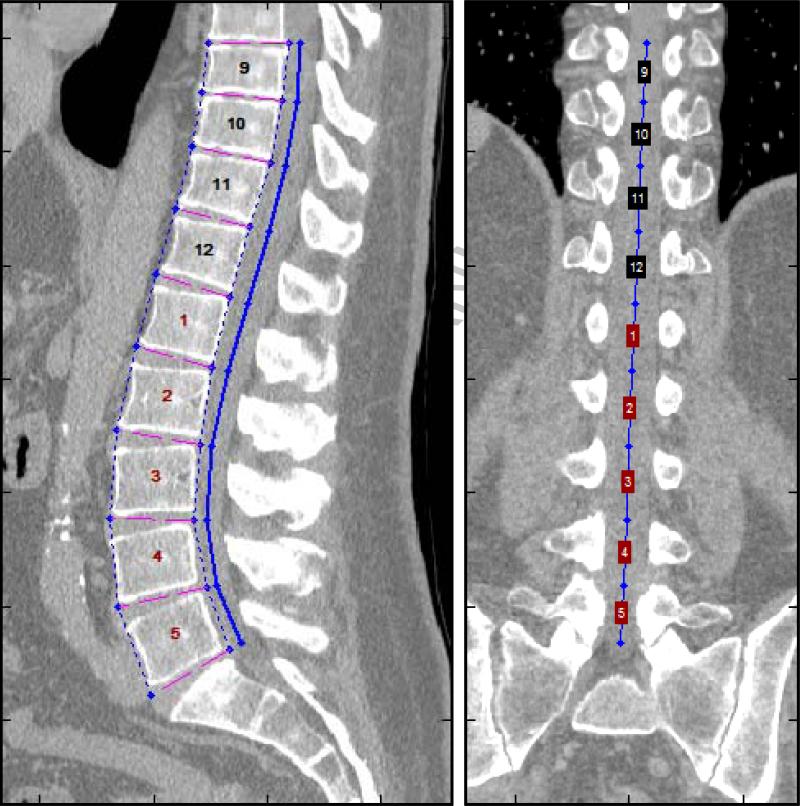
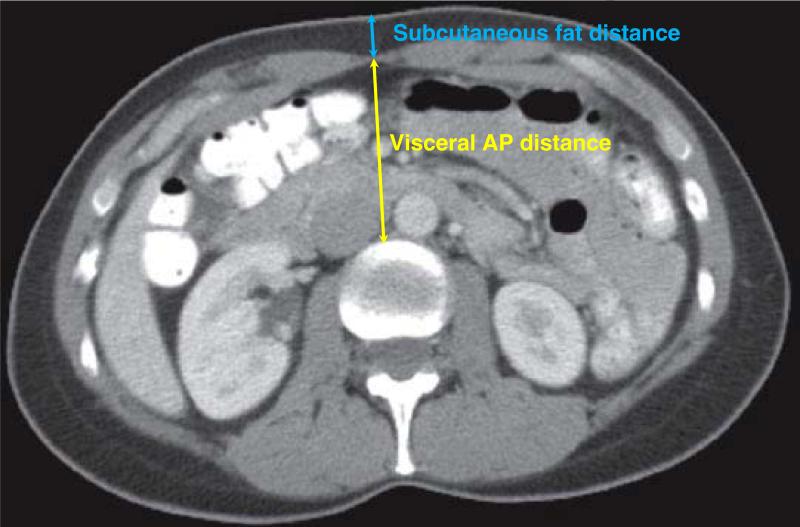
CT scans were processed using semi-automated algorithms programmed into MATLAB v13.0 as described in our previous work. 1,2 These algorithms utilize novel, high-throughput techniques we have named analytic morphomics. The initial processing step identified individual vertebral levels on each patient's scan, as shown in Figure 1. These vertebral levels served as anatomical landmarks for subsequent analysis. The next processing step identified the linea alba and the anterior abdominal skin along the midline at each vertebral level from T12 to L4. The average distance between the linea alba and the anterior skin along T12 to L4 was labeled the subcutaneous fat distance, and the average distance between the anterior aspect of the vertebra and the linea alba was labeled the visceral anterior-to-posterior (AP) distance. The sum of the subcutaneous fat distance and visceral AP distance was labeled the total AP distance. Figure 2 depicts these measurements. In order to control for variations in patient size, the subcutaneous fat distance was normalized as the percentage of the total AP distance. This metric was labeled the normalized subcutaneous fat, and served as the primary exposure variable for this study.
Figure 1.
Vertebral levels were identified on each patient's CT scan. These levels served as anatomic landmarks that allowed for consistent measurement of body composition along the T12 – L4 levels.
Figure 2.
Body composition measurements were obtained using cross sectional images. The subcutaneous fat distance is measured between the anterior skin at the midline and the line alba, while the visceral anterior-to-posterior distance is measured between the linea alba and the spine. These two distances were summed to calculate the total anterior-to-posterior distance.
Total psoas area was measured as described in our previous work.1,2 Briefly, the transverse slice corresponding to the inferior aspect of L4 was selected, and the outlines of the psoas muscles were traced. The enclosed area was then computed as the total psoas area.
Statistical Analysis
Descriptive statistics were computed for the study cohort. Continuous variables were summarized by the mean and standard deviation, while categorical variables were summarized with frequency tables. Continuous variables were compared using a t-test, while chi-square and Fisher's exact tests were used to compare categorical and dichotomous variable, respectively.
Univariate analysis was used to identify potential morphometric measures of interest. Five morphometric measures were evaluated in this initial analysis: visceral AP distance, subcutaneous fat distance, total AP distance, normalized subcutaneous fat, and total psoas area. Three SSI outcomes were also evaluated: any SSI, superficial incisional SSI, and combined deep incisional or organ space SSI. Organ space and deep incisional SSI were combined as a single end point due to the limited number of events for each outcome.
Based on this initial univariate analysis, normalized subcutaneous fat and superficial incisional SSI were chosen as the primary variables of interest. Additional candidate predictors were chosen from risk factors identified by previous investigators.17 These included preoperative demographic and medical risk factors, operative variables, procedure type (colectomy versus all other procedures), and wound classification. Initially, univariate analysis was used to compare these candidate variables for patients with and without superficial incisional SSI. Multivariable logistic regression was then used to identify factors independently associated with the incidence of superficial incisional SSI. All candidate predictors were entered into the logistic regression analysis, and stepwise backward selection was used to select a subset of adjustment covariates. An interaction term for normalized subcutaneous fat and gender was also entered into the selection to account for the observed differences in normalized subcutaneous fat with respect to gender.
Model validity and comparisons were assessed using the Omnibus tests of model coefficients, and the area under the receiver operating curves (AUC). The Omnibus tests provide an absolute measure of model validity by testing the predictive ability of all covariates in the model compared to a constant-only model. Differences in AUC were evaluated using the method described by Hanley and McNeil.18 All statistical computations were performed in SPSS v17.0. A two-sided significance level of α = 0.05 was used for all analyses.
This study was approved by the University of Michigan Institutional Review Board.
Results
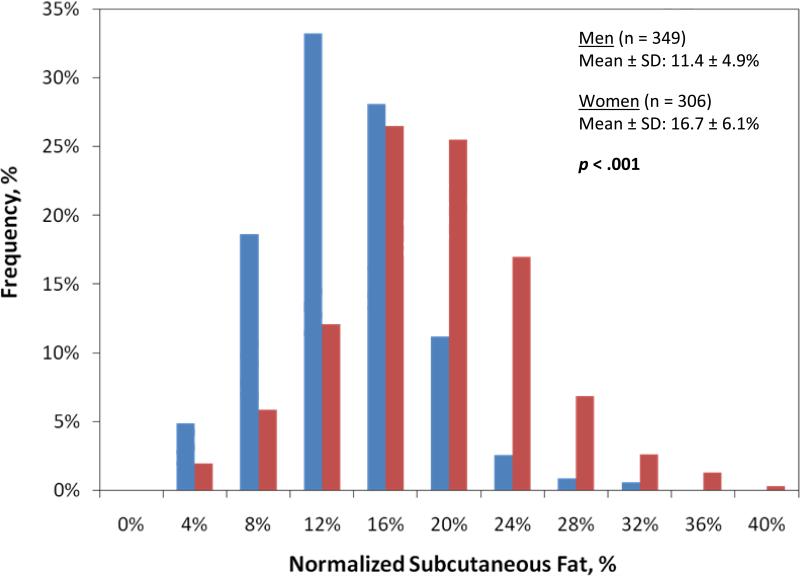
2,355 patients underwent a midline laparotomy during the study period. Of these, 655 had an eligible preoperative CT scan, and this subset of patients served as our study group. Descriptive statistics for the study sample are summarized in Table 1. Overall, SSIs were observed in 12.5% (n = 82) of the sample. The incidence of superficial incisional SSI, deep incisional SSI, and organ space SSI was 7.6% (n = 50), 0.6% (n = 4), and 5.3% (n = 35), respectively. Seven patients experienced two SSIs. The overall incidence of any SSI was greater in women (n = 44) than in men (n = 38), although the difference was not significant (14.4% versus 10.9%; p = 0.19). The average normalized subcutaneous fat for all patients was 13.9 ± 6.1 %. Female patients had significantly greater normalized subcutaneous fat compared to males (16.7% versus 11.4%, p < .001). The distribution of normalized subcutaneous fat for males and females is shown in Figure 3.
Table 1.
Patient Characteristics (n = 655)
| Characteristic | |
|---|---|
| Age at treatment, y, mean ± SD | 59.2 ± 15.5 |
| Height, in, mean ± SD | 169.3 ± 11.1 |
| Weight, kg, mean ± SD | 79.4 ± 21.0 |
| Body Mass Index, kg/m2, mean ± SD | 27.6 ± 6.6 |
| Normalized subcutaneous fat, %, mean ± SD | 13.9 ± 6.1 |
| Race, n (%) | |
| White | 550 (84.0) |
| African-American | 55 (8.4) |
| Other | 16 (2.4) |
| Not specified | 34 (5.2) |
| Sex, n (%) | |
| Male | 349 (53.3) |
| Female | 306 (46.7) |
| Smoker, n (%) | 103 (15.7) |
| Steroid use, n (%) | 57 (8.7) |
| Most common procedures, n (%) | |
| Proximal subtotal pancreatectomy (Whipple-type procedure) | 43 (6.6) |
| Partial colectomy with ileocolostomy | 38 (5.8) |
| Enterectomy (single resection and anastomosis) | 37 (5.6) |
| Partial colectomy with anastomosis | 34 (5.2) |
| Hepatectomy (partial lobectomy) | 25 (3.8) |
| Events, n (%) | |
| All surgical site infections | 82 (12.5) |
| Superficial Incisional SSI | 50 (7.6) |
| Deep Incisional SSI | 4 (0.6) |
| Organ space SSI | 35 (5.3) |
SSI, surgical site infection.
Figure 3.
The distribution of normalized subcutaneous fat in men and women. Women in the study had a significantly greater normalized subcutaneous fat compared to men (16.7% versus 11.4%, p < 0.001). Blue bar, men; red bar, women. AP, anterior-to-posterior.
Results from the initial univariate analysis of morphometric measures and SSI outcomes are shown in Table 2. Patients with superficial incisional SSI had significantly higher subcutaneous fat distance compared to those without superficial incisional SSI (22.8 versus 20.0 mm, p = 0.049). The significance of this difference was strengthened by normalizing this measure to the total AP distance to adjust for variations in overall abdomen size (15.8% versus 13.7%, p = 0.020). This metric was labeled the normalized subcutaneous fat. Of note, normalized subcutaneous fat and subcutaneous fat distance were not significantly increased in patients with organ space or deep incisional SSI (p = 0.66 and 0.58, respectively). In addition, patients with superficial incisional SSI did not have significantly different visceral AP distance, total AP distance, or total psoas area when compared to those with no superficial incisional SSI. We also note that visceral AP distance and total AP distance were not significantly increased in patients with any of the three SSI outcomes evaluated in this study (any SSI, superficial incisional SSI, and deep incisonal/organ space SSI). Based on this analysis, we selected normalized subcutaneous fat and superficial incisional SSI as the primary variables of interest.
Table 2.
Univariate Analysis of Morphometric Measures and Surgical Site Infection Outcomes
| Variable | Any SSI | Superficial incisional SSI | Organ space/deep incisional SSI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Without (n = 573) | With (n = 82) | p Value | Without (n = 605) | With (n = 50) | p Value | Without (n = 617) | With (n = 38) | p Value | |
| BMI, kg/m2, mean ± SD | 27.6 ± 6.6 | 27.7 ± 7.1 | 0.87 | 27.6 ± 6.7 | 27.9 ± 5.9 | 0.73 | 27.6 ± 6.5 | 27.7 ± 8.2 | 0.97 |
| Total AP distance, mm, mean ± SD | 147.2 ± 33.3 | 149.8 ± 35.6 | 0.52 | 147.5 ± 33.5 | 148.4 ± 35.2 | 0.85 | 147.3 ± 33.5 | 151.6 ± 34.5 | 0.45 |
| Visceral AP distance, mm, mean ± SD | 127.3 ± 31.1 | 127.8 ± 34.5 | 0.88 | 127.5 ± 31.4 | 125.6 ± 34.1 | 0.70 | 127.1 ± 31.4 | 130.5 ± 33.8 | 0.52 |
| Subcutaneous fat distance, mm, mean ± SD | 20.0 ± 9.4 | 22.0 ± 9.6 | 0.073 | 20.0 ± 9.4 | 22.8 ± 9.5 | 0.049 | 20.2 ± 9.5 | 21.1 ± 9.4 | 0.58 |
| Normalized subcutaneous fat, %, mean ± SD | 13.7 ± 6.0 | 15.1 ± 6.5 | 0.048 | 13.7 ± 6.0 | 15.8 ± 6.4 | 0.020 | 13.8 ± 6.1 | 14.3 ± 6.4 | 0.66 |
Univariate relationships between candidate predictors and the occurrence of superficial incisional SSI are reported in Table 3. Compared to patients with no superficial incisional SSI, patients with superficial incisional SSI had significantly higher normalized subcutaneous fat and operative times, and were significantly more likely to have smoked in the past year. Significant differences in wound classification were also observed. Steroid use, gender, and ASA class were not significant in the univariate analysis.
Table 3.
Univariate Analysis of Candidate Predictors for Superficial Incisional Surgical Site Infections
| Variable | No Superficial Incisional SSI (n = 605) | Superficial Incisional SSI (n = 50) | p Value |
|---|---|---|---|
| Normalized subcutaneous fat, mean ± SD | 13.7 ± 6.0 | 15.8 ± 6.4 | 0.020 |
| BMI, kg/m2, mean ± SD | 27.6 ± 6.7 | 27.9 ± 5.9 | 0.73 |
| Total psoas area, mm2, mean ± SD | 2064.0 ± 791.4 | 1964.4 ± 823.2 | 0.39 |
| Age, y, mean ± SD | 59.3 ± 15.4 | 58.5 ± 16.5 | 0.73 |
| Women, n (%) | 278 (46.0) | 28 (56.0) | 0.19 |
| Diabetes, n (%) | 108 (17.9) | 11 (22.0) | 0.45 |
| Dyspnea, n (%) | 69 (11.4) | 6 (12.0) | 0.82 |
| Steroid use, n (%) | 50 (8.3) | 7 (14.0) | 0.19 |
| Alcoholism, n (%) | 8 (1.3) | 0 (0.0) | >0.99 |
| Smoker, n (%) | 88 (14.5) | 15 (30.0) | 0.008 |
| Radiotherapy within past 90 d, n (%) | 22 (3.6) | 3 (6.0) | 0.43 |
| ASA class, n (%) | 0.11 | ||
| I | 11 (18) | 0 (0.0) | |
| II | 194 (32.1) | 25 (50.0) | |
| III | 315 (52.1) | 21 (42.0) | |
| IV | 80 (13.2) | 4 (8.0) | |
| V | 5 (0.83) | 0 (0.0) | |
| Procedure type, n (%) | 0.39 | ||
| Colectomy | 145 (24.0) | 15 (30.0) | |
| All other procedures | 460 (76.0) | 35 (70.0) | |
| Wound classification, n (%) | 0.009 | ||
| Clean | 116 (19.3) | 2 (4.0) | |
| Clean/contaminated | 367 (61.0) | 42 (84.0) | |
| Contaminated | 67 (11.1) | 4 (8.0) | |
| Dirty/infected | 52 (8.6) | 2 (4.0) | |
| Operative time, hrs, mean ± SD | 3.7 ± 2.3 | 4.5 ± 2.0 | 0.014 |
| Work RVU, mean ± SD | 28.5 ± 12.4 | 30.1 ± 12.1 | 0.38 |
Table 4 compares the different logistic regression models performed for this analysis. When comparing normalized subcutaneous fat (Model 1) and BMI (Model 2) as the only model variables, the Omnibus tests of model coefficients indicated that normalized subcutaneous fat significantly improved model predictions of superficial incisional SSI (AUC: 0.60, p = 0.023) while BMI did not (AUC: 0.52, p = 0.73). For Model 3, all candidate predictors listed in Table 3 were entered into the logistic regression analysis, and a subset of covariates was selected using stepwise backward selection. Using this approach, it was determined that the only significant covariates in the model were normalized subcutaneous fat, smoking in the year prior to operation, incision-to-close operative time (hrs), steroid use, and ASA class (dichotomized as ASA class 1 – 2 versus 3 – 5). This model was statistically significant (p < 0.001; AUC = 0.69), and provided slightly improved predictions compared to replacing normalized subcutaneous fat with BMI (Model 4: p = 0.002, AUC = 0.68). The difference in AUC between Models 3 and 4, however, was not significant (p = 0.41).
Table 4.
Comparative Summary of Logistic Regression Models for Incidence of Superficial Incisional Surgical Site Infection
| Model | Variables | Omnibus tests of model coefficients* (p value) | AUC |
|---|---|---|---|
| 1 | Normalized Subcutaneous Fat (per 10% increase) | 0.023 | 0.60 ± 0.044 |
| 2 | BMI | 0.73 | 0.52 ± 0.039 |
| 3 | ASA, smoking, steroid use, incision-to-close operative time (h), normalized subcutaneous fat | < 0.001 | 0.69 ± 0.036 |
| 4 | ASA, smoking, steroid use, incision-to-close operative time (h), BMI | 0.002 | 0.68 ± 0.041 |
The Omnibus tests of model coefficients provide an absolute measure of model validity by testing the predictive ability of all covariates in the model compared to a constant-only model
Estimated parameters from the logistic regression model obtained using backward selection (Model 3) are shown in Table 5. This model indicated that patients with increased normalized subcutaneous fat had significantly greater odds of developing superficial incisional SSI (OR = 1.76 per 10% increase, 95% CI: 1.10 – 2.83, p = 0.019). Patients who smoked in the past year were also at increased risk compared to non-smokers (OR = 2.83, 95% CI: 1.45 – 5.53, p = 0.002). Risk of superficial incisional SSI was also significantly higher for steroid use (OR = 2.65, 95% CI: 1.08 – 6.54, p = 0.034) and increased incision-to-close operative time (OR = 1.20 per hour increase, 95% CI: 1.06 – 1.36, p = 0.003). Interestingly, patients in ASA Class 3 – 5 were at lower risk compared to those in ASA 1 – 2 (OR = 0.53, 95% CI:0.29 – 0.98, p = 0.043).
Table 5.
Model Covariates for Incidence of Superficial Incisional Surgical Site Infection
| Model Variable | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Normalized subcutaneous fat (per 10% increase) | 1.76 | 1.10 – 2.83 | 0.019 |
| ASA Classification 3 – 5 (reference: ASA 1 – 2) | 0.53 | 0.29 – 0.98 | 0.043 |
| Steroid use | 2.65 | 1.08 – 6.54 | 0.034 |
| Smoking | 2.83 | 1.45 – 5.53 | 0.002 |
| Incision-to-close operative time, h | 1.20 | 1.06 – 1.36 | 0.003 |
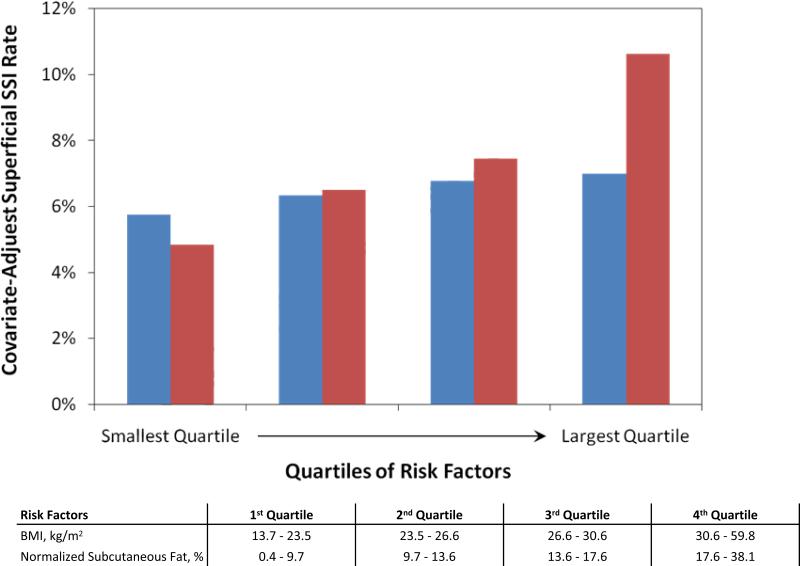
Figure 4 compares BMI and normalized subcutaneous fat as risk factors for superficial incisional SSI. Patients were stratified into quartiles of normalized subcutaneous fat and BMI. Models 3 and 4 were then used to calculate the covariate-adjusted rate of superficial incisional SSI. As shown in Figure 4, the covariate-adjusted rate of superficial incisional SSI increases with normalized subcutaneous fat. Furthermore, patients in the largest quartile of normalized subcutaneous fat had more than twice the risk of superficial incisional SSI compared to those in the smallest quartile (10.6% versus 4.8%, p < 0.05). In contrast, although a similar trend is observed for BMI, patients in the largest quartile of BMI had only a slightly increased risk superficial incisional SSI compared to patients in the smallest quartile (7.0% versus 5.7%, p>0.05).
Figure 4.
Covariate-adjusted incidence of superficial incisional surgical site infection (SSI) with patients stratified into quartiles of normalized subcutaneous fat and BMI. SSI rate was adjusted for gender, steroid use, smoking, operative time, and BMI or normalized subcutaneous fat using the Models 3 and 4 in Table 4. The covariate-adjusted rate of superficial incisional SSI increased with normalized subcutaneous fat. Furthermore, patients in the largest quartile of normalized subcutaneous fat had more than twice the risk of superficial incisional SSI compared to those in the smallest quartile (10.6% versus 4.8%, p < 0.05). In contrast, although a similar trend is observed for BMI, patients in the largest quartile of BMI had only a slightly increased risk of superficial incisional SSI compared to patients in the smallest quartile (7.0% versus 5.7%, p > 0.05). Blue bar, body mass index; red bar, normalized subcutaneous fat.
Discussion
The validation of sensitive and clinically relevant morphometric measures of preoperative risk may further inform both clinical decision-making and our understanding of the pathophysiology of surgical disease. With this work, we introduce analytic morphomics as a novel concept that can be applied to better characterize body composition. Specifically, we have evaluated an objective measure of subcutaneous fat at the surgical site as a risk factor for SSI, and compared it to BMI (a known risk factor for SSI).3,4 We note that an objective measure of subcutaneous fat at the site of the surgical incision significantly informs clinical risk models, and outperforms BMI as a clinical risk factor. These data suggest that analytic morphomics may provide a powerful new approach for preoperative risk assessment.
It is intuitive that the amount of subcutaneous fat at the site of the surgical incision would associate with risk of an infection. In addition, other groups have reported that alternative measures of body composition provide improved assessment of SSI risk compared to BMI.5,19 Potentially, a surgeon may be able to appreciate the amount of subcutaneous fat on a preoperative CT scan, and this could inform intraoperative clinical decision-making and informed consent. For example, if the surgeon knows preoperatively that the procedure involves a high-risk wound, he or she may consider surgical techniques to minimize the risk of major wound complications, such as placing closed suction drains in the subcutaneous space. For higher risk cases, delayed primary closure or leaving a wound open may be appropriate.
Aside from the clinical relevance of this measure, the true power of analytic morphomics in this setting is that it may provide new insight into the pathophysiology of surgical site infection. This work is the first to note that the amount of visceral fat is not associated with increased risk of SSI. Furthermore, although previous work has explored the relationship between subcutaneous fat and SSI risk in patients undergoing elective colorectal surgery,5,19 this study is the first to utilize a measure targeted specifically at the wound site in the context of a wide range of procedures. It is also the first to focus on the potentially unique relationship between subcutaneous fat and superficial incisional SSI. There are several proposed mechanisms that could explain why increased fat at the site of the surgical incision may increase risk of surgical site infection. These include increased technical difficulty, increased tissue trauma, increased tension on the wound, decreased circulation and oxygenation at the local wound site, and local immunosuppression related to large populations of adipocytes.20-22
The findings of this study have several key differences compared to risk factors identified by previous investigators.17 Specifically, wound classification was not a significant risk factor for SSI, while patients with ASA class 1 – 2 were at increased risk compared to those with ASA class 3 – 5. These differences are likely due to the small number of events in the study (n = 50). This limited our analysis of variables with multiple categories due to the small number of events in each category (50% of the events occurred within ASA class 2 while 84% of the events were in the clean/contaminated wound classification). Although ASA class and wound classification were dichotomized in the multivariable analysis to ensure an even distribution of patients in each group, this inevitably limited our ability to evaluate these variables as potential risk factors. Future work will require a larger study with more events to compare subcutaneous fat to ASA class and wound classification as risk factors for SSI. We note that despite differences with respect to ASA class and wound classification, the remaining risk factors identified in this study (steroid use, smoking, and operative time) agree with those identified by previous studies.17,23
This study has several important limitations. First, it is a retrospective study at a single center with a relatively small cohort of patients. Future studies should include a larger sample size and multiple institutions. Second, there is potential selection bias for patient inclusion in the study group, considering the fact that having a preoperative CT scan was among the inclusion criteria for the study group. However, this is likely of less importance when considering that the overall goal of this report was to introduce the concept of a morphometric measure of body composition and that all patients included in the analysis were exposed to this bias (having a preoperative CT scan). Nonetheless, future work focusing on body composition and surgical risk will require prospective data accumulation to prevent such selection bias. Furthermore, relying on CT scans for our analysis has several inherent limitations. Considering the risks of CT imaging, it is unlikely that patients will undergo these studies simply for morphometric analysis. Within this context, we are developing novel techniques using ultrasound imaging to capture the same objective anatomic information. Such methods will also be more conducive to prospective study.
Another limitation of this study is that we only describe a small number of measures for body composition. The analytic morphomic techniques that we utilized may potentially yield hundreds of novel clinical variables, and we have chosen to focus on a relatively small number based on our clinical intuition. More advanced analysis will be needed to better understand the complex physiologic and anatomic relationships between patient morphometry and surgical disease. Furthermore, this work compares measures of subcutaneous fat to a single measure of body composition, BMI. Recent work has suggested that body fat percentage provides improved risk stratification for SSI compared to BMI.5,19 Although measurements of body fat percentage were not available for our study, future work will need to compare body fat percentage and measures of subcutaneous fat as predictors of SSI risk. In addition, we have chosen to limit our study to procedures involving midline laparotomy. To provide further insight into the relationship between subcutaneous fat and surgical site infection, future studies will need to assess this relationship in other procedures, such as total knee replacement.
Our study was also limited due to a lack of data on important process measures associated with reducing the incidence of surgical site infection, such as timing of intravenous antibiotics. Such measures may certainly impact SSI rates, and future studies will need to account for these potential confounders. In addition, as explained earlier, we note that our analysis of variables with multiple categories – such as ASA class and wound classification – was limited by the small number of events in the study (n = 50). For example, half of the events (n = 25) occurred within a single ASA class (ASA 2). This uneven distribution may explain why patients with ASA 3 – 5 had a lower risk of superficial incisional SSI compared to those with ASA 1 – 2 even though no trend was observed in the univariate analysis. Future work will require a larger study with more events to compare subcutaneous fat to ASA class and wound classification as potential risk factors. Finally, we note that subcutaneous fat may not significantly improve specificity or sensitivity for predictions of SSI risk, as adding normalized subcutaneous fat to the model only modestly improved the AUC compared to BMI. Nevertheless, the odds ratio associated with normalized subcutaneous fat indicates that this metric may help identify patients at higher risk for SSI. We note that this work is a pilot study intended to introduce a new technique that may yield novel predictors and perspectives of operative risk. To determine if these measures add predictive value to established models for SSI risk, future work will need to evaluate these metrics more rigorously in the context of a larger prospective study with more events.
As we continue to investigate analytic morphomics, we hope to further inform clinical risk assessment and elucidate the pathophysiology of surgical disease. More specifically, radiologists and surgeons typically focus on pathology when reviewing cross-sectional images for operative planning. This approach to cross-sectional imaging, however, may be too narrow. Cross-sectional images contain vast amounts of additional patient-specific data that are never assessed or appreciated by clinicians. A potential new paradigm is utilizing cross-sectional images not only for the assessment of the specific disease process, but for a more global assessment of the patient. Our initial work assessing core muscle size and surgical outcomes suggests these methods may significantly inform assessments of operative risk.1,2
In summary, with this work we have demonstrated that the relative amount of subcutaneous fat at the site of the incision is a significant independent risk factor for superficial incisional SSI in patients undergoing midline laparotomy. Further, this metric is more strongly associated with superficial incisional SSI when compared to the conventional measure of body composition, BMI. Steroid use, smoking, and incision-to-close operative time were also identified as independent risk factors for SSI, which agrees with previous work utilizing ACS NSQIP data.13 Surgeons may consider directly evaluating the amount of subcutaneous fat on preoperative imaging studies as they assess patient risk. In addition, this work represents our efforts to develop improved measures of preoperative risk by applying the novel approach of analytic morphomics. Additional work in this field may offer significant opportunities to improve the care of surgical patients and to better understand the pathophysiology of surgical disease.
Acknowledgment
The authors would like to formally thank Darrell Campbell, MD and Daniel Kowalsky and for their time and effort with this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Dr Englesbe was supported by NIH – NIDDK (K08 DK0827508) and the Blue Cross and Blue Shield Foundation of Michigan.
References
- 1.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JS, Harbaugh C, He K, et al. Frailty, sarcopenia, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg. 2010 doi: 10.1016/j.jvs.2010.10.111. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Khuri SF, Henderson WG. The patient safety in surgery study. J Am Coll Surg. 2007;204:1087–1088. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Lynch RJ, Ranney DN, Shijie C, et al. Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg. 2009;250:1014–1020. doi: 10.1097/SLA.0b013e3181b4ee9a. [DOI] [PubMed] [Google Scholar]
- 5.Waisbren E, Rosen H, Bader AM, et al. Percent body fat and prediction of surgical site infection. J Am Coll Surg. 210:381–389. doi: 10.1016/j.jamcollsurg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher D, Visser M, Sepulveda D, et al. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 7.Whittle AT, Marshall I, Mortimore IL, et al. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323–328. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heymsfield SB, Gallagher D, Mayer L, et al. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr. 2007;86:82–91. doi: 10.1093/ajcn/86.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell DA, Englesbe MJ, Kubus JJ, et al. Accelerating the pace of surgical quality improvement: the power of hospital collaboration. Arch Surg. 2010 doi: 10.1001/archsurg.2010.220. In Press. [DOI] [PubMed] [Google Scholar]
- 11.Englesbe MJ, Brooks L, Kubus J, et al. A statewide assessment of surgical site infection following colectomy: the role of oral antibiotics. Ann Surg. 252:514–519. doi: 10.1097/SLA.0b013e3181f244f8. discussion 519-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englesbe MJ, Dimick JB, Sonnenday CJ, et al. The Michigan surgical quality collaborative: will a statewide quality improvement initiative pay for itself? Ann Surg. 2007;246:1100–1103. doi: 10.1097/SLA.0b013e31815c3fe5. [DOI] [PubMed] [Google Scholar]
- 13.Henke PK, Kubus J, Englesbe MJ, et al. A statewide consortium of surgical care: a longitudinal investigation of vascular operative procedures at 16 hospitals. Surgery. 148:883–889. doi: 10.1016/j.surg.2010.07.009. discussion 889-892. [DOI] [PubMed] [Google Scholar]
- 14.Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180:519–531. [PubMed] [Google Scholar]
- 15.Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998;228:491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khuri SF, Daley J, Henderson W, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:315–327. [PubMed] [Google Scholar]
- 17.Neumayer L, Hosokawa P, Itani K, et al. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178–1187. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 19.Fujii T, Tsutsumi S, Matsumoto A, et al. Thickness of subcutaneous fat as a strong risk factor for wound infections in elective colorectal surgery: impact of prediction using preoperative CT. Dig Surg. 27:331–335. doi: 10.1159/000297521. [DOI] [PubMed] [Google Scholar]
- 20.Lamas O, Marti A, Martinez JA. Obesity and immunocompetence. Eur J Clin Nutr. 2002;56:S42–45. doi: 10.1038/sj.ejcn.1601484. [DOI] [PubMed] [Google Scholar]
- 21.Elmadfa I, Meyer AL. Body composition, changing physiological functions and nutrient requirements of the elderly. Ann Nutr Metab. 2008;52:2–5. doi: 10.1159/000115339. [DOI] [PubMed] [Google Scholar]
- 22.De Vivo A, Mancuso A, Giacobbe A, et al. Wound length and corticosteroid administration as risk factors for surgical-site complications following cesarean section. Acta Obstet Gynecol Scand. 89:355–359. doi: 10.3109/00016340903568175. [DOI] [PubMed] [Google Scholar]
- 23.Culver DH, Horan TC, Gaynes RP, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:152S–157S. doi: 10.1016/0002-9343(91)90361-z. [DOI] [PubMed] [Google Scholar]