Abstract
We tested whether the resting state functional connectivity of the motor system changed during 4 weeks of motor skill learning using functional magnetic resonance imaging (fMRI). Ten healthy volunteers learned to produce a sequential finger movement by daily practice of the task over a 4 week period. Changes in the resting state motor network were examined before training (Week 0), two weeks after the onset of training (Week 2), and immediately at the end of the training (Week 4). The resting state motor system was analyzed using group independent component analysis (ICA). Statistical Parametric Mapping (SPM) second-level analysis was conducted on independent z-maps generated by the group ICA. Three regions, namely right postcentral gyrus, and bilateral supramaginal gyri were found to be sensitive to the training duration. Specifically, the strength of resting state functional connectivity in the right postcentral gyrus and right supramarginal gyrus increased from Week 0 to Week 2, during which the behavioral performance improved significantly, and decreased from Week 2 to Week 4, during which there was no more significant improvement in behavioral performance. The strength of resting state functional connectivity in left supramarginal gyrus increased throughout the training. These results confirm changes in the resting state network during slow-learning stage of motor skill learning, and support the premise that the resting state networks play a role in improving performance.
Keywords: motor skill learning, resting state network, resting state functional connectivity, independent component analysis, ICA, supramarginal gyrus
1. INTRODUCTION
Motor skill learning (Doyon and Benali, 2005; Halsband and Lange, 2006; Hikosaka et al., 2002; Hotermans et al., 2006; Luft and Buitrago, 2005; Sanes, 2003; Willingham, 1998) contains an early rapid phase in which considerable improvement in performance is achieved in a single training session of a few minutes, an intermediate phase of consolidation of several hours, and a later slow-learning stage in which greater improvement in performance is achieved over relatively long duration, e.g., several weeks (Karni et al., 1998; Ungerleider et al., 2002). In the neuroimaging literature, several studies have investigated the slow-learning stage of motor skill learning. Functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) studies investigating slow-learning stage of hand movement are listed in Table 1. These studies indicate that the slow-learning stage is associated with task related changes in the motor system indexed by blood oxygen level dependent (BOLD) signal measured by fMRI (Floyer-Lea and Matthews, 2005; Hlustik et al., 2004; Karni et al., 1995; Lehericy et al., 2005), regional cerebral blood flow measured by PET (Xiong et al., 2009), as well as task driven functional connectivity (Coynel et al., 2010) or effective connectivity (Ma et al., 2010).
Table 1.
Summary of neuroimaging studies on slow-learning stage of motor skill learning. M1= primary motor cortex; and S1= primary somatosensory cortex.
| Publications | Training duration and trained hand |
Neuroimaging modality and training task |
Major findings |
|---|---|---|---|
| Karni et al. (1995) | Four weeks Left hand | fMRI Sequential finger movement task | Greater activation in right M1 when subjects conducted the learned sequence than when they conducted the unpracticed sequence. |
| Hlustik et al. (2004) | Three weeks Left hand | fMRI Sequential finger movement task | Gradual expansion of right M1 and right S1 areas activated by the practiced sequence. |
| Lehericy et al. (2005) | One month Left hand | fMRI Sequential finger movement task | Activation decreased in rostrodorsal (associative) regions of the putamen, and activation increased in more caudoventral (sensorimotor) regions of the putamen. |
| Floyer-Lea and Matthews (2005) | Three weeks Right hand | fMRI Force-tracking task | Activity induced by the trained task increased in left S1, M1, and right putamen. |
| Xiong et al. (2009) | Four weeks Left hand | PET Sequential finger movement task | Regional cerebral blood flow in right M1 increased during both task and resting states. |
| Ma et al. (2010) | Four weeks Left hand | fMRI Sequential finger movement task | Gradually enhanced cortico-basal ganglionic and gradually weakened cortico-cerebeller connections. |
| Coynel et al. (2010) | Four weeks Left hand | fMRI Sequential finger movement task | Gradually weakened functional connectivity in the associative/premotor network and gradually enhanced functional connectivity between associate/premotor network and the sensorimotor network. |
The changes in brain activity levels in the motor system are thought to follow Hebbian principle (Klintsova and Greenough, 1999), which describes a fundamental mechanism for synaptic plasticity in which an increase in synaptic efficacy is the outcome of one cell's repeated and persistent stimulation of another cell (Hebb, 1949). However, there is no consensus on the pattern of change in the cortical regions following motor practice (Kelly and Garavan, 2005). For example, continued increase in BOLD signal in motor areas (Karni et al., 1995, 1998; Ungerleider et al., 2002) and a waxing and waning pattern (Hlustik et al., 2004; Ma et al., 2010; Xiong et al., 2009) have both been reported. In our previous study, we found that significant training-induced increases in resting state cerebral blood flow were noted in motor areas (Xiong et al., 2009). That is, while the activation-volume analysis for fMRI BOLD and PET cerebral blood flow during task showed an early increase followed by a return pre-training level by Week 4, our regional resting state cerebral blood flow analysis demonstrates that the apparent decrease during task performance was actually due to a substantial increase in baseline cerebral blood flow (Xiong et al., 2009). Similarly, an increase in the BOLD signal in the 0.08 Hz frequency band consistent with a focal, resting state increase in blood flow was noted (Duff et al., 2008; Xiong et al., 2009). We believe that traditional task-based conditional contrast analyses and imaging methods such as fMRI BOLD signals where baseline changes cannot be quantified partly contribute to the confusion. Thus, task independent measures of brain function, i.e., examining resting state networks of the brain may contain rich information on the mechanism of motor skill learning. Therefore, we sought to understand motor skill learning through investigating the resting state networks of the brain.
The study of resting state neural activity is an alternative approach to task related regional activity and likely provides additional information on the neurobiological mechanisms of specific brain systems (Auer, 2008; Fox and Raichle, 2007; Greicius, 2008; van den Heuvel and Hulshoff Pol, 2010; Van Dijk et al., 2010). In the resting state brain, there are spontaneous low frequency (Cordes et al., 2001) neuronal activations that show high temporal cohesion (correlation) among functionally related brain regions (Biswal et al., 1995). The temporal correlation during resting state is termed as resting state functional connectivity (Auer, 2008; Fox and Raichle, 2007; Greicius, 2008; van den Heuvel and Hulshoff Pol, 2010; Van Dijk et al., 2010), and the regions showing temporal correlation constitute a resting state (or intrinsic) network (Albert et al., 2009a; Cole et al., 2010; De Luca et al., 2006; Deco et al., 2011; Miall and Robertson, 2006; Raichle and Snyder, 2007; Uddin et al., 2010). The low frequency fluctuations in resting state are even present during sleep and sedation (Van Dijk et al., 2010). To date, the most commonly defined resting state networks include motor, visual, auditory, executive functioning, auditory processing, memory, and default-mode network systems (Cole et al., 2010). Resting state functional connectivity appears to be constrained by structural connectivity (van den Heuvel and Hulshoff Pol, 2010; Van Dijk et al., 2010). There is close correspondence of the brain’s functional architecture during rest and task elicited activation (Smith et al., 2009). Resting state fMRI is a powerful tool for resting state network analysis (Fox and Raichle, 2007; Van Dijk et al., 2010). It possesses percentage BOLD signal change that is comparable to that found in task-related experiments (Damoiseaux et al., 2006). Importantly, the resting state fMRI studies require relatively short acquisition time (e.g. as brief as 5 minutes) (Van Dijk et al., 2010) and are not confounded by task performance issues. Resting state networks have been found to be consistent across participants (Damoiseaux et al., 2006; Shehzad et al., 2009), recording sessions (Damoiseaux et al., 2006; Meindl et al., 2010; Shehzad et al., 2009), and analysis methods (Fox and Raichle, 2007; van den Heuvel and Hulshoff Pol, 2010). Resting state data have been used to investigate both clinical (see Fox and Greicius, 2010; Greicius, 2008 for review) and non-clinical (e.g., Albert et al., 2009b; Biswal et al., 1995; Hampson et al., 2002; Jiang et al., 2004; Xiong et al., 1999, among many other studies) populations.
Related to the current study, several studies have reported that spontaneous activation in the resting state brain can be affected by learning. A number of studies have used resting state fMRI to index changes in the brain associated with learning. Albert et al. (2009b) found that a fronto-parietal resting state network and a cerebellar resting state network were altered after the participants practiced a visuomotor tracking task for 11 min. Lewis et al. (2009) found that the resting state functional connectivity and directed mutual interaction between trained visual cortex and frontal-parietal areas were significantly modified following several days (2 to 9 days) of training on a shape-indentification task. Daselaar et al. (2010) used a perceptuomotor task and found that the superior parietal cortex activated during the task training was more active during post-training rest duration than pre-training rest duration. In addition, it has been shown that resting state functional connectivity between the left and right supramarginal gyrus (SMG, part of inferior parietal lobule) was stronger for new word learners than for non-learners (Veroude et al. (2010). Critically, the above studies on learning and resting state connectivity suggest that the fronto-parietal regions is involved in learning. However, no study has investigated the resting state network during slow learning stage.
We have investigated local changes in resting state cerebral blood flow associated with slow learning of a sequential finger movement task (Xiong et al., 2009). To extend our previous work, we conducted this study to investigate whether there were changes in the resting state functional connectivity of the motor system during slow learning of the same motor task. We analyzed three sets of resting state fMRI data sequentially acquired in 4 weeks, during which participants regularly practiced a sequential finger movement task and improved their skills significantly (Ma et al., 2010; Xiong et al., 2009). Based on the evidence showing that resting state networks can be altered following learning (Albert et al., 2009b; Daselaar et al., 2010; Lewis et al., 2009; Veroude et al., 2010), we hypothesized that changes will be observed in the resting state network of motor system during slow-learning stage of motor skill learning.
2. METHODS
2.1 Participants
This study was approved by the Institutional Review Board of University of Iowa, Iowa City, Iowa, and University of Texas Health Science Center, San Antonio, Texas. After providing informed consent, thirteen healthy normal volunteers participated in this study, and ten (5 male and 5 female) of them completed the experiment, and were included for final analysis. The participants who completed the experiment had a mean age of 30 years (± 8.33 years, standard derivation), ranging from 18 to 45 years. We used self-report to obtain demographic information that indicated that: (1) all the participants were right-handed; (2) none of the participants was a professional musical instrument player; and (3) none of the participants had known neurological and psychiatric disorders.
2.2 Motor Sequence Learning Paradigm
Each participant was trained to perform a motor task in which the fingers of left hand were opposed to the thumb in specific sequences for a total of 15 min each day. The training lasted for 4 weeks (including weekend) for all participants. The participants were instructed that, during the 15-min practice session, they were to perform the sequence as accurately and quickly as possible. Each practice session was video recorded.
The details of the motor sequence learning task have been described elsewhere (Karni et al., 1995; Ma et al., 2010; Xiong et al., 2009). Briefly, during the task, participants performed one of two specific sequences without visual feedback. The order of finger movement in Sequence A is 5, 2, 4, 3, 5, and the order of finger movement is 5, 3, 4, 2, 5 in Sequence B (a mirror image of Sequence A). The numbers are coded as: 2-index finger, 3-middle finger, 4-ring finger, and 5-little finger. Five participants (Group A) were randomly assigned Sequence A as the training sequence, and the other five participants (Group B) were assigned Sequence B as the training sequence. There was no statistical difference in behavior performance between the two participant groups (see the Results section), therefore Group A and Group B were combined into a single group for this resting state fMRI study.
2.3 MRI Data Acquisition
There were three MRI scanning sessions. The first scanning session was performed just prior to the onset of training (Week 0). After 2 weeks of practice, there was a second scanning session (Week 2). The last scanning session occurred immediately after 4 weeks of practice (Week 4).
The MRI images were acquired on an Elscint Prestige 2-T whole-body MRI scanner (Elscint Prestige, Haifa, Israel). A total of 400 volumes of resting state fMRI images were acquired in a transverse plane using a T2-weighted gradient-echo echo-planar-imaging (EPI) sequence, with the following parameters: repetition time (TR) = 700 ms, echo time (TE) = 45 ms, flip angle = 70°, field of view (FOV) = 411×229 mm2, 72×72 voxels image matrix, 3.28×3.28 mm2 in-plane voxel resolution, 7 contiguous fMRI slices, slice thickness = 5 mm, interslice gap = 1 mm, and receive bandwidth = 12.21 Hz. The 7 contiguous slices cover the areas of primary motor cortex (M1), primary somatosensory cortex (S1), supplementary motor area (SMA), dorsal premotor cortex, posterior parietal somatosensory association area, and dorsal cingulate motor area (Xiong et al., 1999). The scan acquired during task performance always occurred after the resting state scan (240 volumes, transverse plane, T2-weighted gradient-echo EPI sequence, TR = 2 s, TE = 45 ms, flip angle = 90°, FOV = 411×229 mm2, 72×72 voxels image matrix, 3.28×3.28 mm2 in-plane voxel resolution, 16 contiguous fMRI slices, slice thickness=5 mm, interslice gap=1 mm, receive bandwidth=12.21 Hz). T1-weighted anatomical images were also acquired, with the following parameters: repetition time = 33 ms, echo time = 12 ms, flip angle = 60°, 16 contiguous slices, slice thickness = 5 mm, interslice gap = 1 mm, 256×256 voxels image matrix, and 1×1 mm2 voxel resolution.
During MRI scanning, the participants were supine with their heads supported by a foam-padded, hemicylindrical head holder. Each participant's head was immobilized within a tightly fitting thermally molded plastic facial mask extending from hairline to chin. During the resting state fMRI scan, the participants were instructed to remain still, with their eyes closed.
2.4 MRI Image Preprocessing
For each participant and at each test point (Week 0, Week 2, or Week 4), the first 10 fMRI images were discarded to allow MRI signal to reach a steady state. Statistical Parametric Mapping (SPM8) software (http://www.fil.ion.ucl.ac.uk) and Resting state fMRI Data Analysis Toolkit (REST) V1.4 software (http://resting-fmri.sourceforge.net/) were used to preprocess the MRI data. The fMRI images were spatially realigned to the first volume to correct the bias due to head movements (using SPM8 software). Both the resting state fMRI images and the T1-image were co-registered to the task performance fMRI images. After that, the T1 image was transformed to the coordinates of the Montreal Neurological Institute (MNI) standard space using the SPM8 normalization procedure. This transformation was applied to the resting state fMRI images to convert them to MNI space. After spatial normalization, a linear drift correction (Tanabe et al., 2002) was applied to all fMRI images (using REST software). To increase the signal-to-noise-ratio, the fMRI images were spatially smoothed by an isotropic Gaussian filter with a full-width at half-maximum (FWHM) of 8 mm (using SPM8 software). A bandpass filter with cutoff frequencies of 0.01 and 0.12 Hz (Ma et al., 2007) was used to remove high-frequency noise and very low-frequency signals (using REST software). In the literature, different cutoff frequencies have been used or proposed: e.g., 0.08 Hz (Chuang et al., 2008; Kwak et al., 2011), 0.1 Hz (van den Heuvel et al., 2010; Van Dijk et al., 2010), and up to 0.15 Hz (Cole et al., 2010; Smith et al., 2008). In one of our previous studies, we used the same resting state fMRI data and found a significant increase in power spectrum in the 0.08 Hz frequency band in the right M1 area after two weeks and four weeks training periods (Xiong et al., 2009). To avoid losing essential signals at 0.08 Hz frequency band, we chose 0.12 Hz as the cutoff frequency. All the programs were run on a platform of MATLAB 7.1 (The MathWorks, Inc., Natick, MA).
2.5 Group Independent Component Analysis
Group ICA (Calhoun et al., 2009), an extension of the traditional ICA (McKeown et al., 1998), was used to analyze the fMRI data. Among the two broadly-used and performance-comparable group ICA alternatives (Schopf et al., 2010), i.e., Group ICA of fMRI Toolbox (GIFT) (Calhoun et al., 2001), and Probabilistic ICA or PICA (Beckmann et al., 2005), we chose GIFT software (http://icatb.sourceforge.net/). GIFT software results in the detection of consistent resting state networks across multiple sessions at least over a period of several weeks (Chen et al., 2008). Using the ICA algorithm which was proposed by Lin et al. (2010) and implemented in the GIFT software version 2.0d, we analyzed the resting state fMRI data for each test point (Week 0, Week 2, or Week 4). Prior to ICA analysis, principal component analysis (PCA) is used to reduce data. The number of ICA components was set to 20 because one of our previous studies has shown that 20 components can yield robust ICA results for motor network with a moderate computation load for the current data set (Ma et al., 2007). Spatially independent components were back-reconstructed (Calhoun et al., 2001; Calhoun et al., 2009) for all participants and all test points (Week 0, Week 2, and Week 4).
One of the challenges in ICA analysis is the selection of components of interest among several identified independent components (Calhoun et al., 2005; Lin et al., 2010). It has been shown that, the ICA algorithms proposed by Calhoun et al. (2005) and Lin et al. (2010) are able to deal with this challenge. These algorithms use some prior temporal (Calhoun et al., 2005) or spatial information (Lin et al. 2010) on the fMRI data to search the components of interest, and therefore they are referred to as “semi-blind” ICA algorithms (Calhoun et al., 2005; Lin et al., 2010). In this study, to select spatially independent components for motor system resting state network, we chose the semi-blind ICA algorithm with spatial constraint (Lin et al., 2010). Our previous study (Ma et al., 2007) using the same data set showed that the resting state network of motor system essentially contains M1, S1, and premotor cortex (including SMA). Therefore the spatial constraint map was constructed by combining the probabilistic atlases of M1, S1, and premotor cortex (including SMA), provided by the SPM Anatomy toolbox version 1.6 (Eickhoff et al., 2006; Eickhoff et al., 2007; Eickhoff et al., 2005). All the independent components (or maps) generated by the semi-blind ICA algorithm were visually inspected to ensure that they indeed were part of the motor system.
All independent components were converted to z maps (Ma et al., 2007; McKeown et al., 1998). Each z-score represents the fit of a specific voxel BOLD time course to the time course of the group averaged component (Assaf et al., 2010). The z maps were tested for the strength of the connectivity (i.e. signal synchronization) of each voxel to the entire spatial component using standard SPM statistical analysis package.
2.6 SPM Full Factorial Analysis
The main effects of the z-score (averaged across Week 0, Week 2, and Week 4) and planned comparisons among the test points were analyzed using SPM8 second-level full factorial analysis (random effects), with the z maps from ICA analysis as inputs (one independent component for each participant and each test point). The full factorial module included a factor of test point (three levels: Week 0, Week 2, and Week 4). Planned comparisons between test points included Week 2 minus Week 0, Week 0 minus Week 2, Week 4 minus Week 2, Week 2 minus Week 4, Week 4 minus Week 0, and Week 0 minus Week 4. The planned comparisons were masked with significant clusters obtained from the main effects to ensure that only robust signals were considered for the planned comparisons (Van Dijk et al., 2010).
The cluster-defining threshold was t = 3.5, and all P values resulting from the SPM second-level analysis reported in this article are cluster P values that were corrected for multiple comparisons using random field theory and computed by SPM8 to control the family-wise error (FWE) rate to be less than 0.05 (Friston et al., 1996; Friston et al., 1994). Approximate anatomical labels for regions of significant clusters were determined using the SPM Anatomy toolbox version 1.6 (Eickhoff et al., 2005, 2006, 2007).
3. RESULTS
3.1 Motor sequence learning paradigm
The learning curve for the trained sequence has been shown elsewhere (Ma et al., 2010; Xiong et al., 2009). Statistical analysis of the behavioral data has a between-subject factor of group (Group A versus Group B) and a within-subject factor of training duration (Week 0, Week 2, and Week 4). A two by three repeated measures analysis of variance (ANOVA) analysis based on the movement rate revealed significant difference between the training durations (F(2,24)=25.84, p<0.00001). Neither the difference between the two subject groups (F(1,24)=0.39, p=0.54) nor the interaction between the subject group and the training duration (F(2,24)=0.02, p=0.98) were significant. Post hoc unpaired Student t-test analyses indicate that the mean movement rates (pooled across Group A and Group B) at Week 2 (t=5.63, degree of freedom or DF=18, p<0.001, Bonferroni corrected) and Week 4 (t=7.27, DF=18, p<0.00001, Bonferroni corrected) were significantly greater than that at Week 0. No significant difference was found between Week 2 and Week 4 (t=1.08, DF=18, p>0.2).
3.2 Group Independent Component Analysis
The GIFT software using the semi-blind ICA algorithm with spatial constraint (Lin et al., 2010) generated a single independent component for each participant and for each test point (Week 0, Week 2, or Week 4). Visual inspection on the independent components confirmed that the semi-blind ICA algorithm with spatial constraint (Lin et al., 2010) was able to distinctly detect the independent component of motor resting state network from other independent components for all participants and for all test points (Week 0, Week 2, and Week 4).
3.3 SPM Full Factorial Analysis
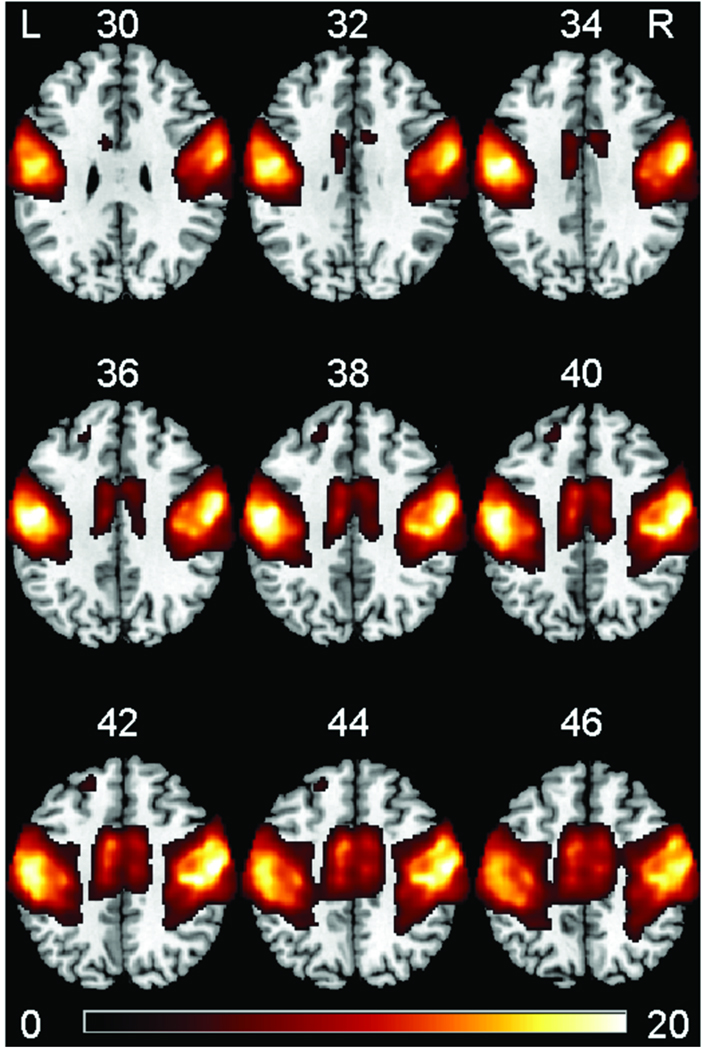
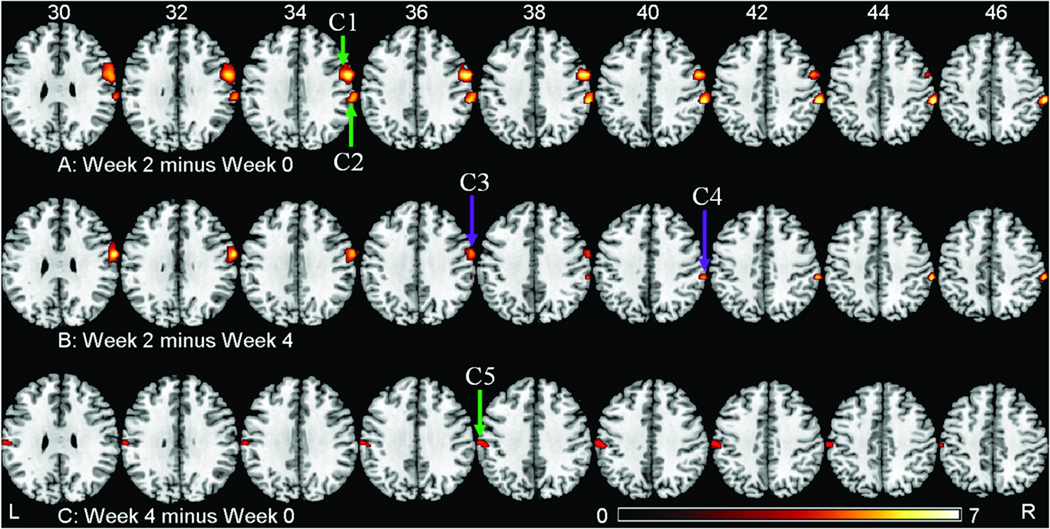
The SPM random effects full-factorial analysis of the independent components found two significant clusters for the main effects of z-score (averaged across Week 0, Week 2, and Week 4). The two significant clusters, which are overlaid in color on axial slices of an MNI template brain, are shown in Figure 1. In addition, two significant clusters (Cluster 1 and Cluster 2 respectively) were found for the planned comparison Week 2 minus Week 0; two significant clusters (Cluster 3 and Cluster 4 respectively) were found for the planned comparison Week 2 minus Week 4; and a significant cluster (Cluster 5) was found for the planned comparison Week 4 minus Week 0. No significant cluster was found for the other planned comparisons (Week 0 minus Week 2, Week 0 minus Week 4, and Week 4 minus Week 2). The five significant clusters (Clusters 1 to 5), which are overlaid in color on axial slices of an MNI template brain, are shown in Figure 2. For each of the seven significant clusters, the cluster-level family-wise error corrected P value, cluster volume, and the MNI coordinate and approximate anatomical location (within 3 mm radius) of the voxel with maximal t value are listed in Table 2.
Figure 1.
Significant clusters (main effects) of resting state network of motor system found by the SPM second-level full-factorial analysis, based on the output (z maps) from ICA analysis. The significant clusters are overlaid in color on axial slices of an MNI template brain. The number above each slice indicates slice location (mm) of MNI z coordinate. Scale on color bar represents voxel t values. Left (L) side in the figure is left hemisphere of the brain, and right (R) side of figure is right hemisphere of brain.
Figure 2.
Significant clusters (planned comparison among the test points) of resting state network of motor system found by the SPM second-level full-factorial analysis, based on the output (z maps) from ICA analysis. The significant clusters are overlaid in color on axial slices of an MNI template brain. The number above each slice indicates slice location (mm) of MNI z coordinate. Scale on color bar represents voxel t values. Left (L) side in the figure is left hemisphere of the brain, and right (R) side of figure is right hemisphere of brain. A: Week 2 minus Week 0 planned comparison. B: Week 2 minus Week 4 planned comparison. C: Week 4 minus Week 0 planned comparison. C1 to C5 denote Cluster 1 to Cluster 5.
Table 2.
SPM full factorial second-level (Random Effects) analysis results. Within each significant cluster, the voxel with maximal t value and its approximate anatomical locations within 3 mm radius were listed. x, y, and z = MNI standard space coordinates (mm). Negative x= left hemisphere and positive x= right hemisphere. Smoothness of residual field (FWHM) = [10.7 10.2 14.1] mm. FWE = family-wise error corrected probability.
| Contrast | Cluster label |
Cluster P [FWE- corrected] |
Cluster volume [mL] |
Max- imal voxel t value |
Voxel MNI coordinate [x, y, z] |
Maximal voxel t location |
|---|---|---|---|---|---|---|
| Week 2 minus Week 0 |
1 | < 0.001 | 3.536 | 6.77 | 60, 0, 36 | Right postcentral gyrus |
| 2 | < 0.001 | 2.200 | 6.97 | 62, −32, 44 | Right supramaginal gyrus | |
| 5.43 | 66, −26, 34 | Right supramaginal gyrus | ||||
| Week 2 minus Week 4 |
3 | 0.001 | 1.584 | 6.54 | 64, −4, 30 | Right postcentral gyrus |
| 4 | 0.038 | 0.552 | 6.08 | 62, −34, 44 | Right supramaginal gyrus | |
| Week 4 minus Week 0 |
5 | 0.008 | 1.024 | 4.32 | −66, −20, 30 | Left supramaginal gyrus |
| 4.21 | −62, −22, 42 | Left supramaginal gyrus | ||||
| Main effect of z-score (across Week 0, Week 2, Week 4) |
6 | < 0.001 | 83.752 | 23.48 | 56, −12, 40 | Right postcentral gyrus |
| 22.00 | −48, −14, 41 | Left postcentral gyrus | ||||
| 20.81 | 46, −20, 42 | Right postcentral gyrus | ||||
| 19.79 | 60, −6, 38 | Right postcentral gyrus | ||||
| 19.52 | 40, −20, 42 | Right postcentral gyrus | ||||
| 17.86 | 52,0, 44 | Right postcentral gyrus | ||||
| 15.01 | −40, −34, 46 | Left postcentral gyrus | ||||
| 14.82 | −6, 0, 44 | Left middle cingulate cortex | ||||
| 14.18 | 38, −10, 46 | Right precentral gyrus | ||||
| 13.26 | −8, −10, 42 | Left middle cingulate cortex | ||||
| 11.97 | 8, −14, 46 | Right middle cingulate cortex | ||||
| 7 | 0.028 | 0.632 | 4.88 | −18, 38, 42 | Left superior frontal gyrus | |
The Anatomy toolbox indicated that Cluster 1 found by the planned comparison Week 2 minus Week 0 represented a portion of the right postcentral gyrus (PCG), and that Cluster 2 found by the planned comparison Week 2 minus Week 0 was in a portion of the right SMG. In addition, Cluster 3 (Week 2 minus Week 4) was in a portion of the right PCG; Cluster 4 (Week 2 minus Week 4) was in a portion of the right SMG; and Cluster 5 (Week 4 minus Week 0) was in a portion of the left SMG.
Cluster 1 and Cluster 3 overlapped with a volume of 1.120 mL, corresponding to 32% of the volume of Cluster 1 and 71% of the volume of Cluster 3. Cluster 2 and Cluster 4 overlapped (volume of 0.552 mL), corresponding to 25% of the volume of Cluster 2 and 100% of the volume of Cluster 4.
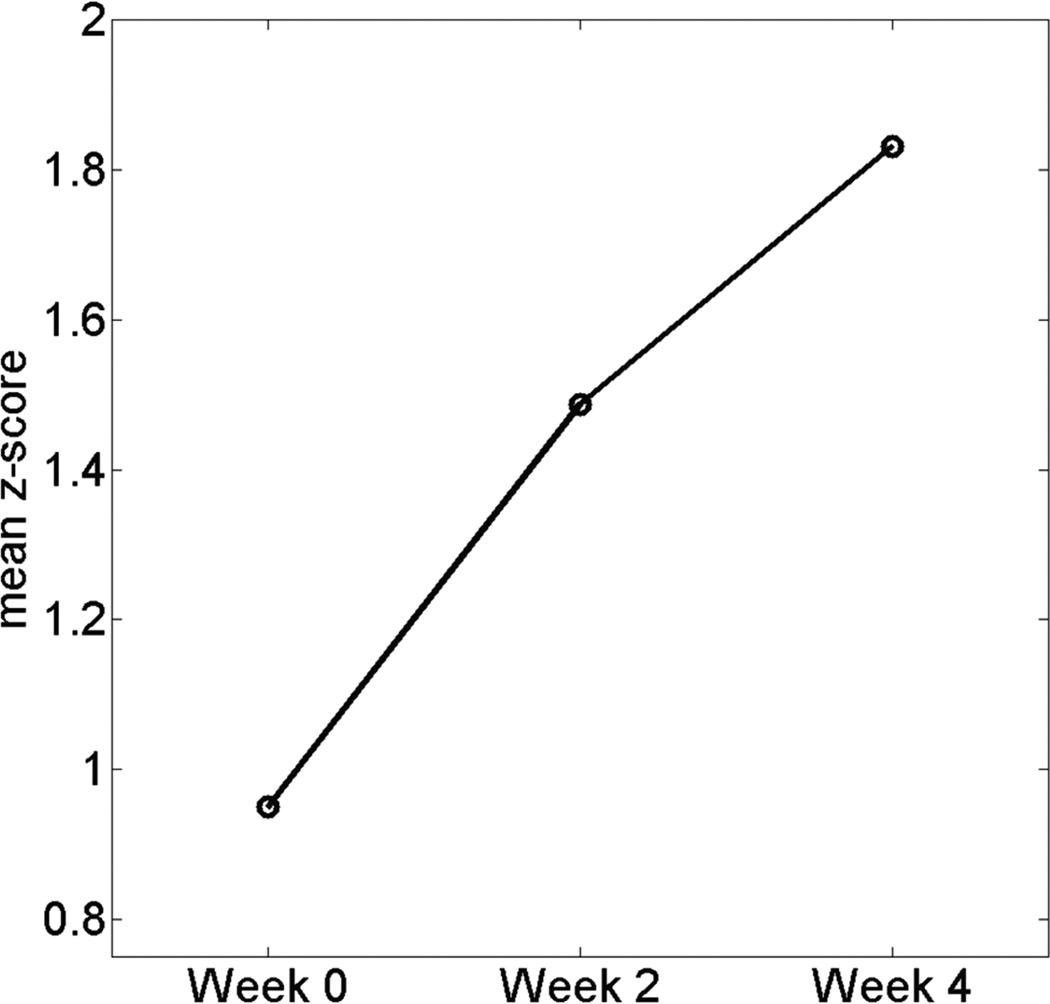
A trend analysis was used to investigate the temporal dynamics of the strength of the resting state functional connectivity in Cluster 5 that showed a significant effect for the planned comparison of Week 4 minus Week 0. The mean z-score (across all voxels within Cluster 5) was computed for each participant and for each test point (Week 0, Week 2, or Week 4). The trend analysis was conducted under an SPSS (SPSS Inc, Chicago, Illinois) one-way ANOVA in which there was a within-group factor of test points (Week 0, Week 2, and Week 4). This trend analysis indicated that the mean z-score in Cluster 5 was well fit by a linear model (P = 0.002), which accounted for 29% of the variance. The mean plot of the mean z-score (averaged across all participants at each test point) in Cluster 5 is depicted in Figure 3.
Figure 3.
Mean plot for the mean z-score in Cluster 5, portion of left supramarginal gyrus.
4. DISCUSSION
We used group ICA and SPM random effects full factorial analysis to test if there are changes in the resting state motor network associated with 4 weeks of motor skill learning. Based on the data (z maps generated by the group ICA), the SPM analysis revealed five clusters that were sensitive to the slow learning. The clusters (right PCG and bilateral SMG) were in the parietal lobe, consistent with the role of the parietal lobe in motor control (Fleming and Crosby, 1955), the findings that the parietal lobe plays an important role in later stages of motor learning (Halsband and Lange, 2006; Sakai et al., 1998), and findings that the fronto-parietal regions may be important for learning (Albert et al., 2009b; Daselaar et al., 2010; Lewis et al., 2009; Veroude et al., 2010). We also found involvement of SMG in the motor skill learning, consistent with the strong anatomical connection of SMG with ventral premotor area and M1 (Koch et al., 2010), and the role of this region in motor cognition (Hanakawa et al., 2008; Tunik et al., 2008). Motor cognition refers the concept that cognition is embodied in action, and that the motor system contributes to mental processing (Sommerville and Decety, 2006).
We found changes associated with different periods of learning. Cluster 1 and Cluster 2, significant for Week 2 minus Week 0, were in right PCG and right SMG respectively. Cluster 3 and Cluster 4, significant for Week 2 minus Week 4, were also in right PCG and right SMG. Therefore, we conducted a further analysis to determine if same the portion of right PCG or SMG was involved in the connectivity changes. To that end, we examined volume of overlap between clusters, and found that the volume of the overlap between Cluster 1 and Cluster 3 was 71% of the volume of Cluster 3, and the volume of the overlap between Cluster 2 and Cluster 4 was 100% of the volume of Cluster 4. Based on these results, we assume that under the current experimental conditions, same regions of right PCG and right SMG were affected by the motor skill learning in the remaining part of this paper.
The PCG and SMG contralateral (right) to the trained hand, exhibited difference for two specific comparisons, Week 2 minus Week 0 and Week 2 minus Week 4. Specifically, the strength of resting state functional connectivity in right PCG and right SMG increased (from Week 0 to Week 2) and then decreased (from Week 2 to Week 4), suggesting that the regions in the right hemisphere are supportive of the learning process in early phases of motor skill learning. This finding supports the literature that suggests the dominance of the right hemispheric parietal regions in the early phases of motor skill learning (Halsband and Lange, 2006). The right SMG is involved visuospatial attention (Gitelman et al., 1999; Kim et al., 1999; Lacquaniti et al., 1997; Loayza et al., 2011; Nobre et al., 1997). It is well known that attention is an important component of motor learning (Perchet and Garcia-Larrea, 2000; Posner et al., 1982). The dynamic changes (increased-first-then-decreased) in resting state functional connectivity in the right PCG and right SMG probably reflect the necessity of sensorimotor integration and visuospatial attention in the earlier phases of motor skill learning which became less necessary in the later phases of motor skill learning.
We also found that the left SMG exhibited a significant effect for the Week 4 minus Week 0 comparison. In addition, the trend analysis showed that the mean z-score in the left SMG was well fit by a linear model (Figure 3). Thus, the strength of resting state functional connectivity in the left SMG systematically increased throughout the 4-week training duration. We interpret these changes as evidence that the dynamic change in the resting state functional connectivity in the left SMG reflects long-term motor skill learning. The role of left SMG in learning is likely associated with retention of learned skills. The role of dominant-hemisphere in the later phases of motor skill learning (Halsband and Lange, 2006), and the role of the left SMG in higher motor cognition (Deiber et al., 1996; Hesse et al., 2006; Kimura, 1993; Krams et al., 1998; Rushworth et al., 2001; Rushworth et al., 1997) strongly support our interpretation of resting state changes in this region. Others have suggested that the left SMG plays a role in motor programming and attention (Deiber et al., 1996; Hesse et al., 2006; Kimura, 1993; Krams et al., 1998; Rushworth et al., 2001; Rushworth et al., 1997), including the storage of visuomotor skills (Heilman et al., 1982; Johnson-Frey et al., 2005). Therefore, we hypothesize that the systematic increase in the strength of resting state functional connectivity in the left SMG is an index of the retention of the learned motor skills.
The findings of the current study have important implications for understanding the role of the resting state networks in behavior. Currently, it is hypothesized that the resting state networks may help to keep functional systems in an active state (Miall and Robertson, 2006; Raichle et al., 2001; van den Heuvel and Hulshoff Pol, 2010), represent a dynamic prediction about expected use of the functionally correlated regions (Fox and Raichle, 2007), make critical contributions to the off-line memory consolidation (an improvement or enhancement in performance observed after a delay) (Miall and Robertson, 2006), and help to improve behavioral performance (van den Heuvel and Hulshoff Pol, 2010). The increased strength of functional connectivity in the right PCG and right SMG and the subsequent decrease from Week 2 to Week 4 accompanied by increases and then stabilization in our behavioral measure of learning, suggesting these regions serve to regulate early stages of learning and in particular the initial enhancement of behavioral performance (van den Heuvel and Hulshoff Pol, 2010).
Previous studies have implicated M1 as critical for the consolidation of motor skills (Muellbacher et al, 2002; Robertson et al, 2005) and for performance changes during the slow learning stage (Wilkinson et al, 2010). It has also been reported that SMA is critical for motor consolidation (in some conditions; Tanaka et al, 2010) but not for slow learning stage (Wilkinson et al, 2010). Strong task-related changes in regional activation in these two regions during the slow-learning stage of motor learning have consistently been reported in the literature (Hlustik et al., 2004; Karni et al., 1995; Ma et al., 2010; Xiong et al., 2009). However, unlike during task, the data from the current study showed that the resting state functional connectivity in M1 and SMA does not vary. This dissociation between task-induced and resting state spontaneous activation likely reflects differences in the neurobiology underlying the task-induced activation and resting state spontaneous activation. As well, there is a clear difference in the physiology underlying task-evoked BOLD responses and spontaneous BOLD fluctuations (Fox and Raichle, 2007) that is also reflected by our data.
One limitation of this study is the partial spatial coverage (only seven contiguous axial slices), therefore other regions modulated by the motor skill training such as basal ganglia, thalamus, and cerebellum were missed. However, the limited spatial coverage is accompanying with a relatively high sampling rate (700 ms), which reduces aliasing of high frequency cardiac or respiratory activity (Fox and Raichle, 2007; Van Dijk et al., 2010). In addition, the participant sample size (ten participants were included in the final analysis) is relatively small and the observed changes in the resting state network are generally quite small, thereby reducing power. Future studies with more participants and/or controlling the effect of order (e.g., by having a group of control participants who perform a matched untrained task or not, and the resting state network is recorded at the same time as the trained group) will help provide greater insight into resting state network changes associated with learning. Furthermore, although we have excluded participants who were professional musical instrument players, we did not question the participants about their history in sports. Despite these limitations, the results observed in this study confirm that there are changes in the resting state network during slow-learning stage of motor skill learning, and support the hypothesis that the resting sate networks help to improve behavioral performance.
Acknowledgements
The authors thank the anonymous reviewers for their constructive comments which have resulted in significant improvement of this manuscript.
Role of funding source
Funding for this study was provided by National Science Foundation (NSF) (BCS 05-09626) and National Institutes of Health (NIH) (5 R01 NS046082) grants. The NSF and NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Abbreviations
- ANOVA
analysis of variance
- BOLD
blood oxygen level dependent
- DF
degree of freedom
- EPI
echo-planar-imaging
- FOV
field of view
- FWE
family-wise error
- FWHM
full-width at half-maximum
- GIFT
Group ICA of fMRI Toolbox
- M1
primary motor cortex
- MDL
minimum descript length
- MNI
Montreal Neurological Institute
- PCA
principal component analysis
- PCG
postcentral gyrus
- PICA
Probabilistic ICA
- REST
Resting state fMRI Data Analysis Toolkit
- S1
primary somatosensory cortex
- SMA
supplementary motor area
- SMG
supramarginal gyrus
- TE
echo time
- TR
repetition time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors has any conflict of interest with regard to the findings presented in this manuscript, financial or otherwise.
REFERENCES
- Albert NB, Robertson EM, Mehta P, Miall RC. Resting state networks and memory consolidation. Commun Integr Biol. 2009a;2:530–532. doi: 10.4161/cib.2.6.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009b;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O'Boyle JG, Schultz RT, Pearlson GD. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the 'resting' brain. Magn Reson Imaging. 2008;26:1055–1064. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Stevens MC, Kiehl KA, Pekar JJ. Semi-blind ICA of fMRI: A method for utilizing hypothesis-derived time courses in a spatial ICA analysis. Neuroimage. 2005;25:527–538. doi: 10.1016/j.neuroimage.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ross TJ, Zhan W, Myers CS, Chuang KS, Heishman SJ, Stein EA, Yang Y. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 2008;1239:141–151. doi: 10.1016/j.brainres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KH, van Gelderen P, Merkle H, Bodurka J, Ikonomidou VN, Koretsky AP, Duyn JH, Talagala SL. Mapping resting-state functional connectivity using perfusion MRI. Neuroimage. 2008;40:1595–1605. doi: 10.1016/j.neuroimage.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Coynel D, Marrelec G, Perlbarg V, Pelegrini-Issac M, Van de Moortele PF, Ugurbil K, Doyon J, Benali H, Lehericy S. Dynamics of motor-related functional integration during motor sequence learning. Neuroimage. 2010;49:759–766. doi: 10.1016/j.neuroimage.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Huijbers W, de Jonge M, Goltstein PM, Pennartz CM. Experience-dependent alterations in conscious resting state activity following perceptuomotor learning. Neurobiol Learn Mem. 2010;93:422–427. doi: 10.1016/j.nlm.2009.12.009. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Sadato N, Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol. 1996;75:233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Duff EP, Johnston LA, Xiong J, Fox PT, Mareels I, Egan GF. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum Brain Mapp. 2008;29:778–790. doi: 10.1002/hbm.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fleming JF, Crosby EC. The parietal lobe as an additional motor area; the motor effects of electrical stimulation and ablation of cortical areas 5 and 7 in monkeys. J Comp Neurol. 1955;103:485–512. doi: 10.1002/cne.901030306. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94:512–518. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the Significance of Focal Activations Using their Spatial Extent. Human Brain Mapping. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122(Pt 6):1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol Paris. 2006;99:414–424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb Cortex. 2008;18:2775–2788. doi: 10.1093/cercor/bhn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: Wiley & Sons; 1949. [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32:342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- Hesse MD, Thiel CM, Stephan KE, Fink GR. The left parietal cortex and motor intention: an event-related functional magnetic resonance imaging study. Neuroscience. 2006;140:1209–1221. doi: 10.1016/j.neuroscience.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Hlustik P, Solodkin A, Noll DC, Small SL. Cortical plasticity during three-week motor skill learning. J Clin Neurophysiol. 2004;21:180–191. doi: 10.1097/00004691-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, Maertens de Noordhout A, Moonen G, Maquet P. Early boost and slow consolidation in motor skill learning. Learn Mem. 2006;13:580–583. doi: 10.1101/lm.239406. [DOI] [PubMed] [Google Scholar]
- Jiang T, He Y, Zang Y, Weng X. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp. 2004;22:63–71. doi: 10.1002/hbm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9:269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Kimura D. Neuromotor Mechanisms in Human Comminucation. New York: Oxford University Press; 1993. [Google Scholar]
- Klintsova AY, Greenough WT. Synaptic plasticity in cortical systems. Curr Opin Neurobiol. 1999;9:203–208. doi: 10.1016/s0959-4388(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Koch G, Cercignani M, Pecchioli C, Versace V, Oliveri M, Caltagirone C, Rothwell J, Bozzali M. In vivo definition of parieto-motor connections involved in planning of grasping movements. Neuroimage. 2010;51:300–312. doi: 10.1016/j.neuroimage.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Krams M, Rushworth MF, Deiber MP, Frackowiak RS, Passingham RE. The preparation, execution and suppression of copied movements in the human brain. Exp Brain Res. 1998;120:386–398. doi: 10.1007/s002210050412. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Muller ML, Dayalu P, Seidler RD. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front Syst Neurosci. 2011;4:143. doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacquaniti F, Perani D, Guigon E, Bettinardi V, Carrozzo M, Grassi F, Rossetti Y, Fazio F. Visuomotor transformations for reaching to memorized targets: a PET study. Neuroimage. 1997;5:129–146. doi: 10.1006/nimg.1996.0254. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin QH, Liu J, Zheng YR, Liang H, Calhoun VD. Semiblind spatial ICA of fMRI using spatial constraints. Hum Brain Mapp. 2010;31:1076–1088. doi: 10.1002/hbm.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loayza FR, Fernandez-Seara MA, Aznarez-Sanado M, Pastor MA. Right parietal dominance in spatial egocentric discrimination. Neuroimage. 2011;55:635–643. doi: 10.1016/j.neuroimage.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM. Stages of motor skill learning. Mol Neurobiol. 2005;32:205–216. doi: 10.1385/MN:32:3:205. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang B, Chen X, Xiong J. Detecting functional connectivity in the resting brain: a comparison between ICA and CCA. Magn Reson Imaging. 2007;25:47–56. doi: 10.1016/j.mri.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, Fox PT, Xiong J. Changes in regional activity are accompanied with changes in inter-regional connectivity during 4 weeks motor learning. Brain Res. 2010;1318:64–76. doi: 10.1016/j.brainres.2009.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl T, Teipel S, Elmouden R, Mueller S, Koch W, Dietrich O, Coates U, Reiser M, Glaser C. Test-retest reproducibility of the default-mode network in healthy individuals. Hum Brain Mapp. 2010;31:237–246. doi: 10.1002/hbm.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Robertson EM. Functional imaging: is the resting brain resting? Curr Biol. 2006;16:R998–R1000. doi: 10.1016/j.cub.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120(Pt 3):515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Perchet C, Garcia-Larrea L. Visuospatial attention and motor reaction in children: an electrophysiological study of the "Posner" paradigm. Psychophysiology. 2000;37:231–241. [PubMed] [Google Scholar]
- Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philos Trans R Soc Lond B Biol Sci. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097-1089. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. The Journal of Neuroscience. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM. From creation to consolidation: a novel framework for memory processing. PLoS Biol. 2009;7:e19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Renowden S, Wade DT, Passingham RE. The left parietal cortex and motor attention. Neuropsychologia. 1997;35:1261–1273. doi: 10.1016/s0028-3932(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Putz B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci. 1998;18:1827–1840. doi: 10.1523/JNEUROSCI.18-05-01827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN. Neocortical mechanisms in motor learning. Curr Opin Neurobiol. 2003;13:225–231. doi: 10.1016/s0959-4388(03)00046-1. [DOI] [PubMed] [Google Scholar]
- Schopf V, Windischberger C, Kasess CH, Lanzenberger R, Moser E. Group ICA of resting-state data: a comparison. MAGMA. 2010;23:317–325. doi: 10.1007/s10334-010-0212-0. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Niazy R, Beckmann C, Miller K. Resting state networks: neither low frequency nor anticorrelated?. 14th Annual Meeting of the Organization for Human Brain Mapping; 15–19 June; Melbourne, Australia. 2008. [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Decety J. Weaving the fabric of social interaction: articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychon Bull Rev. 2006;13:179–200. doi: 10.3758/bf03193831. [DOI] [PubMed] [Google Scholar]
- Song S. Consciousness and the consolidation of motor learning. Behav Brain Res. 2009;196:180–186. doi: 10.1016/j.bbr.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Miller D, Tregellas J, Freedman R, Meyer FG. Comparison of detrending methods for optimal fMRI preprocessing. Neuroimage. 2002;15:902–907. doi: 10.1006/nimg.2002.1053. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Honda M, Hanakawa T, Cohen LG. Differential contribution of the supplementary motor area to stabilization of a procedural motor skill acquired through different practice schedules. Cereb Cortex. 2010;20:2114–2121. doi: 10.1093/cercor/bhp276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Lo OY, Adamovich SV. Transcranial magnetic stimulation to the frontal operculum and supramarginal gyrus disrupts planning of outcome-based hand-object interactions. J Neurosci. 2008;28:14422–14427. doi: 10.1523/JNEUROSCI.4734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4:1–12. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroude K, Norris DG, Shumskaya E, Gullberg M, Indefrey P. Functional connectivity between brain regions involved in learning words of a new language. Brain Lang. 2010;113:21–27. doi: 10.1016/j.bandl.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychol Rev. 1998;105:558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
- Wilkinson L, Teo JT, Obeso I, Rothwell JC, Jahanshahi M. The contribution of primary motor cortex is essential for probabilistic implicit sequence learning: evidence from theta burst magnetic stimulation. J Cogn Neurosci. 2010;22:427–436. doi: 10.1162/jocn.2009.21208. [DOI] [PubMed] [Google Scholar]
- Xiong J, Ma L, Wang B, Narayana S, Duff EP, Egan GF, Fox PT. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. Neuroimage. 2009;45:75–82. doi: 10.1016/j.neuroimage.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp. 1999;8:151–156. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]