Abstract
Background
The third trimester in human fetal development represents a critical time of brain maturation referred to as the “brain growth spurt”. This period occurs in rats postnatally, and exposure to ethanol during this time can increase the risk of impairments on a variety of cognitive and motor tasks. It has been proposed that one potential mechanism for the teratogenic effects of ethanol is NMDA receptor-mediated excitotoxicity during periods of ethanol withdrawal. In neonatal rats, antagonism of NMDA receptors during ethanol withdrawal, with drugs such as MK-801 and eliprodil, has been shown to mitigate some of the behavioral deficits induced by developmental ethanol exposure. The current study examined whether memantine, an NMDA receptor antagonist and a drug used clinically in Alzheimer’s patients, would attenuate impairments associated with binge ethanol exposure in neonatal rats.
Methods
On postnatal day 6, rats were exposed to 6 g/kg ethanol via intubation with controls receiving an isocaloric maltose dextrin solution. Twenty-one hours following the ethanol binge, rats received intraperitoneal injections of memantine at 0, 10, 15, or 20 mg/kg. Ethanol’s teratogenic effects were assessed using multiple behavioral tasks: open field activity, parallel bars and spatial discrimination reversal learning.
Results
Ethanol-treated rats were overactive in the open field and were impaired on both reversal learning and motor performance. Administration of 15 or 20 mg/kg memantine during withdrawal significantly attenuated ethanol’s adverse effects on motor coordination, but did not significantly alter activity levels or improve the spatial learning deficits associated with neonatal alcohol exposure.
Conclusion
These results indicate that a single memantine administration during ethanol withdrawal can mitigate motor impairments but not spatial learning impairments or overactivity observed following a binge ethanol exposure during development in the rat.
Keywords: memantine, fetal alcohol, NMDA receptors, excitoxicity, binge ethanol
1. Introduction
Prenatal alcohol exposure can produce a range of physical, physiological, and behavioral alterations that are referred to as fetal alcohol spectrum disorders (FASD). Brain imaging studies in children with FASD indicate that prenatal alcohol exposure reduces overall brain size, disrupting the development of numerous central nervous system (CNS) areas including the basal ganglia, corpus callosum, and cerebellum, which is disproportionately reduced in volume compared to overall brain size (Riley and McGee, 2005, Sowell et al., 1996). Alcohol-induced neuropathology also includes white matter deficits, increased gray matter densities and asymmetries, and reduced growth in the frontal lobes (Coffin et al., 2005, Riley et al., 2004). Consistent with CNS pathology, children exposed to alcohol prenatally may exhibit reductions in IQ and deficits in visual spatial performance, attention, executive function, motor coordination and social functioning (Mattson et al., 2001). Although there is considerable evidence demonstrating that the behavioral and physical deficits associated with heavy alcohol abuse during pregnancy are completely preventable, the occurrence of FASD continues unabated. As a result, concerted effort needs to be applied to finding treatments that can mitigate the severity of these ethanol-induced impairments.
A period of time when the brain is particularly vulnerable to the teratogenic effects of ethanol is during the third trimester “brain growth spurt” (Dobbing and Sands, 1979). The third trimester equivalent in rats occurs postnatally and provides a time when an ethanol insult causes significant brain injury, affecting activity levels, spatial learning and motor behavior. Ethanol disrupts brain development through many mechanisms, including actions at specific receptor sites. Ethanol at high doses is known to interfere with glutamatergic action at NMDA, AMPA and kainate receptor subtypes (Nevo and Hamon, 1995, Schummers and Browning, 2001). Following chronic ethanol exposure, the withdrawal period is characterized by an upregulation of NMDA receptor function and concurrent increase in receptor activation (Davidson et al., 1995). This upregulation of NMDA receptors may result in NMDA receptor-mediated excitotoxicity due to a dramatic increase in calcium entering the postsynaptic cell and may contribute to many of the observed CNS and behavioral dysfunctions associated not only with adult chronic alcohol exposure, but also with alcohol’s teratogenic effects (Lewis et al., 2007, Ward et al., 2009).
Blockade of NMDA receptors by MK-801 during ethanol withdrawal in the developing rat can attenuate behavioral impairments in a time-dependent manner, that is, only when administered during withdrawal and not concurrent with ethanol (Thomas et al., 2001, Thomas, 2002, Thomas et al., 1997). MK-801 is an noncompetitive NMDA receptor antagonist that binds at the phencyclidine site inside the NMDA receptor ion channel, However, when administered at certain doses, MK-801 can cause acute toxicity and apoptotic cell death (Bittigau et al., 2002, Ikonomidou et al., 1999). In other words, MK-801 and similar drugs can block excitotoxicity, sparing the cell, but can also cause apoptotic cell death, depending on the dose, timing and age of administration.
Memantine, a drug used clinically to treat Alzheimer’s patients (Reisberg et al., 2003), is an uncompetitive voltage-dependent NMDA receptor antagonist. Thus, it acts as a channel blocker when the NMDA receptor is being abnormally activated, as is the case during ethanol withdrawal, but allows for normal receptor function and glutamatergic transmission to occur with low-level tonic stimulation of the receptor (Volbracht et al., 2006). Given the rapid off-rate kinetics and lower affinity properties, it is possible then that memantine, due to a more specific mechanism of action, could prove beneficial in mitigating both excitotoxicity and behavioral impairments associated with developmental ethanol exposure, but remain clinically viable.
The present study examines the effects of administering memantine during the withdrawal phase in neonatal rat pups following ethanol exposure on postnatal day (PD) 6. We hypothesized that memantine delivered during ethanol withdrawal would mitigate ethanol’s effects on behavioral outcomes in a dose-dependent manner. Any reliable attenuation of ethanol’s adverse effects on behavior would have important implications for treating individuals with FASD.
2. Materials and Methods
2.1 Animals
Subjects were 135 male and female Sprague-Dawley rats generated from 17 litters, born onsite at the animal colony at the Center for Behavioral Teratology, San Diego State University. The day of birth (gestational day 22) was recorded as PD 0. On PD 1, litters were pseudorandomly culled to eight pups with the goal of obtaining four males and four females ideally from each litter. Rats were housed in a temperature and humidity controlled room, maintained under a 12:12 h light/dark cycle, and given access to food and water ad libitum. All housing and behavioral tests were conducted in accordance with university and national guidelines and were approved by the San Diego State University Institutional Animal Care and Use Committee.
2.2 Pharmacological Manipulations
Treatment began on PD 6, with pups randomly assigned to one of eight groups based on the combination of ethanol (EtOH) dose (6 g/kg vs 0 g/kg) and dose of memantine hydrochloride (Sigma, St. Louis, MO; dissolved in saline) at 0, 10, 15, or 20 mg/kg (6.67 mL/kg). To control for potential litter effects, no more than one sex pair per litter was assigned to any treatment group. EtOH subjects received 6 g/kg/day (13.6% v/v in a milk formula) ethanol in a binge-like manner via two intragastric intubations (27.5 mL/kg), administered two hours apart (Goodlett and Johnson, 1997, Goodlett et al., 1990, Thomas et al., 2001, Thomas et al., 2002). The control subjects were intubated with a milk formula containing an isocaloric maltose dextrin solution (MC) in the same manner. This procedure assures that there are no differences in caloric intake between EtOH- and MC-treated groups (Livy et al., 2003, Maier et al., 1997, Maier et al., 1999, Thomas et al., 1997). In between each of the two treatment intubations, rats were housed with their dams. As pups treated with this level of binge ethanol do not nurse from their dam while intoxicated, EtOH subjects received two additional intubations of a nutritionally balanced milk formula at two and four hours post-ethanol, while controls received sham intubations, but no additional milk diet. Twenty-one hours following the last ethanol dose, when blood alcohol levels were nearing 0 mg/dL and when administration of MK-801 has proven successful in mitigating ethanol-induced behavioral deficits (Thomas et al., 2001), memantine, via a single intraperitoneal (i.p.) injection, was delivered in a variety of doses (0 [saline], 10, 15 and 20 mg/kg) that have shown protective effects following brain damage e.g. (Rao et al., 2001).
2.3 Blood Ethanol Concentration
In order to determine peak blood ethanol levels, 20 µL of blood was collected from the tail of pups 1.5 hours after the second EtOH intubation (Kelly et al., 1987), samples were centrifuged and plasma was collected. Samples were analyzed by the Analox Alcohol Analyzer (Model AM1, Lunenberg, MA) for blood ethanol content.
2.4 Locomotor Activity
Basic activity levels were assessed from PD 18–21 according to standard operating protocols (Thomas et al., 2001, Thomas et al., 2004). The testing took place in an open field Plexiglas chamber (40 × 40 × 30.5 cm). In order to eliminate the impact of outside noise on activity levels, the open fields were housed within enclosed chambers and white noise was played throughout the testing period. Subjects were acclimated to the activity room for 30 minutes prior to testing, and the chambers were cleaned thoroughly prior to testing to eliminate odor cues. Rats were placed in the center of the chamber and their behavior was collected in five-minute bins for 60 minutes via an automated optical beam activity monitor (Hamilton-Kinder, San Diego, CA) that calculates infrared beam interruptions. Number of beam breaks, total distance traveled, rearing, center time and fine movements served as performance measures. Subjects were tested for four consecutive days during the dark cycle, between 18:00–24:00.
2.5 Parallel Bar Motor Coordination
Parallel bar motor coordination testing took place from PD 30–32 (Thomas et al., 1998, Thomas et al., 1996). The apparatus consisted of two platforms (15.3 × 17.8 cm) spaced 91 cm apart with two parallel steel rods (measuring 0.5 cm diameter each) running between them. The entire apparatus stood 63 cm above a large container of wood shavings into which the rats could fall without injury. Subjects were acclimated to the room for 30 minutes prior to testing. At the start of testing, rats were placed on each platform for 30 sec to acclimate to the height of the apparatus from a stable position. Following acclimation, each subject was carefully placed on the rods halfway between the platforms, with all four paws positioned on the two bars. Each platform had a set of 28 grooved slots spaced 0.5 cm apart into which the rods were placed and could be moved incrementally. The initial distance between the rods was set at 3.5 cm and would increase by 0.5 cm on the following trial if the rat took four successive alternating steps with its hindpaws, which was considered a successful traversal. Subjects were given 5 trials to successfully traverse the rods at a given width, with a maximum of 15 trials in one day; if the subject placed two hind paws on one rod, fell or swung under the rods, the trial was considered unsuccessful and testing was finished for the day. On PD 31 and 32, the initial distance between the rods was set at the last successful distance attained by the subject on the previous day. The maximum gap distance successfully traversed each day, as well as the percent of traversals that were successful, served as motor coordination performance measures.
2.6 Spatial Discrimination Reversal Learning
Serial spatial discrimination reversal learning took place from PD 40–42 (Thomas et al., 2001, Thomas et al., 1997). The apparatus consisted of a partially submerged Plexiglas T-maze (19-cm-wide passages, 71-cm-long stem and a 91-cm arm span with 10-cm-wide cul-de-sac at each end). The tank itself was 121 cm in diameter and was filled with 26°C water made opaque with the addition of a cup of powdered milk. Each of the two arms of the T-maze had an escape ladder hung on a rod at the end. While the stem was constructed of clear Plexiglas, the arms themselves were made from black Plexiglas, which prevented the subjects from seeing the escape ladder.
A 10-trial pretraining session took place on PD 39. The purpose of the pretraining trials was to familiarize the subjects to the apparatus and procedure. For the pretraining trials, one of the two escape arms was blocked and the subject was placed into the starting arm facing the experimenter. Subjects were given 60 seconds to find the escape ladder in the open arm. If the escape ladder was not found within this time, the rat was physically guided to the ladder by the experimenter. Both arms were opened 5 times in a pseudorandom order.
Testing began on PD 40 with a pre-trial screening to determine the side preference for each subject. The preferred side was chosen through one trial in which both arms were open, the subject was allowed to choose which arm to enter, and was then removed from the maze. During the initial position discrimination trials, the escape ladder was placed in the nonpreferred arm. Subjects could enter either arm until they chose the correct side with the escape ladder, and escaped from the water. An error was noted if the subject entered the incorrect arm, re-ntered the starting stem after making an arm choice, or backtracked out of the goal arm without escaping. An initial error was recorded if the subject first entered the incorrect arm. If, after committing the initial error, the subject were to return to the choice point and re-enter the incorrect arm, enter the starting stem, or enter the goal arm without escaping, it was considered a repeated error. Because of the self-correction procedure, a subject could commit multiple errors within a trial before escaping. Trials continued until the subject entered the escape arm six consecutive times without an error. After six successful consecutive trials, the escape ladder was switched to the opposite (previously non-reinforced) arm. Training to that arm continued until six successful consecutive trials was achieved, and the location of the escape platform was reversed again. Thus, every time the subject achieved six consecutive successful trials, the location of the ladder was switched. Subjects were tested for 30 trials a day for 3 consecutive days with a one to two minute inter-trial interval during which time subjects were kept in wire cages under heat-lamps with a temperature of 31°C in order to prevent hypothermia. The number of trials to the first success criterion, the total number of successful discriminations achieved, and the total number of errors (including both initial and repeated errors) served as performance measures.
2.7 Data Analyses
Data were analyzed using SPSS software. Dependent measures included blood alcohol concentration, body weight, activity performance, parallel bar and serial spatial discrimination reversal learning performance. All data were analyzed with analysis of variance (ANOVA) with a 2 (ethanol, maltose) × 4 (0, 10, 15, 20 mg/kg memantine) × 2 (male, female) between-subjects design, with the exception of blood ethanol concentration that was analyzed with a 4 (memantine) × 2 (sex) design. Day served as a repeated within-subject variable for body weight, parallel bar and reversal learning performance. Activity data were measured with day (4) and bin (12) as within-subjects variables. Post hoc comparisons were conducted with Least Significant Differences (LSD) analyses (p <0.05).
3. Results
3.1 Body Weights
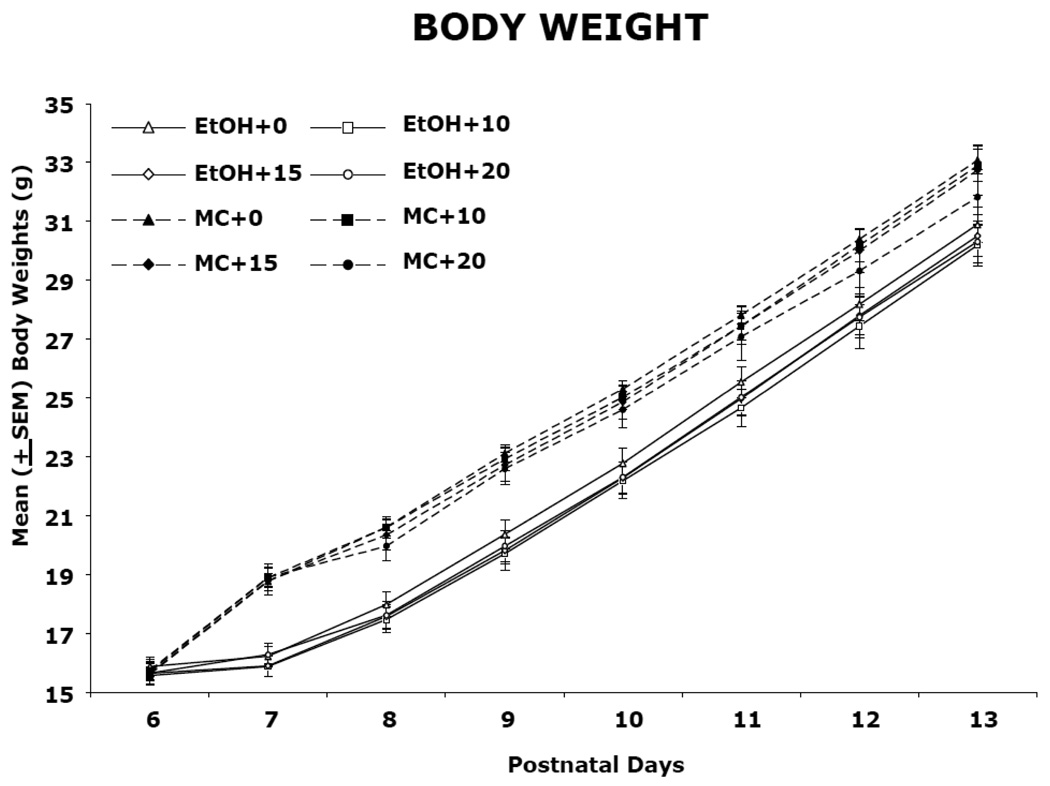
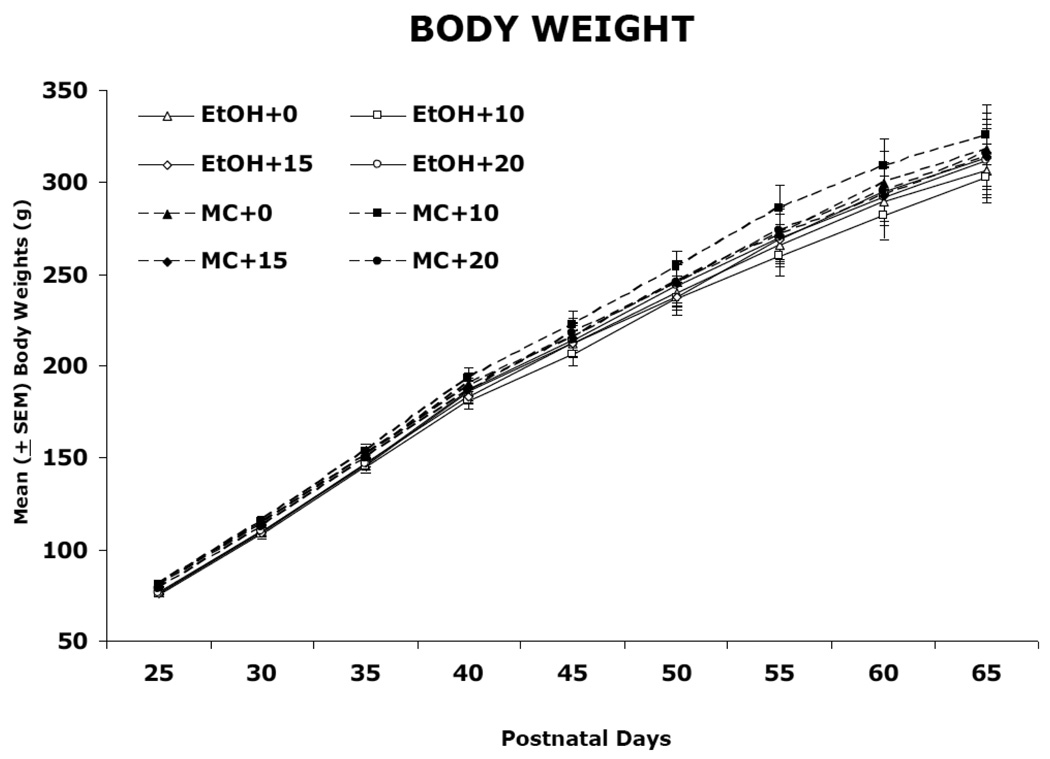
Body weights during ethanol and memantine treatment (PD 6) and following treatment (PD 7–13 and 25–65) are shown in Figure 1 and 2, respectively. During PD 6–13, there was a significant effect of day, due to growth in all groups [F(7,826) = 1194.6, p<0.05], significant effects of sex [F(1,118) = 5.3, p<0.05], and a day by sex interaction [F(14,826) = 2.2, p<0.05], as males grew faster than females. There was also a significant day by ethanol interaction [F(7,826) = 38.33, p<0.05] as well as a main effect of ethanol [F(1,118) = 46.1, p<0.05]. Although there were no significant differences in body weight among groups on PD 6, beginning on PD 7, the ethanol-treated subjects lagged in growth compared to controls. During PD 25–65, ethanol-exposed subjects continued to lag in weight, producing a main effect of ethanol [F(1,118) = 9.0, p<0.05], although the effect was not robust. In addition, significant effects of day [F(10,1180) = 1911.9, p<0.05] and sex [F(2,118) = 292.3, p<0.01], and a significant day by sex interaction [F(20,1180) = 211.7, p<0.05] was observed, which was again due to the faster growth of males compared with females. Memantine treatment had no significant effect on body growth at any dose.
Figure 1.
Mean (±SEM) body weight (g) from postnatal day (PD) 6–13. Ethanol-exposed subjects lagged in growth compared to controls beginning on PD 7. Maltose control subjects are represented by dashed lines while ethanol-treated rats are represented by solid lines.
EtOH + 0, ethanol-exposed, 0 mg/kg memantine; EtOH + 10, ethanol-exposed 10 mg/kg memantine; EtOH + 15, ethanol-exposed, 15 mg/kg memantine; EtOH + 20, ethanol-exposed, 20 mg/kg memantine; MC + 0, maltose control, 0 mg/kg memantine; MC + 10, maltose control, 10 mg/kg memantine; MC + 15, maltose control, 15 mg/kg memantine; MC + 20, maltose control, 20 mg/kg memantine.
Figure 2.
Mean (±SEM) body weight (g) from postnatal day (PD) 25–65. Ethanol-exposed subjects continued to lag in growth compared to controls.
3.2 Blood Ethanol Concentration
Blood ethanol concentrations were 400.4 ± 7.9, 408.8 ± 6.92, 396.8 ± 7.6 and 402.4 ± 8.0 mg/dL for ethanol-exposed rats receiving memantine injections at 0, 10, 15 and 20 mg/kg, respectively. There were no significant differences among the groups [F (3,70) = 0.755, p >0.5].
3.3 Locomotor Activity
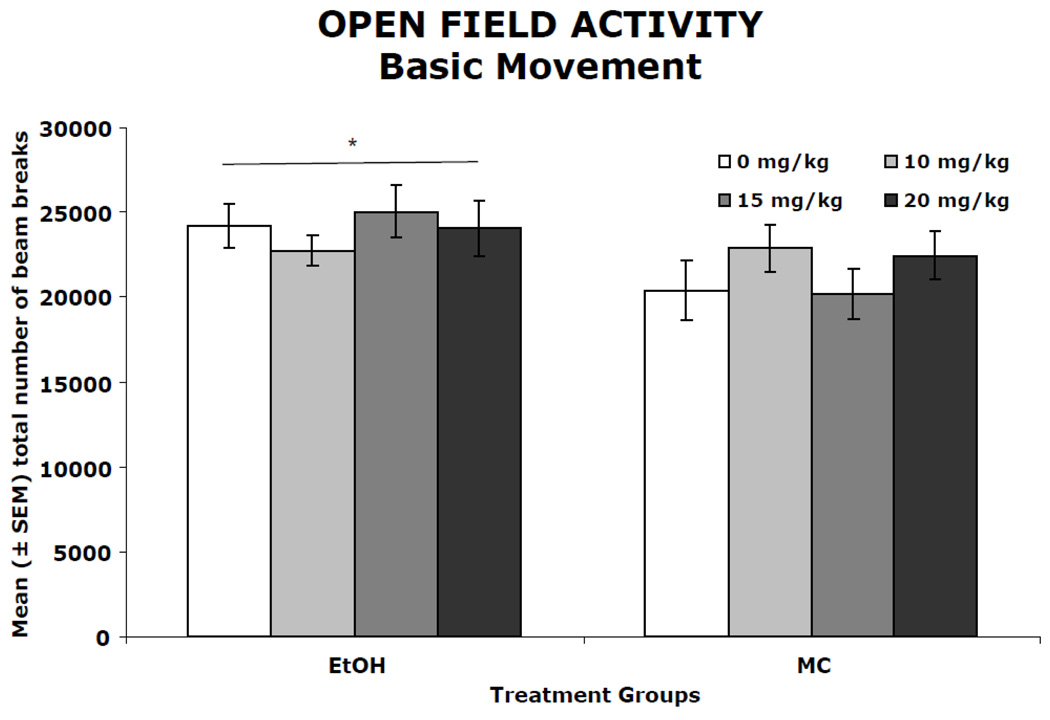
Figure 3 shows the number of total beam breaks in the open field over the course of four days. (Data from seven subjects, distributed evenly amongst treatment groups, was lost due to computer error). During the four-day activity testing, activity levels (number of beam breaks) decreased gradually between and within sessions (main effect of day [F(3,333) = 5.2, p<0.05] and bin [F(11,1221) = 134.3, p<0.05]). The ethanol-exposed subjects had higher activity levels compared to maltose control subjects, producing a significant main effect of ethanol [F(1,111= 6.4, p<0.05]. Memantine treatment did not significantly affect activity levels in either ethanol-treated or control subjects. A similar pattern was observed for total distance traveled, center time, fine movements and rearing (data not shown). On all measures, ethanol exposure during development led to increased activity levels with no significant effect of memantine on either EtOH or MC animals.
Figure 3.
Mean (± SEM) open field activity summed across the four testing days. When collapsed across memantine treatment, ethanol-treated subjects had significantly higher activity levels compared to maltose control (MC) groups as evidenced by total number of beam breaks. Memantine did not significantly alter activity level in either ethanol-treated or control animals.
* ethanol-exposed groups differed significantly from controls
3.4 Parallel Bar Motor Coordination
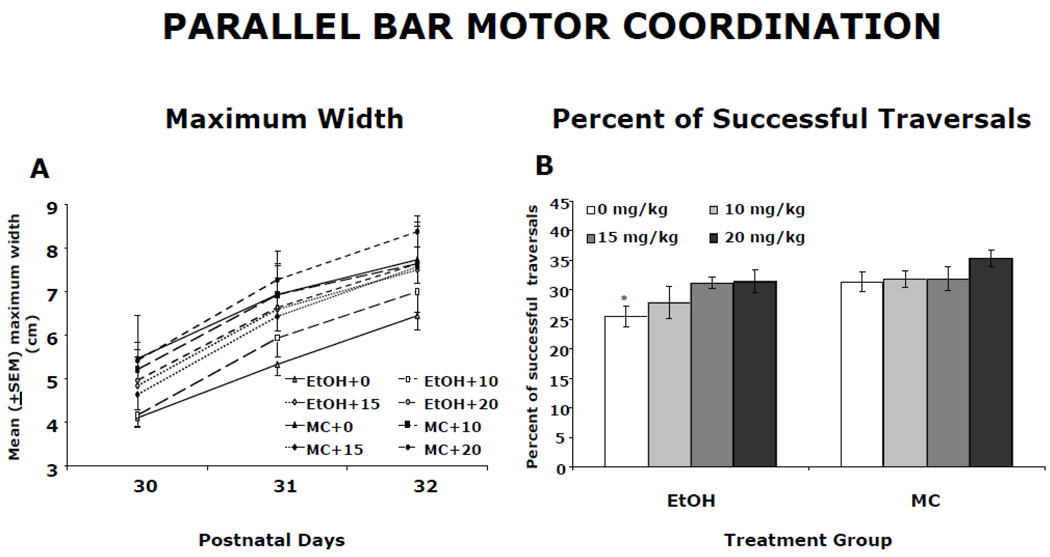
Memantine treatment reduced the severity of ethanol-related motor deficits assessed on the parallel bar task. The maximum gap successfully traversed on the parallel bars for each testing day is shown in Figure 4A. All groups showed gradual improvement over the three days (main effect of day [F(2,202) = 85.6, p< 0.05]). There was also a significant main effect of ethanol [F(1,101) = 11.6, p< 0.05] and a significant ethanol by memantine interaction [F(3,101)=2.8, p<0.05]. Follow-up analyses indicated that EtOH + 0 and EtOH + 10 subjects traversed significantly smaller widths compared to all controls (p’s<0.05). In contrast, EtOH subjects treated with either 15 or 20 mg/kg memantine successfully traversed a larger gap than the EtOH + 0 group, but not the EtOH + 10 group.
Figure 4.
Parallel bar motor coordination. A: Mean (± SEM) maximum gap successfully traversed on the parallel bars. All groups showed improvements in motor performance over the 3 testing days. However, ethanol-exposed subjects traversed significantly smaller maximum gaps when compared to maltose control (MC) rats. Administration of 15 or 20 mg/kg memantine improved motor performance of ethanol-exposed subjects. B: Mean (± SEM) percent of trials that were successful. Ethanol-exposed subjects treated with vehicle were significantly less successful compared to controls. Ethanol-exposed subjects treated with 15 and 20 mg/kg memantine performed at control levels, having a significantly higher percent of successful traversals than EtOH + 0 subjects. The EtOH + 10 group did not differ significantly from any other group.
*significantly different from all groups except EtOH + 10 memantine
Figure 4B shows that memantine treatment also improved the percent of successful traversals by ethanol-treated rats. The percent of trials successfully traversed represents a measure that is particularly sensitive to developmental alcohol exposure. A significant effect of ethanol [F(1,118) = 9.6, P<0.05] and a significant main effect of memantine [F(3,118) = 3.3, p<0.05] was found. Although the interaction of ethanol and memantine failed to reach significance, the ethanol effect was primarily driven by the EtOH + 0 group, as seen in Figure 4B. In fact, post-hoc analyses revealed that EtOH rats treated with 15 or 20 mg/kg memantine performed at control levels, performing significantly more successfully than EtOH + 0 subjects. The performance of the EtOH + 10 group was intermediate, not differing significantly from that of any other group, including the maltose controls. No significant effects of memantine were observed in the maltose control animals for this behavioral task.
3.5 Spatial Discrimination Reversal Learning
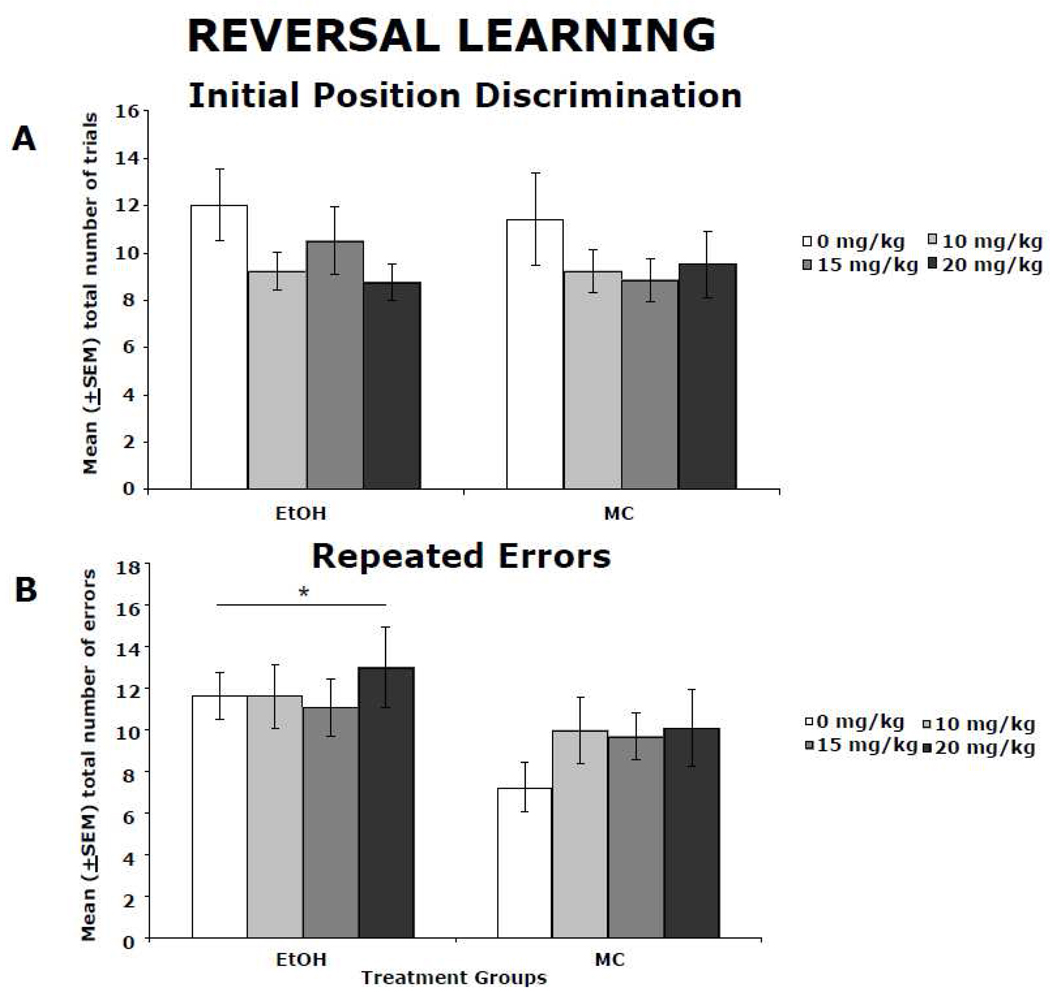
Ethanol-exposed animals performed poorly when compared to controls on the spatial reversal learning task, and memantine did not significantly affect behavior. Significant main effects of ethanol were found on three of the four outcome measures on the reversal learning task (total number of successful discriminations achieved, total number of errors and total number of repeated errors), with ethanol having no significant effect on the number of trials to first success criterion. There were no significant effects of memantine or a significant ethanol by memantine interaction on any of the four performance measures in this task. Although ethanol-exposed subjects were not impaired on the initial position discrimination (Figure 5A), they were unable to achieve the same number of successful discriminations as maltose controls, committing significantly more errors. In particular, ethanol-exposed rats committed more repeated errors than control animals, resulting in a significant main effect of ethanol [F(1,115) = 5.8, p<0.05]. The average number of total perseverative-type errors over the course of the three testing days is shown in Figure 5B. No significant effect of memantine was observed in either ethanol-treated or control animals in any of the performance measures.
Figure 5.
Spatial discrimination reversal learning. Ethanol-exposed subjects were not impaired on the initial position discrimination, as shown by the mean (± SEM) number of trials to the first success (A); however, they were unable to achieve the same number of successful discrimination reversals as maltose controls, committing significantly more errors. Specifically, ethanol-exposed subjects committed significantly more perseverative-type errors than MC animals (mean ± SEM perseverative-type errors committed during testing (B)). Memantine treatment did not significantly improve performance in the ethanol-treated subjects, or have a significant effect on control subjects.
* ethanol-exposed groups differed significantly from controls
4. Discussion
Binge exposure to ethanol during critical time points in brain development can cause significant changes in activity level, motor and learning impairments. Surprisingly little is known about treatments that can mitigate these impairments (see (Lupton et al., 2004)). In this study, we demonstrated that a single administration of memantine during alcohol withdrawal could attenuate motor deficits, but not learning deficits or overactivity, caused by developmental ethanol exposure.
One day of ethanol exposure, on PD 6, produced significant increases in activity levels and deficits in both motor coordination and reversal learning. This is consistent with earlier reports (Thomas et al., 2001, Thomas et al., 2002, Thomas, 2002, Thomas et al., 1996, Thomas et al., 1997) and further demonstrates how a brief exposure to an ethanol binge can induce long-term disruptions to the developing CNS. Ethanol-exposed subjects were significantly overactive in the open field. Ethanol-exposed subjects also performed poorly when compared to controls on the parallel bar motor coordination and spatial discrimination reversal learning tasks. Similar changes in activity level, spatial learning and motor behavior can be produced by traumatic brain insults such as hypoxia/ischemia (Balduini et al., 2000, Bona et al., 1997). The excitotoxic cell death and neurodegeneration that are observed as a result of percussive head trauma, focal ischemia, epilepsy, hypoxia and glutamate receptor agonists (Gonzalez and Ferriero, 2008, Ishimaru et al., 1999, McDonald et al., 1993) are consistent with damage observed after neonatal binge ethanol exposure (Ikonomidou et al., 2000). Treatment with NMDA receptor antagonists like MK-801 and memantine can dramatically reduce the extent of damage to the developing rat brain following such insults (Idrus et al., 2011, Olney et al., 1989). However, unlike MK-801, memantine is less likely to result in unintended apoptotic cell death and/or exacerbate ethanol-related behavioral deficits.
The present study demonstrated that administration of 15 or 20 mg/kg memantine during withdrawal significantly attenuated ethanol’s adverse effects on motor coordination. A single binge exposure to ethanol during the critical brain growth spurt period caused significant motor coordination impairments on the parallel bar task, a task that is highly sensitive to deficits caused by developmental ethanol exposure (Thomas et al., 1996). However, a single memantine treatment 21 hours after the binge ethanol exposure was able to mitigate the ethanol-induced impairments. Ethanol-exposed subjects treated with 15 or 20 mg/kg memantine had more overall successful traversals and traversed bars that were further apart compared to ethanol-exposed subjects treated with vehicle. In fact, performance of ethanol-exposed subjects treated with the higher doses of memantine did not differ from that of controls. The motor performance improvement following memantine administration suggests that memantine can reduce NMDA receptor-mediated excitotoxicity in areas of the brain that contribute to motor behavior, such as the cerebellum and/or motor cortex. NMDA receptors are expressed throughout the brain, including the developing rat cerebellum (Watanabe et al., 1994), during this period of neonatal development. NMDA receptor expression in the cerebellum, specifically in the Purkinje cells, begins on embryonic day 13 in the developing rat and increases, reaching a peak on PD 14. The granule cells of the internal and external granular layers begin expressing NMDA receptors at PD 1 and PD 7, respectively. From PD 14, NMDA receptor expression decreases to adult levels (Watanabe et al., 1992, Watanabe et al., 1994, Zhong et al., 1995). It is therefore possible that memantine improved the motor incoordination of ethanol-exposed rats by blocking excitotoxicity in the cerebellum. In fact, we recently demonstrated that administration of memantine during ethanol withdrawal can protect against cerebellar Purkinje cell loss (Idrus et al., 2011).
In contrast to motor performance, memantine was not effective in attenuating ethanol’s effects on open field activity or spatial reversal learning. Interestingly, previous studies have shown that administration of MK-801 or eliprodil, NMDA receptor antagonists, can reduce alcohol’s adverse effects on these behaviors (Lewis et al., 2007, Thomas et al., 2001, Thomas et al., 2002, Thomas et al., 2004, Thomas, 2002, Thomas et al., 1997). Deficits in spatial reversal learning and overactivity in the open field may be related to hippocampal dysfunction (Mumby et al., 2002, Zolamorgan and Squire, 1993). It is also well established that the hippocampus is particularly vulnerable to neonatal ethanol exposure as demonstrated by reduced hippocampal cell numbers, decreased morphological plasticity, altered synaptic activity, and deficits in hippocampal-associated behavioral deficits (Berman and Hannigan, 2000, Chen et al., 2003, Gonzalez-Burgos et al., 2006, Livy et al., 2003, Thomas et al., 2002, Tran and Kelly, 2003). Ethanol withdrawal-related excitoxicity is thought to also contribute to these deficits. For example, upregulation of NMDA receptors and increases in glutamate release can be found up to 36 hours after ethanol exposure in both embryonic hippocampal cell cultures and adult animals in vivo (Maler et al., 2005, Rossetti and Carboni, 1995, Sanna et al., 1993). The developing hippocampus also displays greater sensitivity to excitotoxic insults during ethanol withdrawal (Prendergast et al., 2004). Memantine, when administered during the withdrawal period, can prevent increases in NMDA receptor number in the hippocampus (Maler et al., 2005), and protect against hippocampal cell death, as well as ethanol-withdrawal induced seizures (Stepanyan et al., 2008). Surprisingly, memantine did not improve performance on the behavioral tasks that depend on the functional integrity of the hippocampus, suggesting that the hippocampus was not afforded neuroprotection in the present study. It should be noted, however, that behavioral performance on these tasks may also depend on the functional integrity of other CNS regions, like the prefrontal cortex (Stefani and Moghaddam, 2005), especially since there were no ethanol-related deficits in the initial spatial discrimination but there were deficits in reversal learning and response inhibition. Thus, it is possible that there was some protection in the hippocampus, but not in other brain areas.
Given previous studies, it is not clear why memantine did not mitigate behavioral deficits associated with the hippocampus, especially given the robust effects observed on motor behavior. One possibility is that the optimal dose of memantine to block ethanol’s effects may vary among CNS regions and that higher doses of memantine would impact hippocampal-based behaviors. Another possibility is that the precise timing of NMDA overactivation may depend on CNS region and that memantine was not maximally active at the time of ethanol withdrawal when the hippocampus would be most vulnerable to NMDA receptor-mediated excitotoxic cell death. With concentrations of memantine peaking 20–30 minutes post-injection and having a relatively short half-life of 3–5 hours (Parsons et al., 2007), memantine, when administered 21 hours post-ethanol binge, might have lost its peak binding potential by the time NMDA receptor-mediated excitotoxicity was at its highest levels in the hippocampus. Administration of MK-801, which has a much longer half-life, is able to mitigate ethanol-related behavioral deficits associated with the hippocampus when administered either 21 or 33 hours after ethanol (Thomas et al., 2001). Similarly, eliprodil mitigated ethanol-induced spatial reversal learning deficits (Thomas et al., 2004) when administered 21 hours after ethanol, but this drug also exhibits a longer half-life than memantine (Garrigou-Gadenne et al., 1995). To date, it is not yet known exactly when hippocampal excitotoxicity would be occurring in this model; however, we do know that upregulation of NMDA receptors and increases in glutamate release can be found up to 36 hours after ethanol exposure in vitro. Therefore, it may be that NMDA receptor antagonists should be administered not only at the appropriate time, but must block NMDA receptors for an extended period of time during ethanol withdrawal.
It is also possible that memantine is working via alternative mechanisms. For example, memantine’s effects on glia include reducing inflammatory responses and increasing the release of neurotrophic factors, which can be neuroprotective (Jantas and Lason, 2009, Wu et al., 2009). Other neurotransmitter systems, such as the serotonergic system can also be influenced by memantine. This is particularly intriguing as administration of serotonergic agonists are known to be protective against alcohol’s neurotoxic effects (Druse et al., 2004). However, attenuation of alcohol-related deficits in motor coordination has also been demonstrated with administration of another NMDA receptor modulator, agmatine, during ethanol withdrawal (Lewis et al., 2007). Here, agmatine was administered following 7 consecutive days of binge-like exposure to ethanol from PD 1 to 8, thus reflecting the clinical setting of a newborn undergoing withdrawal at birth. Taken together, these studies suggest that blocking NMDA receptors during ethanol withdrawal can mitigate alcohol-related motor deficits.
It is important to note that there were no significant effects of memantine at any dose in the performance of the controls in any of the behaviors assessed. This suggests that memantine administration still allows for normal glutamatergic neurotransmission without disrupting normal CNS development. This is clearly advantageous in a clinical setting when other NMDA receptor antagonists could potentially induce toxic effects.
In summary, we demonstrated that memantine mitigates motor coordination deficits when administered 21 hours after a binge ethanol treatment in neonatal rat pups. This finding supports the hypothesis that some of the behavioral deficits observed in FASD could be attributable to NMDA receptor-mediated excitotoxicity that occurs during ethanol withdrawal. The present findings provide further support that ethanol withdrawal may contribute to fetal alcohol spectrum disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
The authors have no conflicts of interest.
References
- Balduini W, De Angelis V, Mazzoni E, Cimino M. Long-lasting behavioral alterations following a hypoxic/ischemic brain injury in neonatal rats. Brain Res. 2000;859:318–325. doi: 10.1016/s0006-8993(00)01997-1. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: Spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona E, Johansson BB, Hagberg H. Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatr Res. 1997;42:678–683. doi: 10.1203/00006450-199711000-00021. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Maier SE, Parnell SE, West JR. Alcohol and the developing brain: Neuroanatomical studies. Alcohol Res Health. 2003;27:174–180. [PMC free article] [PubMed] [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O'Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: A comparative study of eyeblink conditioning in children with adhd and dyslexia. Cortex. 2005;41:389–398. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Davidson M, Shanley B, Wilce P. Increased nmda-induced excitability during ethanol withdrawal - a behavioral and histological study. Brain Research. 1995;674:91–96. doi: 10.1016/0006-8993(94)01440-s. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin NF, Gillespie RA, Dickson E, Atieh M, Pietrzak CA, et al. The serotonin-1a agonist ipsapirone prevents ethanol-associated death of total rhombencephalic neurons and prevents the reduction of fetal serotonin neurons. Brain Res Dev Brain Res. 2004;150:79–88. doi: 10.1016/j.devbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Garrigou-Gadenne D, Thenot JP, Morselli PL. Influence of the rate of intravenous administration of eliprodil (sl 82.0715), a new anti-ischaemic agent, on its distribution in rat plasma and tissues. J Pharmacokinet Biopharm. 1995;23:147–161. doi: 10.1007/BF02354269. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M, Olvera-Cortes ME, Perez-Vega MI, Evans S, Feria-Velasco A. Prenatal-through-postnatal exposure to moderate levels of ethanol leads to damage on the hippocampal ca1 field of juvenile rats: A stereology and golgi study. Neurosci Res. 2006;56:400–408. doi: 10.1016/j.neures.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, Ferriero DM. Therapeutics for neonatal brain injury. Pharmacol Ther. 2008;120:43–53. doi: 10.1016/j.pharmthera.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: Timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar purkinje cell loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Idrus NM, McGough NN, Riley EP, Thomas JD. Administration of memantine during ethanol withdrawal in neonatal rats: Effects on long-term ethanol-induced motor incoordination and cerebellar purkinje cell loss. Alcohol Clin Exp Res. 2011;35:355–364. doi: 10.1111/j.1530-0277.2010.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, et al. Blockade of nmda receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Ishimaru MJ, Ikonomidou C, Tenkova TI, Der TC, Dikranian K, Sesma MA, et al. Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J Comp Neurol. 1999;408:461–476. [PubMed] [Google Scholar]
- Jantas D, Lason W. Anti-apoptotic effect of memantine against staurosporine- and low-potassium-induced cell death in cerebellar granule cells: A development-dependent effect. Pharmacol Rep. 2009;61:827–937. doi: 10.1016/s1734-1140(09)70138-0. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Bonthius DJ, West JR. Developmental changes in alcohol pharmacokinetics in rats. Alcohol Clin Exp Res. 1987;11:281–286. doi: 10.1111/j.1530-0277.1987.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Lewis B, Wellmann KA, Barron S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol Biochem Behav. 2007;88:114–121. doi: 10.1016/j.pbb.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. 2004;127C:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- Maier SE, Chen WJ, Miller JA, West JR. Fetal alcohol exposure and temporal vulnerability regional differences in alcohol-induced microencephaly as a function of the timing of binge-like alcohol exposure during rat brain development. Alcohol Clin Exp Res. 1997;21:1418–1428. doi: 10.1111/j.1530-0277.1997.tb04471.x. [DOI] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: Regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res. 1999;23:726–734. doi: 10.1111/j.1530-0277.1999.tb04176.x. [DOI] [PubMed] [Google Scholar]
- Maler JM, Esselmann H, Wiltfang J, Kunz N, Lewczuk P, Reulbach U, et al. Memantine inhibits ethanol-induced nmda receptor up-regulation in rat hippocampal neurons. Brain Res. 2005;1052:156–162. doi: 10.1016/j.brainres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Fix AS, Tizzano JP, Schoepp DD. Seizures and brain injury in neonatal rats induced by 1s,3r-acpd, a metabotropic glutamate receptor agonist. J Neurosci. 1993;13:4445–4455. doi: 10.1523/JNEUROSCI.13-10-04445.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learning & Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol-abuse and alcoholism. Neurochemistry International. 1995;26:305–336. doi: 10.1016/0197-0186(94)00139-l. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ikonomidou C, Mosinger JL, Frierdich G. Mk-801 prevents hypobaric-ischemic neuronal degeneration in infant rat brain. J Neurosci. 1989;9:1701–1704. doi: 10.1523/JNEUROSCI.09-05-01701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Stoffler A, Danysz W. Memantine: A nmda receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, 2nd, Gibson DA, Holley RC, et al. Hippocampal ca1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of n-methyl-d-aspartate receptors. Neuroscience. 2004;124:869–877. doi: 10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Todd KG, Bowen KK, Dempsey RJ. Neuroprotection by memantine, a non-competitive nmda receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Doody R, Mobius HJ. Memantine in moderate-to-severe alzheimer's disease - reply. New England Journal of Medicine. 2003;349:610–610. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: A decade of brain imaging. Am J Med Genet C Semin Med Genet. 2004;127C:35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, et al. Chronic ethanol intoxication induces differential effects on gabaa and nmda receptor function in the rat brain. Alcohol Clin Exp Res. 1993;17:115–123. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Schummers J, Browning MD. Evidence for a role for gaba(a) and nmda receptors in ethanol inhibition of long-term potentiation. Molecular Brain Research. 2001;94:9–14. doi: 10.1016/s0169-328x(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: Size reduction in lobules i-v. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical nmda receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Stepanyan TD, Farook JM, Kowalski A, Kaplan E, Barron S, Littleton JM. Alcohol withdrawal-induced hippocampal neurotoxicity in vitro and seizures in vivo are both reduced by memantine. Alcohol Clin Exp Res. 2008;32:2128–2135. doi: 10.1111/j.1530-0277.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Fleming S, Riley EP. Mk-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcohol Clin Exp Res. 2001;25:764–773. [PubMed] [Google Scholar]
- Thomas JD, Fleming SL, Riley EP. Administration of low doses of mk-801 during ethanol withdrawal in the developing rat pup attenuates alcohol's teratogenic effects. Alcohol Clin Exp Res. 2002;26:1307–1313. doi: 10.1097/01.ALC.0000025888.60664.D9. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004;175:189–195. doi: 10.1007/s00213-004-1806-x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res. 1998;105:159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Edwards RB, Chen W-JA. Mk-801 admnistered during withdrawal attenuates hippocampal ca1 pyramidal cell loss in rats neonatally exposed to alcohol. Alcohol. 2002;26:134A. [Google Scholar]
- Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: Importance of developmental timing and number of episodes. Dev Psychobiol. 1996;29:433–452. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Weinert SP, Sharif S, Riley EP. Mk-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcohol Clin Exp Res. 1997;21:1218–1225. [PubMed] [Google Scholar]
- Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol Teratol. 2003;25:519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006;23:2611–2622. doi: 10.1111/j.1460-9568.2006.04787.x. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F, de Witte P. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or 'binge drinking' alcohol abuse. Alcohol Alcohol. 2009;44:128–135. doi: 10.1093/alcalc/agn100. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of nmda receptor channel subunit mrnas. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal expressions of five nmda receptor channel subunit mrnas in the cerebellum. J Comp Neurol. 1994;343:513–519. doi: 10.1002/cne.903430402. [DOI] [PubMed] [Google Scholar]
- Wu HM, Tzeng NS, Qian L, Wei SJ, Hu X, Chen SH, et al. Novel neuroprotective mechanisms of memantine: Increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology. 2009;34:2344–2357. doi: 10.1038/npp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB. Expression of mrnas encoding subunits of the nmda receptor in developing rat brain. J Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]
- Zolamorgan S, Squire LR. Neuroanatomy of memory. Annual Review of Neuroscience. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]