Abstract
Hypertension afflicts 25% of the general population and over 50% of the elderly. In the present work, arterial spin labeling MRI was used to non-invasively quantify regional cerebral blood flow (CBF), cerebrovascular resistance and CO2 reactivity in spontaneously hypertensive rats (SHR) and in normotensive Wistar Kyoto rats (WKY), at two different ages (3 months and 10 months) and under the effects of two anesthetics, α-chloralose and 2% isoflurane (1.5 MAC). Repeated CBF measurements were highly consistent, differing by less than 10% and 18% within and across animals, respectively. Under α-chloralose, whole brain CBF at normocapnia did not differ between groups (young WKY: 61±3ml/100g/min; adult WKY: 62±4ml/100g/min; young SHR: 70±9ml/100g/min; adult SHR: 69±8ml/100g/min), indicating normal cerebral autoregulation in SHR. At hypercapnia, CBF values increased significantly, and a linear relationship between CBF and PaCO2 levels was observed. In contrast, 2% isoflurane impaired cerebral autoregulation. Whole brain CBF in SHR was significantly higher than in WKY rats at normocapnia (young SHR: 139±25ml/100g/min; adult SHR: 104±23ml/100g/min; young WKY: 55±9ml/100g/min; adult WKY: 71±19ml/100g/min). CBF values increased significantly with increasing CO2; however, there was a clear saturation of CBF at PaCO2 levels greater than 70 mmHg in both young and adult rats, regardless of absolute CBF values, suggesting that isoflurane interferes with the vasodilatory mechanisms of CO2. This behavior was observed for both cortical and subcortical structures. Under either anesthetic, CO2 reactivity values in adult SHR were decreased, confirming that hypertension, when combined with age, increases cerebrovascular resistance and reduces cerebrovascular compliance.
Keywords: arterial spin labeling, hypercapnia, magnetic resonance imaging, perfusion, spontaneously hypertensive rat
1. Introduction
Hypertension is a disease that afflicts 25% of the general population and more than half of the elderly population (Pedelty and Gorelick, 2008). It is the most important modifiable risk factor for cerebrovascular diseases and the second most important risk factor, after age, for hemorrhagic and ischemic stroke (Veglio et al., 2009). It is a leading cause of cognitive decline and dementia and an important risk factor for Alzheimer’s disease. Hypertension modifies the intricate mechanisms of cerebral blood flow (CBF) regulation, including functional hyperemia, cerebrovascular autoregulation, and endothelial regulation (Iadecola and Davisson, 2008). In patients with symptomatic atherosclerotic disease, hypertension has been shown to be associated with elevated CBF (van Laar et al., 2008). For all of the above, continued research on the effects of hypertension on cerebrovascular function is a crucial step in the design of preventive therapies aimed at minimizing the risk of development of cerebrovascular disease.
The spontaneously hypertensive rat (SHR) is an important experimental model in the study of stroke and other cerebrovascular diseases (Amenta et al., 2003). The presence of chronic hypertension in this rat strain results in vascular remodeling, specifically in form of thickening of the arterial wall (hypertrophy), narrowing of the lumen inner diameter (eutrophy) and vascular stiffening (Amenta et al., 2003). This remodeling causes alterations in the limits of autoregulation (Fujishima et al., 1984; Harper and Bohlen, 1984) and leads to lower cerebral rate of glucose utilization (Katsuta, 1997; Wei et al., 1992), impaired cerebrovascular reactivity to carbon dioxide (CO2) (Nakajima et al., 2007; Tamaki et al., 1995) and higher susceptibility to stroke (Barone et al., 1992; Duverger and MacKenzie, 1988; Hom et al., 2007) in SHR compared to the normotensive Wistar-Kyoto (WKY) rat. However, the chronic effects of hypertension on resting CBF in this animal model are less clear. Contradictory findings of similar (Tamaki et al., 1995; Wei et al., 1992), smaller (Grabowski et al., 1993; Katsuta, 1997), or larger (Heinert et al., 1998) basal CBF values in SHR versus WKY rats have been reported. A possible reason for the significant discrepancy of findings in animal models is methodological. The various studies were conducted under diverse experimental conditions, including the use of animals of different ages, sedated under different anesthetics. Both age (Dai et al., 2008; Martin et al., 1991) and anesthesia (Hoffman et al., 1991; Scheller et al., 1986; Strebel et al., 1995) are known to have a strong influence on CBF. In addition, previous studies of the effect of hypertension on the functional status of the cerebral vasculature in SHR have utilized invasive techniques to measure CBF, including the hydrogen clearance method (Fujishima et al., 1984; Heinert et al., 1998), quantitative autoradiography (Grabowski et al., 1993; Ito et al., 2002; Katsuta, 1997; Wei et al., 1992), or laser Doppler flowmetry (Barone et al., 1992; Dupuis et al., 2005). These techniques are impractical for repeated measurements, making it difficult to carryout longitudinal studies.
The purpose of the present study was to investigate the effects of hypertension and aging on the regional cerebrovascular status of the SHR and WKY rats. CBF was quantified in the whole brain and in the cortex, caudate putamen, and thalamus, with continuous arterial spin labeling (CASL), using a separate labeling coil. In addition to CBF, cerebrovascular resistance (CVR) and cerebrovascular reactivity to CO2 were assessed during graded hypercapnia under the effects of two commonly used anesthetic agents, α-chloralose and isoflurane.
2. Materials and Methods
2.1. Animal Preparation
All experiments were approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke, National Institutes of Health. Male spontaneously-hypertensive rats (SHR) and normotensive rats (Wistar Kyoto – WKY) were acquired from a commercial vendor (Harlan Laboratories, Somerville, NJ) and aged to a minimum of 12 weeks. Measurements of whole brain CBF were performed in 32 rats (8 WKY – 235 ± 21 g, 8 SHR – 285 ± 33 g, 3 – 4 months old; 8 WKY – 418 ± 23 g, 8 SHR – 402 ± 46 g, 10 months old) in association with a hypercapnic (CO2) challenge.
On the day of the experiments, the rats were anesthetized with isoflurane (5% induction, 2% maintenance), orally intubated, mechanically ventilated in oxygen-enriched air and equipped with indwelling femoral arterial and venous lines. Following surgery, animals were secured to an MRI-compatible stereotaxic head frame by means of ear-pieces and a bite bar. Rectal temperature was monitored and maintained at 37 ± 1 °C using circulating warm water. Heart rate (HR), respiration rate (RR), end-tidal CO2 (EtCO2) and arterial oxygen saturation (spO2) were continuously monitored by means of a pulse oximeter (Surgivet, Waukesha, WI, USA).
The animals were further divided into two different anesthetic regimens. Four rats of each strain and age were kept anesthetized under 2% isoflurane anesthesia (1.5 MAC – minimum alveolar concentration) delivered in the inhaled gas mixture. This concentration was found necessary to maintain the 10-month-old SHR rats properly anesthetized in the MRI scanner in the presence of the loud MRI sounds. Therefore, to avoid confounds introduced by the use of different levels of anesthesia across the different groups, this concentration was uniformly applied to both WKY and SHR rats of all ages. For the remaining rats, isoflurane was discontinued and anesthesia was switched to α-chloralose (80 mg/kg 6 initial bolus, 27 mg/kg/h continuous infusion) injected intravenously. Hypercapnia was achieved by adding 1.5%, 3%, 4.5%, 6% and 10% CO2 to the inhaled gas mixture. A 5-minute adjustment was allowed between switching the gas and starting the imaging acquisition. Arterial blood gases were sampled at the end of each CO2 level through the PE-50 catheter inserted into the right femoral artery.
2.2. MRI Methods
MRI experiments were conducted in a horizontal 7T/30cm AVIII MRI system (Bruker-Biospin, Inc., Billerica, MA, USA), equipped with gradients capable of 450 mT/m amplitude (Resonance Research Inc., Billerica, MA, USA). RF excitation was accomplished via a home-built, transmit-only birdcage volume RF coil, 12 cm internal diameter. For signal reception, a home-built single surface coil, equipped with dedicated RF preamplifiers, was used. For ASL, a small home-built figure-8 labeling coil (Silva et al., 1995) was positioned under the neck of the animal, approximately 2 cm away from the magnet’s isocenter, and connected to the second RF transmit channel of the spectrometer. All coils were equipped with active decoupling circuits to minimize coil-to-coil interferences during the labeling and imaging stages of the experiment, and to minimize off-resonance saturation of water in the acquisition region (Zhang et al., 1995).
Coronal ASL perfusion-weighted images were acquired using a spin-echo echo-planar-imaging (EPI) sequence with the following parameters: TR = 10000 ms, TE = 28 ms, FOV = 32 × 32 mm2, matrix size = 64 × 64, slice thickness = 2 mm, number of slices = 5. These parameters yielded an in-plane spatial resolution of 500 μm. A post-labeling delay of 994 ms was employed to prevent ASL signals in the feeding arteries from causing overestimation of CBF (Alsop and Detre, 1996). A labeling RF pulse of 8183 ms was applied in the presence of a longitudinal gradient Gz = 1 G/cm at the appropriate labeling frequency offset. The RF power used for ASL was optimized in a subset of animals to produce the highest labeling efficiency. The optimal power required to achieve the maximum labeling efficiency of 75 ± 2% in the brain was 2 W. The 5 contiguous slices were acquired according to a sequential anterior-posterior order to avoid interferences from the acquisition in the magnetization of labeled blood. Sixteen averages (NA = 16) were acquired resulting in an acquisition time of 5 min 20 s.
2.3. Data Analysis
Quantitative CBF maps were calculated using MATLAB R2009a (The MathWorks, Natick, MA, USA). Control and labeled images were separately averaged and then subtracted. CBF values (ml/100g/min) were calculated for each CO2 level using the following equation (Zhang et al., 1995):
| [1] |
where λ is the brain/blood partition coefficient, T1 is the longitudinal relaxation time of the brain water magnetization in the absence of perfusion and cross-relaxation, SC is the equilibrium signal intensity, SL is the brain water signal intensity from the labeled image, and α is the labeling efficiency. The labeling efficiency was determined experimentally and the values of SC and SL were measured for all subjects; λ = 0.9 ml/g and T1 = 1.75 s were used in all calculations.
Cerebrovascular reactivity to CO2 (%/mmHg) was calculated using the following equation:
| [2] |
where CBF0 is the resting cerebral blood flow measured before adding CO2 to the inhaled gas mixture, and CBF and PaCO2 are, respectively, the cerebral blood flow and the arterial pressure CO2 for each level of CO2 added to the gas mixture.
Cerebrovascular resistance (mmHg/ml/100g/min) was calculated using Eq. [3]:
| [3] |
where MABP is the mean arterial blood pressure.
Four regions of interest (ROIs) were analyzed: whole brain, cortex, caudate putamen and thalamus (Fig. 1a). For each rat, CBF, CO2 reactivity and CVR values of the four ROIs were calculated for each level of CO2 (six levels, from 0 to 10%). Then, the values were divided in two groups (normocapnia, for PaCO2 ≤ 45 mmHg; and hypercapnia, for PaCO2 > 60 mmHg), and averaged across individuals.
Figure 1.
(a) Coronal EPI images of a spontaneously hypertensive rat under isoflurane. Five adjacent 2 mm thick slices are shown from posterior (left) to anterior (right). Overlaid on the EPI images are three regions of interest: cortex (blue), caudate putamen (red), and thalamus (green). (b) Corresponding cerebral blood flow (CBF) images acquired under normocapnia show equal sensitivity to perfusion on both cerebral hemispheres, good gray versus white matter contrast, and heterogeneous distribution of CBF across the brain. Grayscale bar expresses the CBF values in ml/100g/min.
2.4. Statistics
Physiologic parameters, CBF, CO2 reactivity, and CVR were compared by analysis of variance (ANOVA) and post-hoc Bonferroni’s test, or corresponding non-parametric test, using SYSTAT 12 2007 (Systat Software, Inc., Chicago, IL, USA). All values and error bars are reported as mean ± standard deviation. Statistical significance was set at P < 0.05.
3. Results
To assess the effect of hypertension and age on the cerebrovascular reactivity and resistance, graded CO2 was added to the breathing gas mixture of the rats. Initially, once the animals were in the magnet, the respiration rate and the amount of oxygen in the breathing gas mixture were adjusted for each animal to bring arterial blood gases to normal physiological conditions. Thereafter the respiration parameters were fixed and, throughout the remainder of the experiments, heart rate, rectal temperature, oxygen saturation and end-tidal CO2 were monitored continuously. Arterial blood gases were sampled periodically after the CBF measurements at each CO2 level. Physiologic measurements under basal (PaCO2 ≤ 45 mmHg) and hypercapnic (PaCO2 > 60 mmHg) conditions are listed in Table 1. Mean arterial blood pressure (MABP) was higher in SHR than in WKY (P < 0.05), and for all groups of rats the MABP values under α-chloralose were higher than under isoflurane (P < 0.05). During hypercapnia, PaCO2 increased and pH decreased compared to normocapnia (P < 0.05).
Table 1.
Physiologic parameters at basal conditions (PaCO2 ≤ 45 mmHg) and at hypercapnia (PaCO2 > 60 mmHg) under two different anesthetics for all groups of rats.
| Group | CO2 Level | HR | RR | pH | PaCO2 (mmHg) | PaO2 (mmHg) | SPO2 | MABP (mmHg) |
|---|---|---|---|---|---|---|---|---|
| α-chloralose | ||||||||
| young WKY | Normocapnia | 350 ± 1 | 58 ± 1 | 7.4 ± 0.1 | 42 ± 3 | 111 ± 7 | 88 ± 5 | 137 ± 11 |
| Hypercapnia | 350 ± 2 | 58 ± 1 | 7.2 ± 0.1§ | 83 ± 19§ | 120 ± 9 | 84 ± 11 | 117 ± 15 | |
| adult WKY | Normocapnia | 344 ± 6 | 59 ± 1 | 7.5 ± 0.1 | 38 ± 5 | 134 ± 24 | 92 ± 1 | 150 ± 6 |
| Hypercapnia | 350 ± 1 | 59 ± 1 | 7.3 ± 0.1§ | 81 ± 16§ | 130 ± 20 | 93 ± 4 | 118 ± 9§ | |
| young SHR | Normocapnia | 347 ± 4 | 59 ± 1 | 7.4 ± 0.1 | 38 ± 5 | 141 ± 14 | 95 ± 2 | 176 ± 11ŧ |
| Hypercapnia | 349 ± 2 | 58 ± 1 | 7.2 ± 0.1§ | 81 ± 17§ | 135 ± 11 | 92 ± 1 | 143 ± 12ŧ§ | |
| adult SHR | Normocapnia | 350 ± 1 | 56 ± 1 | 7.4 ± 0.1 | 35 ± 15 | 136 ± 9 | 95 ± 1 | 193 ± 17ŧ |
| Hypercapnia | 333 ± 8 | 57 ± 1 | 7.2 ± 0.1§ | 79 ± 14§ | 128 ± 12 | 93 ± 2 | 159 ± 15ŧ§ | |
| Isoflurane | ||||||||
| young WKY | Normocapnia | 271 ± 14† | 54 ± 5 | 7.4 ± 0.1 | 37 ± 5 | 152 ± 16 | 94 ± 4 | 68 ± 8† |
| Hypercapnia | 296 ± 14† | 48 ± 8† | 7.2 ± 0.1§ | 79 ± 17§ | 159 ± 12† | 90 ± 5 | 64 ± 7† | |
| adult WKY | Normocapnia | 261 ± 22† | 56 ± 1 | 7.4 ± 0.1 | 37 ± 4 | 146 ± 15 | 94 ± 4 | 93 ± 18† |
| Hypercapnia | 297 ± 19† | 57 ± 3 | 7.2 ± 0.1§ | 78 ± 16§ | 137 ± 9 | 94 ± 3 | 83 ± 8† | |
| young SHR | Normocapnia | 329 ± 23ŧ | 54 ± 13 | 7.4 ± 0.1 | 36 ± 3 | 156 ± 14 | 94 ± 1 | 116 ± 25ŧ† |
| Hypercapnia | 329 ± 28ŧ | 49 ± 13 | 7.2 ± 0.1§ | 72 ± 15§ | 134 ± 19ŧ§ | 90 ± 4 | 114 ± 12ŧ† | |
| adult SHR | Normocapnia | 321 ± 22ŧ | 53 ± 2 | 7.4 ± 0.1 | 38 ± 4 | 127 ± 11* | 93 ± 2 | 104 ± 22† |
| Hypercapnia | 321 ± 30 | 48 ± 6† | 7.2 ± 0.1§ | 73 ± 12§ | 146 ± 7 | 94 ± 1 | 107 ± 11† | |
Heart rate (HR), respiration rate (RR), pH, PaCO2, PaO2, oxygen saturation (spO2), mean arterial blood pressure (MABP). (
compared to young rats of the same strain;
compared to normotensive rats of the same age;
compared to the same rat group during normocapnia;
compared to α-chloralose; P < 0.05).
Representative coronal EPI images from a SHR under isoflurane are shown in Fig. 1a. Five adjacent 2 mm thick slices are shown from posterior to anterior. Overlaid on the EPI images are three representative ROIs, which include the cortex, caudate putamen and thalamus. Corresponding whole-brain CBF maps obtained under normocapnia are shown in Fig. 1b. To ensure maximum SNR, we acquired MRI data under fully relaxed conditions both for the control and the labeling phases of the CASL experiment. There was equal sensitivity to perfusion of both cerebral hemispheres, with good gray versus white matter contrast, and CBF distributed heterogeneously across the brain.
To evaluate the reproducibility of CBF measurements within the same animal, each rat was measured twice under normocapnia. The average percentage differences were similar between rat strains and anesthesias, with whole brain averages of 9% and 13% for rats under α-chloralose and isoflurane, respectively. Consistency of CBF measurements across animals in the same group was also similar between rat strains and anesthesias. It was 16% for rats under α-chloralose and 20% for rats under isoflurane, which are comparable with previous studies (Danker and Duong, 2007; Duong et al., 2000).
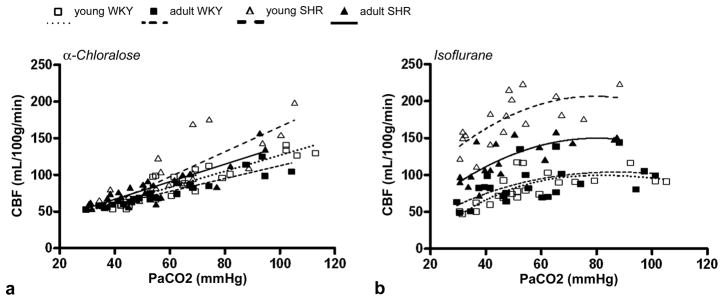
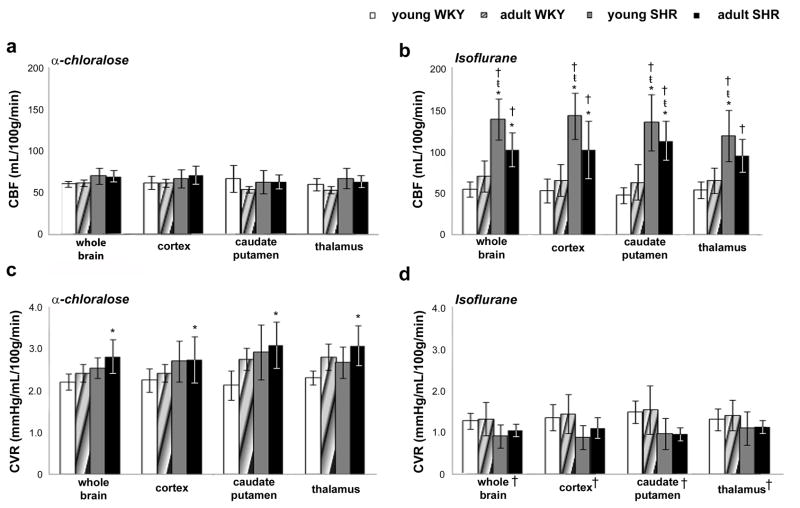
Addition of CO2 significantly increased CBF in all rat groups under either anesthetic (Figs. 2). Under α-chloralose, a linear relationship between CBF and PaCO2 levels was observed for all analyzed brain regions (Fig. 2a shows the whole brain CBF × PaCO2 curves for all groups). At normocapnia, there was no difference in CBF values between brain regions or between rat groups under α-chloralose (Fig. 3a), which indicates normal cerebral autoregulation in SHR. Average whole brain CBF values were 61 ± 3 ml/100g/min for young WKY, 62 ± 4 ml/100g/min for adult WKY; 70 ± 9 ml/100g/min for young SHR, and 69 ± 8 ml/100g/min for adult SHR. At hypercapnia, CBF values for all brain regions significantly increased when compared to normocapnia, but still did not differ between rat groups (whole brain CBF - young WKY: 108 ± 21 ml/100g/min; adult WKY: 98 ± 16 ml/100g/min; young SHR: 131 ± 39 ml/100g/min; adult SHR: 114 ± 25 ml/100g/min).
Figure 2.
Relationship between whole brain cerebral blood flow (CBF) and PaCO2 measured in normotensive (Wistar Kyoto – WKY) and spontaneously hypertensive (SHR) rats under (a) α-chloralose and (b) isoflurane. While a linear increase in CBF with PaCO2 levels is obtained under α-chloralose, there is a clear CBF saturation at PaCO2 levels greater than 70mmHg under isoflurane (polynomial fit).
Figure 3.
Mean CBF (a–b) and CVR (c–d) for normotensive (WKY) and hypertensive (SHR) rats at normocapnia, under α-chloralose and isoflurane. (* compared to young WKY; ŧ compared to adult WKY; P < 0.05).
Under isoflurane, there was a clear saturation of CBF at PaCO2 levels greater than 70 mmHg in both young and adult rats (Fig. 2b), regardless of absolute CBF values, which suggests isoflurane interferes with the vasodilatory mechanisms of CO2. The same behavior was observed for the cortex, caudate putamen and thalamus. Furthermore, under isoflurane, CBF in both young and adult SHR were significantly higher than the respective groups under α-chloralose (Fig. 2b), demonstrating that isoflurane impairs cerebral autoregulation. There was no difference in CBF in WKY rats between the two anesthetics, which suggests that the effects of isoflurane on autoregulation are specific to the hypertensive animals. Young SHR presented significantly higher CBF than WKY rats in all brain regions, both at normocapnia (Fig. 3b) (whole brain CBF - young SHR: 139 ± 25 ml/100g/min; young WKY: 55 ± 9 ml/100g/min; adult WKY: 71 ± 19 ml/100g/min, P< 0.05), as well as at hypercapnia (whole brain CBF - young SHR: 194 ± 22 ml/100g/min; young WKY: 91 ± 12 ml/100g/min; adult WKY: 94 ± 26 ml/100g/min, P < 0.05). On the other hand, adult SHR presented CBF values that were not different from young SHR at normocapnia (104 ± 23 ml/100g/min, for whole brain), but significantly lower at hypercapnia (135 ± 15 ml/100g/min, for whole brain; P < 0.05), which indicates that aging further augments the effects of hypertension in compromising the cerebrovascular CO2 reactivity in SHR.
Mean CVR values were calculated for both ages and strains and averaged across individuals. No significant differences were observed between ROIs for both α-chloralose (Fig. 3c) and isoflurane (Fig. 3d). Under α-chloralose, mean whole brain CVR value in adult SHR at normocapnia (2.8 ± 0.4 mmHg/ml/100g/min) was significantly higher than in young normotensive rats (2.2 ± 0.2 mmHg/ml/100g/min, P < 0.05), however it was not different from other groups (adult WKY: 2.4 ± 0.2 mmHg/ml/100g/min; young SHR: 2.5 ± 0.2 mmHg/ml/100g/min). At hypercapnia, MABP decreased while CBF increased, significantly decreasing CVR when compared to normocapnia (whole brain CVR - young WKY: 1.1 ± 0.3 mmHg/ml/100g/min; adult WKY: 1.2 ± 0.3 mmHg/ml/100g/min; young SHR: 1.2 ± 0.4 mmHg/ml/100g/min; adult SHR: 1.5 ± 0.4 mmHg/ml/100g/min, P < 0.05). Under isoflurane (Fig. 3d), CVR values were significantly lower when compared to the values in rats under α-chloralose (P < 0.05). Moreover, CVR values for SHR and WKY rats of both ages were not different either at normocapnia (Fig. 3d) or at hypercapnia, showing that 2% isoflurane completely masks the effects of hypertension in elevating CVR.
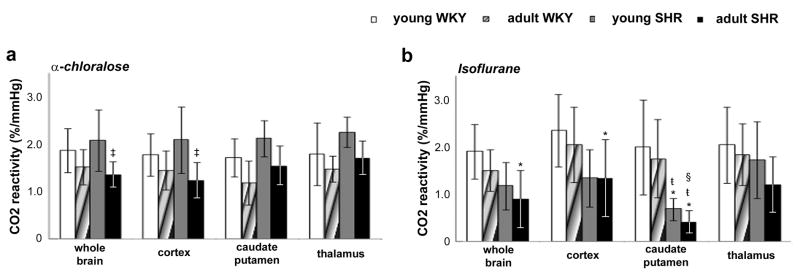
Mean CO2 reactivity values were calculated for both ages and strains using Eq. [2], and averaged across individuals (Fig. 4). Under α-chloralose, no differences were observed between brain regions. However, under isoflurane, CO2 reactivity values in caudate putamen were significantly lower than in cortex (P < 0.05) for adult SHR. Moreover, under both anesthetics, CO2 reactivity in adult SHR were significantly decreased (P < 0.05), suggesting an age-dependent impairment of cerebrovascular reactivity in SHR.
Figure 4.
Mean CO2 reactivity for normotensive (WKY) and hypertensive (SHR) rats, under (a) α-chloralose and (b) isoflurane. (* compared to young WKY; ŧ compared to adult WKY; † compared to young SHR; § compared to cortex region of the same rat group; P < 0.05).
4. Discussion
In the present work, we have successfully used ASL to investigate the combined effects of hypertension and aging on the functional status of the cerebral vasculature in a well-established rat model of chronic hypertension. CBF, CVR and CO2 reactivity were quantified in SHR and their normotensive controls, the WKY rats, under two commonly used anesthetics, α-chloralose and isoflurane. We demonstrated that hypertension, when combined with age, significantly alters cerebrovascular resistance and, impairs cerebrovascular reactivity. Furthermore, our data also shows that, in contrast to α-chloralose, 2% isoflurane causes impairment of cerebral autoregulation, masks the effects of hypertension on CVR and interferes with the vasodilatory mechanisms of CO2.
4.1. Differential Physiological Effects of α-chloralose versus Isoflurane
Anesthesia is a necessary tool in experimental preparations to ensure compliance and to relieve pain and distress. However, anesthetic agents have a profound effect on both systemic physiology as well as on the physiological state of the brain, introducing potential confounds and making interpretability of the data difficult. While isoflurane is a commonly used anesthetic in experimental research, it decreases systemic blood pressure by significantly reducing peripheral vascular resistance (Eger, 1984). Consistently with the potent systemic effects of isoflurane, we observed lower blood pressure in both strains and age groups, when compared to α-chloralose, a hypnotic anesthetic that preserves systemic blood pressure (Table 1). Moreover, isoflurane eliminated the blood pressure difference between SHR and WKY in the adult age group. On the other hand, under α-chloralose, SHR rats of either age group presented significantly higher blood pressure than their respective WKY controls, demonstrating that, at 3 months of age, the hypertensive state in SHR is already fully established.
4.2. Differential Cerebrovascular Effects of α-chloralose versus Isoflurane
The divergent influence of the two anesthetics on systemic physiology translated into distinct cerebrovascular effects. Under α-chloralose, there was no difference in basal CBF values between normotensive and hypertensive rats (Fig. 3a), which indicates this anesthetic does not impair autoregulation in SHR. This finding is in agreement with previous studies that used chloralose-urethane (Alson et al., 1985) or barbiturates (Tamaki et al., 1995). In addition, under α-chloralose, CBF increased linearly with CO2 (Fig. 2a), and CO2 reactivity values are in good agreement with previous LDF studies (Bakalova et al., 2001). On the other hand, under isoflurane, CBF values in young SHR were significantly higher than in WKY rats (Fig. 3b). Moreover, CBF values in SHR of either age group under isoflurane were significantly higher than the respective groups under α-chloralose (Fig. 3b). Isoflurane significantly reduced CVR in all rats and masked the effects of hypertension in elevating CVR (Fig. 3d). Taken together, these findings indicate impairment in autoregulatory mechanisms caused by isoflurane, in agreement with previous reports in rats (Hoffman et al., 1991), dogs (McPherson et al., 1989; McPherson and Traystman, 1988), monkeys (Van Aken et al., 1986) and humans (Strebel et al., 1995), and urge the use of caution when interpreting results obtained under this anesthetic.
The basal CBF values found here under isoflurane are consistent with those measured in previous studies (Danker and Duong, 2007; Heinert et al., 1998; Kusuda et al., 1996). The fact that saturation is observed in all strains and age groups regardless of the absolute value of CBF, as shown in Fig. 2b, suggests that isoflurane at 1.5 MAC interferes with the vasodilatory mechanisms of CO2. In support of our results, Sicard et al observed a much attenuated CBF response to 5% and 10% CO2 challenges in rats anesthetized with 1.5 MAC isoflurane compared to awake rats (Sicard et al., 2003), and concluded that the cerebrovascular reactivity to CO2 was markedly suppressed by isoflurane. As well, McPherson et al observed no CBF response to hypercapnia and markedly decreased CVR and CO2 reactivity in dogs anesthetized under isoflurane at 2 MAC (McPherson et al., 1989). In our study, CBF saturation was observed for PaCO2 levels greater than 70 mmHg, suggesting that isoflurane interferes most with nitric oxide independent vasodilatory mechanisms, which have been shown to affect the CBF response to hypercapnia at high PaCO2 levels (Iadecola and Zhang, 1994). However, further studies are necessary to address this issue.
4.3. Effects of Hypertension and Aging on Cerebrovascular Resistance and Reactivity
When left untreated, the chronic presence of hypertension leads to vascular remodeling in the form of hypertrophy, eutrophy, and reduced vascular compliance (Tuttle et al., 2002). In agreement with previous work (Kusuda et al., 1996), adult SHRs presented increased cerebrovascular resistance under elevated arterial blood pressure (Fig. 3c). There has been a suggestion that the increased CVR may be a mechanism to keep CBF within normal values and to protect the brain against the development of hemorrhagic stroke (Gonzalez et al., 2008). Indeed, when CVR was reduced due to the use of isoflurane anesthesia, CBF values increased substantially in SHRs (Fig. 3b).
It has been shown that normal aging leads to decreased vascular response to vasodilator stimuli (Riecker et al., 2003). Compared to their young counterparts, adult normotensive animals showed a trend towards elevated CVR and decreased CO2 reactivity, but these were not significant. However, adult SHR presented significantly decreased CO2 reactivity at either anesthetic agent. There is significant evidence that aging compounds the impairment in vascular relaxations induced by hypertension (Tamaki et al., 1995). As well, hypertension accelerates the vascular stiffening that naturally occurs due normal aging (Feihl et al., 2009). The data obtained in adult SHR can be fully explained by a combined effect of aging and hypertension in limiting the compliance of the cerebral vessels.
Regional cerebral blood flow of hypertensive rats under isoflurane was significantly higher than those from normotensive controls in all regions analyzed (Danker and Duong, 2007); however, no difference was observed under α-chloralose. Contrary to a previous study that showed greater CBF response to hypercapnia in the cortex when compared to subcortical structures of normotensive rats (Lu et al., 2009), our data did not show regional CO2 reactivity differences in WKY under both anesthesias. However, in hypertensive rats under isoflurane, CO2 reactivity was lower in the caudate putamen than in the cortex. These findings suggest that cerebral blood flow response to hypercapnia is dependent to region, rat strain and anesthesia.
4.4. Conclusions
In conclusion, we successfully used ASL to assess and characterize the compliance of the cerebral vasculature of an important animal model used in modeling stroke and other cerebrovascular diseases, the spontaneously hypertensive rat, under different anesthetics. Contrary to α-chloralose, 2% isoflurane (1.% MAC) had profound effects on the animals’ systemic blood pressure and caused impairment of the mechanisms of CBF regulation, which may introduce potential confounds on studies of cerebrovascular diseases. Chronic hypertension, when compounded by age, resulted in increased cerebrovascular resistance and reduced cerebrovascular reactivity to CO2.
Research highlights.
Effects of hypertension and aging on functional status of cerebral vasculature.
Differential effects of α-chloralose and isoflurane on brain physiology.
Increased cerebrovascular resistance in hypertension.
Decreased CO2 reactivity in aged hypertensive rats.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NINDS (Alan P. Koretsky, Scientific Director), and FAPESP (2006/05706-5, 2003/13399-7, 2005/56663-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alson RL, Dusseau JW, Hutchins PM. Arteriolar and systemic autoregulatory responses during the development of hypertension in the spontaneously hypertensive rat. Proc Soc Exp Biol Med. 1985;180:62–71. doi: 10.3181/00379727-180-42144. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Amenta F, Di Tullio MA, Tomassoni D. Arterial hypertension and brain damage--evidence from animal models (review) Clin Exp Hypertens. 2003;25:359–380. doi: 10.1081/ceh-120023545. [DOI] [PubMed] [Google Scholar]
- Bakalova R, Matsuura T, Kanno I. Frequency dependence of local cerebral blood flow induced by somatosensory hind paw stimulation in rat under normo- and hypercapnia. Jpn J Physiol. 2001;51:201–208. doi: 10.2170/jjphysiol.51.201. [DOI] [PubMed] [Google Scholar]
- Barone FC, Price WJ, White RF, Willette RN, Feuerstein GZ. Genetic hypertension and increased susceptibility to cerebral ischemia. Neurosci Biobehav Rev. 1992;16:219–233. doi: 10.1016/s0149-7634(05)80182-4. [DOI] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–354. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Duong TQ. Quantitative regional cerebral blood flow MRI of animal model of attention-deficit/hyperactivity disorder. Brain Res. 2007;1150:217–224. doi: 10.1016/j.brainres.2007.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn Reson Med. 2000;43:383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Dupuis F, Atkinson J, Liminana P, Chillon JM. Comparative effects of the angiotensin II receptor blocker, telmisartan, and the angiotensin-converting enzyme inhibitor, ramipril, on cerebrovascular structure in spontaneously hypertensive rats. J Hypertens. 2005;23:1061–1066. doi: 10.1097/01.hjh.0000166848.95592.a5. [DOI] [PubMed] [Google Scholar]
- Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8:449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- Eger EI., 2nd The pharmacology of isoflurane. Br J Anaesth. 1984;56(Suppl 1):71S–99S. [PubMed] [Google Scholar]
- Feihl F, Liaudet L, Waeber B. The macrocirculation and microcirculation of hypertension. Curr Hypertens Rep. 2009;11:182–189. doi: 10.1007/s11906-009-0033-6. [DOI] [PubMed] [Google Scholar]
- Fujishima M, Sadoshima S, Ogata J, Yoshida F, Shiokawa O, Ibayashi S, Omae T. Autoregulation of cerebral blood flow in young and aged spontaneously hypertensive rats (SHR) Gerontology. 1984;30:30–36. doi: 10.1159/000212604. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Somoza B, Conde MV, Fernandez-Alfonso MS, Gonzalez MC, Arribas SM. Hypertension increases middle cerebral artery resting tone in spontaneously hypertensive rats: role of tonic vasoactive factor availability. Clin Sci (Lond) 2008;114:651–659. doi: 10.1042/CS20070361. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Mattsson B, Nordborg C, Johansson BB. Brain capillary density and cerebral blood flow after occlusion of the middle cerebral artery in normotensive Wistar-Kyoto rats and spontaneously hypertensive rats. J Hypertens. 1993;11:1363–1368. doi: 10.1097/00004872-199312000-00007. [DOI] [PubMed] [Google Scholar]
- Harper SL, Bohlen HG. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension. 1984;6:408–419. doi: 10.1161/01.hyp.6.3.408. [DOI] [PubMed] [Google Scholar]
- Heinert G, Casadei B, Paterson DJ. Hypercapnic cerebral blood flow in spontaneously hypertensive rats. J Hypertens. 1998;16:1491–1498. doi: 10.1097/00004872-199816100-00014. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Edelman G, Kochs E, Werner C, Segil L, Albrecht RF. Cerebral autoregulation in awake versus isoflurane-anesthetized rats. Anesth Analg. 1991;73:753–757. doi: 10.1213/00000539-199112000-00013. [DOI] [PubMed] [Google Scholar]
- Hom S, Fleegal MA, Egleton RD, Campos CR, Hawkins BT, Davis TP. Comparative changes in the blood-brain barrier and cerebral infarction of SHR and WKY rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1881–1892. doi: 10.1152/ajpregu.00761.2005. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F. Nitric oxide-dependent and -independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol. 1994;266:R546–552. doi: 10.1152/ajpregu.1994.266.2.R546. [DOI] [PubMed] [Google Scholar]
- Ito T, Yamakawa H, Bregonzio C, Terron JA, Falcon-Neri A, Saavedra JM. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002;33:2297–2303. doi: 10.1161/01.str.0000027274.03779.f3. [DOI] [PubMed] [Google Scholar]
- Katsuta T. Decreased local cerebral blood flow in young and aged spontaneously hypertensive rats. Fukuoka Igaku Zasshi. 1997;88:65–74. [PubMed] [Google Scholar]
- Kusuda K, Ibayashi S, Sadoshima S, Ishitsuka T, Fujishima M. Brain ischemia following bilateral carotid occlusion during development of hypertension in young spontaneously hypertensive rats--importance of morphologic changes of the arteries of the circle of Willis. Angiology. 1996;47:455–465. doi: 10.1177/000331979604700504. [DOI] [PubMed] [Google Scholar]
- Lu J, Dai G, Egi Y, Huang S, Kwon SJ, Lo EH, Kim YR. Characterization of cerebrovascular responses to hyperoxia and hypercapnia using MRI in rat. Neuroimage. 2009;45:1126–1134. doi: 10.1016/j.neuroimage.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11:684–689. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- McPherson RW, Briar JE, Traystman RJ. Cerebrovascular responsiveness to carbon dioxide in dogs with 1.4% and 2.8% isoflurane. Anesthesiology. 1989;70:843–850. doi: 10.1097/00000542-198905000-00022. [DOI] [PubMed] [Google Scholar]
- McPherson RW, Traystman RJ. Effects of isoflurane on cerebral autoregulation in dogs. Anesthesiology. 1988;69:493–499. doi: 10.1097/00000542-198810000-00008. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Kondoh T, Morishita A, Yamashita H, Kohmura E, Sakurai T, Yokono K, Umetani K. Loss of CO2-induced distensibility in cerebral arteries with chronic hypertension or vasospasm after subarachnoid hemorrhage. Kobe J Med Sci. 2007;53:317–326. [PubMed] [Google Scholar]
- Pedelty L, Gorelick PB. Management of hypertension and cerebrovascular disease in the elderly. Am J Med. 2008;121:S23–31. doi: 10.1016/j.amjmed.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Riecker A, Grodd W, Klose U, Schulz JB, Groschel K, Erb M, Ackermann H, Kastrup A. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab. 2003;23:565–573. doi: 10.1097/01.WCB.0000056063.25434.04. [DOI] [PubMed] [Google Scholar]
- Scheller MS, Todd MM, Drummond JC. Isoflurane, halothane, and regional cerebral blood flow at various levels of PaCO2 in rabbits. Anesthesiology. 1986;64:598–604. doi: 10.1097/00000542-198605000-00009. [DOI] [PubMed] [Google Scholar]
- Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23:472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Zhang W, Williams DS, Koretsky AP. Multi-slice MRI of rat brain perfusion during amphetamine stimulation using arterial spin labeling. Magn Reson Med. 1995;33:209–214. doi: 10.1002/mrm.1910330210. [DOI] [PubMed] [Google Scholar]
- Strebel S, Lam AM, Matta B, Mayberg TS, Aaslid R, Newell DW. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995;83:66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- Tamaki K, Nakai M, Yokota T, Ogata J. Effects of aging and chronic hypertension on cerebral blood flow and cerebrovascular CO2 reactivity in the rat. Gerontology. 1995;41:11–17. doi: 10.1159/000213657. [DOI] [PubMed] [Google Scholar]
- Tuttle JL, Sanders BM, Burkhart HM, Fath SW, Kerr KA, Watson WC, Herring BP, Dalsing MC, Unthank JL. Impaired collateral artery development in spontaneously hypertensive rats. Microcirculation. 2002;9:343–351. doi: 10.1038/sj.mn.7800151. [DOI] [PubMed] [Google Scholar]
- Van Aken H, Fitch W, Graham DI, Brussel T, Themann H. Cardiovascular and cerebrovascular effects of isoflurane-induced hypotension in the baboon. Anesth Analg. 1986;65:565–574. [PubMed] [Google Scholar]
- van Laar PJ, van der Graaf Y, Mali WP, van der Grond J, Hendrikse J. Effect of cerebrovascular risk factors on regional cerebral blood flow. Radiology. 2008;246:198–204. doi: 10.1148/radiol.2453061932. [DOI] [PubMed] [Google Scholar]
- Veglio F, Paglieri C, Rabbia F, Bisbocci D, Bergui M, Cerrato P. Hypertension and cerebrovascular damage. Atherosclerosis. 2009;205:331–341. doi: 10.1016/j.atherosclerosis.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Wei L, Lin SZ, Tajima A, Nakata H, Acuff V, Patlak C, Pettigrew K, Fenstermacher J. Cerebral glucose utilization and blood flow in adult spontaneously hypertensive rats. Hypertension. 1992;20:501–510. doi: 10.1161/01.hyp.20.4.501. [DOI] [PubMed] [Google Scholar]
- Zhang W, Silva AC, Williams DS, Koretsky AP. NMR measurement of perfusion using arterial spin labeling without saturation of macromolecular spins. Magn Reson Med. 1995;33:370–376. doi: 10.1002/mrm.1910330310. [DOI] [PubMed] [Google Scholar]