Abstract
Objective
To test the hypothesis that gamma interferon (IFN-γ) promotes MHC class II expression on bone marrow (BM) cell targets that facilitates T cell-mediated BM destruction in immune-mediated BM failure.
Materials and Methods
Allogeneic lymph node (LN) cells were infused into MHC or minor histocompatibility antigen (minor-H) mismatched hosts to induce BM failure. MHC class II and Fas expression and cell apoptosis were analyzed by flow cytometry. MHC class II-Fas co-localization was detected by ImageStream Imaging Flow Cytometry and other cell-cell associations were visualized by confocal microscopy. T cell-mediated BM cell apoptosis and effects of IFN-γ on MHC class II-Fas co-localization on normal BM cells were studied using cell culture in vitro followed by conventional and imaging flow cytometry.
Results
BM failure animals had significantly up-regulated MHC class II expression on CD4−CD8−CD11b−CD45R− residual BM cells and significantly increased MHC class II-Fas co-localization on BM CD150+ and CD34+ hematopoietic cells. MHC class II+Fas+ BM cells were closely associated with CD4+ T cells in the BM of affected animals, and they were significantly more responsive to T-cell mediated cell apoptosis relative to MHC class II−Fas− BM cells. Infusion of IFN-γ-deficient LN cells into minor-H mismatched recipients resulted in no MHC class II-Fas up-regulation and no clinically overt BM failure. Treatment with recombinant IFN-γ significantly increased both MHC class II-Fas co-expression and co-localization on normal BM cells.
Conclusion
Elevation of the inflammatory cytokine IFN-γ stimulated MHC class II expression and MHC class II-Fas co-localization, which may facilitate T-cell mediated cell destruction.
In the human disease aplastic anemia (AA), the paradigm of bone marrow (BM) failure syndromes, expansion of self-reactive T cells producing cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) that causes the destruction of hematopoeitic stem and progenitor cells and the development of marrow hypocellularity and pancytopenia [1–4]. This disease process was reproduced in murine models by infusing allogeneic lymph node (LN) cells into major histocompatibility complex (MHC) or minor-histocompatibility (minor-H) antigen mismatched recipients [5–8]. In these models, host animals have a similar disease course as do human patients showing expansion of T cells, secretion of inflammatory cytokines, and rapid destruction of BM hematopoietic stem and progenitor cells [7,8]. The involvement of Th1 immune response in the disease is evident since deficiency of the Th1 regulatory gene T-bet from donor lymphocytes abrogates hematopoietic cell destruction [9]. Fas-mediated cell apoptosis is the major pathway leading to active BM cell destruction [10].
A role of CD4 T cells in the development of AA was suggested when a specific CD4 T cell clone, NT4.2, isolated from an AA patient, had extensive cytotoxic activity against autologous and allogeneic target cells [11,12]. More recent study found that some AA patients have an increased CD4/CD8 T cell ratio in the course of the disease [13]. Studies of T cell clonality and function through T cell receptor beta variable region classification [14], spectrotyping [15], gene expression array analyses [16], and cell apoptosis assays [17,18], also implicated CD4 T cells in human marrow failure. In murine models, depletion of CD4 T cells abrogated the ability of allogeneic lymphocytes to induce marrow damage, supporting the idea that CD4 T cells also play some role in immune-mediated BM failure [19]. Many previous studies also have indicated that CD4 T cells kill target cells in an MHC class II-dependent manner [20–22].
Under normal circumstances, MHC class II expression is restricted to antigen presenting cells. However, inflammation and autoimmunity may stimulate MHC class II expression on other cell types, as observed in animal models of arthritis, hepatitis, and diabetes [23–28]. Since IFN-γ stimulates the expression of MHC class II [24,25] and Fas [29], we reasoned that IFN-γ could also augment MHC class II and Fas expression on BM cell targets, contributing to BM failure. In the current study, we infused allogeneic LN cells to MHC and minor-H mismatched hosts to reproduce BM failure, examined MHC class II and Fas expression on BM cells that are CD4−CD8−CD11b−CD45R− (residual BM cells), detected MHC class II and Fas co-localization on BM hematopoietic cells, visualized the association between MHC class II+Fas+ BM cells and CD4+ T cells, and analyzed MHC class II+Fas+ BM cell apoptosis. We also tested the ability of IFN-γ-deficient LN cells to induce BM failure and analyzed the effectiveness of IFN-γ on MHC class II and Fas co-expression and co-localization on normal BM cells. Our results support an active role of MHC class II in the development of murine BM failure through co-localization with the apoptotic receptor Fas and the interaction with effector T cells. Our data also suggest that IFN-γ-stimulated MHC class II and Fas co-localization could be a general mechanism for cell destruction during inflammatory responses.
Materials and Methods
Induction of BM failure
Inguinal, axillary, and lateral axillary LN cells were isolated from C57BL/6 (B6) or B6.129S7-Ifngtm1Ts/J (IFN-γ−/−) donors and infused into C.B10-H2b/LilMcd (C.B10) or CByB6F1 recipients at 5 × 106 LN cells/recipient through lateral tail vein injection. All recipients received 5 Gy total body irradiation (TBI) from a Shepherd Mark 1 137cesium gamma source (J. L. Shepherd & Associates, Glendale CA) four to six hours before LN cell infusion. Mice that had received 5 Gy TBI without LN cells were used as controls. All mice were originally obtained from the Jackson Laboratory (Bar Harbor, ME) and were bred at the National Institutes of Health’s animal facility with standard care and nutrition. All mouse study protocols were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee.
Cell preparation and flow cytometry
Recipient mice and TBI-only control animals were bled from the retro-orbital sinus and were euthanized by CO2 inhalation two to three weeks post LN cell infusion. Complete blood counts were measured by a Hemavet 950 analyzer (Drew Scientific, Oxford, CT). BM cells were extracted from bilateral tibiae and femurs of each animal into IMDM, counted using a ViCell counter, and processed for various analyses.
Procedures for flow cytometry analyses were as detailed earlier [7,8]. Antibodies for mouse CD4 (clones RM4-5 and H129.19), CD8 (clone 53-6.7), CD11b (clone M1/70), CD40 (clone 3/23), CD45R (B220, clone RA3-6B2), CD80 (clone 16-10A1), and CD95 (Fas, clone Jo2) were from BD Biosciences (San Diego, CA), while antibodies for mouse CD34 (clone MEC14.7) and CD150 (clone TC15-12F12.2) were from Biolegend (San Diego, CA). Two types of monoclonal antibodies to mouse I-A/I-E (MHC class II), clone 2G9 (PE-conjugated) and clone M5/114.15.2 (PE-Cy5 conjugated), were obtained from BD Biosciences and eBiosciences respectively. Stained cells were acquired using a BD LSR II flow cytometer at twenty thousand cells per sample, and data were analyzed with the FACSDiva software (Beckton Dickinson, San Jose, CA). Staining of apoptotic cells was carried out using the Annexin V and 7AAD staining kit (BD Biosciences) along with antibodies for CD95, MHC class II, CD4, CD8, CD11b and CD45R.
Amnis ImageStream imaging flow cytometry
BM cells from TBI only control and LN cell-infused C.B10 mice were stained with CD95 (Fas)-FITC + MHC class II-PE + CD150 (CD34)-PE-Cy5 antibody cocktail using 3 × 107 cells/mL. MHC class II and Fas antigens on residual BM cells were localized using the Amnis ImageStream Imaging flow cytometer (Amnis Corporation, Seattle, WA), as described by others [30]. Cells were stained with Hoechst 33342 (Invitrogen, Carlsband, CA) at a 3.2 μM final concentration for 30 minutes at 37°C. Co-localization of Fas and MHC class II was assessed on CD150+ and CD34+ cells in which data were acquired in INSPIRE and were analyzed using the IDEAS analysis software (Amnis, Seattle, WA). The degree of co-localization was calculated using the similarity feature, a pixel by pixel comparison of Fas and MHC class II, which is based on the log transformed Pearson’s coefficient. Cells with a similarity score of 2.5 or higher were classified as co-localized.
Immunofluorescence labeling for wholemounts and confocal imaging
Sternebrae from TBI-only and LN cell-infused C.B10 mice were transected sagittally to obtain 2–3 mm fragments and were fixed and stained as wholemounts with anti-mouse MHC class II (BMA Biomedicals), CD95-Biotin and CD4-APC (BD Biosciences) antibodies followed by FITC-conjugated anti-American hamster IgG (for MHC class II, Jackson ImmunoResearch) and rhodamine Red-X-conjugated streptavidin (for CD95, Jackson ImmunoResearch) at room temperature for 45 min after blocking of non-specific binding using normal IgG matching the primary antibody’s species and washing with PBS. All images were acquired by a confocal laser scanning microscope (CLSM) with the Zeiss LSM 510 system (Carl Zeiss MicroImaging). Fluorescence images of 4-colors were captured sequentially, using laser lines at 360–405 nm (for DAPI), 488 nm (for FITC), 561 nm (for Rhodamine Red-X), and 633 nm l (for APC). Series of x-y-z images were collected along the z-axis at 1–4 μm intervals to ~40–150 μm depths throughout the BM tissue, using 20× NA 0.75 objectives. Higher resolution images were also obtained using a 40 x C-Apochromat, NA=1.2 water immersion objective. The volume data were used to create 3D renderings of the BM architecture using Imaris software (Bitplane, Zurich, Switzerland).
T-cell-mediated cell apoptosis
BM cells obtained from CByB6F1 mice at 14 days following 5 Gy TBI and 6 × 106 B6 LN cell infusion were stained with an antibody cocktail containing CD11b-FITC + MHC class II-PE + CD45R-PE-Cy5 + CD95-PE-Cy7 + CD4-APC + CD8-APC-Cy7. Stained cells were sorted by a FACSAria cell sorter to collect CD4+ and CD8+ T cells as effector cells. The remaining CD4−CD8−CD11b−CD45R− residual BM cell population was further divided based on MHC class II and Fas staining to sort out MHC class II+/Fas+ and MHC class II−/Fas− cell fractions as target cells. CD4+ or CD8+ T cells were mixed 10:1 with MHC class II+/Fas+ or MHC class II−/Fas− residual BM cell targets, and then cultured at 37°C with 5% CO2 in supplemented RPMI-1640 media plus 50U/mL mIL-2 for 60 minutes. Cells were then harvested and stained with Annexin V-FITC + 7AAD + CD4-APC, or Annexin V-FITC + 7AAD + CD8-APC-Cy7, respectively, and analyzed on a LSR II flow cytometer to define cell apoptosis.
Cell culture in vitro
To test the effectiveness of IFN-γ on MHC class II and Fas co-expression, we extracted BM cells from normal B6 mice and cultured cells in vitro in supplemented RPMI-1640 media (10% fetal calve serum, 100 U/mL penicillin, 100 μg/mL streptomycin; 2 mM L-glutamine, and 50 μM β-mercaptoethanol) at 37°C with 5%CO2 in the presence of 0, 20, 40 or 60 ng/mL of IFN-γ, respectively, for 24–72 hours in 6-well culture plates. Cells were then harvested, stained, and analyzed for the expression of MHC class II and Fas on total BM cells and residual BM cells. We further tested the effectiveness of IFN-γ and other cytokines on MHC class II and Fas co-localization by culturing normal B6 BM cells under the same conditions for 48 hours with or without 50 ng/mL of IFN-γ, IL-3 or stem cell factor (SCF) respectively. Harvested cells were stained and analyzed with the Amnis ImageStream Imaging flow cytometer to study MHC class II and Fas co-expression and co-localization as described above.
Data analysis
Statistical analyses were performed using JMP Statistical Discovery Software [31]. Significance was claimed at P<0.05 and P<0.01 levels, respectively.
Results
Up-regulated MHC class II expression and co-expression of Fas in experimental BM failure
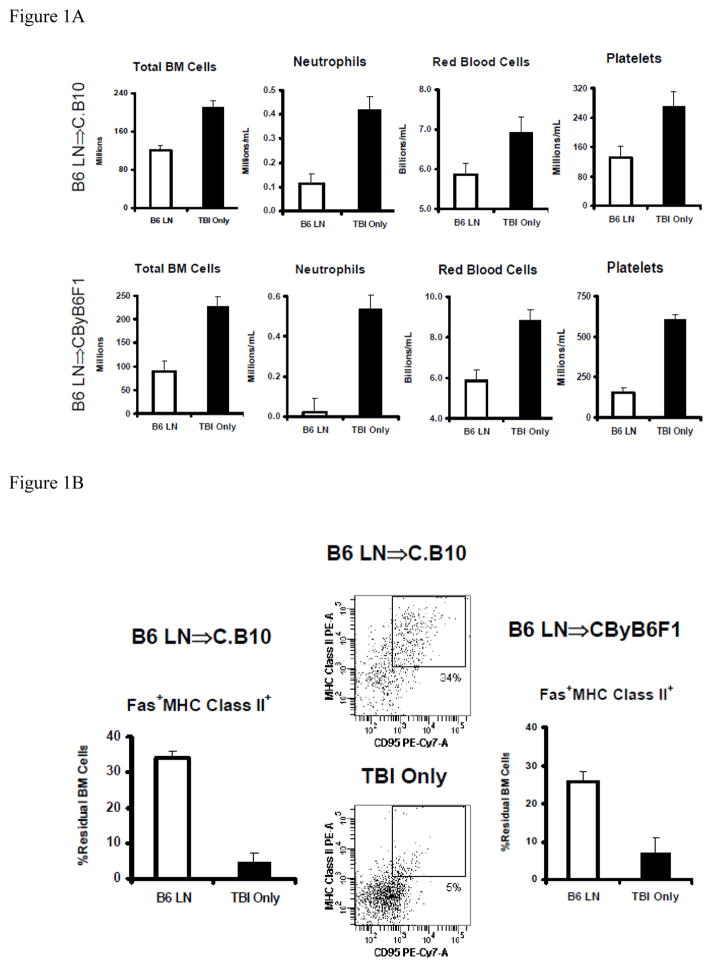
Following the infusion of 5 × 106 B6 LN cells into minor-H mismatched C.B10 recipients pre-treated with 5 Gy TBI, we observed BM hypoplasia and pancytopenia within two to three weeks: LN-cell-infused animals had significant declines in total BM cells (P<0.01), blood neutrophils (P<0.01), red blood cells (P<0.05) and platelets (P<0.01) in comparison to animals that received TBI-only without LN-cell infusion (Fig. 1A). We also observed severe pancytopenia and BM hypoplasia when B6 LN cells were infused into MHC-mismatched pre-irradiated CByB6F1 recipients (Fig. 1A).
Figure 1.
Up-regulation in MHC class II and Fas co-expression on CD4−CD8−CD11b−CD45R− residual BM cells in experimental models of BM failure. Congenic C.B10 mice or hybrid CByB6F1 mice were pre-irradiated with 5 Gy TBI followed by the infusion of 5 × 106 B6 LN cells (B6 LN, n=29 for C.B10 and N=8 for CByB6F1). Mice that received 5 Gy TBI without LN cell infusion (TBI-only, N=16 for C.B10 and N=9 for CByB6F1) were used as controls. A, B6 LN cell infused animals developed sever marrow hypoplasia and pancytopenia showing significant declines in Total BM cells (P<0.01), neutrophils (P<0.01), red blood cells (P<0.05) and platelts (P<0.01) in both C.B10 and CByB6F1 hosts. B, BM cells from animals that received LN cell infusion had significantly (P<0.01) higher proportion of residual BM with MHC class II and Fas co-expression than BM cells from TBI-only controls in both C.B10 and CByB6F1 recipients. Residual BM (residual BM) cells were defined as CD4−CD8−CD11b−CD45R− BM cells.
We then specifically analyzed MHC class II expression and its co-expression with Fas on residual BM cells. In the minor-H mismatch B6⇒C.B10 LN cell infusion model, we observed a significantly increased proportion of MHC class II+ cells (49% ± 5%, P<0.05) from LN-cell-infused animals relative to TBI-only controls (21% ± 4%). As a result, there was a significantly higher proportion of MHC class II+Fas+ cells (34% ± 2%, P<0.01) in residual BM cells in comparison to TBI-only control (7% ± 2%) animals (Fig. 1B). Similar changes were also observed in the MHC mismatch B6⇒CByB6F1 LN cell infusion model, in which proportions of MHC class II+ cells and MHC class II+Fas+ cells in residual BM cells were 55% ± 8% and 26% ± 3% in LN cell-infused animals, both significantly higher (P<0.01) than in TBI-only controls, at 21% ± 9% and 7% ± 4% respectively (Fig. 1B).
Infusion of allogeneic LN cells was associated with significant increases in the proportions of CD4 and CD8 T cells in the BM of both C.B10 and CByB6F1 recipients (Table 1), consistent with our earlier observations [5–8]. The significant decline in total BM cells (Fig. 1A) resulted in marked reduction in total residual BM cells in both B6⇒C.B10 and B6⇒CByB6F1 LN cell infusion models (Table 1). However, when the decline in total BM cells was compounded with the significant increase in the proportion of MHC class II+Fas+ cells (Fig. 1B), we observed mixed results as total MHC class II+Fas+ residual BM cell number was mildly increased in LN-cell infused C.B10 mice but significantly reduced in LN-cell infused CByB6F1 mice, when compared to their TBI-only controls respectively (Table 1).
Table 1.
BM cellular changes during immune-mediated BM failure
| Measurements | N | TBI + B6 LN | N | TBI-onl;y | P< |
|---|---|---|---|---|---|
| CD4 (%) | |||||
| C.B10 | 5 | 17.7 ± 4.1 | 4 | 0.9 ± 0.3 | 0.0083 |
| CByB6F1 | 8 | 13.4 ± 3.5 | 6 | 0.8 ± 0.1 | 0.0094 |
| CD8 (%) | |||||
| C.B10 | 5 | 25.5 ± 6.4 | 4 | 0.8 ± 0.1 | 0.0114 |
| CByB6F1 | 8 | 42.1 ± 8.5 | 6 | 1.0 ± 0.2 | 0.0013 |
| Residual BM cells (106) | |||||
| C.B10 | 21 | 19.0 ± 3.7 | 13 | 36.4 ± 7.4 | 0.0253 |
| CByB6F1 | 6 | 4.6 ± 0.8 | 3 | 86.1 ± 9.3 | 0.0001 |
| MHC class II+Fas+ residual BM cells (106) | |||||
| C.B10 | 21 | 4.0 ± 1.1 | 13 | 1.6 ± 0.5 | 0.1024 |
| CByB6F1 | 6 | 1.2 ± 0.3 | 3 | 5.9 ± 0.9 | 0.0004 |
TBI + B6 LN: mice received 5 Gy TBI plus infusion of 5 × 106 B6 LN cells; TBI-only: mice received 5 Gy TBI only without LN cell infusion. Data were collected at 2–3 weeks after LN cell infusion and presented as Mean ± standard error.
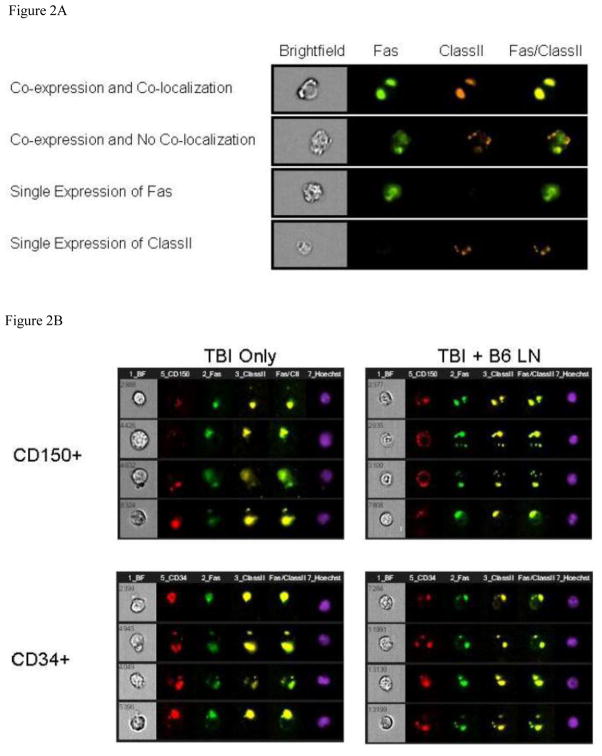
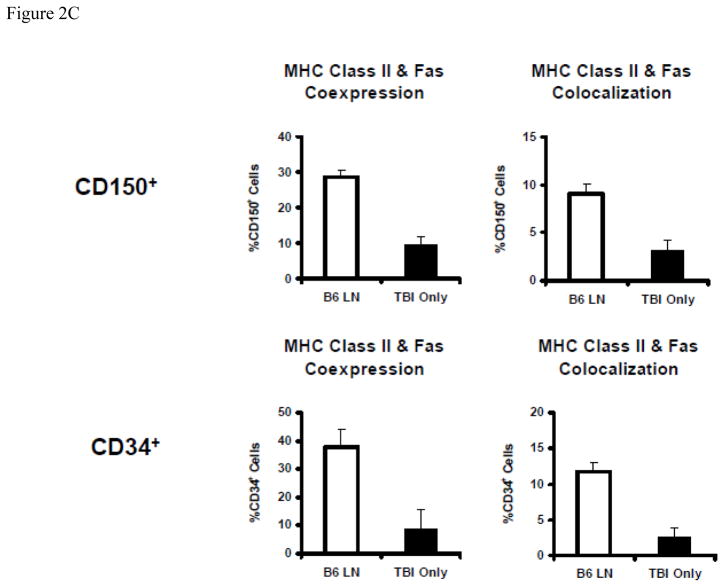
MHC class II and Fas co-localization on BM hematopoietic cells
Because MHC class II and Fas expression were up-regulated on residual BM cells in mice with immune-mediated BM failure, we next visualized MHC class II and Fas antigen expression patterns on BM CD150+ and CD34+ cells using the Amnis ImageStream imaging flow cytometer, as described earlier [30]. The combination of flow cytometry and fluorescence microscopy enabled the detection of physical association between two or more molecules, by analyzing the similarity of their expression patterns to define their relationship as being co-localized, co-expressed but not co-localized, or independently expressed (Figure 2A). Cells were gates by eliminating saturating events to identify single cells that are nucleated and in focus. Under this gating conditions, we found only insignificant declines in CD34+ (23.0 ± 8.2 × 106 vs 32.5 ± 5.9 × 106, P<0.4074) and CD150+ (16.2 ± 5.8 × 106 vs 29.9 ± 12.4 × 106, P<0.3133) hematopoietic cells in TBI + B6 LN treated animals than in TBI-only controls. MHC class II and Fas had seemingly identical expression patterns on a large proportion of CD150+ and CD34+ hematopoietic cells (Fig. 2B). Statistical analyses on 1–5 × 104 cells per sample of 5–7 animal samples per treatment group found significantly higher (all at P<0.01) proportions of CD150+ and CD34+ cells with MHC class II & Fas co-expression and co-localization in LN-cell-infused animals than in TBI only controls (Fig. 2C).
Figure 2.
Co-localization of MHC class II and Fas on BM hematopoietic cells. BM cells from TBI + B6 LN treated and TBI-only C.B10 mice were stained with antibody cocktails containing CD95-FITC + MHC class II-PE + CD150 (or CD34)-PE-Cy5, and were analyzed using an Amnis ImageStream Imaging Flow Cytometer. A, Gated CD150+ and CD34+ BM cells were evaluated for the expression of Fas (CD95) and MHC class II to characterize their co-localization when the similarity score is above 2.5, or co-expression without co-localization when the expression similarity score is below 2.5, or single expression. B, Co-localization of Fas and MHC class II on BM CD150+ (n=5 for TBI-only and n=5 for TBI + B6 LN) and CD34+ (n=6 for TBI-only and n=7 for TBI + B6 LN) cells were shown as representatives. C, Percentages of CD150+ and CD34+ cells with Fas-MHC class II co-expression (P<0.01) and Fas-MHC class II co-localization (P<0.01) were significantly higher in TBI + B6 LN treated animals than in TBI-only controls. CD150+ or CD34+ cells displayed similar elevated levels of Fas and MHC class II co-expression and co-localization in B6-LN-cell-infused animals and similar low levels in TBI-only control animals.
Association of MHC class II+Fas+ BM cells with CD4+ T cells
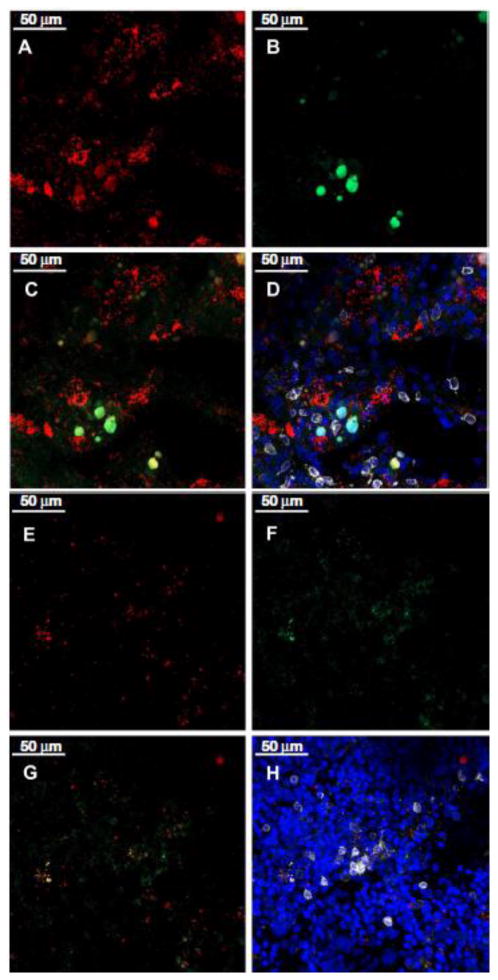
The increased MHC class II and Fas co-localization on residual BM cells, especially on CD150+ and CD34+ hematopoietic stem and progenitor cells, in BM failure mice led us to hypothesize that the association of these two molecules may facilitate BM cell destruction by immune effectors, especially CD4+ effector T cells, since previous studies indicated that effector CD4 T cells can be cytotoxic in an MHC class II dependent manner [20–22]. We stained wholemount sternebrae from LN cell-infused (Fig. 3A, B, C, D) and TBI-only (Fig. 3E, F, G, H) animals with antibodies specific for MHC class II (red), Fas (green), and CD4 (white), and acquired images by confocal microscopy. In LN-cell infused animals, the BM contained MHC class II+ cells (Fig. 3A) and Fas+ cells (Fig. 3B), with clusters of cells staining double positive for both MHC class II and Fas (Fig. 3C). In the same view, we also found some CD4+ T cells closely associated with the cluster of MHC class II+Fas+ BM cells (Fig. 3D). Under the same conditions, there was only very few MHC class II+ (Fig. 3E), Fas+ (Fig. 3F) or MHC class II and Fas double positive (Fig. 3G) cells in BM of control animals that had received TBI without LN-cell infusion. Only rare CD4+ T cells (Fig. 3H) were present in the BM of these control animals. The close association between MHC class II+Fas+ BM cells and CD4+ T cells in BM failure mice but not in control animals suggested that MHC class II+ BM cells could be targets for CD4 T cells.
Figure 3.
MHC class II and Fas co-localization on BM cells and their relationship with CD4 T cells during BM failure. Sternebrae from C.B10 mice that received TBI + B6 LN (A–D, n=3) or TBI only (E–H, n=2) treatment were cut sagittally, fixed and stained with antibodies for mouse MHC class II (red), Fas (green), CD4 (white) and DAPI (blue). Stained wholemount sternebrae tissues were analyzed by a Zeiss LSM 510 confocal microscope. Data shown are representative views of MHC class II single staining (A and E), Fas single staining (B and F), MHC class II and Fas overlap showing co-expression/co-localization (C and G), and the close encounter of MHC class II+Fas+ cells with CD4+ T cells within the BM (D and H).
T cell-mediated apoptosis in BM cell targets
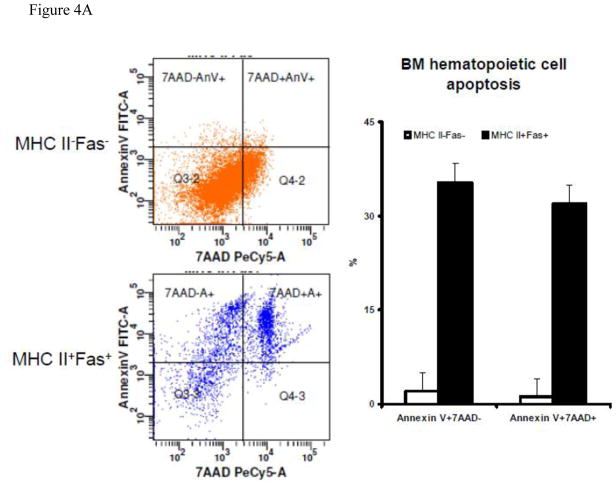
We next examined BM cell apoptosis in BM cells from C.B10 mice that received TBI + LN-cell-infusion. In MHC class II+Fas+ residual BM cells we detected 35% ± 3% cells as Annexin V+7AAD− and 32% ± 3% cells as Annexin V+7AAD+, both significantly (P<0.01) higher than those in MHC class II−Fas− residual BM cells (2.0% ± 3.0% and 1.2% ± 2.9%), indicating that MHC class II+Fas+ residual BM cells are much more apoptotic than MHC class II−Fas− residual BM cells (Fig 4A).
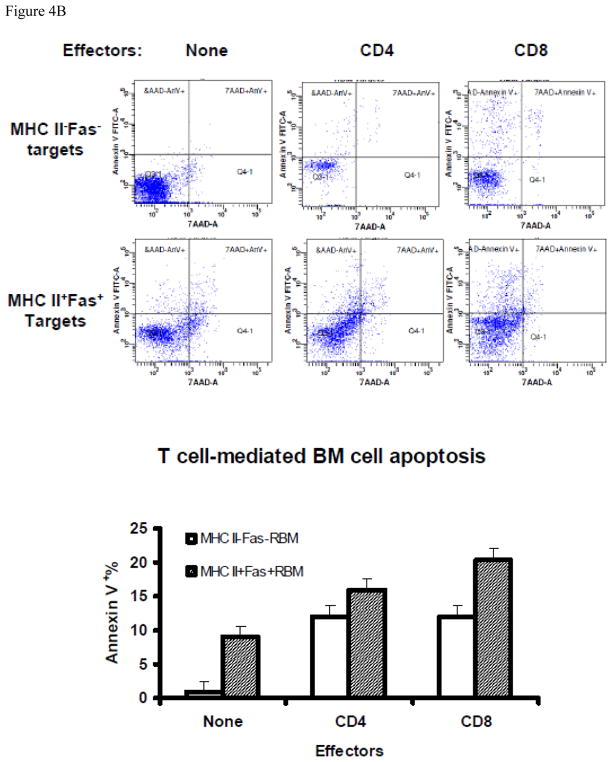
Figure 4.
T-cell-mediated apoptosis in BM cells from mice with experimental BM failure. A, BM cells from CByB6F1 mice that received TBI + B6 LN (N =3) 14 days earlier were stained with a mixture of Annexin-V-FITC + MHC class II-PE + CD95 (Fas)-PE-Cy7 + CD4/CD8/CD11b/CD45R-APC and analyzed by an LSR II flow cytometer. MHC II−Fas− and MHC II+Fas+ cells were gated out from CD4/CD8/CD11b/CD45R negative residual BM cells and were analyzed for the binding to Annexin V and staining for 7AAD. Proportions of Annexin V+7AAD− and Annxin V+7AAD+ cells were shown as representative dot plots and as means with standard errors. B, in a separate study, BM cells from CByB6F1 mice that received TBI + B6 LN treatment 14 day earlier (N=3) were stained with an antibody mixture of CD11b-FITC + MHC class II-PE + CD45R-PE-Cy5 + CD95 (Fas)-PE-Cy7 + CD4-APC + CD8-APC-Cy7, and were then sorted by a FACSAria cell sorter to collect CD4+ T cells and CD8+ T cells as effectors. The remaining CD4−CD8−CD11b−CD45R− residual BM cells were divided into MHC class II+Fas+ and MHC class II−Fas− fractions as targets. CD4+ or CD8+ effector T cells were mixed 10:1 with MHC class II+Fas+ or MHC class II−Fas− residual BM cell targets, cultured at 37°C with 5% CO2 for 60 minutes, and then analyzed for Annexin V binding and 7AAD staining to define cell apoptosis, Data shown as representatives and means with standard errors.
To further examine the functional relationship between effector T cells and target BM cells, we sorted out CD4 and CD8 T cells as immune effectors, and MHC class II+Fas+ and MHC class II−Fas− residual BM cells as immune targets, from BM failure animals. We then mixed effector and target cells at a 10:1 ratio and incubated at 37°C for 60 minutes to test target cell apoptosis. Both CD4 and CD8 T cells induced target cell apoptosis staining as Annexin V+7AAD− and Annexin V+7AAD+ (Fig 4B). Using a two factor model for statistical analyses, we found that the proportion of Annexin V+ cells (Annexin V+7AAD− and Annexin V+7AAD+ combined) was significantly higher (P<0.05) for MHC class II+Fas+ cells (18.1% ± 1.6%) than that for MHC class II−Fas− cells (12.0% ± 1.6%). Among the two types of effector T cells, CD8 T cells induced more cell apoptosis (16.2% ± 1.6%) than did CD4 T cells (13.9% ± 1.6%), but this difference was not significant (Fig 4B). Thus, both CD4 and CD8 T cells expressed cytotoxicity toward BM hematopoietic cells, with MHC class II+Fas+ residual BM cells being more susceptible than were MHC class II−Fas− residual BM cells.
Augmented MHC class II and Fas up-regulation by IFN-γ
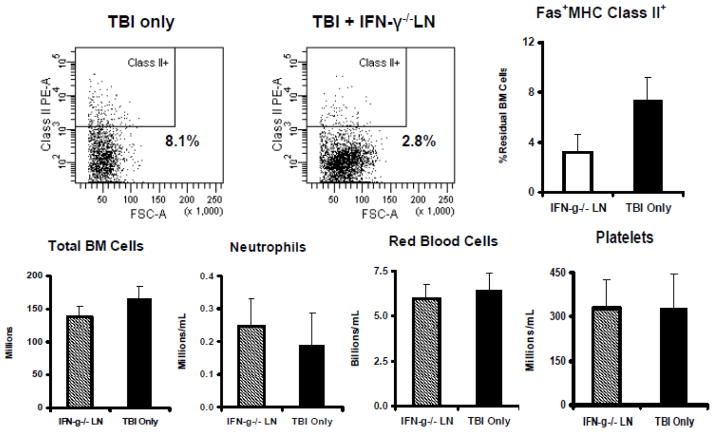
Since MHC class II expression is stimulated by the inflammatory cytokine IFN-γ and IFN-γ production is up-regulated during BM failure, we reasoned that deficiency of IFN-γ might abrogate MHC class II up-regulation and mitigate BM failure. Thus we infused sub-lethally-irradiated C.B10 mice with LN cells from IFN-γ−/− donors [32], and monitored animals for cellular changes and for the development of BM failure. We found that IFN-γ−/− LN cell-infused animals had percentages of MHC class II+, Fas+, and MHC class II+Fas+ residual BM cells similar to those of TBI-only controls, and these animals displayed no sign of BM failure (Fig. 5).
Figure 5.
IFN-γ deficiency reduces immune-mediated BM failure. Infusion of LN cells from B6 mice that carry germline deletion of the IFN-γ gene (IFN-γ−/− LN) into sublethally-irradiated C.B10 hosts (n=6) showed no expansion of MHC class II+Fas+ cells in recipient BM in comparison to TBI-only (n=4) controls. Levels of total BM cells, blood neutrophils, red blood cells and platelets were not different between IFN-γ−/− LN cell-infused animals and TBI-only controls.
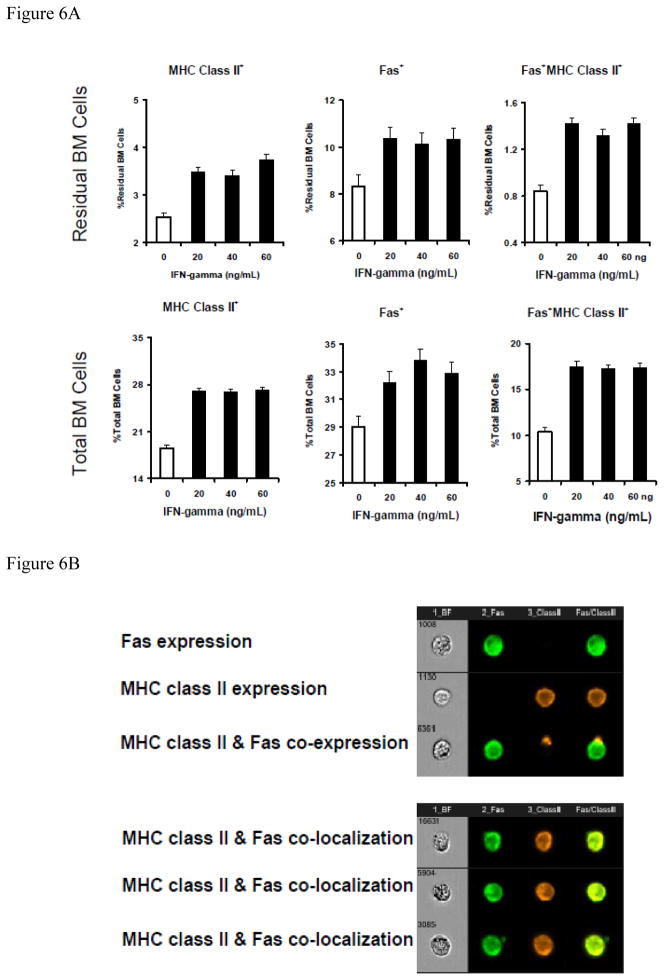
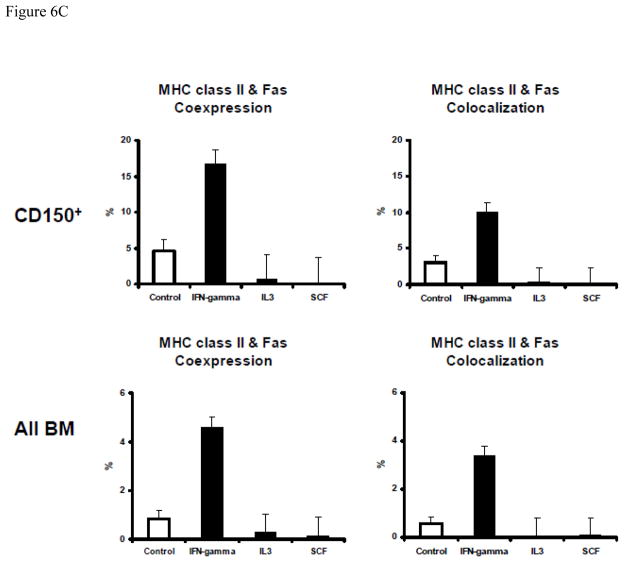
We further examined the role of IFN-γ, reasoning that IFN-γ alone might stimulate MHC class II-Fas co-expression and co-localization in normal BM cells to facilitate cell apoptosis. Toward this end, we first cultured normal B6 BM cells in vitro with or without recombinant murine IFN-γ. Data from multiple cell culture experiments showed that addition of 20–60 ng/mL recombinant IFN-γ caused significant MHC class II (P<0.01) and Fas (P<0.01) up-regulation on total and residual BM cells (Fig. 6A), resulting in significantly increased proportions of MHC class II+Fas+ cells in total (P<0.01) and residual BM cells (P<0.01). In other experiments, we cultured normal B6 BM cells with 50 ng/mL IFN-γ, IL-3 or SCF for 48 hours and found that IFN-γ stimulated MHC class II and Fas co-expression and co-localization on total BM cells as well as on CD150+ hematopoietic cells (Fig. 6, B & C). In contrast, treatment with the same concentrations of IL-3 or SCF had no such effect (Fig. 6C).
Figure 6.
Augmented MHC class II and Fas coexpression and colocalization on normal BM cells by IFN-γ. A, Treatment of normal B6 BM cells with IFN-γ in vitro significantly (P<0.01 for all) increased the proportions of MHC class II+, Fas+ and MHC class II+Fas+ cells in total BM cells as well as in residual BM (CD4−CD8−CD11b−CD45R−) cell fraction. BM cells were cultured in complete RPMI 1640 medium supplemented with 0, 20, 40, and 60 ng/mL of recombinant IFN-γ for 18, 24, 40, 48 and 72 hours respectively in a 37°C incubator with 5% CO2. Cells were cultured in suspension using 6-well flat-bottom plates with three replications per (IFN-γ dose × Time) treatment group. B, In a different study, BM cells from normal B6 mice were cultured for 48 hours under the same conditions with no cytokine as control or with 50 ng/mL of recombinant IFN-γ, IL3 or SCF respectively. Harvested cells were stained with an antibody mixture of CD95-FITC + MHC class II-PE + CD150-PE-Cy5, and were analyzed using an Amnis ImageStream Imaging Flow Cytometer for the analysis of MHC class II & Fas co-localization, shown as representatives of MHC class II and Fas single expression, co-expression, co-expression without colocalization, and co-expression with co-localization. C, summarized data from the above study in B showing that IFN-γ, but not IL3 or SCF, significantly (P<0.01) increased MHC class II & Fas co-expression and co-localization on total BM cells or CD150+ BM cells. Data shown are means and standard errors of cells from one to three animals each cultured in triplicate wells.
Discussion
In the human disease AA, proliferation of oligoclonal pathogenic T cells and up-regulation in Fas expression on BM hematopoietic cells accompanies profound BM destruction [33–35]. This pathology was reproduced in experimental models of BM failure in which expansion of oligoclonal T cells and the activation of Th1 immune response and Fas-mediated cell apoptosis led to massive BM cell destruction [9,10].
In the current study, we observed MHC class II up-regulation and co-expression/co-localization with Fas in both B6⇒C.B10 and B6⇒CByB6F1 LN cell infusion models. We also observed an association of MHC class II+Fas+ BM cells with CD4+ T cells, implicating CD4 cell-mediated cytotoxicity in the destruction of MHC class II+Fas+ BM cells. The infusion of allogeneic LN cells caused significant declines in BM cells (marrow hypoplasia) and mature blood cells (pancytopenia). We did not specifically measure changes in the numbers of CD150+, CD34+ or Lin−Kit+Sca1+ hematopoietic cells in our current study, as we have previously reported that infusion of 5 × 106 B6 LN cells to 5 Gy-irradiated C.B10 or CByB6F1 recipients caused significant declines in radiation-protective hematopoietic cells [19], in Lin−Kit+Sca1+ hematopoietic stem and progenitor cells [7], and in Lin−Kit+ hematopoietic cells [8], along with development of marrow hypoplasia and peripheral blood pancytopenia. We specifically examined MHC class II expression and MHC class II-Fas co-localization on residual BM cells by excluding CD4+, CD8+, CD11b+ and CD45R+ mature cells that do not contain hematopoietic stem or progenitor cells but do include MHC class II-positive antigen presenting cells. Thus, we focused on those less mature hematopoietic cells that are the likely main targets for cell destruction in BM failure.
The co-localization of MHC class II and the apoptotic receptor Fas on residual BM cells, as observed by Amnis ImageStream imaging flow cytometry analysis, provided fresh evidence of an association between these two types of molecules and their potential role in cell destruction. MHC class II molecules have short cytoplasmic tails with no known signaling motifs [36,37]. Physical co-localization with Fas would provide a possibility for MHC class II to transmit signals through Fas to its downstream molecular components to initiate cell destruction. This mechanism of cell killing is consistent with previous reports indicating that Fas-mediated cell apoptosis is the major pathway of cell destruction in AA patients [33,34] as well as in mouse models of immune-mediated BM failure [10]. Ideally we would analyze MHC class II-Fas co-expression and co-localization on residual BM cells since the destruction of residual BM cells is most relevant to immune-mediated BM destruction. However, residual BM cells are based on negative selection of CD4−CD8−CD11b−CD45R− cells. Thus, we examined MHC class II and Fas co-localization on CD150+ and CD34+ cells because these two antibodies provide positive identification of cell populations that are enriched in hematopoietic stem and progenitor cells.
Confocal microscopy revealed a close association between CD4+ T cells and MHC class II+Fas+ BM cells in the BM cavity of mice that had developed BM failure (Fig 4). Our observation is concordant with previous reports showing CD4 T cell-mediated cell destruction [20–22]. In a particular AA patient, a specific CD4 T cell clone was isolated and was found to have cytotoxic activity toward autologous and allogeneic cell targets [11,38]. It has also been reported that AA patients show oligo-clonal CD4 T cell expansion in blood and BM [14,15]. Thus, the infusion of allogeneic LN cells may cause initial T cell expansion and IFN-γ up-regulation in the BM of affected animals. Elevated IFN-γ then up-regulates MHC class II and Fas co-expression/co-localization on residual BM cells, including hemaopoietic progenitor and stem cells, that may attract activated CD4 and CD8 T cells. Engagement of CD4 and MHC class II between effector and target cells transduces signals through the co-localized Fas receptor to trigger a chain of molecular responses leading to target cell destruction. Indeed, the majority of MHC class II+Fas+ residual BM cells in LN-cell infused animals were Annexin V+, indicating cell apoptosis, and only a very small fraction of MHC class II−Fas− residual BM cells were Annexin V+ (Fig. 4A). Incubation of CD4 and CD8 T cells caused more cell apoptosis in MHC class II+Fas+ residual BM cells than in MHC class II−Fas− residual BM cells, further indicating that the expression of MHC class II and Fas facilitated target cell apoptosis. While overall the CD4 T cell effect was less than for CD8 T cells in the induction of target cell death in vitro, CD4 T cells did express slightly higher cytotoxicity toward MHC class II+Fas+ residual BM cells than did CD8 T cells (Fig 4B); further supporting an MHC class II engagement with CD4 T cells in immune-mediated cell destruction of hematopoietic targets.
Since IFN-γ is a key cytokine up-regulated during inflammation, we speculate that the IFN-γ-driven MHC class II up-regulation, and the associated elevation in MHC class II/Fas co-localization on target cells and T-cell-mediated target cell apoptosis, might represent a general mechanism for cell destruction in BM failure as well as in other inflammatory conditions. Indeed, we found that IFN-γ-deficient LN cells failed to cause BM failure (Fig. 5). Lack of IFN-γ may have abrogated BM destruction through (at least) two mechanisms: 1) by preventing expansion and activation of cytotoxic T cells as immune effectors; 2) by reducing IFN-γ-mediated bystander destruction of BM cells.
Data from our cell culture studies in vitro supported the hypothesis that IFN-γ was an active regulator of MHC class II and Fas, since culturing normal BM cells with recombinant IFN-γ resulted in increased MHC class II and Fas co-expression and co-localization. In direct comparison, cytokines that promote hematopoiesis, such as IL3 and SCF, lacked these effects (Fig. 6). Notably, IFN-γ-induced MHC class II and Fas co-expression/co-localization in vitro (Fig 6) was less than seen in residual BM cells in the animal models (Fig 1 & 2), suggesting that the interaction of IFN-γ with other cellular components in the in vivo settings, such as CD4 and CD8 T cells, are important in the induction of BM failure. It is possible that other inflammatory cytokines, such as IL-5, IL-15 and IL-17, might also be able to induce MHC class II-Fas co-expression and co-localization on target cells during inflammation. Future studies are certainly needed to reveal the roles of each specific cytokine.
Acknowledgments
We thank Dr. Jan Joseph Melenhorst from Hematology Branch, NHLBI, NIH, for helpful scientific discussions, and NHLBI Light Microscopy Core Facility for Confocal microscopy image acquisition and analyses. This research was supported by funds for NHLBI intramural research.
Abbreviations
- AA
aplastic anemia
- B6
C57BL/6
- BM
bone marrow
- C.B10
C.B10-H2b/LilMcd
- CLSM
Confocal Laser Scanning Microscope
- Cy5
Cyanin 5
- Cy7
Cyanin 7
- LN
lymph node
- minor-H
minor histocompatibility antigens
- TBI
total body irradiation
Footnotes
The authors have no conflict of interest in conducting this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15:162–168. doi: 10.1097/MOH.0b013e3282fa7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kojima S, Frickhofen N, Deeg HJ, Okamoto S, Marsh J, Teramura M, Bacigalupo A, Mizoguchi H. Aplastic anemia. Int J Hematol. 2005;82:408–411. doi: 10.1532/IJH97.05138. [DOI] [PubMed] [Google Scholar]
- 3.Nagy SM, Jr, Fisher JJ. Aplastic anemia and immunosuppression. JAMA. 2003;290:193. doi: 10.1001/jama.290.2.193-b. [DOI] [PubMed] [Google Scholar]
- 4.Marsh JC. Results of immunosuppression in aplastic anaemia. Acta Haematol. 2000;103:26–32. doi: 10.1159/000041001. [DOI] [PubMed] [Google Scholar]
- 5.Morley A, Blake J. An animal model of chronic aplastic marrow failure. I. Late marrow failure after busulfan. Blood. 1974;44:49–56. [PubMed] [Google Scholar]
- 6.Knospe WH, Husseini SG, Chiu KM, Fried W. Immunologically mediated aplastic anemia in mice: evidence of hematopoietic stromal injury and injury to hematopoietic stem cells. Exp Hematol. 1994;22:573–581. [PubMed] [Google Scholar]
- 7.Chen J, Lipovsky K, Ellison F, Calado RT, Young NS. Bystander destruction of hematopoietic progenitor and stem cells in a mouse model of infusion-induced bone marrow failure. 2004:1671. doi: 10.1182/blood-2004-03-1115. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Ellison FM, Eckhaus MA, Smith AL, Keyvanfar K, Calado RT, Young NS. Minor antigen H60-mediated aplastic anemia is ameliorated by immunosuppression and the infusion of regulatory T cells. J Immunol. 2007;178:4159–4168. doi: 10.4049/jimmunol.178.7.4159. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Desierto MJ, Chen J, Young NS. The role of the Th1 transcription factor T-bet in a mouse model of immune-mediated bone-marrow failure. Blood. 2010;115:541–548. doi: 10.1182/blood-2009-03-211383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omokaro SO, Desierto MJ, Eckhaus MA, Ellison FM, Chen J, Young NS. Lymphocytes with Aberrant Expression of Fas or Fas-ligand Attenuate Immune Bone Marrow Failure in a Mouse Model. J Immunol. 2009;182:3414–3422. doi: 10.4049/jimmunol.0801430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakao S, Takami A, Takamatsu H, Zeng W, Sugimori N, Yamazaki H, Miura Y, Ueda M, Shiobara S, Yoshioka T, Kaneshige T, Yasukawa M, Matsuda T. Isolation of a T-cell clone showing HLA-DRB1*0405-restricted cytotoxicity for hematopoietic cells in a patient with aplastic anemia. Blood. 1997;89:3691–3699. [PubMed] [Google Scholar]
- 12.Takami A, Zeng W, Wang H, Matsuda T, Nakao S. Cytotoxicity against lymphoblastoid cells mediated by a T-cell clone from an aplastic anaemia patient: role of CD59 on target cells. Br J Haematol. 1999;107:791–796. doi: 10.1046/j.1365-2141.1999.01790.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Li Q, Xu JW, Zhang AM, Xu XC, Zhai ZM. [Clinical significance of detection of T-cell subgroups in patients with aplastic anemia] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:1046–1049. [PubMed] [Google Scholar]
- 14.Zeng W, Maciejewski JP, Chen G, Young NS. Limited heterogeneity of T cell receptor BV usage in aplastic anemia. J Clin Invest. 2001;108:765–773. doi: 10.1172/JCI12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risitano AM, Kook H, Zeng W, Chen G, Young NS, Maciejewski JP. Oligoclonal and polyclonal CD4 and CD8 lymphocytes in aplastic anemia and paroxysmal nocturnal hemoglobinuria measured by V beta CDR3 spectratyping and flow cytometry. Blood. 2002;100:178–183. doi: 10.1182/blood-2002-01-0236. [DOI] [PubMed] [Google Scholar]
- 16.Zeng W, Kajigaya S, Chen G, Risitano AM, Nunez O, Young NS. Transcript profile of CD4+ and CD8+ T cells from the bone marrow of acquired aplastic anemia patients. Exp Hematol. 2004;32:806–814. doi: 10.1016/j.exphem.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Giannakoulas NC, Karakantza M, Theodorou GL, Pagoni M, Galanopoulos A, Kakagianni T, Kouraklis-Symeonidis A, Matsouka P, Maniatis A, Zoumbos NC. Clinical relevance of balance between type 1 and type 2 immune responses of lymphocyte subpopulations in aplastic anaemia patients. Br J Haematol. 2004;124:97–105. doi: 10.1046/j.1365-2141.2003.04729.x. [DOI] [PubMed] [Google Scholar]
- 18.Zheng M, Sun H, Zhou J, Xu H, Huang L, Liu W. Proliferation and apoptosis of bone marrow CD4(+) T cells in patients with aplastic anemia and impacts of the secreted cytokines on hematopoietic stem cells from umbilical cord blood. J Huazhong Univ Sci Technolog Med Sci. 2010;30:37–41. doi: 10.1007/s11596-010-0107-3. [DOI] [PubMed] [Google Scholar]
- 19.Bloom ML, Wolk AG, Simon-Stoos KL, Bard JS, Chen J, Young NS. A mouse model of lymphocyte infusion-induced bone marrow failure. Exp Hematol. 2004;32:1163–1172. doi: 10.1016/j.exphem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Jeon YK, Kang BH, Chung DH, Park CG, Shin HY, Jung KC, Park SH. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J Exp Med. 2010;207:237. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drozina G, Kohoutek J, Jabrane-Ferrat N, Peterlin BM. Expression of MHC II genes. Curr Top Microbiol Immunol. 2005;290:147–170. doi: 10.1007/3-540-26363-2_7. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Flynt FL, Hong M, Chen H, Gilbert CA, Briley NT, Bolick SC, Wright KL, Piskurich JF. MHC class II transactivator (CIITA) expression is upregulated in multiple myeloma cells by IFN-gamma. Mol Immunol. 2007;44:2923–2932. doi: 10.1016/j.molimm.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 26.Kanazawa S, Ota S, Sekine C, Tada T, Otsuka T, Okamoto T, Sonderstrup G, Peterlin BM. Aberrant MHC class II expression in mouse joints leads to arthritis with extraarticular manifestations similar to rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006;103:14465–14470. doi: 10.1073/pnas.0606450103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speeti M, Stahls A, Meri S, Westermarck E. Upregulation of major histocompatibility complex class II antigens in hepatocytes in Doberman hepatitis. Vet Immunol Immunopathol. 2003;96:1–12. doi: 10.1016/s0165-2427(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 28.Amrani A, Verdaguer J, Thiessen S, Bou S, Santamaria P. IL-1alpha, IL-1beta, and IFN-gamma mark beta cells for Fas-dependent destruction by diabetogenic CD4(+) T lymphocytes. J Clin Invest. 2000;105:459–468. doi: 10.1172/JCI8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maciejewski J, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood. 1995;85:11–3183. [PubMed] [Google Scholar]
- 30.Beum PV, Lindorfer MA, Hall BE, George TC, Frost K, Morrissey PJ, Taylor RP. Quantitative analysis of protein co-localization on B cells opsonized with rituximab and complement using the ImageStream multispectral imaging flow cytometer. J Immunol Methods. 2006;317:90–99. doi: 10.1016/j.jim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 31.SAS Institute Inc. JMP Statistics and Graphics Guide, Version 3. SAS Institute; Cary, NC: 1998. [Google Scholar]
- 32.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 33.Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br J Haematol. 1995;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 34.Callera F, Garcia AB, Falcão RP. Fas-mediated apoptosis with normal expression of bcl-2 and p53 in lymphocytes from aplastic anaemia. Br J Haematol. 1998;100:698–703. doi: 10.1046/j.1365-2141.1998.00625.x. [DOI] [PubMed] [Google Scholar]
- 35.Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet. 2004;364:355–364. doi: 10.1016/S0140-6736(04)16724-X. [DOI] [PubMed] [Google Scholar]
- 36.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin L, Stolpa JC, Young RM, Pugh-Bernard AE, Refaeli Y, Cambier JC. MHC class II structural requirements for the association with Igalpha/beta, and signaling of calcium mobilization and cell death. Immunol Lett. 2008;116:184–194. doi: 10.1016/j.imlet.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakao S, Takamatsu H, Yachie A, Itoh T, Yamaguchi M, Ueda M, Shiobara S, Matsuda T. Establishment of a CD4+ T cell clone recognizing autologous hematopoietic progenitor cells from a patient with immune-mediated aplastic anemia. Exp Hematol. 1995;23:433–438. [PubMed] [Google Scholar]