Abstract
Hear rate conditioning is used as an index of conditioned fear and is important for understanding disorders of anxiety and stress including post traumatic stress disorder (PTSD). One important feature of PTSD is that patients generalize conditioned fear from danger signals to safety signals especially when the two signals have overlapping features. What has not been determined is whether generalization occurs between unconditioned stimuli with overlapping features. In the current experiment, heart rate conditioning and conditioning-specific reflex modification of rabbit heart rate were examined as a function of two different unconditioned stimulus locations. Heart rate conditioning occurred at identical terminal levels whether electrical stimulation was presented near the eye or on the back. Despite different heart rate response topographies to electrical stimulation at the two locations, conditioning-specific reflex modification was detected near the eye and on the back and appeared to generalize between the locations. Interestingly, only conditioning-specific reflex modification detected on the back persisted for a week after heart rate conditioning. This persistence may be a model for some features of post traumatic stress disorder. Over-generalization of unconditioned responses to unconditioned stimuli similar to the trauma may also be an important aspect of PTSD modeled here.
Keywords: bradycardia, classical conditioning, fear conditioning, heart rate, heart rate conditioning, rabbit, tachycardia
The study of fear conditioning is important to understanding disorders of anxiety and stress and, in particular, post traumatic stress disorder (Blechert, Michael, Vriends, Margraf, & Wilhelm, 2007; Hofmann, 2008; McNally, 2007; Milad et al., 2009; Norrholm et al., 2011; Siegmund & Wotjak, 2007). One common characteristic of post traumatic stress disorder (PTSD) is the generalization of fear to stimuli and situations beyond those associated with the original traumatic event. This is especially true of stimuli that have overlapping features with the conditioned stimulus (CS) (Lissek et al., 2008). Another aspect of PTSD is stress-induced heightening of physiological responses, such as heart rate (Brunijnzeel, Stam, Croiset, & Wiegant, 2001; Elsesser, Sartory, & Tackenberg, 2004; Orr, Metzger, & Pitman, 2002). To our knowledge, the extent to which generalization of these heightened unconditioned responses (URs) occurs to unconditioned stimuli (US) that have overlapping features with the original traumatic event has not been explicitly tested.
Heart rate (HR) conditioning is used as an index of conditioned fear in many species including humans (Cohen & Pitts, 1968; Fitzgerald & Martin, 1971; Gantt, 1960; Graham, 1978; Schneiderman, Smith, Smith, & Gormezano, 1966; Scobie, 1973; Stebbins & Smith, Jr., 1964; Stiedl, Tovote, Ogren, & Meyer, 2004) and also has been used to assess abnormalities of fear conditioning in patients with PTSD (Burriss, Ayers, & Powell, 2007). Work in our laboratory with rabbits has shown that HR conditioning can be a useful paradigm to assess conditioning-related changes in HR to both cues associated with a fear event and parametric variations of the fear event itself (Burhans, Smith-Bell, & Schreurs, 2008; Burhans, Smith-Bell, & Schreurs, 2010; Schreurs, Crum, Wang, & Smith-Bell, 2005). Heart rate classical conditioning occurs when rabbits that receive CS-US pairings of a tone with electrical stimulation (ES) show a conditioned deceleration in HR (bradycardia) to the tone relative to rabbits that receive explicitly unpaired CS and US presentations (Ghelarducci & Sebastiani, 1997; McEchron, Tseng, & Disterhoft, 2003; Powell & Levine-Bryce, 1988; Schneiderman et al., 1966; Supple, Jr. & Kapp, 1993). As a result of HR conditioning, the rabbit also changes the way it responds to ES when the ES is presented by itself (Burhans et al., 2008; Burhans et al., 2010; Schreurs et al., 2007; Schreurs et al., 2005). Specifically, the acceleration (tachycardia) elicited by ES before HR conditioning changes to bradycardia after HR conditioning – an associative phenomenon we have termed conditioning-specific reflex modification (CRM). A related phenomenon occurs in the rabbit nictitating membrane response (NMR) where the UR becomes larger with peak amplitudes that occur later as a result of NMR conditioning (Buck, Seager, & Schreurs, 2001; Burhans et al., 2008; Gruart & Yeo, 1995; Schreurs, 2003; Schreurs et al., 2005; Schreurs, Oh, Hirashima, & Alkon, 1995; Schreurs, Shi, Pineda, & Buck, 2000; Wikgren, Ruusuvirta, & Korhonen, 2002). We have previously argued that the study of CRM of HR and NMR may be a useful model of some of the reflexive, physiological features of PTSD (Burhans et al., 2008).
In the present study, we sought to further explore the nature of HR CRM to see if it would generalize across USs with overlapping features. With the exception of a few studies using ES to the ear (Sebastiani, La Noce, Paton, & Ghelarducci, 1992; Supple, Jr. & Kapp, 1993) or direct stimulation of the brain (Swadlow & Schneiderman, 1970; VanDercar, Elster, & Schneiderman, 1970), rabbit HR conditioning has employed ES near the eye as the US (Gallagher, Kapp, Frysinger, & Rapp, 1980; Kapp, Frysinger, Gallagher, & Haselton, 1979; Kazis, Milligan, & Powell, 1973; Kehoe, Palmer, Weidemann, & Macrae, 2000; Kim & Jung, 2006; Lavond, McCormick, & Thompson, 1984; McEchron, McCabe, Green, Llabre, & Schneiderman, 1991; Powell & Kazis, 1976; Schneiderman et al., 1966; Schreurs et al., 2005).
In the current experiment we compared HR changes resulting from ES near the eye to that resulting from stimulation on the back. Stimulation of the back was chosen because it is so different from the eye not just because of different sensory innervations but also because it elicits a different physiological response (see below). The purpose of the experiment was to see whether HR conditioning and conditioning specific reflex modification (CRM) of HR could occur with stimulation to the back, compare it to HR conditioning and CRM near the eye and see if CRM generalized between the two locations.
Methods
Subjects
The subjects were 35 male, New Zealand White rabbits (Oryctolagus cuniculus) weighing approximately 2.0–2.2 kg upon delivery from the supplier (Harlan, Indianapolis, IN). The rabbits were housed in individual cages on a 12 hour light-dark cycle and given ad libitum access to food and water. They were maintained and treated in accordance with guidelines issued by the National Institutes of Health, and the research was approved by the West Virginia University Animal Care and Use Committee.
Apparatus
The HR conditioning apparatus has been detailed elsewhere (Schreurs et al., 2005; Schreurs & Smith-Bell, 2005) and was modified from that developed and described by Schneiderman and Gormezano (Schneiderman et al., 1966). Briefly, rabbits were restrained in a Plexiglas box placed inside a sound-attenuating, ventilated chamber (Coulborn Instruments, Allentown, PA; Model E10–20). Inside the chambers, a stimulus panel containing a speaker and houselight (10-W, 120 V) was mounted at a 45° angle 15 cm anterior and dorsal to the rabbit’s head. An exhaust fan created a constant ambient noise level of 65 dB inside the chamber. Electrical stimulation served as a US, and was delivered by a programmable two-pole stimulator (Colbourn Instruments, Whitehall, PA, Model E13–35) via stainless steel Autoclip wound clips (Stoelting, Wood Dale, IL) that were positioned 10 mm ventral and 10 mm posterior to the dorsal canthus of the right eye (Eye) or 10 mm on either side of the midline at a point on the back between the shoulders (Back).
For heart rate recording, three wound clips were placed in shaved skin, two on either side of the breast bone (20 mm lateral of the apex) and one on the right shoulder. For each rabbit, the three clips were coupled to an individual custom amplifier providing a 10,000-fold amplification of the ECG signal which was then routed to an analog-to-digital converter (1-ms sampling rate) and stored on a trial-by-trial basis for subsequent inspection and analysis. A custom circuit isolated the US from the ECG amplifier during US presentations to prevent high-voltage artifacts and subsequent electronic ringing from saturating the system. The custom circuit was crucial for resolving components of the ECG waveform during trials where the US was presented, allowing detection of all heartbeats with the exception of those during or immediately following the US. Stimulus delivery, data collection, and analysis were all accomplished using the LabVIEW software system (National Instruments, Austin, TX).
Procedure
One week after arrival, rabbits were assigned to one of four groups and received one session per day beginning with adaptation, US pretest (Pretest), two sessions of HR conditioning, and then US post test (Post Test 1). A second session of US testing occurred a week later (Post Test 2). Group assignment comprised a 2×2 factorial design that was based on the location of ES during US testing (Test Eye or Test Back) and HR conditioning (Train Eye or Train Back). Thus, rabbits were assigned to groups that received pretesting, conditioning and post testing with ES near the eye (n=8), to the back (n=9), testing with ES near the eye and conditioning with ES to the back (n=9) or testing with ES to the back and conditioning with ES near the eye (n=9). The group with ES to the eye during training and testing served as our standard HR conditioning and HR CRM group (Burhans et al., 2008; Burhans et al., 2010; Schreurs et al., 2005), and the group with ES to the back during training and testing tested whether HR conditioning and HR CRM could be detected when ES was applied to the back. Groups trained in one location (eye or back) and tested in the other location (back or eye) determined whether HR CRM could generalize between locations.
For adaptation, subjects were prepared for ES delivery and EKG recording and then adapted to the training chambers for an amount of time equivalent to subsequent training sessions (80 min). For pretest and post tests, subjects received 80 trials of US presentations with an average inter-trial interval (ITI) of 60 s (range 50–70 s). Each US presentation was one of 20 combinations of ES intensity (0.1, 0.25, 0.5, 1.0, or 2.0 mA) and duration (10, 25, 50, or 100 ms), and these 20 unique USs were presented in four separately randomized sequences with the restriction that the same intensity or duration could not occur more than 3 times in succession during the session. For HR conditioning, paired trials consisted of presentations of a 1000 ms, 1 kHz, 82 dB tone conditioned stimulus (CS) followed by a 100 ms, 2 mA ES US (1000 ms interstimulus interval, 0 trace) that were interspersed with CS-alone test trials following every 9th paired trial. For the first session of HR conditioning, 20 CS-alone trials were presented in order to habituate the bradycardic orienting response that occurs to a novel tone. The remaining 60 trials of the first session and all 80 trials of the second session were paired trials interspersed with CS-alone trials, yielding a total of 54 and 72 paired CS-US presentations on the first and second days of conditioning, respectively. Average ITIs for HR conditioning sessions and all other sessions were the same as US testing, 60 s (range 50–70 s).
Heart Rate Data Collection and Analysis
For all sessions, the ECG signal was recorded for 6 seconds per trial. For all trials, heartbeats were recorded starting with a 2000-ms baseline prior to stimulus onset. Stimulus onset occurred 2,200 ms after trial onset providing a total post-stimulus observation interval of 3,800 ms. Details for detection of heartbeats and components of the ECG signal have been described in detail elsewhere (Burhans et al., 2010; Schreurs et al., 2007; Schreurs et al., 2005). Briefly, heartbeats were detected in the filtered ECG signal with a template-matching algorithm created in LabVIEW software. Visual inspection of the data corrected any false positives or negatives as a result of artifacts such as those related to movement. Data were expressed as a change in IBI (inverse of heart rate) to the CS or US from the pre-stimulus baseline and was used because it is consistent with our previously published studies and with much of the rabbit HR conditioning literature (Burhans et al., 2010; McEchron et al., 2003; Powell, Churchwell, & Burriss, 2005; Schreurs et al., 2007; Schreurs et al., 2005; Schreurs & Smith-Bell, 2005; Supple, Jr., Sebastiani, & Kapp, 1993).
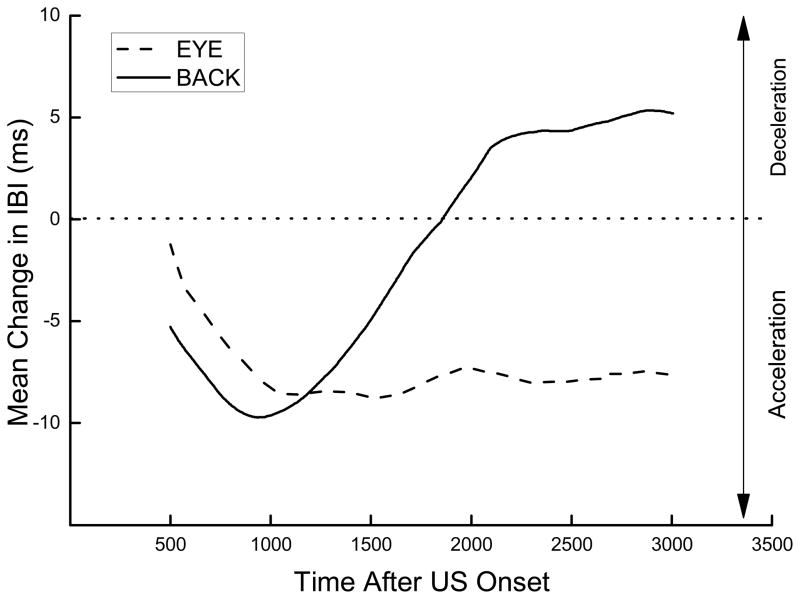
In addition to changes in IBI, average topographies of the IBIs during US testing and HR conditioning were generated and used as a guide to determine the nature of changes in the IBI as a function of time and the appropriate post-stimulus window for analysis of CRM to the two different USs and CRs to the CS. The IBI topographies were averaged across all subjects but the duration was limited to a period that contained complete IBIs for all subjects in the group. Thus, although the observation interval extended for 3,800 ms after stimulus onset, not all subjects had a heart beat that coincided with the beginning or end of the interval so that average topographies normally could not be plotted for the entire interval. Nevertheless, the direction of changes in the IBI (HR acceleration or deceleration) towards the beginning and end of the observation interval was clearly discernable in all average topographies. Figure 1 shows that presentation of a 2.0-mA ES to the back (Test Back) on Pretest, although the same early in the observation interval, produced a different terminal HR topography from ES delivered near the eye (Test Eye). Stimulation near the eye produced a decrease in IBI (HR acceleration) that reached a plateau later in the observation interval whereas stimulation to the back produced a triphasic topographic profile of initial HR acceleration followed by substantial deceleration later in the observation interval. Previous research with ES near the eye has shown that the conditioning-specific changes that characterize HR CRM are associative and most pronounced immediately after US onset (Burhans et al., 2010; Schreurs et al., 2005). Within the first 1000 ms, the initial HR tachycardic response to ES near the eye seen before conditioning becomes bradycardic and then returns to the tachycardic plateau (Burhans et al., 2010). Given the rather different triphasic response topographies resulting from ES to the back, we wanted to capture potential conditioning-induced changes by analyzing changes in IBI during the first and last 1000 ms of the 3,800-ms interval following ES onset separately during US testing.
Figure 1.
Average topography for unconditioned responses to a 2.0-mA electrical stimulus delivered to the back (Test Back) or near the eye (Test Eye) during the first 20 trials on Pretest. Data are expressed as an average change in interbeat interval from a 2,000-ms baseline period before stimulus onset. For these and all other topographies, data were averaged across all subjects but the duration of each curve was limited to the period for which interbeat intervals were available for all subjects in the group. Although the observation interval extended for 3,000 milliseconds after stimulus onset, not all subjects had a heart beat that coincided with the beginning or end of the interval so that average topographies normally could not be plotted for the entire interval. Nevertheless, the direction of changes in the interbeat interval (e.g., heart rate acceleration or deceleration) across the observation interval was clearly discernable in all topographies.
Results
HR conditioning
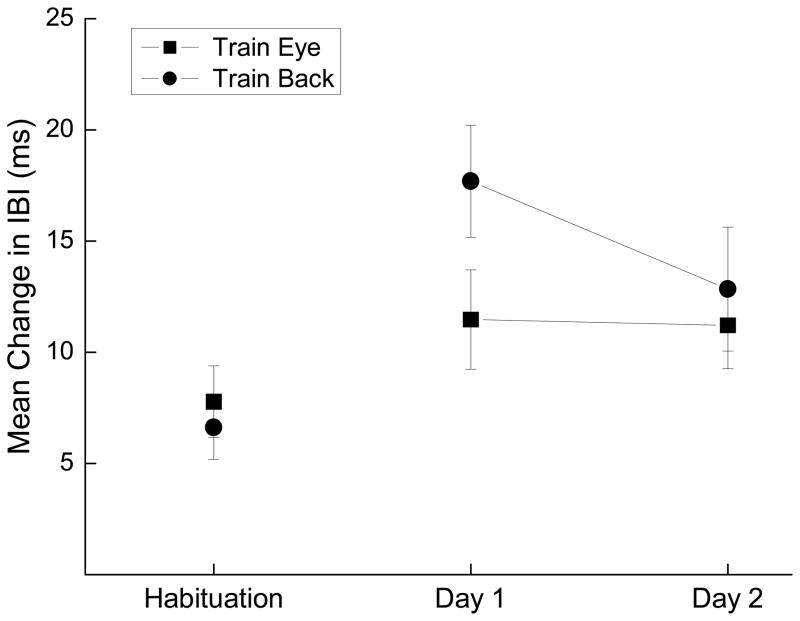
Figure 2 shows the mean change in IBI from baseline to the tone on CS-alone probe trials for Habituation and the two days of HR conditioning combined for all rabbits trained with ES near the eye (Train Eye) compared to all rabbits trained with ES to the back (Train Back). The data are averaged across CS-alone probe trials for each time point. The figure illustrates clearly that relative to the average change in IBI during Habituation, rabbits showed conditioned bradycardia to the tone to almost equal levels by Day 2 of HR conditioning regardless of where the US occurred. Analysis of variance confirmed a significant effect of sessions, (F(2, 66) = 6.00, p < .01), but no effects of US location or interaction of sessions with location (F’s < 1) despite the suggestion of stronger initial HR conditioning with ES to the back.
Figure 2.
Mean and standard error of changes in interbeat interval from baseline to the tone on conditioned stimulus-alone probe trials for Habituation and the two days of heart rate conditioning (Day 1 and Day 2) for rabbits trained with electrical stimulation near the eye (Train Eye) or electrical stimulation to the back (Train Back). The data are averaged across all conditioned stimulus-alone probe trials for each time point.
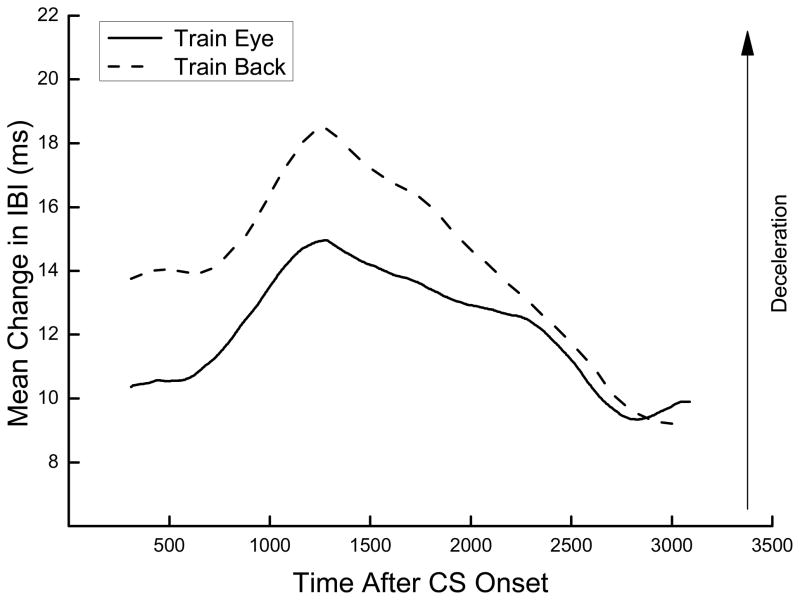
Figure 3 shows the topography of changes in IBI to the tone CS on Day 2 of HR conditioning averaged for all rabbits trained with ES near the eye (Train Eye) or on the back (Train Back). The figure shows a strong biphasic, inverted “U”-shaped bradycardic response to the tone characterized by an initial increase in IBI followed by a decrease we have described previously (Burhans et al., 2010). The similarity of the two topographies provides further support for the observation that stimulation to the eye or the back can support HR conditioning and additionally illustrates that the conditioned HR CRs share a similar shape and timing profile. This strong similarity between HR conditioning levels and response topographies suggests that HR conditioning with ES to the back, like HR conditioning with ES near the eye, is associative in nature (Burhans et al., 2008; Schreurs et al., 2005).
Figure 3.
Average topography of changes in interbeat interval from baseline to the tone conditioned stimulus on Day 2 of heart rate conditioning for rabbits trained with electrical stimulus near the eye (Train Eye) or electrical stimulation to the back (Train Back).
HR CRM
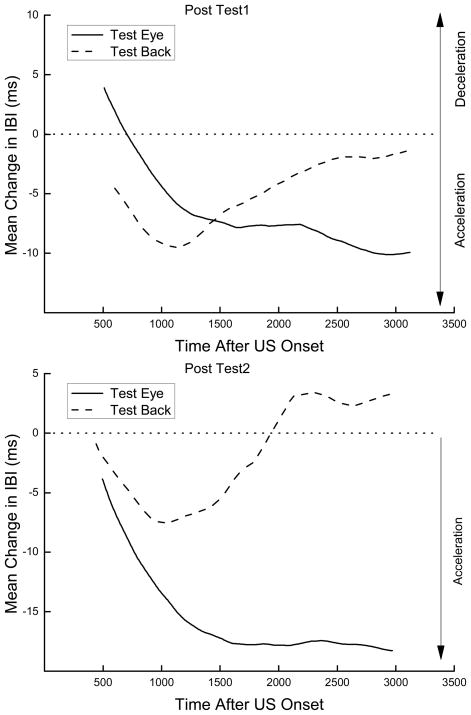
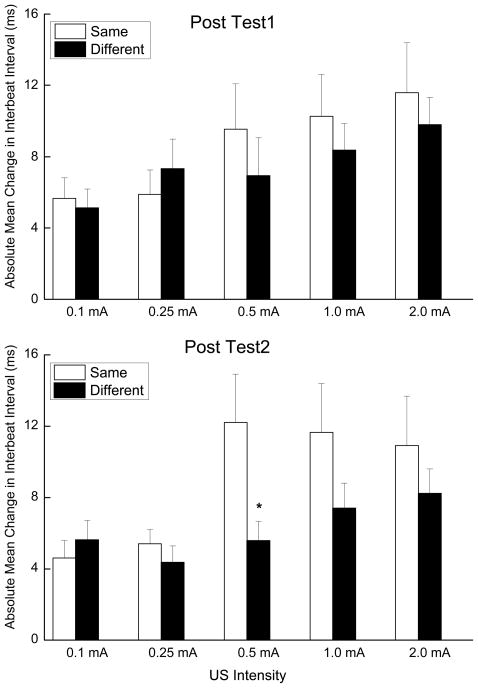
The top and bottom panels of Figure 4 show the averaged topography of changes in IBI to a 2-mA ES tested near the eye (Test Eye) and on the back (Test Back) for Post Test 1 and Post Test 2, respectively, averaged across the training ES location. The figure shows that during Post Test 1 administered the day after HR conditioning, responding to ES changed significantly from that shown in Figure 1. Responding to ES tested near the eye showed an initial bradycardia as a result of HR conditioning – something we have come to expect from HR CRM (Burhans et al., 2008; Burhans et al., 2010; Schreurs et al., 2007; Schreurs et al., 2005). Surprisingly, responding to ES tested on the back showed a reduction in the bradycardia seen on Pretest (Figure 1) as a result of HR conditioning but not until late in the observation interval. The bottom panel shows that during Post Test 2 administered a week later, the initial bradycardia to ES tested near the eye had completely disappeared and, if anything, become even more tachycardic. In contrast, the late reduction in bradycardia to ES tested on the back was still somewhat in evidence a week after HR conditioning. These data are summarized for all ES test intensities in Figure 5.
Figure 4.
Two panels show the averaged topography of changes in interbeat interval from baseline to a 2-mA electrical stimulus tested near the eye (Test Eye) or on the back (Test Back) during unconditioned stimulus testing over the first 20 trials for Post Test 1 (top panel) and Post Test 2 (bottom panel).
Figure 5.
Four panels show mean and standard error of changes in interbeat interval from baseline during unconditioned stimulus testing (Pretest, Post Test 1, Post Test 2) as a function of whether testing took place near the eye (Test Eye, top panels) or on the back (Test Back, bottom panels) during the first 1000 ms (left panels) or last 1000 ms (right panels) of the observation interval after unconditioned stimulus onset. Given the equivalent terminal levels of HR conditioning shown in Figure 1, the US test data were collapsed across the location of the US used during HR conditioning.
The four panels of Figure 5 show mean and SEM of changes in IBI from baseline during US tests (Pretest, Post Test 1, Post Test 2) as a function of whether testing took place near the eye (top) or on the back (bottom) during the first 1000 ms (left) or last 1000 ms (right) of the observation interval after US onset. Given the equivalent terminal levels of HR conditioning shown in Figure 1 and described above, the US test data were collapsed across the location of the US used during HR conditioning. The left panels of Figure 5 show an increase in IBI from Pretest to Post Test 1 characteristic of HR CRM occurred following ES testing near the eye but not after ES testing on the back. Specifically, changes in IBI from baseline on Post Test 1 increased above Pretest levels (bradycardia) during the first 1000 ms as a result of testing near the eye (top panel) but not on the back (bottom panel). Analysis confirmed this observation with a significant interaction of US location (Eye vs. Back) and US test (Pretest vs. Post Test 1), F(1, 31) = 4.81, p < .05). The analysis also yielded a significant interaction of US location and US intensity, F(4,124) = 3.06, p <.05), reflecting the higher overall mean changes in IBI to ES tested near the eye than on the back and a significant interaction of US test and US intensity, F(4,124) = 2.62, p <.05), reflecting the increase in mean change in IBI from Pretest to Post Test 1 at the higher US testing intensities. Finally, there were no significant differences in the mean change in IBI when the US was tested near the eye or on the back a week later (Pretest vs. Post Test 2) suggesting that HR CRM had extinguished. In sum, the left panels of Figure 5 show that during the first 1000 ms after US onset, HR CRM occurred when tested near the eye but not when tested on the back and that HR CRM tested near the eye did not persist when tested a week later. This is consistent with a previous experiment in which rabbits that sat in their home cage for three days after HR conditioning showed diminished HR CRM (Burhans et al., 2010).
The right panels of Figure 5, particularly the bottom right, show a different form of HRM CRM that occurred late in the observation interval when the significant level of bradycardia seen on Pretest (Figure 1) was reduced as a function of HR conditioning. This effect occurs when ES is tested on the back but not when tested near the eye and appears to persist for a week after HR conditioning. Analysis of variance yielded main effects of US location, F(1,31) = 10.19, p < .005, US test, F(2,62) = 3.33, p < .05, US intensity, F(4,124) = 11.49, p < .001, and interactions of US location and US intensity, F(4, 124) = 7.83, p < .001, and US test and US intensity, F(8, 248) = 3.47, p- < .001, confirming the differences in the nature of responding to the US depending on its test location. A separate analysis of mean change in IBI to US testing on the back depicted in the bottom right panel of Figure 5 revealed a significant effect of US test, F(2,34) = 4.29, p < .05, and an interaction of US test and US intensity, F(4,136) = 2.85, p < .01, confirming the persistent reduction in bradycardia after HR conditioning that occurred at the higher US intensities when the US was tested on the back. Taken together, the data depicted in the right panels of Figure 5 show that during the last 1000 ms of the observation interval, HR CRM occurred when tested on the back but not when tested near the eye and that HR CRM that occurred when tested on the back persisted for a week. More importantly, the HR CRM that occurred on the back late in the observation interval was a reduction in or an elimination of a late stage bradycardia rather than the previously observed increase in bradycardia that characterizes HR CRM near the eye early in the observation interval (Burhans et al., 2010; Schreurs et al., 2007; Schreurs et al., 2005). This reduction in bradycardia late in the interval is consistent with the reduction in bradycardia seen late in the observation interval on the second day of HR conditioning shown in Figure 3.
Finally, to determine whether CRM generalized between locations, we analyzed the changes in IBI that resulted from US testing and training in the same location compared to US testing and training in a different location (i.e., test and train near the eye or test and train on the back versus test near the eye and train on the back or test on the back and train near the eye). As noted above, the direction of HR CRM is different depending on where and when CRM is measured with an increase in IBI when measured early near the eye and a decrease in IBI when measured late on the back. To compensate for what would otherwise have been a canceling out of the increase and decrease in IBI when comparing HR CRM across different locations, we calculated the absolute value of the difference in IBI from Pretest to Post Test 1 and Post Test 2 measured near the eye during the first 1000-ms and on the back during the last 1000-ms of the observation interval. The results are shown in the two panels of Figure 6 where the absolute mean change in IBI from Pretest is depicted for Post Test 1 (top) and Post Test 2 (bottom) for the same testing and training location (Same) and for different testing and training locations (Different) as a function of US intensity. The top panel of Figure 6 shows that there were comparable levels of HR CRM (expressed as absolute values above zero) regardless of whether testing and training took place at the same location or a different location. The bottom panel of the figure suggests that a week following HR conditioning, HR CRM was more persistent when testing and training took place at the same location than at different locations.
Figure 6.
Two panels show mean and standard error of absolute value of changes in interbeat interval from Pretest during unconditioned stimulus testing as a function of whether training took place at the same location as during unconditioned stimulus testing (Same) or at a different location as during unconditioned stimulus testing (Different) during the first 1000 ms of the observation interval after unconditioned stimulus onset for testing near the eye or last 1000 ms of the observation interval for testing on the back of the observation interval on Post Test 1 the day after heart rate conditioning (top) and on Post Test 2 a week later (bottom).
Analysis of the Post Test 1 minus Pretest absolute value data yielded a significant effect of US intensity (F(4, 132) = 3.62, p < .01) but no effect of ES location (F’s < 1). The significant effect of intensity provides evidence that changes in IBI were significantly above zero providing an index of HR CRM that increased as a function of US intensity. The lack of a significant effect of location simply means that there were equivalent amounts of CRM regardless of where ES was presented. Analysis of the Post Test 2 minus Pretest absolute value data also yielded a significant effect of US intensity (F(4, 132) = 5.38, p < .001) and although suggestive, there was no overall effect of ES location (F(1,33) = 3.19, p =.08). There was a significant difference between same and different testing and training locations at 0.5 mA (F(1, 33) = 5.69, p < .05). Taken together, the tests comparing locations revealed that similar levels of HR CRM occurred whether CRM was assessed in same location as training or at a different location. There is a suggestion that CRM is less persistent a week later if the testing and training locations are different. Although this measure of generalization is based on comparable levels of CRM, it provides indirect support for the suggestion that CRM can generalize from one location to another because significant HR CRM occurred with stimulation near the eye and on the back, albeit at different time points after the US, regardless of where the ES was administered during HR conditioning (Figures 2 and 3).
Discussion
The principal findings of the present experiment were: (1) ES to the back elicited a different HR response from ES near the eye; (2) ES to the back supported HR conditioning that was comparable to HR conditioning supported by ES near the eye; (3) conditioning-specific modification of HR could be measured when tested on the back as well as when tested near the eye but consisted of a different response – a reduction in bradycardia rather than the previously observed increase in bradycardia; (4) Regardless of which ES location was used to induce HR conditioning, HR CRM could be detected when tested on the back for a week after HR conditioning whereas HR CRM could no longer be detected when tested near the eye; (5) HR CRM generalized to different locations because there was as much HR CRM when tested at locations different from the training location as there was when tested at the same location.
Consistent with previous reports, the current data show that HR CRM occurs following HR conditioning (Burhans et al., 2010; Schreurs et al., 2007; Schreurs et al., 2005) and extend those findings to a new location and a different form of the response. The HR CRM we have reported previously consisted of an increase in bradycardia or even a change from tachycardia to bradycardia early in the response when HR was measured with ES near the eye (Burhans et al., 2010; Schreurs et al., 2007; Schreurs et al., 2005). In the present experiment, we again observed this increase in bradycardia to ES near the eye. When HR was measured with ES to the back, we observed a significant decrease in a late-phase bradycardia. Both forms of HR CRM occurred regardless of whether HR conditioning was obtained with ES near the eye or to the back.
We have suggested previously that CRM may be a generalized conditioned response (Burhans et al., 2008; Burhans et al., 2010; Schreurs et al., 2005). Data consistent with this view come from NMR conditioning where a small, short latency, uniphasic response to ES near the eye becomes a larger, broader and often multi-peaked response resembling an NMR CR (Buck et al., 2001; Burhans et al., 2008; Gruart & Yeo, 1995; Schreurs, 2003; Schreurs, Gonzales-Joekes, & Smith-Bell, 2006; Schreurs et al., 2000; Schreurs, Smith-Bell, Darwish, Stankovic, & Sparks, 2007; Seager, Smith-Bell, & Schreurs, 2003). HR CRM is also consistent with the generalized-CR interpretation because the initial HR increase to ES near the eye becomes more like the decreased HR to the CS that results from HR conditioning (Burhans et al., 2010; Schreurs et al., 2007; Schreurs et al., 2005). The same now appears to be true for HR conditioning when HR is elicited by ES to the back during testing. We see that a bradycardic unconditioned response becomes less bradycardic after HR conditioning. This reduction in the unconditioned response mimics the HR conditioned response which becomes less bradycardic late in the observation interval. Once again, this effect depends upon the location of US during testing not on the location of the US during HR conditioning because HR CRM detected on the back occurred following HR conditioning obtained with ES to the back or near the eye. This result suggests that HR CRM can generalize from the training location to a different testing location.
Although HR conditioning can take place within very few trials relative to conditioning of skeletal responses such as NMR conditioning (Kehoe & Macrae, 1994; Lennartz & Weinberger, 1992; Powell & Levine-Bryce, 1988; Schneiderman, 1972; Weidemann & Kehoe, 2003), it also seems to be more ephemeral. HR conditioning may actually decrease with repeated pairings (Powell & Levine-Bryce, 1988; Schneiderman, 1972) and weaken with no further pairings (Burhans et al., 2010). Although previous data suggest that HR CRM may be equally transient (Burhans et al., 2010), the current data suggest the resilience of the effect may be a function of US location during testing. We found that HR CRM detected near the eye was totally absent a week after conditioning but HR CRM detected on the back persisted. This difference was indifferent to the US location used to obtain HR conditioning.
We have shown previously that both HR and NMR CRM are unequivocally associative in nature. In the present experiment, HR conditioning with shock to the back produced identical terminal HR conditioning levels to those seen as a result of shock near the eye suggesting HR conditioning was associative. Given the strong suggestion, based on topography and unpaired controls, that HR CRM is at least in part a generalized HR CR (Burhans et al., 2008), we believe HR CRM to the back is also associative. Finally, the transfer of HR CRM from one location during training to the other location during testing also suggests that HR CRM is associative.
Hear rate conditioning has been used not only to study autonomic conditioning but also as an index of conditioned fear (Carrive, 2000; McEchron, Cheng, & Gilmartin, 2004; Nijsen et al., 1998; Tovote et al., 2005; Winters, McCabe, & Schneiderman, 2002). The study of fear and its conditioning are important to understanding disorders of anxiety and stress and in particular post traumatic stress disorder (Blechert et al., 2007; Hofmann, 2008; McNally, 2007; Milad et al., 2009; Norrholm et al., 2011; Siegmund & Wotjak, 2007). Indeed, the extinction of conditioned fear has become a focus for strategies to treat the growing incidence of post traumatic stress (Milad et al., 2009; Norrholm et al., 2011; Rauch, Shin, & Phelps, 2006; Yehuda & LeDoux, 2007). We have shown previously that HR conditioning can be extinguished but that there are significant between-subject differences in the extinction of HR CRM (Burhans et al., 2010). Persistence of CRM may be therefore be a model for some features of PTSD such as individual differences in susceptibility (Burhans et al., 2008). The present data suggest that the mode of assessment may be an important determinant of CRM persistence. The data also make the point that over-generalization of URs to USs similar to the trauma may be an important aspect of PTSD and this effect can be modeled by HR CRM.
Acknowledgments
This work was supported by NIMH grant MH081159 and the Blanchette Rockefeller Neurosciences Institute. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIMH.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Reference List
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential and behavioural responses. Behaviour Research and Therapy. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Brunijnzeel AW, Stam R, Croiset G, Wiegant VM. Long-term sensitization of cardiovascular stress responses after a single stressful experience. Physiology & Behavior. 2001;73:81–86. doi: 10.1016/s0031-9384(01)00435-8. [DOI] [PubMed] [Google Scholar]
- Buck DL, Seager MA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: generality and nature of the phenomenon. Behavioral Neuroscience. 2001;115:1039–1047. [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit’s nictitating membrane response and heart rate: behavioral rules, neural substrates, and potential applications to post-traumatic stress disorder. Behavioral Neuroscience. 2008;122:1191–1206. doi: 10.1037/a0013599. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, Schreurs BG. Effects of extinction on classical conditioning and conditioning-specific reflex modification of rabbit heart rate. Behavioural Brain Research. 2010;206:127–134. doi: 10.1016/j.bbr.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burriss L, Ayers E, Powell DA. Combat veterans show normal discrimination during differential trace eyeblink conditioning, but increased responsivity to the conditioned and unconditioned stimulus. Journal of Psychiatric Research. 2007;41:785–794. doi: 10.1016/j.jpsychires.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Carrive P. Conditioned fear to environmental context: cardiovascular and behavioral components in the rat. Brain Research. 2000;858:440–445. doi: 10.1016/s0006-8993(00)02029-1. [DOI] [PubMed] [Google Scholar]
- Cohen DH, Pitts LH. Vagal and sympathetic components of conditioned cardioacceleration in the pigeon. Brain Research. 1968;9:15–31. doi: 10.1016/0006-8993(68)90255-2. [DOI] [PubMed] [Google Scholar]
- Elsesser K, Sartory G, Tackenberg A. Attention, heart rate, and startle response during exposure to trauma-related pictures: a comparison of recent trauma victims and patients with posttraumatic stress disorder. Journal of Abnormal Psychology. 2004;113:289–301. doi: 10.1037/0021-843X.113.2.289. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RD, Martin GK. Heart-rate conditioning in rats as a function of interstimulus interval. Psychological Reports. 1971;29:1103–1110. doi: 10.2466/pr0.1971.29.3f.1103. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Frysinger RC, Rapp PR. B-Adrenergic manipulation in amygdala central N. alters rabbit heart rate conditioning. Pharmacology Biochemistry & Behavior. 1980;12:419–426. doi: 10.1016/0091-3057(80)90047-7. [DOI] [PubMed] [Google Scholar]
- Gantt WH. Cardiovascular component of the conditional reflex to pain, food and other stimuli. Physiological Reviews Supplement. 1960;4:266–291. [PubMed] [Google Scholar]
- Ghelarducci B, Sebastiani L. Classical heart rate conditioning and affective behavior: the role of the cerebellar vermis. Archives Italiennes de Biologie. 1997;135:369–384. [PubMed] [Google Scholar]
- Graham FK. Constraints on measuring heart rate and period sequentially through real and cardiac time. Psychophysiology. 1978;15:492–495. doi: 10.1111/j.1469-8986.1978.tb01422.x. [DOI] [PubMed] [Google Scholar]
- Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Experimental Brain Research. 1995;104:431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear conditioning and extinction in animals and humans: implications for exposure therapy for anxiety disorders. Clinical Psychology Review. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiology & Behavior. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Kazis E, Milligan WL, Powell DA. Autonomic-somatic relationships: blockade of heart rate and corneo-retinal potential responses. Journal of Comparative and Physiological Psychology. 1973;84:98–110. doi: 10.1037/h0035027. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Macrae M. Classical conditioning of the rabbit nictitating membrane can be fast or slow: implications for Lennartz and Weinberger’s (1992) two-factor theory. Psychobiology. 1994;22:1–4. [Google Scholar]
- Kehoe EJ, Palmer N, Weidemann G, Macrae M. The effect of feature-target intervals in conditional discriminations on acquisition and expression of conditioned nictitating membrane and heart rate responses in the rabbit. Animal Learning & Behavior. 2000;28:80–91. [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neuroscience and Biobehavioral Reviews. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavond DG, McCormick DA, Thompson RF. A nonrecoverable learning deficit. Physiological Psychology. 1984;12:103–110. [Google Scholar]
- Lennartz RC, Weinberger NM. Analysis of response systems in Pavlovian conditioning reveals rapidly versus slowly acquired conditioned responses: support for two factors, implications for behavior and neurobiology. Psychobiology. 1992;20:93–119. [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, et al. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behaviour Research and Therapy. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Cheng AY, Gilmartin MR. Trace fear conditioning is reduced in the aging rat. Neurobiology of Learning and Memory. 2004;82:71–76. doi: 10.1016/j.nlm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N. Air puff versus shock unconditioned stimuli in rabbit heart rate conditioning. Physiology & Behavior. 1991;51:195–199. doi: 10.1016/0031-9384(92)90223-o. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. Journal of Neuroscience. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clinical Psychology Review. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;12:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijsen MJMA, Croiset G, Diamant M, Stam R, Delsing D, de Wied D, et al. Conditioned fear-induced tachycardia in the rat; vagal involvement. European Journal of Pharmacology. 1998;350:211–222. doi: 10.1016/s0014-2999(98)00261-1. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in truamatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic tress disorder. Psychiatric Clinics of North America. 2002;25:271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Powell DA, Churchwell J, Burriss L. Medial prefrontal lesions and Pavlovian eyeblink and heart rate conditioning: effects of partial reinforcement on delay and trace conditioning in rabbits (Oryctolagus cuniculus) Behavioral Neuroscience. 2005;119:180–189. doi: 10.1037/0735-7044.119.1.180. [DOI] [PubMed] [Google Scholar]
- Powell DA, Kazis E. Blood pressure and heart rate changes accompanying classical eyeblink conditioning in the rabbit (Oryctolagus cuniculus) Psychophysiology. 1976;13:441–447. doi: 10.1111/j.1469-8986.1976.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Powell DA, Levine-Bryce D. A comparison of two model systems of associative learning: heart rate and eyeblink conditioning in the rabbit. Psychophysiology. 1988;25:672–682. doi: 10.1111/j.1469-8986.1988.tb01906.x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research - past, present, and future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Schneiderman N. Response system divergencies in aversive classical conditioning. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 341–376. [Google Scholar]
- Schneiderman N, Smith MC, Smith AC, Gormezano I. Heart rate classical conditioning in rabbits. Psychonomic Science. 1966;6:241–242. [Google Scholar]
- Schreurs BG. Classical conditioning and modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Behavioral and Cognitive Neuroscience Reviews. 2003;2:83–96. doi: 10.1177/1534582303255014. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Crum JM, Wang D, Smith-Bell CA. Conditioning-specific reflex modification of rabbit (Oryctolagus cuniculus) heart rate. Behavioral Neuroscience. 2005;119:1484–1495. doi: 10.1037/0735-7044.119.6.1484. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gonzales-Joekes J, Smith-Bell CA. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response is sensitive to context. Learning & Behavior. 2006;34:315–324. doi: 10.3758/bf03192886. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Oh MM, Hirashima C, Alkon DL. Conditioning-specific modification of the rabbit’s unconditioned nictitating membrane response. Behavioral Neuroscience. 1995;109:24–33. doi: 10.1037//0735-7044.109.1.24. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Shi T, Pineda SI, Buck DL. Conditioning the unconditioned response: modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:144–156. doi: 10.1037//0097-7403.26.2.144. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA. Heart rate changes during conditioning-specific reflex modification of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response. Neurobiology of Learning and Memory. 2005;84:148–158. doi: 10.1016/j.nlm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Darwish DS, Stankovic G, Sparks DL. High dietary cholesterol facilitates classical conditioning of the rabbit’s nictitating membrane response. Nutritional Neuroscience. 2007;10:31–43. doi: 10.1080/10284150701232034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Darwish DS, Wang D, Burhans L, Gonzales-Joekes J, et al. Cholesterol enhances classical conditioning of the rabbit heart rate response. Behavioural Brain Research. 2007;181:52–63. doi: 10.1016/j.bbr.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie SR. Unconditional stimulus intensity and cardiac conditioning in the goldfish (Carassius auratus) Physiology & Behavior. 1973;11:31–34. doi: 10.1016/0031-9384(73)90118-2. [DOI] [PubMed] [Google Scholar]
- Seager MA, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: US intensity effects. Learning & Behavior. 2003;31:292–298. doi: 10.3758/bf03195990. [DOI] [PubMed] [Google Scholar]
- Sebastiani L, La Noce A, Paton JFR, Ghelarducci B. Influence of the cerebellar posterior vermis on the acquisition of the classically conditioned bradycardic response in the rabbit. Experimental Brain Research. 1992;88:193–198. doi: 10.1007/BF02259141. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. Journal of Psychiatric Research. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Stebbins WC, Smith OA., Jr Cardiovascular concomitants of the conditioned emotional response in the monkey. Science. 1964;144:881–883. doi: 10.1126/science.144.3620.881. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Tovote P, Ogren SO, Meyer M. Behavioral and autonomic dynamics during contextual fear conditioning in mice. Autonomic Neuroscience: Basic and Clinical. 2004;115:15–27. doi: 10.1016/j.autneu.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Supple WF, Jr, Kapp BS. The anterior cerebellar vermis: Essential involvement in classically conditioned bradycardia in the rabbit. Journal of Neuroscience. 1993;13:3705–3711. doi: 10.1523/JNEUROSCI.13-09-03705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supple WF, Jr, Sebastiani L, Kapp BS. Purkinje cell responses in the anterior cerebellar vermis during Pavlovian fear conditioning in the rabbit. Neuro Report. 1993;4:975–978. doi: 10.1097/00001756-199307000-00035. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Schneiderman N. Stimulus generalization and transfer of training in rabbits conditioned to electrical stimulation of lateral geniculate nucleus. Physiology & Behavior. 1970;5:841–847. doi: 10.1016/0031-9384(70)90169-1. [DOI] [PubMed] [Google Scholar]
- Tovote P, Meyer M, Pilz PKD, Ronnenberg A, Ogren SO, Spiess J, et al. Dissociation of temporal dynamics of heart rate and blood pressure responses elicited by conditioned fear but not acoustic startle. Behavioral Neuroscience. 2005;119:55–65. doi: 10.1037/0735-7044.119.1.55. [DOI] [PubMed] [Google Scholar]
- VanDercar DH, Elster AJ, Schneiderman N. Heart-rate classical conditioning in rabbits to hypothalamic or septal US stimulation. Journal of Comparative and Physiological Psychology. 1970;72:145–152. doi: 10.1037/h0029309. [DOI] [PubMed] [Google Scholar]
- Weidemann G, Kehoe EJ. Savings in classical conditioning in the rabbit as a function of extended extinction. Learning & Behavior. 2003;31:49–68. doi: 10.3758/bf03195970. [DOI] [PubMed] [Google Scholar]
- Wikgren J, Ruusuvirta T, Korhonen T. Reflex facilitation during eyeblink conditioning and subsequent interpositus nucleus inactivation in the rabbit (Oryctolagus cuniculus) Behavioral Neuroscience. 2002;116:1052–1058. doi: 10.1037//0735-7044.116.6.1052. [DOI] [PubMed] [Google Scholar]
- Winters RW, McCabe PM, Schneiderman N. Functional utility and neurobiology of conditioned autonomic responses. In: Moore JW, editor. A neuroscientist’s guide to classical conditioning. New York: Springer; 2002. pp. 46–85. [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]