Abstract
Background
Mortality among long-term hemodialysis patients is high, mostly attributed to cardiovascular events, and may be related to chronic inflammation. We hypothesized that the anti-inflammatory benefits of higher dietary omega-3, compared to omega-6, poly-unsaturated fatty acids may modulate the inflammatory processes and reduce death risk.
Study design
Prospective cohort study using linear and Cox proportional regressions.
Setting and Participants
145 hemodialysis patients from 8 DaVita dialysis clinics in Southern California during 2001-2007
Predictors
Intake of dietary omega-3 and ratio of omega-6 to omega-3 using 3-day food record supplemented by dietary interview.
Outcomes
One-year change in serum C-reactive protein (CRP) and 6-year survival.
Results
Patients were 53±14 years old (mean±SD) and included 43% women and 42% African-Americans. Median (25th-75 percentile) of dietary omega-3 intake, ratio of omega-6 to omega-3 intake, baseline serum CRP, and change in CRP over one year were 1.1(0.8-1.6) g/day, 9.3(7.6-11.3), 3.1(0.8-6.8) mg/L, and +0.2(−0.4 to +0.8) mg/L, respectively. In regression models adjusted for case-mix, dietary calorie and fat intake, body mass index and history of hypertension, each 1-unit higher ratio of omega-6 to omega-3 intake was associated with 0.55 mg/L increase in serum CRP (p=0.03). In fully adjusted model, the death hazard ratios (95% confidence interval) for the 1st(1.7-<7.6) 2nd(7.6-<9.3), 3rd(9.3-<11.3) and 4th(11.3-17.4) quartiles of dietary omega-6 to omega-3 ratio were 0.39(0.14-1.18), 0.30(0.09-0.99), 0.67(0.25-1.79) and 1.00(reference), respectively (p-for-trend=0.06).
Limitations
Three-day food record may underestimate actual dietary fat intake at individual level.
Conclusions
Higher dietary omega-6 to omega-3 ratio appears associated with both worsening inflammation over time and a trend towards higher death risk in hemodialysis patients. Additional studies including interventional trials are needed to examine the association of dietary fatty acids with clinical outcomes in these patients.
Keywords: Chronic kidney disease (CKD), hemodialysis, nutritional status, inflammation, mortality, dietary omega-3, omega-6 to omega-3 ratio, poly-unsaturated fatty acids
The mortality rate among individuals with chronic kidney disease (CKD) who receive maintenance hemodialysis (HD) remains high.(1) Cardiovascular disease, protein energy wasting (PEW), and inflammation are among the strongest predictors of mortality in these patients.(2) Maintenance HD patients with lower serum albumin levels have higher serum concentrations of C-reactive protein (CRP), suggesting the potential role of pro-inflammatory processes in the pathogenesis of PEW.(3) Although PEW is a multifactorial disorder, chronic inflammation seems to have an important role in its development.
Dietary omega-3 polyunsaturated fatty acids (PUFAs) have anti-inflammatory properties that can be protective against atherosclerosis and its consequences of myocardial infarction, sudden death, and stroke.(4) Additionally, omega-3 may have a broad range of beneficial effects on levels of triglycerides, high-density lipoprotein (HDL) cholesterol, and oxidative stress; blood pressure; cardiac excitability; and function of platelets, endothelium and vasculature, and the immune system.(4, 5) A study by Friedman et al showed that dietary fish consumption, a main source of omega-3, in maintenance HD patients was far below current American Heart Association (AHA) recommendations, leading to suboptimal omega-3 levels.(6) Given the evidence relating CKD to chronic inflammation(7, 8) and given that half of all CKD deaths are attributed to cardiovascular disease,(9) the anti-inflammatory and cardio-protective benefits of dietary omega-3 may play an important role in modifying the inflammatory processes and thus improving outcomes.
Anthropological, epidemiological, and biochemical studies indicate that human beings evolved on a diet with an equal proportion of omega-6 to omega-3 PUFAs. Over the past 100 years, however, the ratio of omega-6 to omega-3 in Western diets has dramatically increased to ranges of 15/1 or even higher.(10-14) A high dietary omega-6 to omega-3 ratio may contribute to cardiovascular disease, cancer, osteoporosis, and autoimmune disorders. In contrast, higher omega-3 intake or lower omega-6 to omega-3 ratio in diet may exert suppressive effects on the same disease states.(13, 14) To the best of our knowledge, the amount of dietary omega-3 intake or the ratio of omega-6 to omega-3 and their associations with clinical outcomes including survival in CKD patients have not been evaluated. We hypothesized that the anti-inflammatory and cardio-protective benefits of dietary omega-3, compared to omega-6 PUFAs, may modulate the inflammatory processes and reduce death risk in maintenance HD patients. The present study was designed to determine the associations of dietary omega-3 and ratio of omega-6 to omega-3 and mortality in a cohort of maintenance HD patients.
Methods
Patient Population
We studied maintenance HD patients who participated in the NIH-funded NIED (Nutritional and Inflammatory Evaluation in Dialysis) Study.(15-22) The original patient cohort was derived from a pool of approximately 1,300 maintenance HD outpatients in eight DaVita dialysis clinics from the South Bay Los Angeles area. Inclusion criteria were outpatients who had been undergoing maintenance HD for at least 8 weeks, who were 18 years or older and who signed a local Institutional Review Board approved consent form. From October 1, 2001, through September 30, 2007, a total of 893 maintenance HD patients from the eight dialysis clinics gave written informed consent form and underwent the study. Dietary intakes of 145 randomly selected patients were assessed with a 3-day dietary record, accompanied by a diet interview, during the first 6 months of the study (see below). These 145 patients were followed for up to 72 months, i.e. until September 30, 2007. The medical chart of each maintenance HD patient was extensively reviewed by a collaborating physician, and data pertaining to underlying kidney disease and other comorbid conditions were extracted. A modified version of the Charlson comorbidity index was used to assess the severity of comorbidities.(23)
Anthropometric and Body Composition Measures
Anthropometric measurements were performed while patients were undergoing a maintenance HD treatment. Biceps and triceps skinfold thickness was measured with a conventional skinfold caliper using standard techniques as previously described.(20, 21) To estimate the percentage of body fat, portable near infrared (NIR) interactance was utilized at the same time as the anthropometric measurements.(20, 21, 24)
Laboratory Tests
Predialysis blood samples and postdialysis serum urea nitrogen were obtained on a mid-week day. The single-pool Kt/V was used to represent the weekly dialysis dose.(25) Except as indicated below, all laboratory measurements were performed by DaVita Laboratories (www.davita.com) using automated methods. In this study, 3-month averaged values were used, and all laboratory measurements used established assays with well-known coefficients of variation. Serum high sensitivity CRP was measured by a turbidimetric immunoassay (WPCI, Osaka, Japan; reference range: < 3.0 mg/l).(26, 27) Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) were measured with immunoassay kits (R&D Systems, www.rndsystems.com); reference ranges, < 9.9 pg/ml for IL-6 and < 4.7 pg/ml for TNF-α).(28, 29)
Three-Day Food Record with Diet Interview
A 3-day diet diary with diet interviews by a trained dietitian was used to assess usual dietary intake of the subjects participating in the NIED Sub-Study in GCRC. Dietary intake was recorded over the last hemodialysis treatment day of the week and the two adjacent non-dialysis days.(30, 31) All participants received standardized instructions on how to complete the 3 day diet record by trained dietitians. The reliability of the estimates of the dietary omega-3 intake derived from food records has been judged acceptable according to the EURopean micronutrient RECommendations Aligned (EURRECA) scoring system.(32) The instructions included the viewing of a 14 minute videotape, entitled on the Record.(33) The dietitians also reviewed additional instructional material with the participants to guide the details and types of information to be reported. The participants completed the 3 day food record and then returned to the study center for a supplementary dietary interview, in which the trained dietitian reviewed the records and obtained additional relevant information. The American Dietetic Association (ADA) Portion Photos of Popular Foods book was used to assist the dietitian in the review of the food records for accuracy. The dietitian made edits, corrections, and additions on the food record and used the Minnesota Nutrient Data System software (version 2005 Nutrition Coordinating Center, Minneapolis, Min) to complete the nutrient analysis.
Statistical Methods
Changes in serum CRP over the first year and mortality over 6 years were the main outcome measures. We used linear regression analyses to examine the coefficient of change in CRP over time based on dietary omega-3 or the ratio of omega-6 to 3 intake. Cox-regression-based restricted cubic splines graph with two degrees of freedom were employed to illustrate systematic relations between dietary intakes and mortality. This method also served to examine the non-linear associations as continuous mortality predictors as an alternative to potential inappropriate assumptions concerning linearity.(34) Death hazard ratios were obtained using Cox proportional hazard models after controlling for covariates. We performed incremental levels of multivariate adjustment: (A) Case-mix variables included age, gender, race/ethnicity, diabetes mellitus, dialysis vintage, insurance (Medicare vs. others), marital status, modified Charlson comorbidity score and dialysis dose (single pool Kt/V). (B) Dietary intake variables included dietary intake of energy, saturated fat, trans fatty acids, cholesterol and fiber. (C) History of hypertension and body mass index (BMI). Descriptive and multivariate statistics were carried out with the statistical software Stata (version 10.0; Stata Corporation, www.stata.com).
Results
Baseline demographic, clinical, and laboratory values in the 145 maintenance HD patients are shown in Table 1. The patients’ mean age (±SD) was 53±14 years; 43% of patients were women (n=62) and 42% (n=61) African-American. The mean dialysis vintage was 31±33 months (median, 19 months). Median of baseline serum CRP, was 3.1 (25th-75th percentile, 0.8-6.8) mg/L. After ranking subjects according to their dietary intakes, we categorized them into quartiles of dietary omega-3 and also quartiles of the ratio of omega-6 to omega-3. Table 1 shows relevant demographic, clinical and laboratory measures for the four quartiles. The proportions of women and African Americans were higher in the groups with lower and higher dietary omega-3 intake, respectively.
Table 1.
Baseline demographic, clinical, and laboratory values in study participants
| Dietary Omega-3 Intake | ||||||
|---|---|---|---|---|---|---|
| Quartile of Omega-3 intake | P for trend |
|||||
| All Patients | 1 (0.1<0.8 mg/d) |
2 (0.8-<1.1 mg/d) |
3 (1.1-<1.6 mg/d) |
4 (1.6-3.5 mg/d) |
||
| No. | 145 | 37 | 36 | 37 | 35 | |
| Age, years | 53±14 | 52±15 | 52±15 | 54±14 | 53±12 | 0.8 |
| Women | 43 | 51 | 42 | 40 | 37 | 0.2 |
| African-American | 42 | 32 | 45 | 38 | 54 | 0.1 |
| History of hypertension | 16 | 16 | 18 | 11 | 17 | 0.8 |
| Diabetes mellitus | 55 | 51 | 55 | 57 | 57 | 0.5 |
| Charlson comorbidity score | 1.9±1.7 | 1.6±1.5 | 2.0±1.6 | 2.2±2.0 | 1.9±1.5 | 0.8 |
| Body mass index, kg/m2 | 26.1±5.9 | 24.5±4.4 | 27.5±7.4 | 26.9±5.9 | 25.6±5.0 | 0.4 |
| Percentage body fat* | 25.5±10.2 | 24.5±10.0 | 27.2±11.2 | 26.2±10.0 | 24.0±9.6 | 0.6 |
| Dialysis vintage, months | 31±33 | 29±27 | 36±36 | 31±34 | 29±35 | 0.7 |
| Dialysis dose, spKt/Vurea | 1.67±0.30 | 1.68±0.33 | 1.71±0.28 | 1.63±0.25 | 1.64±0.35 | 0.2 |
| Serum albumin, g/dl | 3.97±0.31 | 3.91±0.32 | 4.02±0.21 | 4.02±0.22 | 4.04±0.31 | 0.07 |
| Serum creatinine, mg/dl | 10.7±3.2 | 10.6±2.7 | 10.7±3.1 | 10.6±3.5 | 11.0±3.5 | 0.4 |
| Serum total homocysteine, μmol/l | 25.6±8.7 | 25.3±10.2 | 25.4±8.1 | 24.0±8.7 | 27.6±7.6 | 0.3 |
| Serum CRP, mg/l | 5.4±7.3 | 5.1±5.1 | 7.0±11.6 | 5.1±5.3 | 4.4±4.4 | 0.6 |
| Serum IL-6, pg/ml | 18.4±60.9 | 11.7±16.6 | 30.0±27.0 | 12.7±31.0 | 18.8±37.3 | 0.6 |
| Serum TNF-α , pg/ml | 7.4±7.7 | 7.0±6.8 | 7.5±6.5 | 9.0±11.2 | 5.8±4.7 | 0.4 |
| Dietary Omega-6 to Omega-3 Ratio | |||||
|---|---|---|---|---|---|
| Quartile of Omega-6 to Omega-3 Ratio | P for trend |
||||
| 1 (1.7-<7.6) |
2 (7.6-<9.3) |
3 (9.3-<11.3) |
4 (11.3-17.4) |
||
| No. | 37 | 36 | 36 | 36 | |
| Age, years | 55±12 | 53±15 | 53±13 | 50±14 | 0.1 |
| Women | 43 | 47 | 42 | 39 | 0.6 |
| African-American | 32 | 47 | 53 | 39 | 0.5 |
| History of hypertension | 11 | 19 | 11 | 19 | 0.5 |
| Diabetes mellitus | 62 | 56 | 53 | 50 | 0.3 |
| Charlson comorbidity score | 1.8±1.4 | 2.1±1.8 | 1.7±1.8 | 1.8±1.6 | 0.6 |
| Body mass index, kg/m2 | 25.6±5.2 | 26.5±6.4 | 27.2±6.0 | 25.2±6.2 | 0.6 |
| Percentage body fat* | 26.1±10.1 | 26.6±10.4 | 25.8±10.5 | 23.9±9.9 | 0.3 |
| Dialysis vintage, months | 19.6±15.5 | 27.5±42.4 | 33.1±40.6 | 34.5±25.5 | 0.01 |
| Dialysis dose, spKt/Vurea | 1.7±0.3 | 1.7±0.3 | 1.6±0.2 | 1.6±0.3 | 0.3 |
| Serum albumin, g/dl | 3.96±0.32 | 3.96±0.32 | 3.98±0.22 | 3.98±0.25 | 0.7 |
| Serum creatinine, mg/dl | 10.5±3.8 | 11.0±3.3 | 10.7±3.2 | 11.0±2.5 | 0.6 |
| Serum total homocysteine, μmol/l | 26.8±7.9 | 25.4±7.8 | 25.1±9.1 | 24.6±9.1 | 0.1 |
| Serum CRP, mg/l | 3.8±3.3 | 4.2±3.7 | 8.2±12.2 | 5.1±6.0 | 0.4 |
| Serum IL-6, pg/ml | 25.5±10.7 | 25.1±25.3 | 13.9±23.7 | 8.2±8.6 | 0.4 |
| Serum TNF-α , pg/ml | 6.3±3.9 | 9.3±12.9 | 6.3±4.0 | 7.8±6.7 | 0.5 |
Note: values shown are percentage or mean +/− SD.
measured by NIR interactance
Abbreviations: IL-6: interleukin-6, TNF: tumor necrosis factor; CRP, C-reactive protein.
Conversion factors for units: albumin in g/dL to g/L, ×10; creatinine in mg/dL to umol/L, ×88.4; total homocysteine in umol/L to mg/dL, ×0.14.
Table 2 shows the dietary intakes of 145 maintenance HD patients according to the quartiles of their dietary omega-3 intakes and ratio of omega-6 to omega-3. Median of dietary omega-3 intake and the ratio of omega-6 to omega-3 intake were 1.1 (25th-75th percentile, 0.8-1.6) g/day and 9.3 (25th-75th percentile, 7.6-11.3), respectively. Subjects with higher omega-3 intakes had higher intakes of energy, total fat, saturated fatty acids, monounsaturated fatty acids, PUFAs, omega-6 fatty acids, cholesterol and fiber, but lower omega-6 to omega-3 ratios. Subjects with higher ratio of omega-6 to omega-3 had higher intakes of monounsaturated fatty acids and omega-6 and lower intakes of omega-3.
Table 2.
Baseline dietary intakes of study participants
| Dietary Omega-3 Intake | ||||||
|---|---|---|---|---|---|---|
| All Patients | Quartile of Omega-3 intake | P for trend |
||||
| 1 (0.1<0.8 mg/d) |
2 (0.8-<1.1 mg/d) |
3 (1.1-<1.6 mg/d) |
4 (1.6-3.5 mg/d) |
|||
| No. | 145 | 37 | 36 | 37 | 35 | |
| Energy intake(kilocalorie/day) | 1657±639 | 1151±352 | 1558±502 | 1700±450 | 2254±687 | 0.002 |
| Total fat intake (g/day) | 64±29 | 39±15 | 58±21 | 66±20 | 92±30 | 0.003 |
| Saturated fat intake (g/day) | 20±11 | 12±5 | 19±9 | 21±9 | 27±13 | 0.003 |
| PUFA intake (g/day) | 13.2±6.7 | 7.3±2.6 | 10.8±0.1 | 13.5±3.7 | 21.5±6.7 | 0.006 |
| Omega-3 intake (g/day) | 1.3±0.7 | 0.6±0.2 | 1.0±0.1 | 1.4±0.2 | 2.3±0.5 | 0.001 |
| Omega-6 intake (g/day) | 11.9±6.0 | 6.7±2.4 | 10.2±2.7 | 12.1±3.7 | 19.1±6.5 | 0.009 |
| Ratio of omega-6 to omega-3 | 9.7±3.1 | 11.7±3.6 | 10.3±2.7 | 8.7±2.6 | 8.3±2.3 | 0.009 |
| MUFA intake(g/day) | 24.3±11.2 | 15.4±6.4 | 22.5±9.2 | 25.1±7.5 | 34.6±12.0 | 0.002 |
| Cholesterol intake(mg/day) | 287±154 | 221±133 | 242±128 | 321±143 | 368±169 | 0.001 |
| Fiber intake(g/day) | 13.1±6.7 | 11.2±5.8 | 12.8±7.3 | 11.6±4.9 | 16.9±7.2 | 0.009 |
| Dietary Omega-6 to Omega-3 Ratio | |||||
|---|---|---|---|---|---|
| Quartile of Omega-6 to Omega-3 Ratio | P for trend |
||||
| 1 (1.7-<7.6) |
2 (7.6-<9.3) |
3 (9.3-<11.3) |
4 (11.3-17.4) |
||
| No. | 37 | 36 | 36 | 36 | |
| Energy intake(kilocalorie/day) | 1592±525 | 1652±715 | 1710±585 | 1716±714 | 0.5 |
| Total fat intake (g/day) | 58±26 | 65±33 | 67±23 | 68±32 | 0.09 |
| Saturated fat intake (g/day) | 18.0±9.0 | 20.0±12.0 | 20.4±7.9 | 21.9±12.8 | 0.08 |
| PUFA intake (g/day) | 12.0±5.5 | 13.5±7.2 | 14.1±5.4 | 13.8±8.2 | 0.1 |
| Omega-3 intake (g/day) | 2.0±1.6 | 1.4±0.8 | 1.3±0.5 | 1.0±0.6 | 0.001 |
| Omega-6 intake (g/day) | 10.3±4.8 | 12.0±6.4 | 12.7±4.9 | 13.2±7.4 | 0.03 |
| Ratio of omega-6 to omega-3 | 6.0±1.6 | 8.5±0.4 | 10.1±0.5 | 13.6±1.7 | <0.001 |
| MUFA intake(g/day) | 22.2±10.9 | 24.7±12.9 | 25.6±9.6 | 26.0±11.1 | 0.05 |
| Cholesterol intake(mg/day) | 327±112 | 266±161 | 280±159 | 289±179 | 0.1 |
| Fiber intake(g/day) | 12.4±6.7 | 13.2±6.0 | 11.8±5.8 | 14.9±7.9 | 0.3 |
Note: values shown are percentage or mean +/− SD.
PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid
In order to examine the association of omega-3 intake and the ratio of omega-6 to omega-3 with changes in inflammatory status over time, we studied changes in CRP levels over the first 12 months across quartiles of omega-3 and the ratio of omega-6 to omega-3 and examined the regression coefficients between these quartiles with changes in serum CRP. Median change in CRP over the first 12 months was +0.2 (25th-75th percentile, −0.4 to +0.8) mg/L. As shown in Table 3, in fully adjusted linear regression models each unit higher dietary omega-6 to omega-3 ratio was associated with 0.55 mg/L increase in serum CRP (p=0.03). Higher dietary omega-3 intake alone was associated with a non-significant trend towards a decrease in serum CRP.
Table 3.
association of omega 3 intake and the ratio of omega 6 to omega 3 with changes in inflammatory status
| Association of Omega-3 with Inflammatory Status | ||||
|---|---|---|---|---|
| Quartile of Omega-3 intake | ||||
| 1 (<0.8 mg/d) |
2 (0.8-<1.1 mg/d) |
3 (1.1-<1.6 mg/d) |
4 (1.6-<3.5 mg/d) |
|
| No. of patients | 37 | 36 | 37 | 35 |
| CRP change * (mg/dl) | +1.0 (+0.2 to +1.8) | +0.6 (−0.6 to +1.8) | −0.7 (−1.7 to +0.3) | +0.9 (−0.4 to +1.9) |
| Association of Omega-6 to Omega-3 Ratio with Inflammatory Status | ||||
|---|---|---|---|---|
| Quartile of Omega-6 to Omega-3 Ratio | ||||
| 1 (1.7<7.6) |
2 (7.6-<9.3) |
3 (9.3-<11.3) |
4 (11.3-17.4) |
|
| No. of patients | 37 | 36 | 36 | 36 |
| CRP change* (mg/dl) | −0.4 (−1.0 to +0.2) | +0.4 (−0.4 to +1.2) | −0.2 (−1.4 to +1.0) | +1.5 (+0.3 to +2.7) |
| ANCOVA and Correlation Coefficients | ||
|---|---|---|
| P for ANCOVA | Beta for regression (p-value) | |
| Dietary Omega-3 | ||
| after adjustment for case-mix** | 0.4 | −0.58 (0.2) |
| after adjustment for case-mix and diet | 0.7 | −0.62 (0.3) |
| after adjustment for case-mix , diet, BMI, HTN, and baseline CRP |
0.9 | −0.58 (0.3) |
| Dietary Omega-6 to Omega-3 Ratio | ||
| after adjustment for case-mix | 0.2 | +0.52 (0.09) |
| after adjustment for case-mix and diet | 0.1 | +0.50 (0.04) |
| after adjustment for case-mix , diet, BMI, HTN, and baseline CRP |
0.5 | +0.55 (0.03) |
CRP change= CRP after one year minus baseline CRP; values shown are mean (95% confidence interval).
Case-mix = age, gender, race/ethnicity, diabetes, dialysis vintage, modified Charlson comorbidity score, dialysis dose (Kt/V); diet, intakes of energy, saturated fat, trans fat, cholesterol and fiber.
Abbreviations and definitions: HTN= history of hypertension; BMI, body mass index; HR, hazard ratio; CI, confidence interval; CRP, C-reactive protein; ANCOVA, analysis of covariance
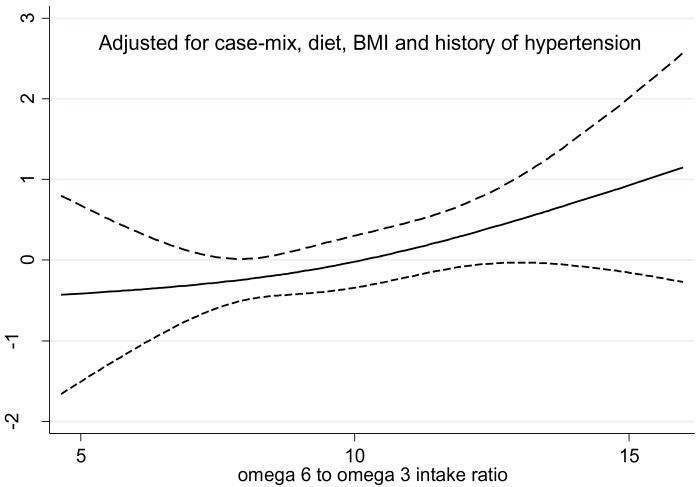
Over the 6 years of the cohort follow up, 42 (29%) patients died. We examined death hazard ratios across the quartiles as shown in Table 4 and 5. Taking the highest quartile as the reference, the lower quartile of omega-6 to omega-3 ratio was associated with lower death risk (p for trend of 0.06 in fully adjusted models, Table 5). We also adjusted for the interaction between dialysis vintage and the ratio of omega-6 to omega-3, which showed similar associations (data not shown). Figure 1 shows the cubic splines graph illustrating the multivariate adjusted association between baseline the ratio of omega-6 to omega-3 intake ratio and mortality. A trend towards increased risk of death was observed in maintenance HD patients with higher omega-6 to omega-3 intake ratios.
Table 4.
HR of 6-year mortality by quartile of dietary omega-3
| Quartile of omega-3 intake | Across quartiles | P for trend |
||||
|---|---|---|---|---|---|---|
| 1 (n=37) |
2 (n=36) |
3 (n=36) |
4 (n=36) |
|||
| Age and gender adjusted |
||||||
| HR (95% CI) | 1.00 (ref) | 1.02(0.47-2.23) | 0.39(0.15-0.98) | 0.65(0.28-1.54) | 0.78(0.59-1.04) | |
| P value | 0.9 | 0.04 | 0.3 | 0.09 | ||
| Case-mix + diet adjusted |
||||||
| HR (95% CI) | 1.00 (ref | 1.39(0.60-3.23) | 0.28(0.10-0.87) | 0.65(0.21-1.97) | 0.75(0.52-1.09) | |
| P value | 0.4 | 0.02 | 0.2 | 0.1 | ||
| Case-mix + BMI + HTN |
||||||
| HR (95% CI) | 1.00 (ref) | 1.69(0.69-4.13) | 0.30(0.10-0.93) | 1.10(0.22-2.21) | 0.76(0.52-1.11) | |
| P value | 0.2 | 0.04 | 0.5 | 0.2 | ||
Note: for 145 maintenance hemodialysis patients in October 2011 to September 2007. Case-mix= age, gender, race/ethnicity, diabetes, dialysis vintage, modified Charlson comorbidity score, dialysis dose (Kt/V); diet, intakes of energy, saturated fat, trans fat, cholesterol, and fiber.
Abbreviations and defintions: HTN= history of hypertension; BMI, body mass index; HR, hazard ratio; CI, confidence interval
Table 5.
HR of 6-year mortality by quartile of dietary omega-6 to omega-3 ratio
| Quartile of omega-6 to omega-3 ratio | Across quartiles | P for trend |
||||
|---|---|---|---|---|---|---|
| 1 (n=37) |
2 (n=36) |
3 (n=36) |
4 (n=36) |
|||
| Case-mix | ||||||
| HR (95% CI) | 0.54(0.21-1.38)] | 0.58(0.22-1.49) | 0.84(0.36-1.96) | 1.00 (ref) | 1.25(0.93-1.69) | |
| P value | 0.2 | 0.3 | 0.7 | 0.1 | ||
| Case-mix and diet adjusted |
||||||
| HR (95% CI) | 0.37(0.14-1.08) | 0.29(0.09-0.93) | 0.61(0.24-1.57) | 1.00 (ref) | 1.40(0.98-1.02) | |
| P value | 0.08 | 0.04 | 0.3 | 0.04 | ||
| Case-mix, diet, BMI, and HTN |
||||||
| HR (95% CI) | 0.39(0.14-1.18) | 0.30(0.09-0.99) | 0.67(0.25-1.79) | 1.00 (ref) | 1.38(0.96-1.99) | |
| P value | 0.1 | 0.01 | 0.4 | 0.06 | ||
Note: for 145 maintenance hemodialysis patients in October 2011 to September 2007. Case-mix= age, gender, race/ethnicity, diabetes, dialysis vintage, modified Charlson comorbidity score, dialysis dose (Kt/V); diet, intakes of energy, saturated fat, trans fat, cholesterol, and fiber.
Abbreviations: HTN= history of hypertension; BMI, body mass index; HR, hazard ratio; CI, confidence interval
Figure 1.
Cubic spline models of the Cox proportional regression analyses reflecting adjusted mortality-predictability (with 95% CI) according to omega 6 to omega 3 intake ratio in the entire cohort of 145 maintenance hemodialysis patients followed for over 6 years (from Oct. 2001 to Sep. 2007). Spline models are with 2 degrees of freedom. Case-mix variables include age, gender, race/ethnicity, diabetes mellitus, dialysis vintage, insurance, marital status, modified Charlson comorbidity score, dialysis dose (Kt/V). Diet includes intakes of saturated fat, trans-fat, cholesterol and fiber.
Discussion
Examining the association of daily intake of dietary omega-3 and the ratio of omega-6 to omega-3 PUFAs with changes in CRP over one year and survival over 6 years in 145 maintenance HD patients, we found that higher omega-6 to omega-3 ratio correlated with increased serum CRP and increased mortality. If our findings can be verified in additional studies, interventional trials are warranted to examine the effect of dietary PUFA in modulating inflammation and improving clinical outcomes of maintenance HD patients. The association of dietary omega-6 and omega-ratio and the risk of cardiovascular disease and mortality has been studied in the general population without apparent CKD.(35-39) Our study is one of the first ones to evaluate this association in advanced CKD patients who undergo maintenance HD. Although we did not directly measure markers of dietary fatty acid in plasma such as circulating omega-3 or omega-6 levels, a study by Svensson et al (40) showed a clinically meaningful correlation between dietary intake and plasma levels of omega-3 in maintenance HD patients.
In an observational study by Kutner et al higher dietary fish consumption, a rich source of omega-3 fatty acids, independently predicted patient survival in 3000 maintenance HD patients.(41) The focus of our current study, on the other hand, was the type and ratios of fatty acids rather than the kind of the ingested food such as fish. We did not find any significant association between dietary omega-3 intake per se and inflammation or mortality. There could be several reasons for our negative findings in this regard. First, there was low statistical power to find meaningful assocaitions because of the small population size and number of deaths overall. Second, the range of omega-3 levels in the dietary patterns of our study population may have been too narrow. This is due to the fact that intake was based on natural foods and not supplements. Third, our study was based on a single self-report of usual intake, a limitation that is common to most studies similar to ours. Mistakes in the record of food intake or changes in diet over time may have made it difficult to find any association between omega-3 PUFA intake and mortality. Fourth, subjects with higher omega-3 intake also consumed higher amounts of omega-6, thereby mitigating the benefit of increased omega-3 intake per se. This underscores the importance of examining the relative consumption of omega-3 vs. omega-6 PUFAs in determining the potential benefits of omega-3 intake.
There are a number of studies of the association of omega-3 and omega-6 PUFAs with inflammation. Ferruci et al.(42) suggested that a higher omega-6 to omega-3 ratio was associated with higher serum interleukin-10 concentrations and concluded that omega-3 is protective against diseases characterized by active inflammation. In observational studies of the secondary prevention of cardiovascular disease, a low omega-6 to omega-3 ratio of 2:1 to 3:1 was associated with less severe inflammation in patients with rheumatoid arthritis, and a ratio of 5:1 was associated with better outcomes in patients with asthma, whereas a ratio of 10:1 or higher was associated with adverse consequences.(12) These studies suggest that the optimal dietary ratio of omega-6 to omega-3 may vary with the disease under consideration.(43)
The effect of dietary omega-3 intake on inflammatory response has been examined in only a few studies in maintenance HD patients, and all of them have focused on omega-3 administered as a dietary supplement rather than in the natural food. Saifullah et al.(44) studied maintenance HD patients who received 1.3 g of oral eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) daily for three months. Only a modest reduction in serum CRP levels was evident in the PUFA-treated group (p=0.03).(44) Perunicic-Pekovic et al.(45) reported omega-3 PUFA depletion in the membrane phospholipid composition of maintenance HD patients. Treatment with 2.4 g of omega-3 PUFAs daily for 2 months resulted in a significant increase in erythrocyte phospholipid fatty-acid composition, a decrease in serum TNFα and IL-6, and an increase in serum albumin and hemoglobin levels (p<0.001). Kalantar-Zadeh et al.(46) demonstrated that 4-weeks of supplementation with omega-3 that contained 1.08 g EPA per day was associated with an increase in patients’ serum albumin, an important marker of nutritional or inflammatory status. In our study, an omega-6 to omega-3 PUFA ratio around 6 was associated with reduced inflammation and all-cause mortality in maintenance HD patients, providing evidence for a beneficial effect of a lower omega-6 to omega-3 PUFA ratio.
The biological mechanisms that may explain this finding are still incompletely understood. Mammalian cells cannot convert omega-6 into omega-3 PUFAs, because they lack the converting enzyme, omega-3 desaturase.(12) Vegetable oils rich in omega-6 have displaced other fats in the US diet because of the evidence of their hypocholesterolemic properties. Based on estimates derived from studies of Paleolithic nutrition and modern-day hunter-gatherers, humans evolved on a diet with substantially lower omega-6 to omega-3 PUFA ratios, indeed roughly equal amounts of omega-6 and 3.(47-49) When humans ingest omega-3, it replaces such omega-6 PUFAs as arachidonic acid in the membranes of a variety of cells, including platelets, erythrocytes, neutrophils, monocytes, and liver cells.(10)
Although the inventory of cellular proteins is determined at the gene level, the contributions of various PUFAs to cell membranes is largely dependent on dietary intake. The metabolites of PUFAs, arachidonic acid and EPA, are parent compounds for the production of mediators of immunity and inflammation, known as eicosanoids.(10) On account of the Western diet’s higher levels of omega-6 PUFAs, eicosanoid metabolic products from arachidonic acid (eg, prostaglandins, thromboxanes, leukotrienes, hydroxy fatty acids and lipoxins) are produced in greater amounts than in diets that include more omega-3 PUFAs, specifically EPA.(10) The eicosanoids from arachidonic acid have biological activity even in minute amounts, and if they occur in greater quantities they can help lead to thrombi and atheromata, the development of allergic and inflammatory disorders, and to the proliferation of cells. These changes can result in vasospasm, vasoconstriction, increased blood viscosity, and decreased bleeding time. In the study of Cleland et al.(50), 30 healthy male subjects consumed omega-3 derivatives. After 4 weeks, the incorporation of omega-3 derivatives in phospholipids of the cell membranes neutrophils was greatest in the group which consumed the lowest levels of omega-6 derivatives, suggesting that consuming omega-6 PUFAs is important in setting EPA incorporation into neutrophil membranes.
Some limitations should be considered in interpreting our findings. First, although our major interest was all-cause mortality, our ability to determine the specific causes of death associated with increased mortality were greatly diminished by the relatively small sample size and low numbers of deaths in this study. The present study lacked statistical power to conduct meaningful analyses of omega-6 to omega-3 intake and the specific cause of mortality for each. Second is our limited statistical power which may have resulted in large confidence intervals. Third is potential selection bias during enrollment, in that patients with PEW were less likely to enroll. However, selection bias in this direction would lead to bias toward the null; therefore, without this bias our results may have been even stronger. Fourth is the lack of information regarding dialysis access, dialysis membranes used, and several other known confounders, such as healthy life-styles or socioeconomic status, as well as unknown confounders. Fifth, we did not assess the food pattern of these patients, whose lower omega-6 to omega-3 ratio may have been associated with a healthier dietary pattern which could confound our results. There are several strengths to this study including the relatively long follow-up period (72 months), the comprehensive laboratory evaluations and the detailed evaluation of the clinical and comorbid states. Our cohort has been extensively characterized for markers of inflammation and nutritional status, energy intake, types of fat intakes and direct total body fat measurements. Finally, participants were selected randomly without the investigators or the patients having prior knowledge of their inflammatory status.
In conclusion our study suggests that a lower dietary omega-6 to omega-3 ratio in the ingested food may be associated with both decreased inflammation and lower mortality risk in maintenance HD patients even after adjusting for intakes of energy, saturated fatty acid, trans-fats, cholesterol and fiber. These findings, if verified in additional studies, and particularly in randomized prospective clinical trials, may imply that the lower ratios of omega-6 to omega-3 PUFA intakes should be recommended to maintenance HD patients. Future studies should also examine the association of different kinds of omega-3 PUFAs; i.e., EPA and DHA, with inflammatory status and mortality in maintenance HD patients.
Acknowledgement
Support: This study was supported by a National Institutes of Health grant (K23 DK61162 and R21 1R21DK078012-01) to Dr Kalantar-Zadeh. The authors are thankful to DaVita Wild West, Gold Coast and Surf and Sun dietitians for supporting the study and the staff at Harbor-UCLA GCRC Core Laboratories for the management of blood samples and measuring inflammatory markers.
Footnotes
Financial Disclosure: Dr Kalantar-Zadeh has been involved in studies that include Omega-3 supplements for malnourished patients and is partially supported by Abbott Nutrition (www.abbottnutrition.com; manufacturer of Oxepa, which contains Omega-3 fatty acids).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunner FP, Selwood NH. Profile of patients on RRT in Europe and death rates due to major causes of death groups. The EDTA Registration Committee. Kidney Int Suppl. 1992;38:S4–15. [PubMed] [Google Scholar]
- 2.Szklarek-Kubicka M, Fijalkowska-Morawska J, Zaremba-Drobnik D, Ucinski A, Czekalski S, Nowicki M. Effect of intradialytic intravenous administration of omega-3 fatty acids on nutritional status and inflammatory response in hemodialysis patients: a pilot study. J Ren Nutr. 2009;19:487–493. doi: 10.1053/j.jrn.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AR, Alvestrand A, Danielsson A, et al. Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int. 1998;53:773–782. doi: 10.1046/j.1523-1755.1998.00812.x. [DOI] [PubMed] [Google Scholar]
- 4.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–467. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 5.Mori TA, Beilin LJ. Long-chain omega 3 fatty acids, blood lipids and cardiovascular risk reduction. Curr Opin Lipidol. 2001;12:11–17. doi: 10.1097/00041433-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Friedman AN, Moe SM, Perkins SM, Li Y, Watkins BA. Fish consumption and omega-3 fatty acid status and determinants in long-term hemodialysis. Am J Kidney Dis. 2006;47:1064–1071. doi: 10.1053/j.ajkd.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Filiopoulos V, Hadjiyannakos D, Takouli L, Metaxaki P, Sideris V, Vlassopoulos D. Inflammation and oxidative stress in end-stage renal disease patients treated with hemodialysis or peritoneal dialysis. Int J Artif Organs. 2009;32:872–882. doi: 10.1177/039139880903201206. [DOI] [PubMed] [Google Scholar]
- 8.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos AP. Is insulin resistance influenced by dietary linoleic acid and trans fatty acids? Free Radic Biol Med. 1994;17:367–372. doi: 10.1016/0891-5849(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 12.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 13.Simopoulos AP, CL, editors. Omega-6/omega-3 essential fatty acid ratio: the scientific evidence. Karger; Basel: 2003. [Google Scholar]
- 14.Simopoulos AP, OJ, editors. Nutrigenetics and nutrigenomics. Karger; Basel: 2004. [Google Scholar]
- 15.Colman S, Bross R, Benner D, et al. The Nutritional and Inflammatory Evaluation in Dialysis patients (NIED) study: overview of the NIED study and the role of dietitians. J Ren Nutr. 2005;15:231–243. doi: 10.1053/j.jrn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Bross R, Chandramohan G, Kovesdy CP, et al. Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis. 2010;55:885–896. doi: 10.1053/j.ajkd.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shantouf RS, Budoff MJ, Ahmadi N, et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31:419–425. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shantouf R, Ahmadi N, Flores F, et al. Impact of phosphate binder type on coronary artery calcification in hemodialysis patients. Clin Nephrol. 2010;74:12–18. doi: 10.5414/cnp74012. [DOI] [PubMed] [Google Scholar]
- 19.Park JC, Kovesdy CP, Duong U, et al. Association of serum alkaline phosphatase and bone mineral density in maintenance hemodialysis patients. Hemodial Int. 2010;14:182–192. doi: 10.1111/j.1542-4758.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noori N, Kovesdy CP, Dukkipati R, et al. Survival predictability of lean and fat mass in men and women undergoing maintenance hemodialysis. Am J Clin Nutr. 2010;92:1060–1070. doi: 10.3945/ajcn.2010.29188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noori N, Kalantar-Zadeh K, Kovesdy CP, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56:338–347. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar Outcomes With Hemodialysis and Peritoneal Dialysis in Patients With End-Stage Renal Disease. Arch Intern Med. 2010 doi: 10.1001/archinternmed.2010.352. [DOI] [PubMed] [Google Scholar]
- 24.Noori N, Kovesdy CP, Bross R, et al. Novel Equations to Estimate Lean Body Mass in Maintenance Hemodialysis Patients. Am J Kid Dis. 2010 doi: 10.1053/j.ajkd.2010.10.003. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JE, Kovesdy CP, Nissenson AR, et al. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55:100–112. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 27.Erbagci AB, Tarakcioglu M, Aksoy M, et al. Diagnostic value of CRP and Lp(a) in coronary heart disease. Acta Cardiol. 2002;57:197–204. doi: 10.2143/AC.57.3.2005389. [DOI] [PubMed] [Google Scholar]
- 28.Pecoits-Filho R, Barany P, Lindholm B, Heimburger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–1688. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 29.Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 30.Bross R, Noori N, Kovesdy CP, et al. Dietary assessment of individuals with chronic kidney disease. Semin Dial. 2010;23:359–364. doi: 10.1111/j.1525-139X.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Kovesdy CP, Bross R, et al. Design and Development of a Dialysis Food Frequency Questionnaire. J Ren Nutr. 2010 doi: 10.1053/j.jrn.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overby NC, Serra-Majem L, Andersen LF. Dietary assessment methods on n-3 fatty acid intake: a systematic review. Br J Nutr. 2009;102(Suppl 1):S56–63. doi: 10.1017/S000711450999314X. [DOI] [PubMed] [Google Scholar]
- 33.Burrowes JD, Larive B, Cockram DB, et al. Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: cross-sectional results from the HEMO study. J Ren Nutr. 2003;13:191–198. doi: 10.1016/s1051-2276(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 34.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 35.Yamagishi K, Iso H, Date C, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 36.He J, Wang T, Sun JQ, Gu LF, Li SP. Isolation and characteristic of a moderately halophilic bacterium accumulated ectoine as main compatible solute. Wei Sheng Wu Xue Bao. 2005;45:900–904. [PubMed] [Google Scholar]
- 37.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–816. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y, Ueshima H, Okamura T, et al. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980-99. Am J Med. 2005;118:239–245. doi: 10.1016/j.amjmed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Iso H, Kobayashi M, Ishihara J, et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 40.Svensson M, Schmidt EB, Jorgensen KA, Christensen JH. The effect of n-3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: a randomized placebo-controlled intervention study. Nephrol Dial Transplant. 2008;23:2918–2924. doi: 10.1093/ndt/gfn180. [DOI] [PubMed] [Google Scholar]
- 41.Kutner NG, Clow PW, Zhang R, Aviles X. Association of fish intake and survival in a cohort of incident dialysis patients. Am J Kidney Dis. 2002;39:1018–1024. doi: 10.1053/ajkd.2002.32775. [DOI] [PubMed] [Google Scholar]
- 42.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 43.Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet. 2003;92:1–22. doi: 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- 44.Saifullah A, Watkins BA, Saha C, Li Y, Moe SM, Friedman AN. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients--a pilot study. Nephrol Dial Transplant. 2007;22:3561–3567. doi: 10.1093/ndt/gfm422. [DOI] [PubMed] [Google Scholar]
- 45.Perunicic-Pekovic GB, Rasic ZR, Pljesa SI, et al. Effect of n-3 fatty acids on nutritional status and inflammatory markers in haemodialysis patients. Nephrology (Carlton) 2007;12:331–336. doi: 10.1111/j.1440-1797.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 46.Kalantar-Zadeh K, Braglia A, Chow J, et al. An anti-inflammatory and antioxidant nutritional supplement for hypoalbuminemic hemodialysis patients: a pilot/feasibility study. J Ren Nutr. 2005;15:318–331. doi: 10.1016/j.jrn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Leaf A, Weber PC. A new era for science in nutrition. Am J Clin Nutr. 1987;45:1048–1053. doi: 10.1093/ajcn/45.5.1048. [DOI] [PubMed] [Google Scholar]
- 48.Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 49.Simopoulos AP. Genetics and nutrition: or what your genes can tell you about nutrition. World Rev Nutr Diet. 1990;63:25–34. doi: 10.1159/000418495. [DOI] [PubMed] [Google Scholar]
- 50.Cleland LG, James MJ, Neumann MA, D’Angelo M, Gibson RA. Linoleate inhibits EPA incorporation from dietary fish-oil supplements in human subjects. Am J Clin Nutr. 1992;55:395–399. doi: 10.1093/ajcn/55.2.395. [DOI] [PubMed] [Google Scholar]