Abstract
Age-specific behavioral and neural characteristics may predispose adolescents to initiate and escalate use of alcohol and drugs. Adolescents may avidly seek novel experiences, including drugs of abuse, because of enhanced incentive motivation for drugs and natural rewards, perhaps especially when that incentive motivation is sensitized by prior drug exposure. Using a Pavlovian conditioned approach (PCA) procedure, sign-tracking (ST) and goal-tracking (GT) behavior was examined in amphetamine-sensitized and control adolescent and adult female Sprague-Dawley rats, with expression of elevated ST behavior used to index enhanced incentive motivation for reward-associated cues. Rats were first exposed to a sensitizing regimen of amphetamine injections (3.0 mg/kg/ml d-amphetamine per day) or given saline (0.9% w/v) once daily for 4 days. Expression of ST and GT was then examined over 8 days of PCA training consisting of 25 pairings of an 8-sec presentation of an illuminated lever immediately followed by response-independent delivery of a banana-flavored food pellet. Results showed that adults clearly displayed more ST behavior than adolescents, reflected via both more contacts with, and shorter latencies to approach, the lever. Prior amphetamine sensitization increased ST (but not GT) behaviors regardless of age. Thus, when indexed via ST, incentive motivation was found to be greater in adults than adolescents, with a prior history of amphetamine exposure generally sensitizing incentive motivation for cues predicting a food reward regardless of age.
Keywords: adolescent, amphetamine, sign-tracking, incentive sensitization, rats
Adolescents differ from individuals at other ages in the way they respond to and interact with natural rewards and drugs, exhibiting age-related increases in social behavior, novelty-seeking/risk-taking and consummatory behaviors. It is also during adolescence that most drug users have their first drug-related experiences, with almost 50% of high school seniors reporting use of an illicit drug during their lifetime (Johnston, O’Malley, Bachman, & Schulenberg, 2010). Given ethical constraints regarding research that can be conducted with human youth, animal models provide an important tool for researchers to investigate factors contributing to age differences in risk-taking behaviors, including substance abuse. Similar to humans, a variety of other mammalian species exhibit a maturational period equivalent to adolescence, with comparable neural, hormonal and behavioral alterations (Adriani & Laviola, 2004; Spear, 2000). In rodents, postnatal days (P) 28 to 42 have been identified as prototypic adolescence (Adriani & Laviola, 2004; Spear, 2000; Spear & Brake, 1983), although sex differences in timing are prevalent, with adolescent-typical characteristics often emerging in females beginning at slightly younger ages and persisting longer in males (see Spear, 2000 for review).
Studies using animal models of adolescence have revealed this ontogenetic phase to be a unique developmental period of sensitivity to addictive substances such as alcohol and nicotine (e.g., Rezvani & Levin, 2004; Slawecki, Thorsell, & Ehlers, 2004), although more work is needed to identify factors contributing to the propensity for drug use during adolescence. In the present study, ontogenetic alterations in incentive salience were investigated as a possible contributor to drug-seeking behavior. As previously characterized by Robinson & Berridge (Robinson & Berridge, 1993, 2003), incentive salience, or “wanting,” refers to the motivational drive directing approach behavior towards natural or drug rewards as well as toward cues repetitively associated with these rewarding stimuli. Such incentive drive is distinguishable functionally and in its neuroanatomical substrates from hedonic processes or “liking” (Berridge & Robinson, 2003). Drugs of abuse have been hypothesized to usurp processes of incentive motivation through alterations in reward-related circuitry – a process of “incentive sensitization” thought to strengthen the salience of drug-associated cues with repeated drug exposures (Robinson & Berridge, 1993). Behavioral sensitization (increased psychomotor activation and/or stereotypies) induced by repeated intermittent exposure to psychostimulants has been suggested to reflect sensitization of reward-related neural processes underlying attribution of incentive salience to drugs and their associated cues (Robinson & Berridge, 2003). Given the complex developmental changes seen in these reward systems during adolescence (for review see Crews, He, & Hodge, 2007; Spear, 2000), it is possible that the increased likelihood of drug use and other risky behaviors during adolescence may be in part associated with an age-related propensity to attribute greater incentive salience or develop greater drug-driven incentive sensitization towards reward-related cues. The present study was designed to investigate this hypothesis by comparing sign-tracking and goal-tracking behavior in stimulant sensitized and non-stimulant-exposed adolescent and adult rats using a Pavlovian Conditioned Approach (PCA) procedure.
PCA is an autoshaping procedure involving repeated exposure to a conditioned stimulus (CS) followed immediately by response-independent presentation of an unconditioned stimulus (US), such as a food reward. When the CS consists of a combination of a cue (e.g. a stimulus light) and a manipulandum (e.g. a response lever) that spatially coincide, previous research has shown that some animals direct substantial attention to the CS prior to reinforcement delivery, often excessively approaching and manipulating the CS (sign-trackers: STs), whereas other animals focus their attention on the location of US delivery (goal-trackers: GTs). Individual differences in ST versus GT behavior (Flagel, Akil, & Robinson, 2009; Flagel, Watson, Akil, & Robinson, 2008; Flagel, Watson, Robinson, & Akil, 2007) have been postulated to be attributable to differences in incentive salience, with STs thought to attach greater incentive salience to the CS. Accordingly, increased ST behavior (to even natural rewards) has been argued to be a marker for vulnerability to drug addiction (Tomie, 1995), with recent studies showing that higher levels of ST behavior in adult rats is associated with a greater propensity to develop cocaine sensitization (Flagel et al., 2008). To the extent that adolescents might pursue drugs and other rewards because of enhanced incentive salience, it would be expected that animals in this developmental phase would exhibit greater ST behavior relative to adult animals, with perhaps the propensity to express ST being elevated further among stimulant sensitized adolescents relative to similarly drug-exposed adults. The present study assessed these predictions using female rats given that females have been shown to develop more marked stimulant sensitization than males (Robinson, 1984; Robinson & Becker, 1986; Robinson, Becker, & Presty, 1982), and have been used in some previous studies investigating amphetamine-induced incentive sensitization (Wyvell & Berridge, 2000, 2001).
Sprague-Dawley female rats derived from our breeding colony (see Doremus-Fitzwater, Varlinskaya, & Spear, 2009 for details) were used in the 2 (age: adolescent or adult) × 2 [sensitization: repeated saline (SAL) or repeated amphetamine (AMPH )] factorial design of this project (n = 12/group), with no more than one animal per litter placed into each group (Holson & Pearce, 1992). Animals were maintained in a temperature-controlled vivarium on a 14:10 hr light:dark cycle (lights on at 0700 hr), with ad libitum access to water and food (Purina Rat Chow, Lowell, MA). At all times, rats were treated in accordance with the guidelines for animal care established by the National Institutes of Health (Institute of Laboratory Animal Research, 1996), using protocols approved by the Binghamton University Institutional Animal Care and Use Committee (IACUC).
On P21 (weaning) for adolescents and at P64 for adults, animals were re-housed with a same-aged, non-littermate partner in a holding room near the testing chambers. Beginning 1 day thereafter and continuing daily for the next four days, animals were weighed, injected intraperitoneally (i.p.) with 3.0 mg/kg/ml D-amphetamine sulfate (Sigma, St. Louis, MO; dissolved in saline and mixed fresh daily) or an equal volume of 0/9% saline, and placed into standard rat-sized operant chambers (30.5 × 24.1 × 21 cm) (Med Associates, St. Albans, VT) housed within sound-attenuating cubicles and equipped with red house lights that remained on during all sessions. The first and fourth days of drug exposure were videotaped and later scored for locomotion and stereotypy. General locomotion was indexed via counting the number of quadrant crossings (based on division of each chamber into four equally-sized quadrants on the videomonitor), whereas stereotypy (i.e., repetitive bobbing, scanning or weaving movements of the head directed at the wall, corner, or floor of the chamber) was measured using a time-sampling procedure. At 5, 15, 25, 35, 45 and 55 min into the session, the number of 10 consecutive 3-sec intervals in which stereotypy occurred was counted, yielding a score of 0-10 for every 30-sec observation period (and a maximum score of 60 over the session).
Following the last drug exposure, animals remained in their home cage for two days, each day receiving a small dish containing approximately 10 gm of banana pellets (45 mg, Bio-Serv, Frenchtown, NJ). On each of the next two days, animals were given pretraining sessions in the operant chambers during which 25 banana pellets were dispensed, using a variable interval (VI) 90 sec schedule, into a trough-style food receptacle mounted in the center of the right-side wall, 3 cm from the floor.
Immediately after these pretraining days, animals received 8 days of PCA training. Each session, animals were given 25 pairings of an illuminated lever (CS) with delivery of a banana pellet (US). This retractable lever was mounted 3 cm from the floor on either the left or right side of the food trough (counterbalanced across subjects). At the onset of the CS, the lever (4.8 cm paddle for adults, requiring 10-gm of force to register contact; or 1.6 cm ultra-sensitive mouse-sized lever for adolescents requiring 2 gm of force) was inserted into the chamber and illuminated through the slot via a white LED light attached to the inside of the lever casing. Each pairing consisted of an 8-sec presentation of the illuminated lever, followed by response-independent delivery of one pellet, and immediate lever retraction. CS-US presentations were given on a VI 90 sec schedule (ranging from 30 to 150 sec), resulting in session lengths of approximately 35-50 min.
Autoshaping sessions were videotaped and later scored for: latency to approach the lever, number of contacts and time spent in contact with the lever (indices of ST behavior), and latency to approach the food trough (an index of GT behavior). During CS presentations, head entries into the food trough (measured via photocells) and lever presses were registered by the computer. Although computer-detected lever presses were lower in frequency than lever contacts scored via the video records (e.g., animals sometimes touched the lever from the side or underneath without registering as a press by the computer), these two measures were significantly correlated for both adolescents (R = 0.84, R2 = 0.70, p ≤ .000001) and adults (R = 0.71, R2 = 0.50, p ≤ .0001). Therefore, data analysis focused on ST behaviors scored from the video records, since these data allowed for more sensitive assessment of potential behavioral changes across age and drug exposure conditions.
Before analysis, behavioral measures were checked for homogeneity of variance. Data for measures violating this assumption were transformed to produce the most normal distribution prior to analysis with analysis of variance (ANOVA), although non-transformed data are shown in the figures and presented in the text (as means ± standard errors) for ease of interpretation. Fisher’s Least Significant Difference (LSD) post hoc tests were used to explore the locus of significant ANOVA effects and interactions, with emphasis in the Results on significant main effects and interactions involving age and drug exposure.
Analysis of body weights following the 4-day exposure phase and at the end of the autoshaping sessions revealed no significant drug effects, with for instance AMPH adolescents (75.5 g ± 2.0) and adults (241.2 ± 4.6) comparable to their SAL counterparts (78.5 ± 2.4; 244.2 ± 5.9, respectively) at the end of the exposure phase.
Behavior on days 1 and 4 of the drug exposure phase was analyzed for evidence of behavioral sensitization to the repeated amphetamine administrations. In the analysis of frequency of quadrant crossings (using log 10 transformed data), a significant age x drug x day interaction [F(1,44) = 5.07, p ≤.05] revealed that AMPH-exposed adolescent (407.2 ± 47.5) and adult rats (213.3 ± 29.8) crossed significantly more quadrants than their SAL controls (adolescents: 61.1 ± 4.9; adults: 92.8 ± 12.8) on day 1 of administration. By the fourth day of exposure, however, adolescents maintained this amphetamine-induced stimulation of crossings (AMPH: 431.7 ± 67.1; SAL: 50.9 ± 6.9) whereas adults did not (AMPH: 88.3 ± 17.5; SAL: 65.7 ± 11.1). Furthermore, while there were no age differences in locomotor activity among saline-treated animals on either day, AMPH adolescents crossed significantly more than AMPH adults on both days 1 and 4 of exposure. Among both age groups, amphetamine-induced crosses were not significantly greater on day 4 relative to day 1, hence there was no evidence of sensitization to the locomotor stimulatory effects of amphetamine across the 4-day exposure period at either age.
Since saline-exposed animals exhibited virtually no stereotypic head scanning/weaving, only AMPH animals were examined for sensitization of stereotypy. A significant age x day interaction [F(1,22) = 12.92, p ≤ .01] emerged in the analysis of stereotypy, with both AMPH adolescents and adults exhibiting significantly more stereotypy on day 4 (adults: 37.92 ± 3.1; adolescents:18.3 ± 5.2) than on day 1 of drug administration (adults: 4.92 ± 1.8; adolescents: 7.0 ± 1.8), and with adults demonstrating significantly more stereotypy than adolescents on day 4. Therefore, evidence of significant sensitization of stereotypy was seen at both ages, with this enhanced stereotypy particularly pronounced in adults and likely competing with potential expression of locomotor sensitization.
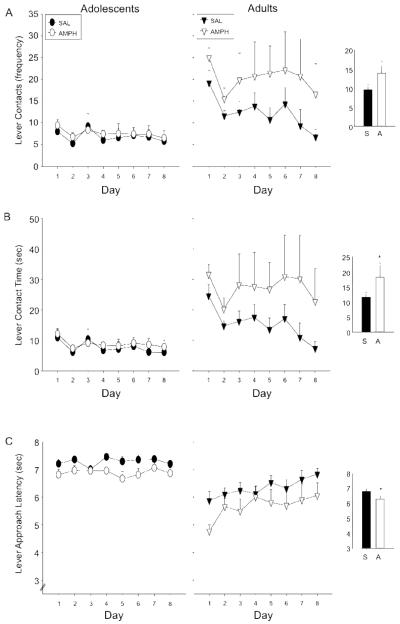
Data for the mean number of lever contacts exhibited by SAL and AMPH adolescents and adults during each PCA session were transformed [√(lever contacts + 0.5)] prior to analysis. Results (Figure 1a) showed that adults exhibited significantly more lever contacts than adolescents [main effect of age: F(1,44) = 12.46, p ≤ .001]. The trend for more lever contacts among AMPH than SAL animals did not reach significance (p = .10).
Figure 1.
Frequency of (A) lever contacts, (B) time in contact with the lever, and (C) latency to approach the lever was assessed in adolescents and adults previously given repeated amphetamine (AMPH; white symbols) or saline (SAL; black symbols) injections. Data shown are non-transformed values across the eight days of Pavlovian Conditioned Approach training, with statistical analyses performed on transformed data. Data points represent means, with error bars showing standard error of the mean. Inserts are included for each measure to emphasize the main effect of drug exposure, with saline-exposed (S) animals represented by the black bars and amphetamine-exposed animals (A) represented by the white bars. Asterisks (*) denote a significant difference from the SAL group.
Analysis of transformed data for the mean amount of time spent in contact with the lever [log (lever time + 0.5)] again showed that adults spent more time in contact with the lever than adolescents [main effect of age: F(1,44) = 15.66, p ≤ .001]. Additionally, a main effect of drug exposure [F(1,44) = 4.95, p ≤ .05] was also observed, with AMPH animals spending significantly more time in contact with the lever than SAL rats. While this effect was seemingly driven by adults, the interaction of age and drug exposure did not reach significance.
Transformed data (√(9 − X) for latency to approach the lever (Figure 1c) ] were analyzed and revealed AMPH animals to have significantly shorter latencies to approach the lever than SAL animals [main effect of drug exposure: F(1,44) = 5.79, p ≤ .05] (see Fig. 1c insert). Overall, adults exhibited significantly shorter latencies to contact the lever than adolescents on all days of PCA training [main effect of age: F(1,44) = 25.0, p ≤ .0001], although this effect did interact with day [day x age interaction: [F(7,308) = 3.46, p ≤ .01].
No effects of drug exposure emerged with GT behaviors (Figure 2) when indexed via frequency of food trough entries during CS presentation or latency to approach the food trough [transformation = √(9 – X)]. Although not obvious from the figure, adults approached the food trough slightly but significantly faster during the CS exposure period (5.78 ± .21) than adolescents (6.46 ± .46) [main effect of age: F(1,44) = 6.72, p ≤ .05].
Figure 2.
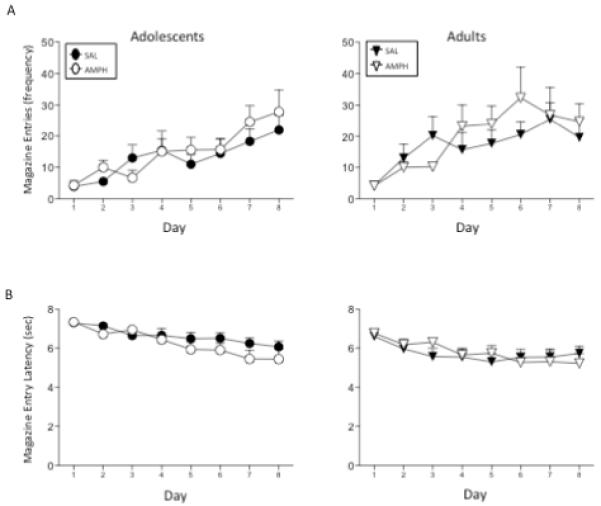
Frequency of (A) trough entries and (B) latency to enter the food trough was assessed in adolescents and adults previously given repeated injections of amphetamine (AMPH; white symbols) or saline (SAL; black symbols). Data shown are non-transformed values across the eight days of Pavlovian Conditioned Approach training, although statistical analysis of latency to food trough entry was performed on transformed data. Data points represent means, with error bars showing standard error of the mean.
Overall, the results of this experiment revealed that adolescents exhibited less sign-tracking (indexed via fewer CS contacts, less CS contact time, and longer latencies to approach the CS) and hence showed less cue-directed incentive salience than adults. Furthermore, the current experiment also revealed that prior psychostimulant sensitization resulted in enhanced incentive salience for a reward-related cue, with increased time spent in contact with the CS and a decreased latency to approach the CS observed in previously sensitized rats.
While acute amphetamine has been reported to shift responding in the direction of GT behavior (Holden & Peoples, 2010), findings have been mixed regarding the influence of prior amphetamine sensitization on incentive salience for cues predicting natural rewards in a drug-free state. Similar to the results observed in the current study, a previous report demonstrated that prior stimulant sensitization increased subsequent incentive salience for reward-related cues using the Pavlovian-to-Instrumental Transfer (PIT) task (Wyvell & Berridge, 2001) in adult female rats. In contrast, a recent study using adult male Long-Evans rats reported that prior sensitization with amphetamine increased GT and not ST in an autoshaping procedure (Simon, Mendez, & Setlow, 2009). Along with differences in sexes and strains used, procedural differences could have contributed to the opposing findings between that experiment and the current study. In the Simon (2009) study, rats were food deprived and given grain-based pellets during autoshaping, whereas non-deprived animals given highly palatable banana pellets were assessed in the current study. Nature and location of the CS cue also differed, with the light and lever components of the cue spatially separated and located 7 cm from the food trough in the Simon (2009) study, but contained within a single compound cue located 3 cm from the food trough in our study, as in prior studies of sign-tracking behavior (Flagel et al., 2009; Flagel et al., 2008; Flagel et al., 2007; Uslaner, Acerbo, Jones, & Robinson, 2006). Sign-tracking behavior is said to be more robust when the cues spatially coincide and are located relatively close to the area of reinforcer delivery (Flagel et al., 2009; Tomie, 1995), with these CS characteristics perhaps helping to promote sensitization of ST behavior in the current study.
Though this sensitization of incentive salience did not differ statistically with age, there was an obvious tendency for adults to show more drug-induced sensitization of ST behavior than adolescents. Furthermore, even among drug-exposed adults, expression of ST was notably variable. Since individual differences in both baseline levels of ST (e.g. Flagel et al., 2009), as well as amphetamine sensitization (Scholl, Feng, Watt, Renner, & Forster, 2009) have been reported, it is not surprising that some animals would be more likely than others to show drug-induced sensitization of ST. Perhaps if larger sample sizes had been utilized, individual differences in sensitization to the ST cue could have been explored at each age, potentially permitting detection of age differences in incentive sensitization following amphetamine exposure.
That adolescents overall exhibited significantly less ST than adults throughout the autoshaping procedure is a result that has been repeatedly observed in our laboratory, in both male and female adolescent and adult rats (Anderson & Spear, submitted). The reasons underlying these consistent age differences in expression of ST are unclear, although there are several possibilities. One likely contributor is possible differences across age in the incentive salience attributed to different rewards. That is, although food appears to be highly reinforcing to adolescents given their general hyperphagia (Nance, 1983) and increased consumption of food relative to adults (Vetter & Spear, November, 2007), in this type of task food may not have provided as salient a reward as other natural rewards (e.g. social stimuli) or drugs. In support of this notion, a recent study (Rubinow, Hagerbaumer, & Juraska, 2009) demonstrated that, whereas food-deprived adult rats exhibited a significant conditioned place preference for an environment paired with food, similarly deprived adolescents did not.
Age-related differences in the neurobiological substrates of sign-tracking may also contribute to the attenuated ST seen in adolescents relative to adults. One area thought to be key for attribution of incentive salience (Berridge & Robinson, 1998; Robinson & Berridge, 1993) and for individual differences in the expression of ST behavior (for reviews see Flagel et al., 2008; Flagel et al., 2007; Tomie, Grimes, & Pohorecky, 2008) is the mesocorticolimbic dopamine system. Apparent attenuations of accumbens-related dopaminergic activity among adolescents as indexed, for example, by lower basal DA levels in adolescent compared to adult striatum (Andersen & Gazzara, 1993) and lower levels of DA synthesis and turnover (Andersen, Dumont, & Teicher, 1997; Teicher et al., 1993) might lead to decreased incentive salience at this age and, therefore, attenuated ST in adolescent rats. Clearly, more research is needed to elucidate factors influencing expression of ST behavior among adolescents, including procedural/contextual variables, motivational state, and their neural correlates.
Characterization of age-related differences in motivational systems, such as hedonic states and incentive salience, are important for understanding the proclivity of adolescents to pursue risks and initiate drug and alcohol use. The data presented in the current experiment suggest that non-deprived adolescents do not show enhanced incentive salience towards reward-related cues, at least when indexed via a cue predicting a palatable food reward. To the extent that ST represents a viable index of incentive salience across age and that this index of incentive motivation generalizes to cues predicting other rewards, adolescents do not appear more vulnerable to cue-induced craving for drug rewards as originally expected. However, given that prior stimulant sensitization enhanced the incentive salience for a reward-related cue, the influence of predictive cues on the development of drug addiction and the persistence of cue-induced relapse remains an important focus for addiction research.
Acknowledgments
Supported by NIH grant R01-DA019071 to LPS
Contributor Information
Tamara L. Doremus-Fitzwater, Center for Development and Behavioral Neuroscience, Department of Psychology, Binghamton University, Binghamton, New York 13902-6000.
Linda P. Spear, Center for Development and Behavioral Neuroscience, Department of Psychology, Binghamton University, Binghamton, New York 13902-6000.
REFERENCES
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behavioural Pharmacology. 2004;15(5-6):341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/−)-7-OH-DPAT. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1997;356(2):173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Gazzara RA. The ontogeny of apomorphine-induced alterations of neostriatal dopamine release: effects on spontaneous release. Journal of Neurochemistry. 1993;61(6):2247–2255. doi: 10.1111/j.1471-4159.1993.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Spear LP. Sign-tracking behavior and ethanol intake in adolescent and adult male and female Sprague-Dawley rats. Manuscript submitted for publication. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Effects of pretest manipulation on elevated plus-maze behavior in adolescent and adult male and female Sprague-Dawley rats. Pharmacology Biochemistry and Behavior. 2009;92(3):413–423. doi: 10.1016/j.pbb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behavioural Brain Research. 2008;186(1):48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191(3):599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Holden JM, Peoples LL. Effects of acute amphetamine exposure on two kinds of Pavlovian approach behavior. Behavioural Brain Research. 2010;208(1):270–273. doi: 10.1016/j.bbr.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and Teratology. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research. C. o. L. S. National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Presso; Washington, D.C.: 1996. Document Number. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2009. National Institute on Drug Abuse; Bethesda, MD: 2008. p. 77. NIH Publication No. 10-7583. [Google Scholar]
- Nance DM. The developmental and neural determinants of the effects of estrogen on feeding behavior in the rat: a theoretical perspective. Neuroscience and Biobehavioral Reviews. 1983;7(2):189–211. doi: 10.1016/0149-7634(83)90015-5. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Adolescent and adult rats respond differently to nicotine and alcohol: motor activity and body temperature. International Journal of Developmental Neuroscience. 2004;22(5-6):349–354. doi: 10.1016/j.ijdevneu.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berl) 1984;84(4):466–475. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Research. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Research. 1982;253(1-2):231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Hagerbaumer DA, Juraska JM. The food-conditioned place preference task in adolescent, adult and aged rats of both sexes. Behavioural Brain Research. 2009;198(1):263–266. doi: 10.1016/j.bbr.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl JL, Feng N, Watt MJ, Renner KJ, Forster GL. Individual differences in amphetamine sensitization, behavior and central monoamines. Physiology and Behavior. 2009;96(3):493–504. doi: 10.1016/j.physbeh.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology (Berl) 2009;202(4):699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Annals of the New York Academy of Sciences. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Developmental Psychobiology. 1983;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Barber NI, Gelbard HA, Gallitano AL, Campbell A, Marsh E, et al. Developmental differences in acute nigrostriatal and mesocorticolimbic system response to haloperidol. Neuropsychopharmacology. 1993;9(2):147–156. doi: 10.1038/npp.1993.53. [DOI] [PubMed] [Google Scholar]
- Tomie A. CAM: An animal learning model of excessive and compulsive implement-assisted drug-taking in humans. Clinical Psychology Review. 1995;15(3):145–167. [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58(1):121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behavioural Brain Research. 2006;169(2):320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Spear LP. Age-associated trajectories of consumption and body weight gain in pair- and isolate-housed adolescent and adult Sprague-Dawley rats; Paper presented at the International Society for Developmental Psychobiology; San Diego, CA. November, 2007. [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. Journal of Neuroscience. 2000;20(21):8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. Journal of Neuroscience. 2001;21(19):7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]