Abstract
A major goal in the study of motor learning is to improve the extent to which subjects adapt their movements in response to errors. Recent attention has focused on the gradual-adaptation paradigm, in which an adaptive stimulus is introduced incrementally, rather than all at once as in conventional adaptation paradigms. However, there is disagreement – even among studies involving the same sensorimotor-learning task – as to the robustness of this approach. In particular, although all studies confirm that retention of learning is improved, not all agree that exposure to a gradual-adaptation paradigm can improve the extent of adaptation that takes place. Also, the paradigm has not previously been studied with saccadic eye movements, which are unique in that they typically lack online error feedback during each movement. To determine the effectiveness of gradual adaptation in this system, we compared saccadic adaptation performed with gradual and conventional adaptation paradigms. We find evidence consistent with more robust adaptation – in the sense of greater extent of adaptation as well as greater retention of learning (larger aftereffects) – in response to a gradual adaptation stimulus. The results suggest the need to develop alternative models of motor learning, as current error-based modeling efforts are unable to account for the increased extent of adaptation when subjects are only exposed to the full adaptive stimulus for a brief time.
Keywords: gradual adaptation, saccades, motor learning
Introduction
Sensorimotor responses are calibrated by a continuous process of motor learning, in which movement errors are used to adjust the parameters of the system to maintain performance. In the laboratory, adaptation is studied by perturbing the movement stimulus to induce artificial errors, then examining how the motor system responds to such changes. Recently, much attention has been focused on the type of paradigm utilized to induce such adaptation, with the aim of enhancing the adaptive response by improving its rate, extent, or retention. Of particular interest is the difference between introducing a large adaptive stimulus all at once (conventional) and introducing the adaptive stimulus slowly across many trials such that subjects observe only small errors on any trial (gradual). In the former case, subjects have a longer period of time in which they experience the full stimulus perturbation (largest errors), so they have the greatest opportunity to learn more about the intended gain change. In the latter case, subjects never experience large errors, and it has been suggested that this may cause learning to proceed more effectively [1, 5] – one hypothesis being that experiencing small, incremental errors is more realistic than suddenly observing a large, albeit consistent, error.
Compared to a conventional-adaptation paradigm, there is clear evidence that following adaptation, gradual-adaptation paradigms yield larger aftereffects (errors in the opposite direction as a result of learned compensation for a stimulus change that is no longer present) or slower washout. In other words, a gradual-adaptation paradigm causes the acquired gain change to be retained more completely and across more trials after the adapting stimulus has been removed. This suggests that there is less of a strategic component to what has been learned; cognitive strategies are often employed to quickly reduce errors in response to an adaptive stimulus, but this rapid performance improvement also vanishes quickly after that stimulus is removed, leading to a faster decline of aftereffects during the washout phase. On the other hand, there remains some debate about whether the gradual introduction of a stimulus perturbation is also able to yield greater overall learning (that is, increase the extent to which subjects adapt). Some studies indeed find improved adaptation following the application of a gradual perturbation stimulus instead of the conventional paradigm [4, 8, 10], whereas other investigations do not [2–3, 5]. Such contradictions are particularly puzzling because disagreements occur even when examining the same motor learning task, such as adaptation to reaching in a force field that perturbs the movement in a direction perpendicular to the reach [2, 4]. Thus, there is unlikely to be a motor-task dependence of the effects of gradual adaptation.
Knowing the extent to which adaptation occurs is important to understanding how the conventional and the gradual paradigms differ in their effects on motor learning. In general, error-based models are difficult to apply to the situation of gradual adaptation because they state that larger observed errors (as in the case of conventional adaptation paradigms) should produce greater adaptation and larger aftereffects, which contradicts some of the previous findings. However, the two-state model of motor learning, also an error-based model, is capable of explaining larger adaptation aftereffects in the gradual condition [2]. In this model, adaptation is thought to be the result of two learning states: a fast process that rapidly corrects for errors but does not retain much information from trial to trial, and a slow process that gradually adapts but retains information over long periods of time. Larger aftereffects in the gradual paradigm may arise because, for the same number of trials, a gradual-adaptation paradigm primarily engages the slow system such that there is greater retention of learning following the adaptation phase. As a consequence, though, less total learning occurs because the fast system contributes far less to overall performance. In general the motor system does not adapt strictly in response to the observed error [6, 13], calling this model into question. If, additionally, the extent of adaptation actually improves with a gradual introduction of the movement perturbation, such a finding would confirm that the two-state model is incomplete as a description of motor adaptation.
While this type of gradual adaptation protocol has been applied to many motor systems, it has not been examined for the classical saccadic double-step adaptation paradigm [7]. Saccadic eye movements rapidly redirect gaze to bring visual targets onto the fovea; hence, it is critical to understand how saccadic accuracy is controlled. Since saccades are so fast and have such a short duration, it is typically thought that they are free of online feedback corrections during each movement. Furthermore, vision is suppressed during saccades, which masks perturbations, and proprioception is not thought to play a significant role for saccades. Thus, saccade gain – at least as far as error-based learning is concerned – is primarily adjusted in response to observations of post-saccadic errors [12–13]. This is an important distinction from the case of limb control, for example, where at least some form of online performance error – visual, proprioceptive, or both – is available during the course of each movement. It is important to test adaptation paradigms in systems that lack the online-correction phase of the movement, to determine if the observed learning effects are due to changes in the initial motor plan or if they arise as part of a late online correction after some movement error information has been acquired.
To that end, we carefully assessed how subjects adapt when they experience both conventional and gradual saccade-adaptation paradigms. We chose to study gain-increase adaptation as it is generally slower and less complete, and therefore will be more sensitive to any effects of the paradigm used to induce the adaptation. This enabled us to address two important questions. First, do saccades respond to gradual-adaptation paradigms in a similar manner as do limb movements, even though such movements lack online feedback? Second, does the gradual introduction of a stimulus perturbation really lead to more robust learning, in the sense of greater overall extent of adaptation? The answers to both questions provide important insight into motor learning and the ways in which we model the underlying adaptive process.
Material and methods
Eye movements were recorded in two sessions spaced at least two weeks apart. Of the five subjects who participated, three performed the gradual-adaptation experiment in the first session and the conventional paradigm in the second session, and this order was reversed for the remaining two subjects. All subjects were naïve as to the purposes of the experiment and were not informed of any differences between the two sessions. Informed consent, according to the local institutional review board, was obtained from each participant on each day of recording.
Subjects sat in a dark room in a stationary chair, and a bite bar was used to minimize head movements. Targets were rear-projected onto a screen 1 m in front of subjects using a mirror-controlled laser dot 2 mm in diameter. Data were acquired on a PC-compatible Pentium 166-MHz computer running real-time experiment control software developed in-house. Eye movements were recorded using a directional scleral search coil (Skalar Medical BV, Delft, The Netherlands; Chronos Vision, Berlin, Germany) to record horizontal and vertical eye movements at 1000 Hz from either the right or left eye [9].
Two conditions were presented: conventional and gradual adaptation (Fig. 1). For either condition, subjects performed six adaptation blocks of 60 trials each. The adaptation trials were modeled after the standard double-step paradigm [7], with the size of the intra-saccadic double step on each trial depending on the session type. The first block began with 30 baseline trials, each consisting of a 10 degree saccade to the right or left (targets were located at ±5° along the horizontal midline) in which the target stepped by 0° during the saccade; that is the target was blanked during the saccade and immediately reappeared in its original location. This transitioned smoothly without a break into 30 trials in which a nonzero intra-saccadic step occurred. During the conventional-adaptation condition, subjects were presented with a target that consistently stepped farther away from the midline by 2.5° during each primary saccade (both rightward and leftward saccades were adapted symmetrically); this stimulus remained constant for the remaining five blocks of adaptation trials (a constant 25% gain-increase stimulus). The gradual-adaptation condition, on the other hand, consisted of an intra-saccadic step size that increased by 0.125° every third trial from the middle of the first block until halfway through the last block, when the intra-saccadic step size reached 2.5°. At this point, the intra-saccadic step size remained constant for the last 30 trials of the block. This represents a linearly-increasing adaptation stimulus over five blocks (the first half of block one and the last half of block six contained a fixed target intra-saccadic step size; see Fig. 1B), ending at a level of 25% gain increase. Gain was measured before and after each adaptation session using open-loop trials, in which the target disappeared during the saccade and the fixation target for the following trial did not appear until 600 ms later [see 13]. A final washout block, containing trials identical to those in the first half of block one in which a 0° target intra-saccadic step occurred, was used to assess de-adaptation by measuring the rate at which gains returned to baseline after the double-step perturbation was removed.
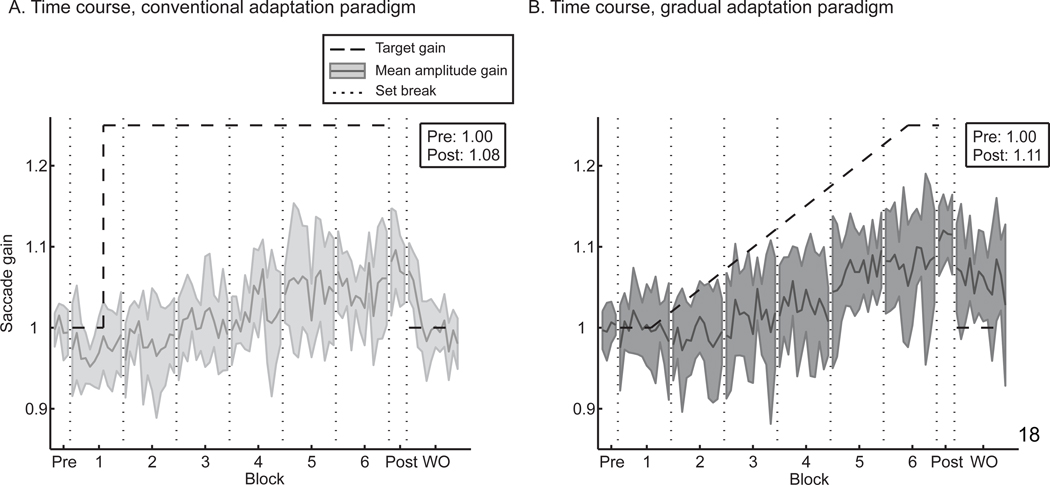
Fig. 1.
Normalized, average time courses of adaptation for the conventional and gradual paradigms. (A) In response to a constant 2.5° target intra-saccadic step (dashed black line), subjects increase their gain by about 8% (comparing pre-adaptation to post-adaptation trials; solid line is the average saccade gain; shaded region is the standard deviation). Subjects quickly de-adapt during the washout (WO) block. (B) In contrast, these same subjects exhibit on average an 11% gain increase (solid gray line) in response to a 2.5° target perturbation that is introduced gradually (dashed black line), and this learning is retained to a greater extent across the washout block. Breaks between blocks are indicated with vertical dotted black lines.
Eye-tracking data were analyzed off-line via an interactive computer program that selected primary saccade start- and end-points using a velocity threshold (> 15°/sec). When subjects blinked during a saccade, that trial was discarded from analysis. Gain was calculated as primary-saccade amplitude divided by target-displacement amplitude. To measure the amount of adaptation achieved in each session, the percent gain change was calculated as:
De-adaptation rate was measured as the slope of a linear-regression fit to primary-saccade amplitudes during the washout block.
Results and Discussion
All subjects, regardless of the paradigm, experienced a change in saccade gain after the adaptation session. Data from each subject were normalized to that subject’s mean pre-adaptation saccade amplitude, and data across subjects were then averaged to examine the time course of learning in each of the two paradigms. The dashed black line in Fig. 1 represents the requested saccade gain at the conclusion of the intra-saccadic step; at the end of adaptation, both paradigms call for a saccade gain of 1.25. Subjects responded by adapting in a gain-increase manner in both the gradual and conventional paradigms. Comparing the change in saccade amplitude before and after conventional adaptation using a two-way ANOVA showed a significant effect of subject (p < 0.001) and pre- versus post-adaptation (p < 0.001) but a non-significant interaction between factors. Identical results were found in the case of gradual adaptation. Thus, in either paradigm, subjects significantly modified their saccade gains.
However, greater saccade adaptation was observed during the gradual paradigm as compared to the conventional paradigm. In a three-way ANOVA comparing the change in saccade amplitudes for both paradigms, there was a significant effect of subject (p < 0.001), pre-versus post-adaptation (p < 0.001), and paradigm type (p < 0.001). There was also a significant interaction between subject and paradigm type (p < 0.001), indicating that the adaptation achieved for the gradual or conventional paradigm varied among subjects. In other words, subjects did not exhibit the same amount of improvement in adaptation from experiencing the gradual-adaptation paradigm (improvement in adaptation varied from a 12% to 108% increase); more importantly, however, consistent across all subjects there was some improvement in performance with the gradual paradigm.
Additionally, saccade gain increased more consistently and at a faster rate throughout the gradual paradigm. It is difficult to determine from these plots whether the saccade gain increases exponentially to an asymptote, as is typically observed in adaptation experiments, or if the change in gain is more linear. In comparing linear and exponential regression fits to the data, the sum of squared errors (SSE) for the two fits were the same in both the conventional adaptation (SSE = 0.018) and gradual adaptation (SSE = 0.017) paradigms. When considering linear regression fits, the regressions for the two paradigms were significantly different (F test, p < 0.001). The gradual adaptation paradigm – despite having smaller target perturbations throughout the majority of the adaptation blocks – produced a greater rate of adaptation (regression slopes, in units of gain change per trial: gradual = 0.002, conventional = 0.001).
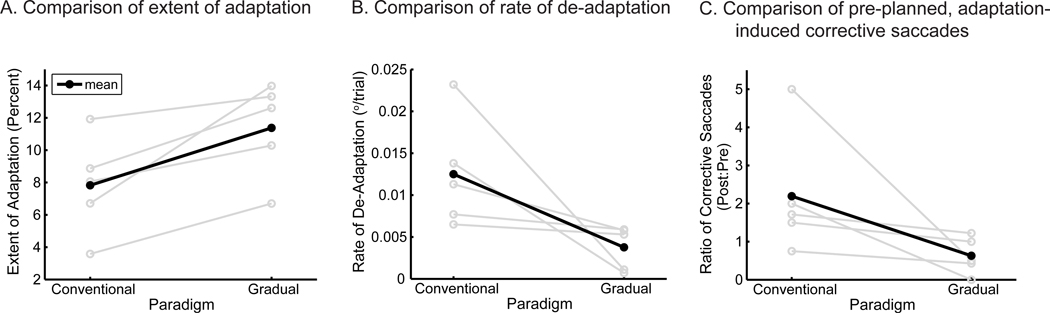
A similar improvement in adaptation with the gradual paradigm can be observed when comparing the adaptation achieved in each paradigm on an individual-subject level, as plotted in Fig. 2A. Here, the acquired adaptation, plotted as the percent gain change, is shown for each subject in the conventional and gradual paradigms (gray lines) along with the average change across all subjects (black line). For every subject, the change in saccade gain was greater for the gradual paradigm (repeated measures ANOVA, p = 0.024). This increase in adaptation occurred regardless of whether the gradual paradigm was experienced first or second, suggesting that there was little retention of adaptation between sessions. Thus, the greater change in saccade gain achieved in the gradual paradigm is not the result of retention from a prior adaptation session (savings); recall that more subjects experienced the gradual-adaptation paradigm first, so any carryover of learning would have been in favor of enhanced adaptation during the conventional paradigm. These data indicate a clear benefit to the extent of adaptation achieved by experiencing the gradual adaptation paradigm instead of the conventional paradigm.
Fig. 2.
Intra-subject comparison of conventional and gradual adaptation. (A) The extent to which each subject adapted is contrasted (gray lines; thick black line is the average across all subjects). In all cases, subjects adapt to a greater extent in the gradual adaptation paradigm. (B) In comparing the rate of de-adaptation during the washout block, all subjects retain their adaptation better (de-adapt slower) in the gradual paradigm. (C) Corrective saccades made during post- and pre-adaptation trials suggest anticipatory planning of error corrections. Each subject was consistent in the number of corrective saccades they made prior to both adaptation sessions; during post-adaptation trials, all subjects tended to make more corrective saccades in the dark following the conventional adaptation paradigm as compared to the gradual paradigm.
The washout rate of the learned adaptation averaged across all subjects can also be observed in Fig. 1. Whereas saccade gains rapidly returned to baseline in the conventional paradigm in about 20 saccades, they remained greater than 1.00 throughout the entire washout block following the gradual paradigm. Therefore, subjects overall tend to retain what they learned to a greater extent following a gradual adaptation paradigm, as the change in gain is more resistant to washout. This trend is also apparent on an individual-subject basis. The washout blocks for each subject in both the conventional and gradual paradigms were fit with linear regressions whose slopes captured the rate of washout; these slopes are compared in Fig. 2B (gray lines) along with the average across subjects (black line). Smaller values mean better retention of learning. Subjects exhibited slower washout for the gradual paradigm (t test, p = 0.026), indicating that they not only learned the adaptation to a greater extent, but what they learned was more resistant to unlearning.
Finally, the number of corrective saccades made during the pre- and post-adaptation trials was analyzed to examine if subjects implemented different learning strategies in the two paradigms (Fig. 2C). During an open-loop trial, subjects received no post-saccadic feedback regarding performance errors for some time after the end of the saccade; any corrective saccades made during this interval must therefore be at least partially pre-planned by the motor system since they are not driven by visual information. Therefore, a subject who made many corrective saccades in the dark may have relied upon a motor plan that always included producing a corrective movement to reach the target, rather than adapting the saccade gain to reduce post-saccadic errors.
For each subject, the ratio of corrective saccades made before and after the conventional and gradual paradigms was calculated, and these ratios were plotted in Fig. 2C (gray lines). Individually, each subject made approximately the same number of corrective saccades during the pre-adaptation blocks of the two paradigms (paired t-test, p = 0.54); on average, subjects made 0.40 ± 1.3 more saccades prior to the gradual paradigm. To compare across subjects, we computed the ratio of corrective saccades made before and after each of the adaptation paradigms. For all subjects, this ratio was slightly lower for the gradual paradigm than the conventional one, although this difference does not quite reach significance (t test, p = 0.075). Nevertheless, these data suggest that the motor system could employ a compensation strategy (in the form of pre-planned corrective movements) in the conventional adaptation condition but not in the gradual adaptation condition. To do so requires that the target perturbation be anticipated, which is more likely to occur in the conventional adaptation condition when perturbations are large and consistent in size across many trials.
Conclusions
Saccade adaptation is found to be more robust in response to a gradually introduced target perturbation, compared to the conventional condition where the full magnitude of the target step is presented on each trial of the paradigm. This robustness is marked in part by a consistent enhancement in the extent to which subjects adapt, although the magnitude of this overall improvement is quite small so it could have easily been missed in previous studies. Additionally, individual subject differences or online corrective feedback during the movement – which would tend to happen more often for larger, noticeable errors – could have obscured this result in prior studies. Still, these results imply that providing a large and consistent error signal is not as effective as a small but continuously varying error signal, in terms of driving a change in the gain of the motor system. Such findings agree with previous evidence suggesting that the error signal driving adaptation does not have to be consistent on every trial [11].
Furthermore, these data confirm the results from studies involving other motor systems, such as the control of reaching or walking, by finding an increase in the amount of learning retained after a gradual-adaptation paradigm. This improvement occurs in the sense that washout of learning is slower (learning is more resilient) following the removal of the adapting stimulus. However, in these other systems, the ability to make corrections late in the movement – or at least to receive online feedback during the movement itself – allows for the possibility of a more complex motor learning process. It is uncertain for the case of limb movements if learning will occur through a change in the initial movement plan or via a motor strategy to make more online corrections in response to feedback during the movement. Both means of adaptation are reasonable, and it is unclear what may prompt the motor system to choose one strategy over the other. By testing this paradigm in the saccadic system, we eliminate this confusion since performance errors are only available at the conclusion of the saccade, preventing online feedback during the movement. We demonstrate that under these conditions, subjects adapt their initial motor plans to a greater extent in response to a gradual-adaptation stimulus.
These data conflict with error-based models of motor learning, which generally exhibit more robust adaptation in response to a large, consistent retinal error signal that is stable across many trials. Even the two-state model, which can explain the larger aftereffects observed for gradual adaptation, cannot simultaneously account for a greater extent of achieved adaptation. Thus, an alternative motor learning model appears to be necessary to explain how more robust adaptation can be achieved in response to a gradually changing perturbation.
The need for a new computational model of motor learning is important because it influences the manner in which future adaptation studies are interpreted. For example, recent work has demonstrated that subjects with damage to the cerebellum – the portion of the brain thought to be critical for adaptation – can learn in response to a gradual paradigm but minimally or not at all with a conventional paradigm [1]. Such findings have important implications for rehabilitation, as they suggest that when impaired, the motor system is still capable of learning as long as observed errors are small. However, the hypothesized reason for this learning – that gradual adaptation primarily engages the slow system of the two-state model, which therefore is likely to be an extra-cerebellar process – may not be correct since this model cannot explain our data in healthy controls. Perhaps an alternative model might incorporate the ability for the motor system to tune its response in relation to the size and predictability of the stimulus perturbation – an ability that may be modulated by the cerebellum – such that when the perturbation is large and constant (that is, a conventional-adaptation paradigm) alternative compensation methods may be employed including pre-planning anticipatory corrective saccades. When the cerebellum is damaged, the brain may be forced to rely on alternative learning strategies that compensate robustly only for small errors. Thus, additional work is necessary to explain the dynamics of motor learning in light of the gradual-adaptation paradigm.
Highlights.
We compare adaptation in response to a gradual or conventional paradigm.
Saccades, lacking online feedback, are adapted under both conditions.
More robust adaptation (extent and retention) is found with gradual adaptation.
This finding cannot be explained by the two-state model of motor learning.
Acknowledgements
This work was supported by the National Institutes of Health Grants R21-EY019713 and T32-DC000023.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J Neurophysiol. 2009;102:931–940. doi: 10.1152/jn.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res. 1997;115:557–561. doi: 10.1007/pl00005727. [DOI] [PubMed] [Google Scholar]
- 4.Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res. 2005;164:250–259. doi: 10.1007/s00221-005-2247-4. [DOI] [PubMed] [Google Scholar]
- 5.Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol. 2008;100:1455–1464. doi: 10.1152/jn.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–3645. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mclaughlin SC. Parametric adjustment in saccadic eye movements. Percept Psychophys. 1967;2:359–362. [Google Scholar]
- 8.Michel C, Pisella L, Prablanc C, Rode G, Rossetti Y. Enhancing visuomotor adaptation by reducing error signals: single-step (aware) versus multiple-step (unaware) exposure to wedge prisms. J Cogn Neurosci. 2007;19:341–350. doi: 10.1162/jocn.2007.19.2.341. [DOI] [PubMed] [Google Scholar]
- 9.Robinson DA. A Method of Measuring Eye Movement Using a Scleral Search Coil in a Magnetic Field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- 10.Schubert MC, Della Santina CC, Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008;191:435–446. doi: 10.1007/s00221-008-1537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srimal R, Diedrichsen J, Ryklin EB, Curtis CE. Obligatory adaptation of saccade gains. J Neurophysiol. 2008;99:1554–1558. doi: 10.1152/jn.01024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallman J, Fuchs AF. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol. 1998;80:2405–2416. doi: 10.1152/jn.1998.80.5.2405. [DOI] [PubMed] [Google Scholar]
- 13.Wong AL, Shelhamer M. Sensorimotor adaptation error signals are derived from realistic predictions of movement outcomes. J Neurophysiol. 2011;105:1130–1140. doi: 10.1152/jn.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]