Figure 4.
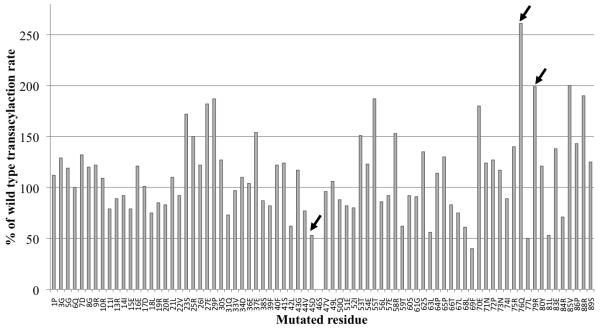
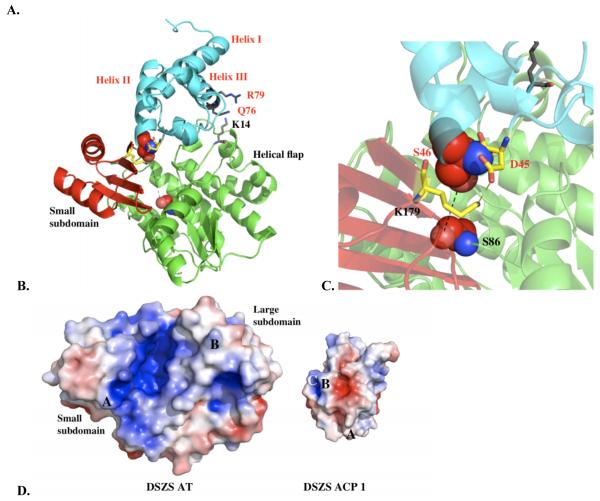
A. Quantification of transacylation rate of alanine-scanning DSZS ACP1 mutants by DSZS AT. The transacylation rates are expressed as a percentage of wild-type DSZS ACP1 tranascylation rate. Mutation in the phosphopantetheinylation site (Ser46) resulted in an inactive ACP. Wild type alanine residues are not represented. (Arrows denote surface residues with 2-fold or greater increase or decrease in transacylation rates) B - D. Docking model of DSZS AT and DSZS ACP1 B. DSZS ACP1 homology model (cyan, red labels) was used as a ligand protein to dock with DSZS AT structure (green with the small subdomain colored red, black labels). The active site serine residues of the AT (Ser86) and the pantetheinate attachment site of the ACP (Ser46) are shown as spheres. The Asp45 - Lys179 pair is colored yellow. Surface residues Gln76 and Arg79 (ACP), along with Lys14 (AT), are colored grey. C. Interaction between helix II (Asp45) of the ACP with Lys179 on the small subdomain of the AT (red). D. Electrostatic surface maps of ACP and AT docking interfaces, generated with the adaptive Poisson-Boltzmann solver (APBS) in PyMOL [35, 36]. Colors range from blue (positive) to white to red (negative). Docked ACP is rotated 180° from AT-ACP interface such that annotations of A – C on AT and ACP interfaces should match up. On the ACP, A = Asp45, B = Gln76, C = Arg79. On the AT, A = Lys179, B = Lys14.