Abstract
Previous studies have shown that microinjections of the GABA-A agonist muscimol into the median raphe nucleus (MR) result in large increases in the intake of solid foods. In the current study, we used microstructural techniques to characterize the effects of intra-MR muscimol injections on the consumption of either a 0.05 M or a 0.29 M sucrose solution. After injections of either saline or muscimol, animals consumed more of the 0.29 M than the 0.05 M solution, an effect which resulted primarily from increases in the initial rate of consumption with no change in the rate at which licking decayed across the test session. In contrast, intra-MR muscimol injections had little effect on the initial licking rate, but greatly increased meal duration, indicating that this treatment affected ingestion in a different way than did altering the sucrose concentration. Muscimol injections produced a significantly larger increase in the intake of the 0.29 M than of the 0.05 M solution. Intra-MR muscimol injections did not alter the within burst rate of licking, suggesting that they did not affect the functioning of the licking pattern generator. In contrast, these injections did increase the number of licks contained within “clusters”, that is groups of licks separated from each other by intervals of more than 0.5 sec. These findings show that inactivation of the MR produces a powerful effect on the intake of liquid diets, and that the nature of this effect is different than that produced here by changes in sucrose concentration and from those reported after pharmacological manipulations of a number of other brain systems. We additionally discuss several theoretical issues arising in the interpretation of microstructural data
Keywords: Feeding, Ingestive behavior, serotonin, GABA, nucleus centralis superior, microstructure, lickometer
Introduction
The median raphe nucleus (MR) is a prominent structure located on the midline of the midbrain and pons that sends both serotonergic and nonserotonergic projections to the hypothalamus and other forebrain sites (Vertes, Fortin & Crane, 1999). In previous studies, we have shown that pronounced alterations in food intake can be produced by injections of a variety of drugs into the MR (Wirtshafter, 2000). For example, marked hyperphagia can be produced by intra-MR microinjections of several glutamate antagonists (Wirtshafter & Krebs, 1990; Wirtshafter & Trifunovic, 1988), whereas injections of glutamate agonists suppress deprivation induced feeding (Wirtshafter & Krebs, 1990). Increases in food intake are also seen following injections of both the inhibitory GABA-A agonist muscimol (Fletcher, 1994; Klitenick & Wirtshafter, 1988; Klitenick & Wirtshafter, 1989; Paris, Mitsushio & Lorens, 1991) and the GABA-B agonist baclofen (Wirtshafter et al, 1993). The response to muscimol has been studied in the greatest detail, and we have found that the effects obtained from the MR are much larger than those seen after injections into a number of adjacent regions, including the ventral tegmental area and the dorsal raphe nucleus (Klitenick & Wirtshafter, 1988). These findings all suggest that treatments which decrease neural activity within the MR, or its immediate vicinity, lead to large increases in food intake. Although the MR is a major source of serotonergic projections to the forebrain (Moore, 1981; Steinbusch & Nieuwenhuys, 1983), several lines of evidence suggest that the orexigenic effects of muscimol at this site cannot be entirely accounted for by their effects on serotonin containing neurons (Wirtshafter, 2000). It is likely, therefore, that cells using other transmitters play a major role in these effects.
The ability of various pharmacological treatments to influence the amount of food eaten must be secondary to the effects of these drugs on the ingestive behavior of animals. It is clear, however, that simply measuring the amount of food eaten over a given period of time does not provide direct information about the way in which the behavior of the animal has been altered. For example, it has been demonstrated that a variety of pharmacological agents, at doses which produce very similar changes in total intakes, may affect ingestive behavior in very different ways (Asin, Davis & Bednarz, 1992; Hsiao & Deupree, 1983; Hsiao & Spencer, 1983; Moran, Carrigan, Schwartz & Ladenheim, 1996). In order to determine exactly what behavioral effects are produced by a given experimental manipulation, it is necessary to actually examine the subject’s behavior, rather than relying on inferences from the amount consumed. In the current study, we attempted to characterize the behavioral basis of the hyperphagia produced by injections of muscimol into the MR using microstructural analysis. In this method, our approach to which has been described in detail in a number of earlier publications (Davis, 1989; Davis & Perez, 1993; Davis & Smith, 1992), the time of occurrence of individual licks is recorded and their distribution over the test period analyzed. We examined the effects of muscimol injections on the intakes of solutions containing two different concentrations of sucrose in order to determine whether the muscimol treatments interacted with the properties of the ingestate. In particular, we were interested in determining whether the effects of muscimol resembled those produced by alterations in sucrose concentration, or those reported after administration of several other orexigenic agents.
Methods
Subjects
Subjects were 17 adult male Sprague-Dawley derived rats obtained from a colony maintained by the University of Illinois at Chicago. Animals weighted between 270 and 330 g at the time of surgery and were housed individually in wire mesh cages on a 12:12 hr light dark cycle with food (Wayne LabBlox) and water available ad libitum, except as noted. All procedures were in accordance with NIH Guidelines for the Care and Use of Laboratory Animals using methods approved by the IACUC.
Surgery
Animals were anesthetized with sodium pentobarbital (50 mg/kg) and prepared with chronic 22 gauge stainless steel guide cannula aimed to terminate 2 mm dorsal to the MR (AP: −0.3, H: 3.2, L: 0.0; mm from interaural line with incisor bar at +6.8 mm). The cannulae were lowered in the sagittal plane following retraction of the superior sagittal sinus (Wirtshafter et al, 1979), and were fixed in place with skull screws and dental cement. A 28 gauge stainless steel obturator which extended 2 mm past the end of the guide cannula was then inserted.
Apparatus
Testing was conducted in wire mesh cages, identical to those in which the rats were housed, measuring 24 cm wide X 29 cm deep X 20 cm high. A stainless steel drinking spout, attached to a 60 ml calibrated tube, was mounted 6 cm above the floor with the tip flush with the inside of the chamber. The tube was connected to an amplifier such that a current of less than 60 nA was passed through the spout each time the animal licked the tube (DiLog Instruments, Tallahassee, FL). Times of licks were recorded to the nearest millisecond and analyzed using Quick Lick software (DiLog Instruments).
Microinjection procedure
Obturators were gently removed and replaced by 28 gauge stainless steel injection cannulae, trimmed to terminate 2 mm beyond the end of the guide cannulae. Injections were made in a volume of 0.5 ul at a rate of 0.25 ul/min using a motor driven microsyringe connected to the injectors by means of a length of polyethylene tubing. Injectors were removed 30 sec following the completion of the injection, and the obturators replaced. Subjects received injections of either normal saline, or 25 ng of muscimol (Klitenick & Wirtshafter, 1988; Klitenick & Wirtshafter, 1989) (Sigma, St. Louis) dissolved in saline. After the injection was completed, subjects were placed in the test cages and 2–3 min later the drinking spouts were inserted.
Procedure
Animals were allowed 10 days to recover from surgery, during which time they were frequently handled. Animals were divided randomly into high and low concentration sucrose groups containing 8 and 9 animals respectively. On the following day, food was removed from their cages and 24 hr later the animals were placed in the test cages for a 1 hr session; animals in the low concentration sucrose group received a 0.05 M sucrose solution, and subjects in the high concentration sucrose group a 0.29 M solution. These concentrations were chosen based on previous experience in our laboratory indicating that they produced dependably different intakes and that the 0.05M solution was near the lower limit for reliably inducing drinking. At the end of the hour, animals were returned to their home cages and the volume consumed measured to the nearest ml. On subsequent days, food was removed 2 hr prior to testing, to ensure that animals had not just eaten a meal. Subjects in both the low and high concentration sucrose groups received 7 daily sessions to allow their intakes to become stable. At this time, approximately half of the subjects in each of the sucrose conditions received an injection of saline and the remaining subjects an injection of muscimol prior to being placed in the lickometer for their 60 min. test session. The next day, they received another baseline run and, the day after that, animals were tested following injections of the solutions complimentary to those given on the first treatment day.
Histology
Following the completion of behavioral testing, all subjects were transcardially perfused, under deep sodium pentobarbital anesthesia, with saline followed by 10% formalin. Several days later, the brainstems were sectioned at a thickness of 60 um, and the sections stained with cresyl violet to evaluate the injection site.
Results
Histology
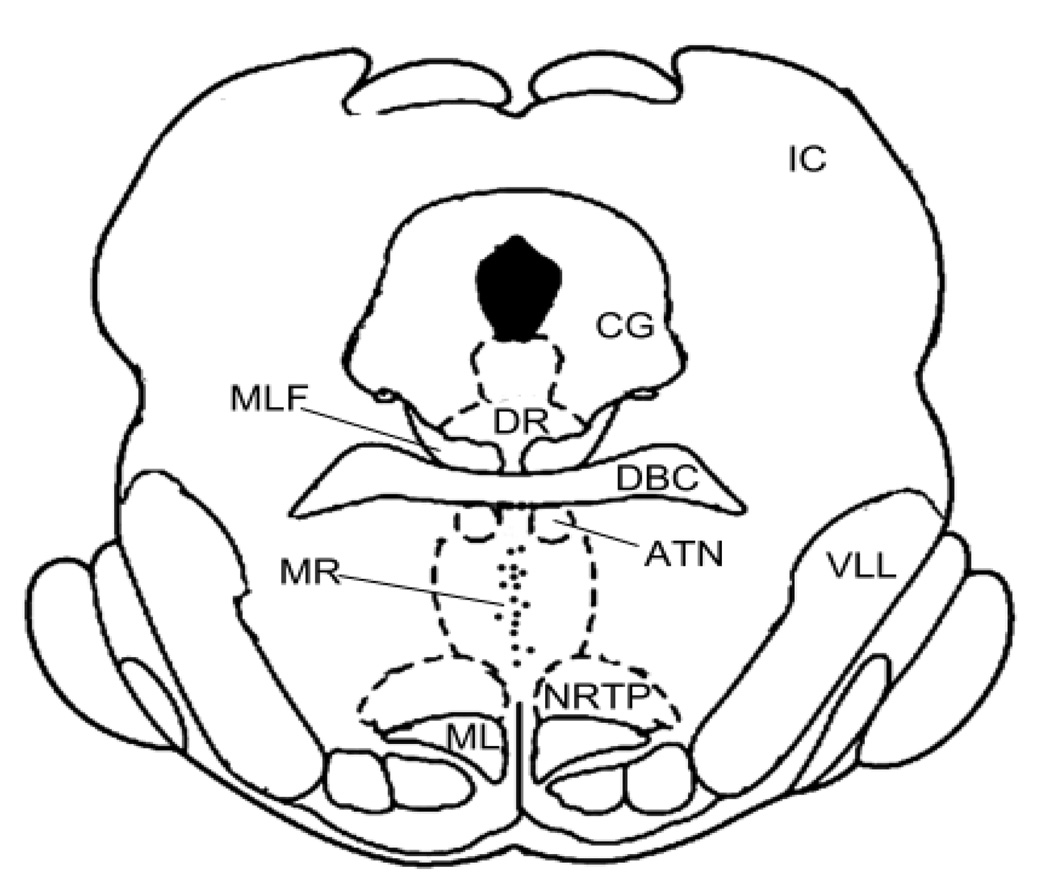
Histological examination indicated that all cannula tracks terminated within the median raphe nucleus at sites similar to those we have examined in previous studies (Fig. 1).
Fig. 1.
Histologically determined location of cannula tips for subjects in the current study. All cannulae tips (small dots) were located in the medial portion of the MR. Tip locations were within 0.3 mm of the coronal plane depicted in this illustration. Abbreviations: ATN=anterior tegmental nucleus, CG=central grey substance, DBC=decussation of the brachium conjunctivum, DR=dorsal raphe nucleus, IC=inferior colliculus, MLF=medial longitudinal fasciculus, ML=medial lemniscus, MR=median raphe nucleus, NRTP=nucleus reticularis tegmenti pontis, VLL=ventral nucleus of the lateral lemniscus. Dashed lines extending from the ATN to the NRTP represent the medial borders of the predorsal bundle.
Macrostructural variables
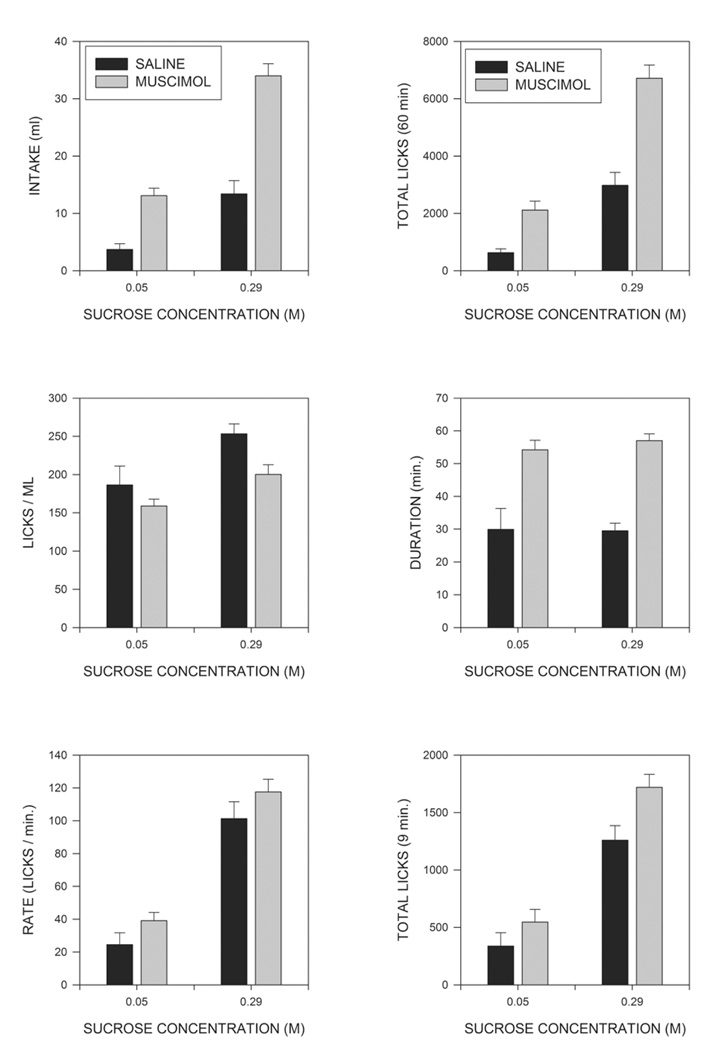
Total intakes across the test period for animals drinking the low and high concentrations of sucrose are shown in the upper left panel of Fig. 2 where it can be seen that rats consumed more of the 0.29 M than of the 0.05 M sucrose solution, and that muscimol injections increased intakes of both solutions, but tended to produce a larger increase of the more concentrated ingestate. These conclusions were supported by a 2-way (sucrose concentration X muscimol) analysis of variance (ANOVA), with repeated measures on the muscimol factor, which indicated significant effects of sucrose concentration (F(1,15)=99.9; p<0.001), of muscimol (F(1,15)=65.1; p<0.001) and of the sucrose X muscimol interaction (F(1,15)=9.0; p<0.01), indicating that muscimol produced a significantly larger increase in feeding in animals consuming the more concentrated than the less concentrated solution. A test of simple main indicated that muscimol significantly increased intake even in subjects tested on the low concentration of sucrose (F(1,15)=13.6; p<0.002). As can be seen in the upper right panel of Fig. 2, a similar pattern was seen when total numbers of licks were examined, and ANOVA again indicated significant effects of both sucrose (F91,15)=153.5; p<0.001)), muscimol (F(1,15)=38.7; p<0.001), and of the sucrose X muscimol interaction F(1,15)=7.2; p<0.02).
Fig. 2.
Effects of sucrose concentration and intra-MR muscimol injections on macrostructural aspects of feeding. The upper left panel shows effects of sucrose and muscimol on the volume of intake across the 60 min test session. The upper right panel shows effects on the total numbers of licks. The middle left panel displays mean numbers of licks needed to consume 1 ml of fluid. The middle right panel shows the mean duration of eating, i.e., the mean interval between the first and last licks made in the test session. The lower left panel shows mean rates of intake over the duration of the eating, i.e., the mean ratios of total licks to the duration of eating. The lower right panel shows the numbers of licks made in the first 9 min of the test session. See text for statistical details.
Although the muscimol-induced increase in fluid consumption resulted primarily from increases in the number of licks executed, this may not have been the only factor involved. The middle left panel of Fig. 2 indicates that muscimol injections produced a small, but consistent, decrease in the number of licks required to consume a milliliter of fluid (F(1,15)=5.38; p<0.05). This effect was of similar magnitude at both concentrations of sucrose, as indicated by the failure of the sucrose X muscimol interaction to approach significance (F>1). In contrast, licks/milliliter was actually reduced slightly at the high, as compared to the low, concentration of sucrose (F(1,15)=6.27; p<0.025). Analogous statistical results were obtained when lick efficiency (i.e., mls/lick) was analyzed (not shown).
The increases in total fluid consumption observed could result from increases in the amount of time that animals spent drinking or from increases in the overall rate at which fluid was consumed. The middle right hand panel of Fig. 2 displays the total duration of licking; that is the time from the first lick to the last lick made within the session, irrespective of any pauses taken. It can be seen that muscimol injections greatly prolonged the period of time over which animals licked (F(1,15)=50.4; P<0.001), whereas sucrose concentration was without effect on this variable (F<1). The lower left hand panel of Fig. 2 shows the average rate at which animals drank; that is, the total number of licks divided by duration of the period during which licking occurred. Overall licking rate was significantly greater for animals consuming the higher concentration of sucrose (F(1,15)=63.5; p<0.001), but was also slightly increased by injections of muscimol (F(1,15)=4.5; p≤0.05). The sucrose X muscimol interaction was not significant (F<1), however, indicating that the effects of sucrose concentration and of muscimol injections on overall licking rates were additive. Latency to initiate licking was not significantly affected either by sucrose concentration or muscimol injections (data not shown).
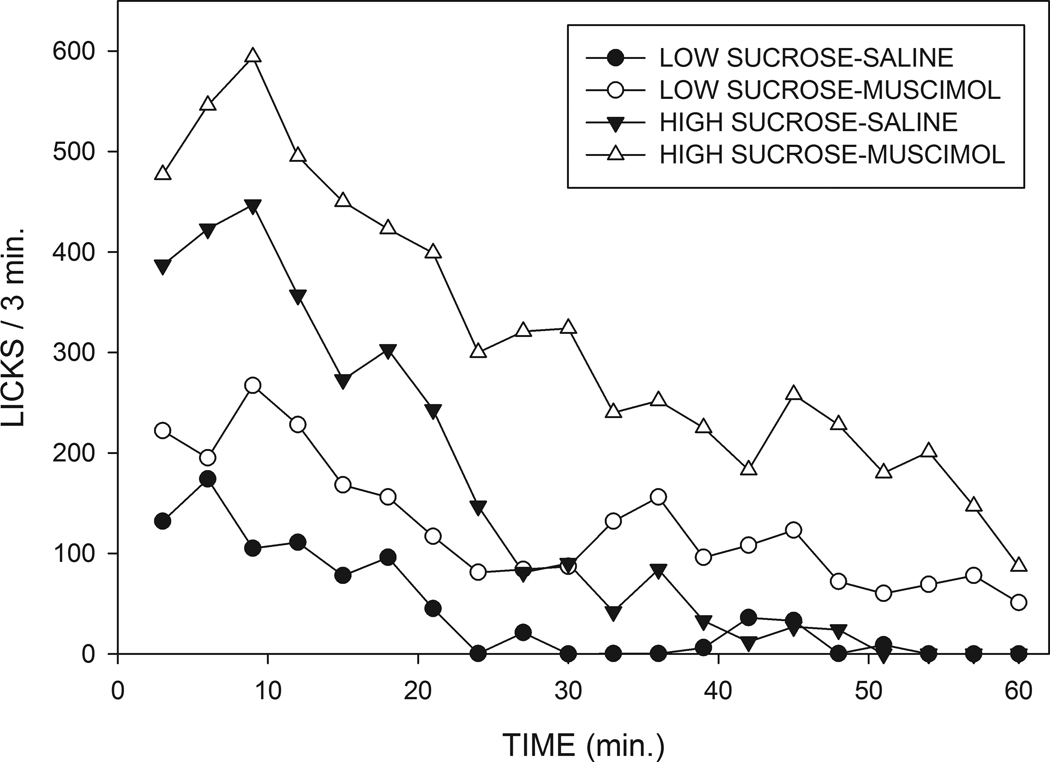
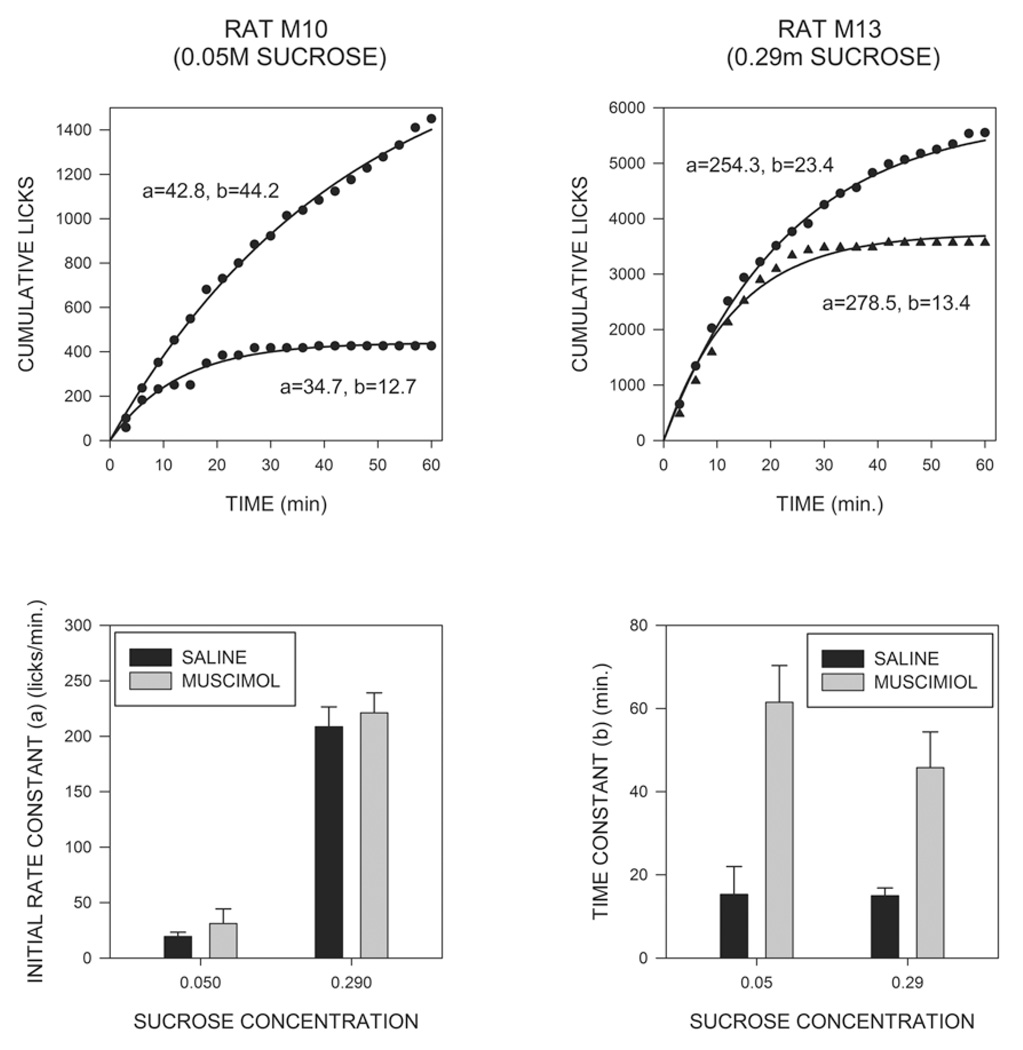
These effects on the temporal pattern of licking can best be appreciated by examining the rate across time curves shown in Fig. 3. For both the low and the high concentrations of sucrose, muscimol injections tend to produce increases in rates of responding which persisted over the course of the 60 min test periods. In order to analyze the temporal pattern of licking behavior, cumulative intake curves for individual animals were fit, using a nonlinear least squares technique, to the exponential decay equation “cumulative licks = ab(1-e−t/b)” where, “t” represents time in minutes, “a” represents slope of the cumulative intake curve at t=0, i.e., the estimated initial rate of licking, “b” represents the time constant of decay, and “e” the base of natural logarithms. The value of b was constrained so as not to exceed 60 minutes, and the mean r2 value for goodness of fit was 0.994. Examples of curve fits and mean values for the “a” and “b” parameters are displayed in Fig. 4, which shows that the initial rate parameter was strongly affected by changes in sucrose concentration (F(1,14)=143.2; p<0.001), but was unaffected by muscimol injections (F<1). In contrast, the decay constant “b” was significantly increased by muscimol injections (F(1,14)=71.5; p<0.001), but was unaltered by changes in sucrose concentration (p>0.3). None of the interactions were significant. In order to examine the initial rate of licking by a different approach, we also analyzed the number of responses made during the first three minutes of consumption. This analysis again indicated a very marked potentiating effect of sucrose concentration (F(1,15)=36.0; p<.001), and, although there was a trend for an effect of muscimol injections, this fell short of statistical significance (F(1,15)=3.50; p<.08).
Fig. 3.
Mean numbers of licks made in three min. time bins for animals injected with saline or 25 ng muscimol into the MR who were allowed to drink sucrose at concentrations of 0.5 or 0.29M.
Fig. 4.
The upper two panels show examples of exponential curve fits for cumulative licking data for a typical subject in the low sucrose condition (upper left panel) and one in the high sucrose condition (upper right panel). Licking after saline injections is shown in triangles, and after muscimol injections in circles. Data were fit to the equation “cumulative licks = ab(1-e−t/b)”. Note the difference in scales between the two figures. The large difference in overall intakes between the two animals is due primarily to the more than 5 fold increase in the “a” parameter, which reflects the initial rate of drinking, whereas the effects of muscimol were due primarily to changes in the time constant “b”. The lower two panels show mean values of the initial rate constant and the time constant after saline and muscimol injections for animals in the two sucrose conditions.
Microstructural variables
The great majority of interlick intervals for all of the animals examined here were located in an approximately normally distributed cluster with a mean of about 150 ms. In order to characterize possible treatment effects on this distribution of “within burst ILIs”, we determined, for each subject, the mean duration and the standard deviation of ILIs less than 250 ms (“within burst ILIs”). These numbers were virtually identical in all of the treatment conditions (F<1, data not shown)
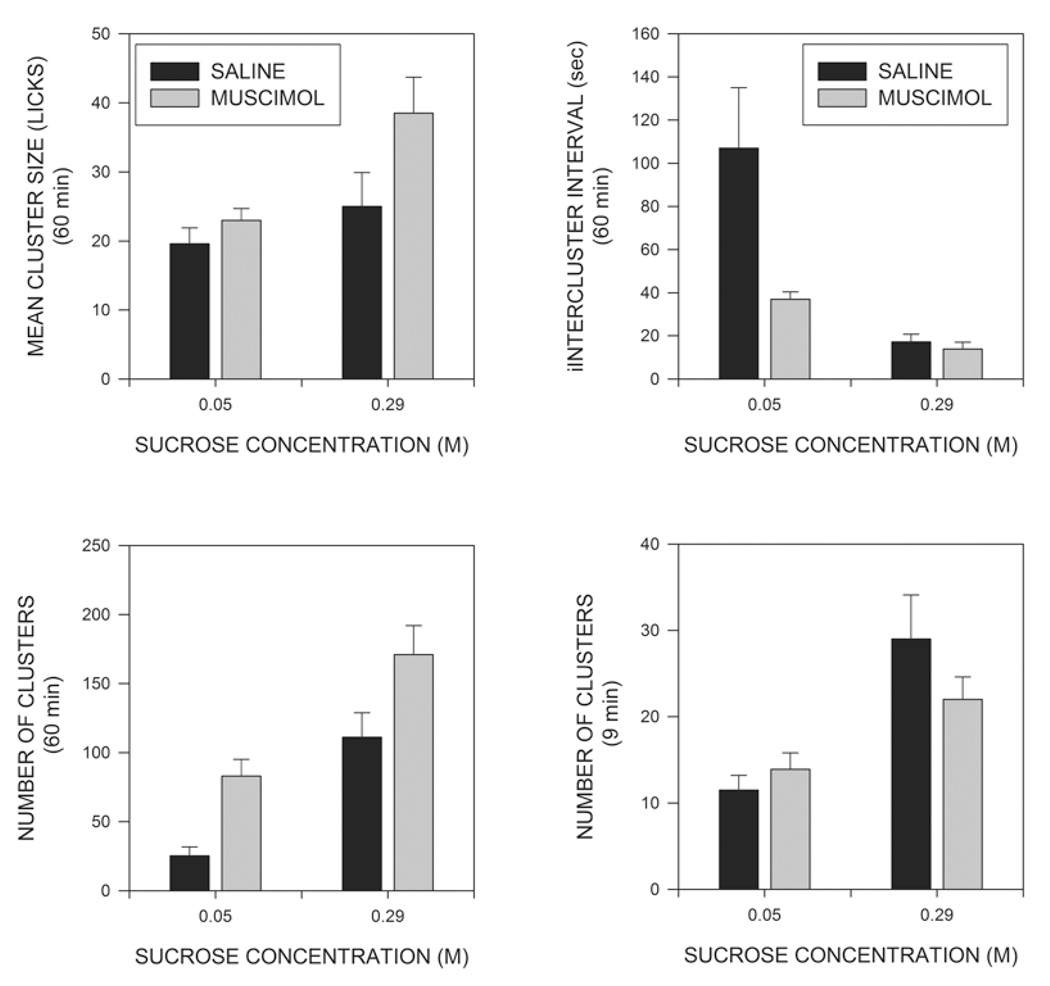
Although the majority of ILIs are less than 250ms in duration, a relatively small number of ILIs are longer and serve to break the stream of licks into relatively discrete groups. As we have done in previous studies (Asin et al, 1992; Breslin et al, 1996; Davis & Perez, 1993; Davis & Smith, 1992; Torregrossa et al, 2006), we will refer to groups of at least three licks separated from other licks by more than 500ms as clusters. The mean size of clusters is shown in the upper left panel of Fig. 5 where it can be seen that cluster size tended to be increased both by increases in the concentration of sucrose and by the administration of muscimol. A 2 × 2 ANOVA indicated significant effects both of sucrose concentration (F(1,15)=6,183; p<0.025) and of muscimol injections (F(1,15)=7.098; p<0.020). Although the effect of muscimol on cluster size tended to be larger at the higher concentration of sucrose, the muscimol X sucrose interaction was not significant (F(1,15)=2.54; p>0.1). The upper right panel of Fig. 5 shows the mean intervals separating clusters from each other (intercluster intervals, ICIs). A 2 × 2 ANOVA demonstrated a significant effect of sucrose (F(1,15)=15.5; p<0.002), and of muscimol (F(1,15)=6.5, p<0.05). The muscimol X sucrose interaction was also significant (F(1,15)=5.29; p<0.05), indicating that muscimol had a larger effect at the low than the high sucrose condition. In fact, analysis of simple main effects indicated that muscimol injections did not even significantly reduce mean ICIs in animals receiving the high concentration of sucrose (F<1). As can be seen in the lower left panel of Fig. 5, the number of clusters generated across the 60 min test period was significantly increased both by muscimol injections (F(1,15)=12.27; p<0.01) and by increases in sucrose concentration (F(1,15)=48.7; p<0.001). The muscimol X sucrose interaction, however, did not approach significance (F<1) indicating that the effects of sucrose concentration and muscimol combined in a near additive fashion.
Fig. 5.
Effects of sucrose concentration and muscimol injections on the size of clusters (upper left panel), interval between clusters (upper right panel), and number of clusters (lower left panel) across the 60 min test session. The lower right panel shows numbers of clusters across the first 9 min of the test. (See text for details.)
The stream of licking is interrupted not only by ICIs that are longer than 500 ms, but also by shorter pauses between 250 and 500 ms in duration; ILIs in this time range form a second mode with a mean of about 300 ms, about which ILIs are again distributed in an approximately normal fashion. In order to determine whether sucrose concentration or muscimol injections altered this portion of the ILI distribution, we determined, for each subject, the mean duration and the standard deviation of ILIs between 250 and 500 ms in duration (“interburst intervals”, IBIs). As was the case for within burst ILIs, the mean IBIs, and their standard deviation, were very similar in all of the treatment conditions (F<1, data not shown).
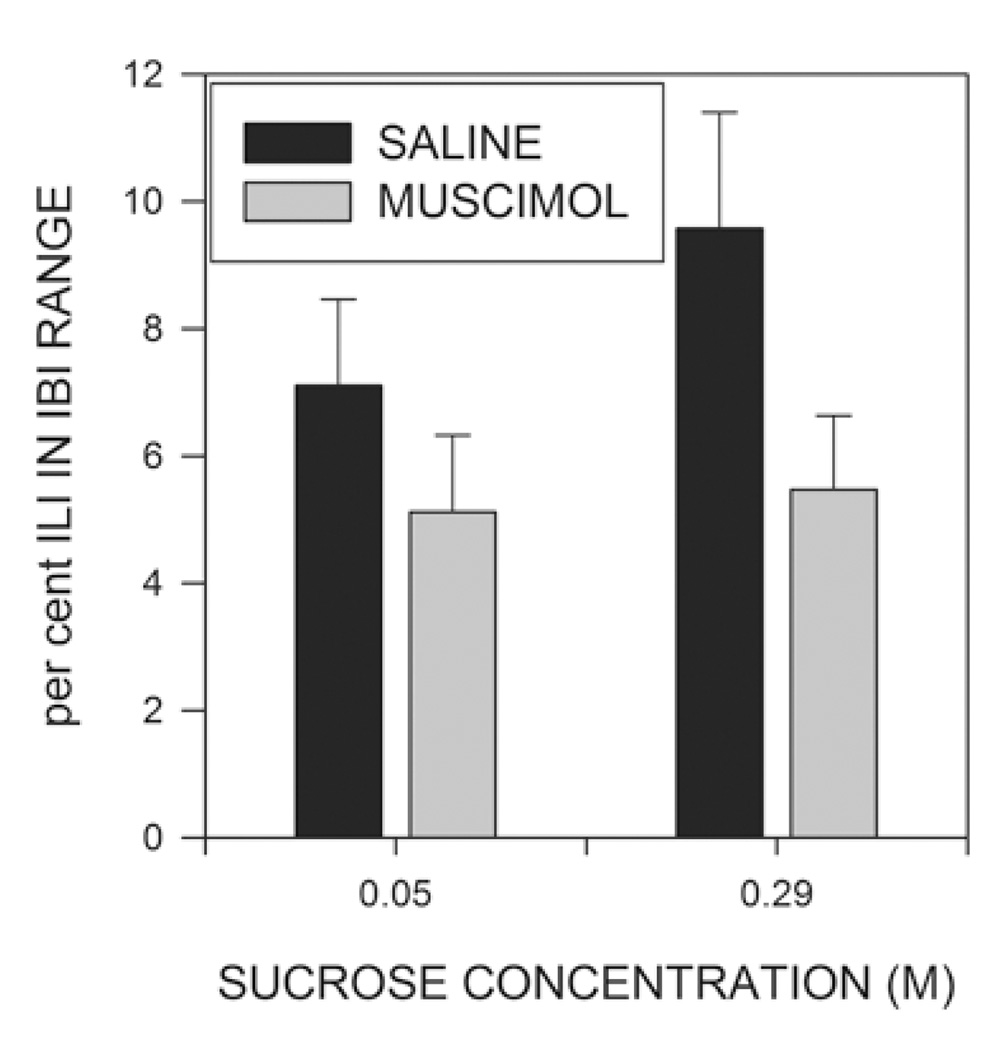
The mean and standard deviation characterize the basic shape of the distribution of IBIs, but do not provide information about the relative frequency of ILIs in this time range. We therefore examined, for each rat, the proportion of the total number of ILIs which were between 250 and 500 ms in length. As can be seen in Fig. 6, the proportion of ILIs in this time range was significantly lower in muscimol treated than control rats (F(1,15)=8.647; p<0.01), but neither the sucrose effect nor the sucrose X muscimol interactions were significant (F<1). The effect of muscimol was still significant if the data were subjected to either arcsin or logit transformations prior to analysis (Warton & Hui, 2011; Winer, Brown & Michels, 1991).
Fig. 6.
The percentage of the total number of interlick intervals (ILIs) which fell between 0.25 and 0.5 sec in the interburst interval (IBI) range. The effect of muscimol was statistically significant, whereas that of sucrose concentration was not. (See text for details.)
ILIs in the 250–500 ms range break the stream of licking into groupings that we previously have called bursts (Davis, 1989; Davis & Perez, 1993; Davis & Smith, 1992). Formally, bursts are defined as runs of at least three licks separated from each other by no more than 250 ms. The experimental manipulations in the current study tended to affect burst size in a fashion similar to that in which they affected cluster size, and we will thus simply review the statistical outcomes. Mean burst size was increased by muscimol injections (F(1,15)=14.998; p<0.002), and tended strongly to be larger in animals tested with the higher concentration of sucrose, although this effect fell short of statistical significance (p=0.15). Mean numbers of bursts were significantly larger following muscimol injections (F(1,15)=10.55; p<0.005) and in animals tested with the higher sucrose concentration (F(1,15)=44.72; p<0.001). The muscimol X sucrose interaction was not significant (F<1) indicating that the effects of muscimol and sucrose were roughly additive.
Some authors (Baird et al, 2006a; Narayanan et al, 2010; Taha et al, 2009) have found it useful to define ingestive structures on a longer time scale than clusters by considering groups of licks separated by intervals of 10 min or more as constituting meals. Using this criterion all subjects in the high sucrose condition displayed only a single meal, whereas many animals under the low sucrose condition injected with saline had more than one meal. Considering just the animals in the low sucrose condition, injections of muscimol significantly reduced the mean number of meals from 1.8±0.3 to 1.1±0.1, (F(1,8)=5.33; p<0.05), reflecting the fact that most muscimol treated animals ate continuously through the entire test session. In order to determine whether the ability of muscimol to reduce ICIs noted above was due entirely to a reduction in the number of these very long intervals, we recalculated ICI, for the subjects in the low sucrose group, eliminating ILIs longer than 600 sec. Even under these conditions, however, muscimol still significantly reduced mean ICI (F(1,8)=14.4; p<0.01).
Since the duration of eating was dramatically increased by muscimol injections, the possibility must be considered that the microstructural alterations reported above somehow resulted from the longer period of time over which measurements were averaged in drug treated animals. The simplest way to investigate this possibility is to examine licking patterns across a fixed time period that is short enough that it is within duration of licking of all of the animals. Preliminary examination indicated that the meal duration of all but one of the animals in the current study was more than 9 minutes; we therefore eliminated this one animal in the low sucrose condition and analyzed licking in the remaining animals across the first 9 minutes of the test session. As can be seen in the lower right panel of Fig. 2, the numbers of licks across this period were significantly affected by sucrose concentration (F(1,14)=72.87; p<0.001) and, to a lesser extent, by muscimol (F(1,14)=7.69; P<0.02) but, in marked contrast to the situation across the entire 60 min test period, the sucrose X muscimol interaction did not approach significance (F<1). (It should be noted that the interaction was still significant at 60 min. (p<0.03) even when the data were analyzed leaving out the one rat whose meal duration under saline was less than 9 min.) Most of the effects observed over 60 min., however, were also present at 9 min. Thus the proportion of ILIs occurring in the IBI range (0.25-0.5 sec) was again decreased by muscimol (p<0.05), but not sucrose, and the mean durations of IBIs, and of within burst ILIs, were again unaltered (p>0.1)(data not shown). Burst and cluster sizes (truncated at the end of 9 min) were again significantly increased by muscimol injections (p<0.01) and, in the latter case, the effect of sucrose was also significant (p<.01). In contrast to the situation across the entire 60 min, however, the number of clusters was not significantly affected by muscimol treatment (p>0.1), although there was a strong effect of sucrose concentration (F(1,14)=19.9; p<.001) (lower right panel of Fig. 5). (Again, cluster number was still significantly increased at 60 min. (p<0.01) when only the animals examined at 9 min were included in the ANOVA.) Mean ICIs were significantly shorter at the high than the low concentration of sucrose (p<.05) whereas the effect of muscimol was not significant (p>0.1), although there was a trend for a decrease.
Discussion
The current results are consistent with previous findings of increased intake of solid foods following injections of muscimol into the MR (Fletcher, 1994; Klitenick & Wirtshafter, 1988; Klitenick & Wirtshafter, 1989; Paris et al, 1991), and extend these results by showing that muscimol injections also increase intakes of sucrose solutions. The detailed macro- and microstructural data obtained here allows for the nature of these effects to be described in considerably more detail than has been the case in previous studies. We discuss these findings at some length because many aspects of the interpretation of the different variables and their interrelations have not been considered in detail by previous authors, even though the general techniques used here have been widely employed.
The increased intake was due largely to increases in the numbers of licks emitted, but increases in lick efficiency (i.e., the number of licks needed to consume one ml of fluid) also played a minor role (Fig. 2, middle left panel). Alterations in lick efficiency have been reported after a number of pharmacological treatments (Baird, Gray & Fisher, 2006a; Hsiao & Deupree, 1983; Hsiao & Spencer, 1983; Knowler & Ukena, 1973; Stratford, Gibbs & Smith, 1995), including intraventricular injections of neuropeptide Y (NPY) (Baird et al, 2006a). The physical basis of the effect observed here is not clear since there is little evidence that muscimol injections alter the basic pattern of licking behavior. It is possible that differences in head positioning or the duration of tongue contact with the spout may have played a role. We also observed that licking was less efficient in subjects consuming the high rather than the low concentration of sucrose. Although not always seen, similar effects have been reported previously (Genn, Higgs & Cooper, 2003) and it is possible that they might reflect alterations in the viscosity or surface tension of the ingestate.
The most dramatic effect of the intra-MR muscimol injections was a prolongation of the amount of time animals spent licking. In contrast, the effect of sucrose concentration was primarily to change the initial rate at which animals drank. It is especially instructive to compare the rate across time as shown in Fig. 3 for the saline injected rats drinking the high concentration of sucrose and the muscimol-injected animals drinking the low concentration of sucrose. Mean intakes under the two conditions were similar, but the temporal pattern of drinking shown by the subjects was drastically different. Thus, animals receiving saline injections and the high concentration of sucrose began licking at a high rate that rapidly decayed, whereas the subjects receiving muscimol injections and the low concentration of sucrose began drinking at a modest rate that declined only slowly throughout the session. This illustrates the point that measures of total amount consumed contain only a small proportion of the available information, and demonstrates that, despite the similarities in overall intake, the behavior of muscimol treated animals consuming a low concentration of sucrose does not resemble that of control subjects consuming a higher concentration of sucrose.
Previous studies have shown that cumulative intake vs. time curves can be well fit by two parameter exponential functions of the form “cumulative licks = ab(1-e−t/b)” (Davis & Levine, 1977; Davis & Perez, 1993; Genn et al, 2003), an observation that was confirmed in the current experiment. Earlier studies have also shown that the value of the initial rate parameter “a” is strongly influenced by the sensory properties of the ingestate (Breslin, Davis & Rosenak, 1996; Davis, 1998; Davis & Levine, 1977; Genn et al, 2003), and this finding was also confirmed here in that values for the rate parameter were substantially higher in animals consuming the more concentrated sucrose solution. In contrast, muscimol injections did not significantly alter the initial rate parameter, or the numbers of licks made in the first 3 min, although there was a small trend in both cases. These results indicate that changes in the initial rate of drinking are not the major mechanism through which intra-MR muscimol exerts its effects on intake.
The time constant parameter “b” reflects the rate at which licking declines from its initial value and is often related to inhibitory feedback arising from the act of licking, or its postingestive consequences (Davis, 1998; Davis & Levine, 1977). In the current study the time constant was not affected by changes in the concentration of sucrose, a result which is somewhat surprising given that previous studies have found that this value is inversely proportional to carbohydrate concentration (Davis & Levine, 1977). It is possible that this difference may reflect the fact that sucrose concentration was a between subject variable in the current study, whereas individual subjects were tested on multiple carbohydrate concentrations in previous reports. At any account, in contrast to the lack of effect of sucrose concentration, muscimol injections produced a marked increase in the time constant, reflecting the fact that licking persisted for a much longer time in these subjects than it did in saline-treated animals. The simplest explanation of these findings is that inactivation of the MR reduced the sensitivity of animals to some form of licking-induced feedback. A similar suggestion has been made with respect to neuropeptide Y (Lynch, Hart & Babcock, 1994), which also increases meal durations. In this case, however, it has been shown that NPY still exerts this effect in sham feeding animals (Torregrossa, Davis & Smith, 2006), suggesting that its actions cannot result entirely from a blockade of post-ingestive feedback. Further studies will be needed to determine whether a similar conclusion holds for the case of intra-MR injections of muscimol. It should also be pointed out that care should be exerted in interpreting the effects of drug injections on the temporal pattern of licking; the actions of most drugs vary as a function of time since injection, and such “time-course effects” might well combine in unknown ways with changes in licking rate naturally occurring across the course of a meal.
In the current experiment, muscimol injections produced a significantly larger increase in intake in animals consuming the high than the low concentration of sucrose. Although one might be tempted to suggest that this reflects a synergistic effect of sucrose concentration and raphe inactivation on the vigor of licking, there is little in the data to support this contention. Analysis of the time course of drinking allows for much simpler explanation. Increasing the sucrose concentration produces a large increase in the mean rate of licking, whereas muscimol injections primarily increase the duration of meals. Since the total amount consumed is equal to the mean rate of ingestion multiplied by the duration of licking, an equivalent increase in meal duration naturally will produce a larger increase in total consumption in animals licking a high rather than a low concentration solution. If this explanation were correct, one would expect the synergy between sucrose concentration and muscimol injections to be greatly reduced under conditions in which meal duration cannot vary; the current results support this viewpoint because the effects of muscimol and sucrose concentration were statistically additive when numbers of licks were measured across just the first 9 min of access. It is interesting that the orexigenic compound NPY has been suggested to alter oral sensory processing based on findings that the magnitude of its effect varies with the palatability of the ingestate. However, since NPY, like intra-MR muscimol, tends to lengthen meals (Lynch et al, 1994; Torregrossa et al, 2006), the considerations discussed here may provide a simpler explanation of these findings.
In normal rats licking sapid solutions, the great majority of interlick intervals (ILIs) fall in the vicinity of 150 ms (Corbit & Luschei, 1969; Stellar & Hill, 1952) and have been proposed to reflect the operation of a brainstem licking pattern generator (Davis & Smith, 1992; Travers, Dinardo & Karimnamaze, 1997; Wiesenfeld, Halpern & Tapper, 1977). The mean of these “within burst ILIs” is typically unaltered by deprivation (Corbit & Luschei, 1969), although small and inconsistent changes have sometimes been observed (Davis & Perez, 1993; Spector et al, 1998). Alterations in within burst ILIs have also been reported after systemic or intracranial administration of a number of compounds (Asin, Davis & Bednarz, 1992; Baird, Rios, Gray, Walsh, Fischer & Pecora, 2006b; Knowler & Ukena, 1973; Stratford et al, 1995). In the current study, however, the shape and location of this distribution were not altered by muscimol injections, suggesting that inactivation of the MR does not directly influence the licking pattern generator,
In agreement with many previous studies (Corbit & Luschei, 1969; Davis & Smith, 1992; Gramling, Fowler & Collins, 1984; Hsiao & Deupree, 1983; Hsiao & Spencer, 1983), we noted that a second, much smaller, peak in the ILI distribution could be observed with a mean close to 300 msec. Some authors have reported that additional peaks can be observed with means close to 450 and 600 msec (Corbit & Luschei, 1969; Gramling et al, 1984), but these were not seen in the current experiment. Previous studies have shown that ILIs between 250 and 500 ms, which we will refer to as inter-burst intervals (IBIs), are distributed normally (Davis & Smith, 1992). Mean IBI has been reported to be unaffected by either deprivation or sucrose concentration (Davis & Perez, 1993), and we confirmed the latter result in the current report. We also found that the mean and standard deviation of the distribution of IBIs was not affected by inactivation of the MR, suggesting that this manipulation did not alter the nature of the behavior responsible for these brief pauses in recorded licking. In contrast, the relative frequency at which IBIs occurred was significantly reduced by muscimol injections. Alterations in the relative frequency of IBIs have also been reported after several other pharmacological treatments (Baird et al, 2006b; Gramling et al, 1984; Hsiao & Deupree, 1983; Hsiao & Spencer, 1983; Taha, Katsura, Noorvaash, Seroussi, & Fields,, 2009). The exact significance of IBIs is not currently understood; one possibility is that they may simply reflect standard licks during which the tongue did not make electrical contact with the spout (Corbit & Luschei, 1969). Alternatively, they might result from alterations in the actual rate of licking (Hsiao & Deupree, 1983), or reflect some other behavior, such as a lateral tongue movement (Davis & Smith, 1992), which coincidentally takes about twice as long as a standard lick. Although not conclusive, the current results are more easily accommodated within the “separate behavior viewpoint”; for example, inactivation of the MR could either reduce the frequency of the behavior responsible for IBIs, or could increase the tendency to lick to the point that any sort of interruption of the licking rhythm became less probable. In contrast, it is not immediately apparent how the probability of missed licks could be altered, although possibly this could result from changes in positioning of the head with respect to the spout. High-speed video analysis of licking would be useful in deciding between these possibilities.
The occurrence of intermittent pauses serves to divide the stream of licks into discrete bouts. Using the terminology of Davis and his coworkers (Davis, 1998; Davis & Perez, 1993; Davis & Smith, 1992), bouts of licking separated from each other by pauses of at least 0.25 sec. are referred to as bursts, whereas bouts isolated by pauses of more than 0.5 seconds (“intercluster intervals,” ICIs) are referred to as “clusters”. (Some workers (Spector, Klumpp & Kaplan, 1998) have suggested that a cutoff of 1.0 sec be used, but this is a matter of little practical importance since, under the current conditions, ILIs between 0.5 and 1.0 seconds were extremely rare.) Alterations in the size of clusters therefore simply reflect alterations in the relative frequency of ILIs longer than 0.5 sec (the mean cluster size being approximately equal to the reciprocal of the probability of an ICI). In contrast, burst size is related to the probabilities of both IBIs and of ICIs, being approximately equal to one over the sum of the probabilities of IBIs and ICIs. As a result of these relations, changes in the frequency of pauses between 0.25 and 0.5 sec in duration would be expected to alter burst size alone, whereas changes in the frequency of pauses longer than 0.5 sec would influence both burst and cluster size. Burst size is thus not independent of cluster size, a consideration which suggests that the relative frequency of IBIs may provide a more discrete measure of these interruptions than does burst size. In the current study, cluster size was significantly increased both by injections of muscimol and by increases in the concentration of sucrose. Burst size was also significantly increased by muscimol injections, as would be expected given the increases in cluster size and the decrease in the relative frequency of IBIs discussed above.
Many previous studies have demonstrated effects of tastant concentration on cluster or burst size, and it has been suggested that these parameters may reflect the palatability of the ingestates. Our current observation that inactivation of the MR increases both cluster and burst size is consistent with the notion that this structure may influence hedonic processing of taste related stimuli. Alterations in the perceived palatability of the test solutions would, however, also be expected to alter the initial rate of licking, an effect which was not observed in the current experiment. Other experiments (Baird et al, 2006a; Torregrossa et al, 2006) have also found that effects on initial rate and cluster size can be dissociated, suggesting that factors in addition to palatability can also influence these variables. It is interesting in this context that lesions of the MR result in perseverative behavior under certain conditions (Asin, Wirtshafter & Kent, 1979; Wirtshafter & Asin, 1983; Wirtshafter & Asin, 1986); if a similar effect occurred after muscimol injections, it is possible that it could play a role in the observed prolongation of the bouts of licking.
Given that the median raphe is a major source of serotonergic projections to the forebrain, it is striking that the serotonergic anorexics fluoxetine and fenfluramine have been reported to decrease burst and cluster size (Asin et al, 1992), effects opposite to those observed here with intra-MR injections of muscimol, which are known to reduce serotonin release (Shim, Javaid & Wirtshafter, 1997; Wirtshafter & Trifunovic, 1992). Although inhibition of serotonin neurons does not appear to be the only mechanism underlying the orexigenic effects of intra-MR muscimol (Wirtshafter, 2000), it is possible that serotonin may be involved in effects on burst and cluster size. A number of feeding-inducing manipulations, including intraventricular injections of either orexin or NPY (Baird et al, 2006a; Baird, Choe, Loveland, Beck, Mahoney, Lord & Grigg, 2009; Torregrossa et al, 2006), and injections of muscimol or DAMGO into the accumbens shell (Stratford & Wirtshafter, 2007; Taha et al., 2009), do not affect cluster size, showing that the these treatments affect feeding in a different way than does intra-MR muscimol. In contrast, increases in cluster size have been reported after systemic administration of benzodiazepines (Higgs & Cooper, 2000). These different patterns illustrate the ability of microstructural analysis to distinguish between different types of effects on ingestive behavior that could not be detected by examining intake data alone. Since intra-MR injections of benzodiazepines have been shown to produce several behavioral effects (Gonzalez, Quagazzal & File, 1998; Sainati & Lorens, 1983), which are presumably mediated through enhanced GABAergic transmission, an interesting possibility is that the MR may be one of the sites at which systemically administered benzodiazepines act to influence feeding.
Increasing the concentration of sucrose from 0.05 to 0.29M significantly increased the numbers of clusters generated across the 60 min session, and a similar effect was found when only the first 9 min were examined. In contrast, muscimol injections significantly increased the number of clusters when measured across the entire test session, but this effect was absent when only the first 9 min were examined. These divergent results highlight certain logical differences between the constraints on microstructural variables that occur when intake is measured over fixed or potentially varying time periods. Since changing the sucrose concentration did not alter the duration of eating, the only way that animals could generate more clusters would be if they were to shorten the time between them. This inference was in fact empirically confirmed, because mean ICIs were significantly shorter at the high than the low sucrose concentration. This effect was apparent both across the entire test session and during the first 9 min. Muscimol, in contrast, produced a large increase in the total duration of eating; increasing meal duration allows for more clusters to be produced even in the absence of changes in mean ICIs. In fact, mean ICIs were significantly reduced by muscimol injections only at the low concentration of sucrose, and even here the effect was much smaller than that produced by increases in sucrose concentration. When, licking is evaluated over the first 9 min alone, a period of time shorter than the smallest observed meal, changes in the total duration of eating can no longer play a role, and the number of clusters generated is related simply to the mean duration of clusters and of ICIs. Under these conditions, sucrose concentration, but not muscimol, had an effect on mean ICIs, with the result that the number of clusters was significantly influenced by sucrose concentration, but not by muscimol. (In fact, muscimol actually tended to reduce the number of clusters seen in the first nine min by animals consuming the high concentration of sucrose, presumably because cluster size had become so large under these conditions that there was insufficient time remaining in the test to accommodate more clusters.) These results further highlight the different nature of the effects of muscimol injections and increased sucrose concentration; the latter manipulation increases the numbers of clusters because it tends to reduce the time between them, whereas muscimol has relatively small effects on the ICI and increases the number of clusters primarily by increasing the duration of eating. In fact, the relatively modest effects of MR inactivation on ICIs during the early part of the drinking period may be one reason why initial rate is affected to a much smaller extent by these injections than by changes in sucrose concentration.
In summary, the current results demonstrate that while intake can be augmented both by increasing sucrose concentrations and by injecting muscimol into the MR, the detailed nature of these two effects differ in a large number of particulars. The data thus provide no support for the view that the MR produces its effects by causing animals to treat sucrose solutions as though they are more concentrated than they really are. Our findings also suggest that the effects of MR inactivation on intake differ substantially from those reported after a number of other orexigenic treatments. For example, intraventricular injections of melanocyte concentrating hormone (MCH) resemble intra-MR muscimol in that they tend to increase cluster size and decrease the proportion of IBIs; in contrast to intra-MR muscimol, however, these injections do not alter meal duration and their effect on intake is primarily due to increases in the within meal rate of eating (Baird et al, 2006b). On the other hand, meal duration is increased by intraventricular injections of orexin or NPY, but these injections either have no effect on, or, in the case of NPY, sometimes actually decrease, cluster size (Baird et al, 2006a; Baird et al, 2009; Torregrossa et al, 2006). Although these comparisons must be made with caution, since the various results have been obtained in different laboratories using varying methods, they do certainly suggest that the effects of inactivation of the MR are distinct from those of these other treatments. In other studies (unpublished observations) we have observed that intra-MR muscimol produces a very large increase in Fos expression within the lateral hypothalamus (LH) which involves both orexinergic cells, and neurons not containing this peptide, and we have suggested elsewhere that some of the effects of MR inactivation on ingestive behavior may be mediated through the LH (Stratford & Wirtshafter, 2000; Wirtshafter, 2000). One interesting possibility is that these injections may simultaneously activate multiple peptidergic cell groups, and perhaps other types of cells as well, to produce a more complex pattern of effects on licking behavior than can be produced by manipulation of any one of these in isolation. Of course, it is possible that projections to targets other than the LH may be critically involved in the effects obtained from the MR – clearly a great deal more work needs to be done to understand the powerful influence of the paramedian tegmentum in the control of ingestive behavior.
Acknowledgement
This publication was supported by grants 0641943 from the National Science Foundation, R01DK071738 from the National Institute of Diabetes and Digestive and Kidney Diseases, and R03DA020802 from the National Institute for Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation or the National Institutes of Health.
Footnotes
The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Asin KE, Davis JD, Bednarz L. Differential effects of serotonergic and catecholaminergic drugs on ingestive behavior. Psychopharmacology. 1992;109:415–421. doi: 10.1007/BF02247717. [DOI] [PubMed] [Google Scholar]
- Asin KE, Wirtshafter D, Kent EW. Straight alley acquisition and extinction and open field activity following discrete electrolytic lesions of the mesencephalic raphe nuclei. Behavioral and Neural Biology. 1979;25:242–256. doi: 10.1016/s0163-1047(79)90597-1. [DOI] [PubMed] [Google Scholar]
- Baird JP, Choe A, Loveland JL, Beck J, Mahoney CE, Lord JS, Grigg LA. Orexin-A hyperphagia: Hindbrain participation in consummatory feeding responses. Endocrinology. 2009;150:1202–1216. doi: 10.1210/en.2008-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JP, Gray NEE, Fischer SG. Effects of neuropeptide Y on feeding microstructure: Dissociation of appetitive and consummatory actions. Behavioral Neuroscience. 2006a;120:937–951. doi: 10.1037/0735-7044.120.4.937. [DOI] [PubMed] [Google Scholar]
- Baird JP, Rios C, Gray NE, Walsh CE, Fischer SG, Pecora AL. Effect of melanin-concentrating hormone on licking microstructure and brief-acess taste responses. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2006b;294 doi: 10.1152/ajpregu.00143.2006. R12656-R1274. [DOI] [PubMed] [Google Scholar]
- Breslin PAS, Davis JD, Rosenak R. Saccharin increases the effectiveness of glucose in stimulating ingestion in rats but has little effect on negative feedback. Physiology and Behavior. 1996;60:411–416. doi: 10.1016/s0031-9384(96)80012-6. [DOI] [PubMed] [Google Scholar]
- Corbit JD, Luschei ES. Invarience of the rat's rate of drinking. Journal of Comparative and Physiological Psychology. 1969;69:119–125. doi: 10.1037/h0027943. [DOI] [PubMed] [Google Scholar]
- Davis JD. The microstructure of ingestive behavior. Annals of the New York Academy of Sciences. 1989;575:106–119. doi: 10.1111/j.1749-6632.1989.tb53236.x. [DOI] [PubMed] [Google Scholar]
- Davis JD. A model for the control of ingestion - 20 years later. Prog.Psychobiol.Physiol.Psychol. 1998;17:127–173. [Google Scholar]
- Davis JD, Levine MW. A model for the control of ingestion. Psychological Review. 1977;84:379–412. [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 1993;264:R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience. 1992;106:217–228. [PubMed] [Google Scholar]
- Fletcher PJ. Effects of 8-OH-DPAT, 5-CT and muscimol on behavior maintained by a DRL20 schedule of reinforcement, following microinjection into the dorsal or median raphe nuclei. Behavioural Pharmacology. 1994;5:326–336. doi: 10.1097/00008877-199406000-00010. [DOI] [PubMed] [Google Scholar]
- Genn RF, Higgs S, Cooper SJ. The effects of 7-OH-DPAT, quinpirole and raclopride on licking for sucrose solutions in the non-deprived rat. Behavioural Pharmacology. 2003;14:609–617. doi: 10.1097/00008877-200312000-00005. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Quagazzal AM, File SE. Stimulation of benzodiazepine receptors in the dorsal hippocampus and median rephe reveals differential GABAergic control in two animal tests of anxiety. European Journal of Neuroscience. 1998;10:3673–3680. doi: 10.1046/j.1460-9568.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Gramling SE, Fowler LJ, Collins KR. Some effects of pimozide on nondeprived rats licking sucrose solutions in an anhedonia paradigm. Pharmacology, Biochemistry and Behavior. 1984;21:617–624. doi: 10.1016/s0091-3057(84)80047-7. [DOI] [PubMed] [Google Scholar]
- Higgs S, Cooper SJ. The effect of the dopamine D2 receptor antagonist raclopride on the pattern of licking microstructure induced by midazolam in the rat. European Journal of Pharmacology. 2000;409:73–80. doi: 10.1016/s0014-2999(00)00802-5. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Deupree D. Cholecystokinin and bombesin effects on rewarded and nonrewarded operants. Peptides. 1983;4:1–3. doi: 10.1016/0196-9781(83)90155-9. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Spencer R. Analysis of licking responses in rats: Effects of cholecystokinin and bombesin. Behavioral Neuroscience. 1983;97:234–235. doi: 10.1037//0735-7044.97.2.234. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Wirtshafter D. Comparative studies of the ingestive behaviors produced by microinjections of muscimol into the midbrain raphe nuclei or the ventral tegmental area of the rat. Life Sci. 1988;42:775–782. doi: 10.1016/0024-3205(88)90650-9. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Wirtshafter D. Elicitation of feeding, drinking, and gnawing following microinjections of muscimol into the median raphe nucleus of rats. Behavioral and Neural Biology. 1989;51:436–441. doi: 10.1016/s0163-1047(89)91078-9. [DOI] [PubMed] [Google Scholar]
- Knowler CW, Ukena TE. The effects of chlorpromazine, pentobarbital, chlordiazepoxide and d-amphetamine on rates of licking in the rat. Journal of Pharmacology and Experimental Therapeutics. 1973;184:385–397. [PubMed] [Google Scholar]
- Lynch WC, Hart P, Babcock AM. Neuropeptide Y attenuates satiety: evidence from a detailed analysis of patterns ingestion. Brain Research. 1994;626:28–34. doi: 10.1016/0006-8993(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Moore KE. The anatomy of central serotonin neuron systems in the rat brain. In: Jacobs BL, Gelpern A, editors. Serotonin Neurotransmission and Behavior. Cambridge: MIT Press; 1981. [Google Scholar]
- Moran TH, Carrigan TS, Schwartz GJ, Ladenheim EE. Bombesin and cholecystokinin differentially affect ingestive microstructural variables whether given alone or in combination. Behavioral Neuroscience. 1996;110:1110–1116. [PubMed] [Google Scholar]
- Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Frontiers in Neuroendocrinology. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JM, Mitsushio H, Lorens SA. Intra-midbrain raphe injections of the neurokinin-3 agonist senktide inhibit food and water intake in the rat. Pharmacology, Biochemistry and Behavior. 1991;38:223–226. doi: 10.1016/0091-3057(91)90616-a. [DOI] [PubMed] [Google Scholar]
- Sainati S, Lorens SA. Intra-raphe benzodiazepines enhance rat locomotor activity: Interactions with GABA. Pharmacology, Biochemistry and Behavior. 1983;18:407–414. doi: 10.1016/0091-3057(83)90463-x. [DOI] [PubMed] [Google Scholar]
- Shim I, Javaid J, Wirtshafter D. Dissociation of hippocampal serotonin release and locomotor activity following pharmacological manipulations of the median raphe nucleus. Behavioural Brain.Research. 1997;89:191–198. doi: 10.1016/s0166-4328(97)00060-0. [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behavioral Neuroscience. 1998;112:678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM, Nieuwenhuys R. The raphe nuclei of the rat brainstem: A cytoarchitectonic and immunohistochemical study. In: Emson PC, editor. Chemical Neuroanatomy. New York: Raven Press; 1983. pp. 131–208. [Google Scholar]
- Stellar E, Hill JH. The rat's rat of drinking as a function of water deprivation. Journal of Comparative and Physiological Psychology. 1952;45:95–102. doi: 10.1037/h0062150. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Gibbs J, Smith GP. Microstructural analysis of licking behavior following peripheral administration of bombesin or gastrin-releasing peptide. Peptides. 1995;16:903–909. doi: 10.1016/0196-9781(95)00051-k. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Forebrain lesions differentially affect drinking elicited by dipsogenic challenges and injections of muscimol into the median raphe nucleus. Behavioral Neuroscience. 2000;114:760–771. [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Activation of GABA-A receptors in the nucleus accumbens shell elicits opposite effects on consumption of sucrose and saccharin solutions; Neuroscience Meeting Planner, Program No. 630.5; 2007. [Google Scholar]
- Taha SA, Katsuura Y, Noorvaash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience. 2009;161:718–733. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa AM, Davis JD, Smith GP. Orosensory stimulation is sufficient and postingestive negative feedback is not necessary for neuropeptide Y to increase sucrose intake. Physiology and Behavior. 2006;87:773–780. doi: 10.1016/j.physbeh.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neuroscience and Biobehavioral Reviews. 1997;21:631–647. doi: 10.1016/s0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. Journal of Comparative Neurology. 1999;407:555–582. [PubMed] [Google Scholar]
- Warton DI, Hui FKC. The arcsin is asinine: the analysis of proportions in ecology. Ecology. 2011;92:3–13. doi: 10.1890/10-0340.1. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld Z, Halpern BP, Tapper DN. Licking behavior: Evidence of hypoglossal oscillator. Science. 1977;196:1122–1124. doi: 10.1126/science.558653. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3rd Ed. New York: McGraw Hill; 1991. pp. 356–357. [Google Scholar]
- Wirtshafter D. The control of ingestive behavior by the median raphe nucleus. Appetite. 2000;36:99–105. doi: 10.1006/appe.2000.0373. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Impaired radial maze peformance in rats with electrolytic median raphe lesions. Experimental Neurology. 1983;79:412–421. doi: 10.1016/0014-4886(83)90222-4. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Discrimination learning and reversal following electrolytic lesions of the median raphe nucleus. Physiology and Behavior. 1986;79:213–219. doi: 10.1016/0031-9384(86)90223-4. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE, Kent EW. Simple technique for midline stereotaxic surgery in the rat. Physiology and Behavior. 1979;23:409–410. doi: 10.1016/0031-9384(79)90388-3. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Krebs J. Control of food intake by kainate/quisqualate receptors in the median raphe nucleus. Psychopharmacology. 1990;101:137–141. doi: 10.1007/BF02253731. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Stratford TR, Pitzer MR. Studies on the behavioral activation produced by stimulation of GABA-B receptors in the median raphe nucleus. Behavioural Brain Research. 1993;59:83–93. doi: 10.1016/0166-4328(93)90154-i. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Trifunovic R. Stimulation of ingestive behaviors following injections of excitatory amino acid antagonists into the median raphe nucleus. Pharmacology, Biochemistry and Behavior. 1988;30:529–533. doi: 10.1016/0091-3057(88)90492-3. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Trifunovic R. Nonserotonergic control of nucleus accumbens dopamine metabolism by the median raphe nucleus. Pharmacology, Biochemistry and Behavior. 1992;41:501–505. doi: 10.1016/0091-3057(92)90364-l. [DOI] [PubMed] [Google Scholar]