Abstract
Arachidonic acid (AA) is metabolized by cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) enzymes into eicosanoids, which are involved in diverse diseases, including type 1 and type 2 diabetes. During the last thirty years, evidence has been accumulated that suggests important functions for eicosanoids in the control of pancreatic β-cell function and destruction. AA metabolites of the COX pathway, especially prostaglandin E2 (PGE2), appear to be significant factors to β-cell dysfunction and destruction, participating in the pathogenesis of diabetes and its complications. Several elegant studies have contributed to the sorting out of the importance of 12-LOX eicosanoids in cytokine-mediated inflammation in pancreatic β cells. The role of CYP eicosanoids in diabetes is yet to be explored. A recent publication has demonstrated that stabilizing the levels of epoxyeicosatrienoic acids (EETs), CYP eicosanoids, by inhibiting or deleting soluble epoxide hydrolase (sEH) improves β-cell function and reduces β-cell apoptosis in diabetes. In this review we summarize recent findings implicating these eicosanoid pathways in diabetes and its complications. We also discuss the development of animal models with targeted gene deletion and specific enzymatic inhibitors in each pathway to identify potential targets for the treatment of diabetes and its complications.
1. Introduction
Between 2000 and 2010, the number of people with diabetes has more than doubled, from 121 million to 285 million. That number is expected to grow to 438 million by 2030 [1]. Currently, 23.4 million Americans have diabetes (American Diabetes Association, National Diabetes Fact Sheet, 2007). The Center for Disease Control (CDC) estimates that the current cost of diabetes is $174 billion annually in the U.S.
Diabetes, which is characterized by hyperglycemia, is divided into type 1 (T1DM) and type 2 diabetes mellitus (T2DM). T1DM is characterized by autoimmune destruction of β-cells [2; 3]. Ultimately, circulating insulin concentrations are negligible or completely absent in patients with T1DM [4]. Obesity, which affects one in three Americans, is a serious health problem because it is often associated with T2DM [5; 6], which occurs because of insufficient generation of insulin and/or the inability of peripheral tissues, including skeletal muscle and adipose tissue, to respond to insulin efficiently. The development of T2DM is firmly associated with insulin resistance, a physiological condition in which insulin becomes less effective at lowering blood glucose [5; 6]. Diabetes shortens the life span as a consequence of cardiovascular disorders, including heart attack, hypertension, and stroke [7; 8]. Accordingly, diabetes-associated complications result in major expense for families and impose a major societal economic burden.
Since pancreatic β-cell dysfunction and destruction are the key events in the onset and progression of diabetes [2; 3; 9; 10], this review will focus on how arachidonic acid (AA)-derived lipid mediators affect insulin secretion and β-cell destruction as well as the role of these eicosanoids in diabetes. AA is a polyunsaturated fatty acid, which is esterified at the sn-2 position of the glycerol backbone of membrane phospholipids or other complex lipids [11]. AA is released from phospholipids by the action of phospholipase A2 (PLA2), which specifically recognizes the sn-2 acyl bond of phospholipids and catalytically hydrolyzes the bond releasing AA and lysophospholipids.
As shown in Figure 1, AA is modified by three major pathways, including cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP), into biologically active eicosanoids (eicosa, Greek for 20, refers to 20 carbon fatty acids) [11–14]. The products of these pathways act as paracrine and autocrine factors and contribute significantly to the regulation of inflammation [13; 15], renal function [16; 17], and vascular function [18; 19]. Since novel receptors and metabolites of COX and LOX pathways are well defined, these two eicosanoid pathways are important therapeutic targets for inflammatory and cardiovascular diseases [13]. This review aims to provide important information related to brief background of biochemistry and function of eicosanoids, regulation of β-cell function by eicosanoids, regulation of β-cell destruction by eicosanoids, and the involvement of eicosanoids in diabetes.
Figure 1.
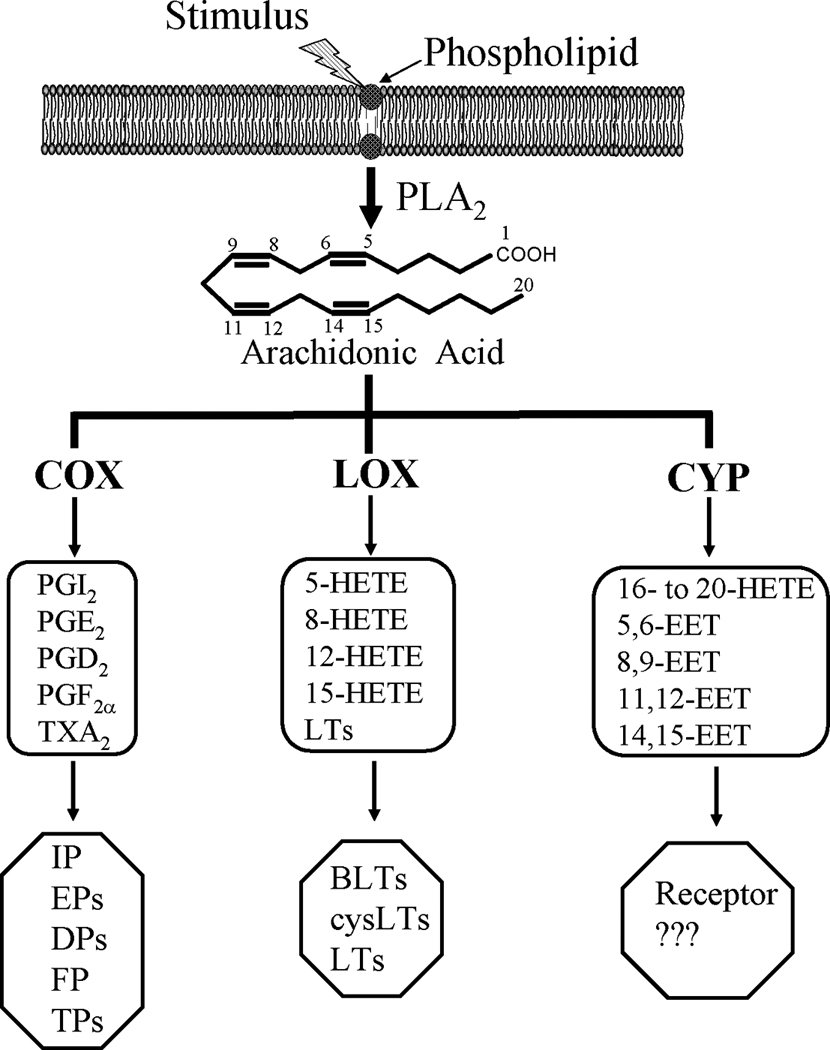
Bioactive eicosanoids derived from the arachidonic acid (AA) cascade. After trigging by inflammatory conditions such as the presence of cytokines and growth factors, AA-containing phospholipids are hydrolyzed by phospholipase A2 (PLA2) resulting in the release of free AA. AA can be further metabolized by three pathways, i.e., the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) pathways. AA cascade generates prostaglandins (PGs), thromboxane A2 (TXA2), and a series of hydroxyeicosatetraenoic acids (HETEs), leukotrienes (LTs), and epoxyeicosatrienoic acids (EETs). The action of these bioactive eicosanoids is mediated through the binding of these substances to their receptors. However, an EET receptor has yet to be identified.
2. Biochemistry and function of eicosanoids
2.1. COX-derived eicosanoids
The major COX-derived eicosanoids are prostaglandins (PGs) and thromboxane (TX). COXs are the enzymes that catalyze the first two steps of the enzymatic reaction to convert AA into PGH2 [20]. Of note, PGH2 is the precursor for the generation of PG2 and TX by the activities of tissue-specific isomerases and synthases [21]. There are two isoforms of COX, COX-1 and COX-2. COX-1 is constitutively expressed in most cells, whereas COX-2 is inducible by inflammatory or stimulatory events in tissues and responds to specific inducers [22; 23]. The major PGs are PGD2, PGE2, PGI2, and PGF2α; the major TX is TXA2. The action of these PGs and TXA2 is mediated through the binding of these products into their membrane-bound receptors, including DP, EPs (EP1 to EP4), IP, FP, and TP receptors (Fig. 1) [14; 23]. In the cardiovascular system, PGI2 is a potent vasodilator and an inhibitor of platelet aggregation, whereas TXA2 is a potent vasoconstrictor and a pro-aggregatory substance [24; 25]. Thus, the balance between PGI2 and TXA2 in the circulation is important for cardiovascular homeostasis [26]. In the kidneys, COX-eicosanoids are important in regulating renal function. Vasodilatory PGs, including PGI2, PGE2, and PGD2, increase renal blood flow and glomerular filtration rate. PGE2 is a key regulator of sodium reabsorption in the distal tubules [21]. Therefore, these COX products are vital in the pathogenesis of cardiovascular [18; 19] and kidney diseases [14; 27].
2.2. LOX-derived eicosanoids
The major LOX-derived eicosanoids are hydroxyeicosatetraenoic acids (HETEs) (12 (S)-HETE, 12 (R)-HETE, and 15 (S)-HETE), as well as leukotrienes (LTs) (LTA4, LTB4, LTC4, LTD4, and LTE4) (Fig. 1). LOXs are enzymes that convert AA into different HETEs. In mammals, LOXs are categorized into 5-LOX, 8-LOX, 12-LOX, and 15-LOX, which are classified according to the insertion site of the hydroperoxy group [28]. LOXs are non-heme iron-containing enzymes that insert a hydroperoxy group into AA [14]. 5-LOX metabolizes AA into 5-HPETE, which is the precursor for the generation of LTA4. Notably, the generation of LTA4 requires the interaction of 5-LOX and 5-LOX-activating protein, which facilitates the transfer of AA to 5-LOX for the enzymatic reaction [27]. LTA4 can be hydrolyzed to form LTB4, which then conjugates with glutathione to form cysteinyl-LTs (cysLTs), LTC4, LTD4, and LTE4 [27]. The action of these LTs is mediated through the binding of these products to their membrane-bound receptors, including BLT, cysLT, and LTE4 receptors.
Of circulating cells, LTs are mainly synthesized by eosinophils, neutrophils, monocytes or macrophages, and mast cells [27]. Since LTs have a relative short half-life, these products act as autacoids near their synthesizing sites [29]. A substantial body of evidence indicates that 5-LOX products exert proinflammatory effects by increasing the production of proinflammatory cytokines; also, 5-LOX products act as chemotaxins to recruit inflammatory cells in the blood vessels [28; 29]. Accordingly, most investigators consider 5-LOX products to be detrimental factors in pathological conditions, including cardiovascular [28–30] and renal diseases [27; 31].
2.3. CYP-derived eicosanoids
The major CYP-derived eicosanoids are HETEs (16-, 17-, 18-, 19-, and 20-HETE) and epoxyeicosatrienoic acids (EETs) (5,6-, 8,9-, 11,12-, and 14,15-EET) (Fig. 1) [32]. EETs are formed endogenously in various tissues and exert potent biological effects on cellular functions. EETs are readily hydrolyzed by soluble epoxide hydrolase (sEH) to the corresponding dihydroxyeicosatrienoic acids (DHETs) [11; 13; 33]. In general, DHETs are much less biologically active than are EETs [13; 33]. Therefore, inhibition of sEH activity has been used as a means of studying the physiological function and cardiovascular benefits of EETs [13; 33]. The rapid development of selective inhibitors of sEH in the past decade is noteworthy. Moreover, sEH inhibitors have been consistently demonstrated to have cardiovascular benefits [13; 33].
Among HETEs, 20-HETE is the major metabolite in blood vessels and the kidneys [32]. Synthesis of 20-HETE is catalyzed by enzymes of CYP4A and CYP4F [32]. Synthesis of EETs is less specific and can be carried out by CYP1A, 2B, 2C, 2D, 2E, and 2J isoforms [13]. Accumulating evidence demonstrates that the major CYP epoxygenases for EETs synthesis are CYP2C and CYP2J isoforms [13]. These CYP products play an important role in the regulation of cardiovascular and renal function [13; 33]. 20-HETE and EETs have been implicated in the regulation of vascular tone [13]. 20-HETE is a vasoconstrictor in the renal and cerebral microcirculation and an important regulator of the myogenic tone [12]. Notably, EETs act directly on the afferent arteriolar smooth muscle cells to elicit relaxation and are considered to be endothelium-derived hyperpolarizing factors [34; 35]. EETs are anti-inflammatory and act as angiogenic factors [13; 33]. Although several studies have suggested that the action of EETs is mediated through an EET receptor [36–38], that receptor has yet to be identified.
In the renal tubules, 20-HETE and EETs are important mediators that inhibit sodium transport in proximal tubules [39; 40], the thick ascending limb [41; 42], and the collecting duct [43]. Accordingly, these CYP eicosanoids play a vital role in the pathogenesis of cardiovascular [13; 44] and renal diseases [27; 33]. The CYP eicosanoid pathway is currently being targeted for the treatment of hypertension and stroke [13].
3. Regulation of β-cell function by eicosanoids
Beta cells are a specific cell type in the pancreatic islets of Langerhans, making up about 50%– 65% of the cells [45]. Pancreatic β cells produce insulin and secrete it at an appropriate rate to maintain blood glucose within a relatively narrow range [46]. In pancreatic β cells, glucose stimulates insulin secretion by two major pathways: the triggering and amplifying pathways [46].
The regulation of insulin secretion by these two pathways is shown schematically in Figure 2. In the triggering pathway, products of glucose metabolism enter the mitochondrial respiratory chain, which then uses them to generate adenosine triphosphate (ATP). Increased ATP levels close the KATP-sensitive channels. This is followed by plasma membrane depolarization and opening of the voltage-sensitive Ca2+ channels, which in turn increase the intracellular Ca2+ concentration and promote insulin secretion [47]. In this pathway, uncoupling protein 2 (UCP2) acts as a negative regulator of glucose-stimulated insulin secretion (GSIS) by decreasing the production of ATP [48]. The amplifying pathway augments insulin secretion independently from its action on KATP-sensitive channels. It is well recognized that the triggering pathway determines insulin secretion under physiological conditions, whereas the amplifying pathway optimizes the secretory response induced by the triggering signal [10]. In the following subsections, we will discuss the influence of COX-metabolites, LOX-metabolites, and CYP-metabolites on β-cell function.
Figure 2.
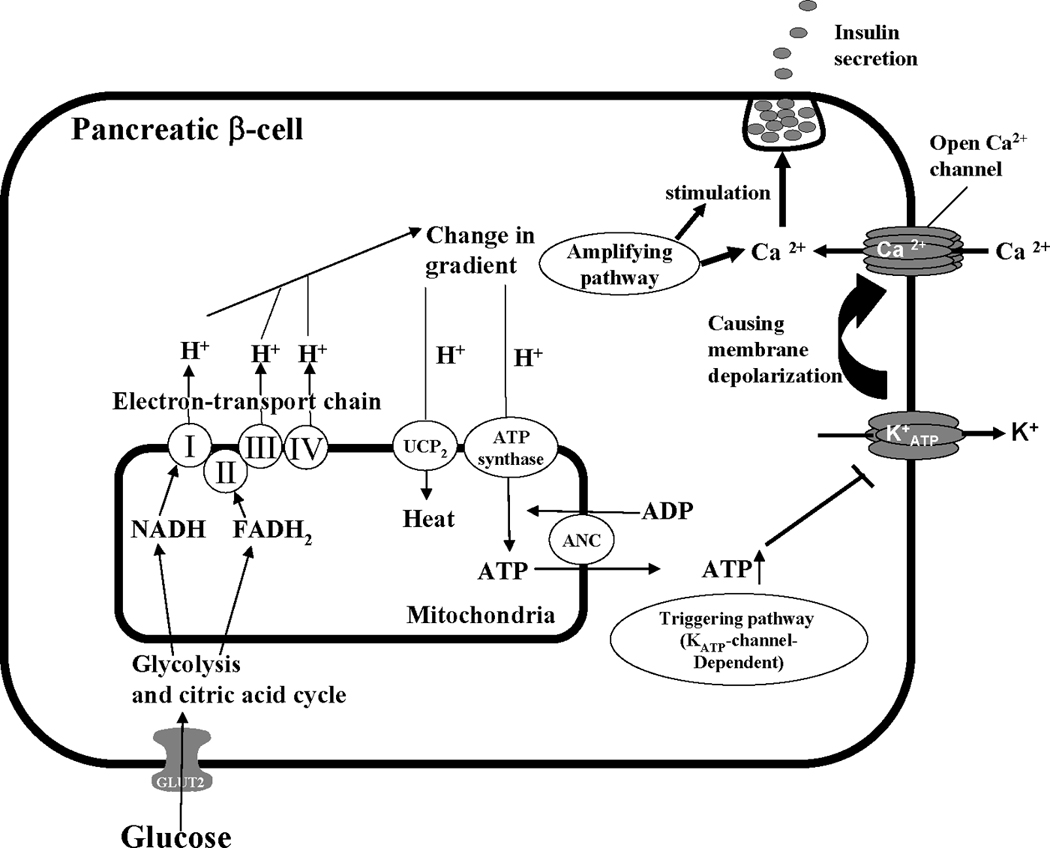
In pancreatic β cells, the uptake of glucose is mediated by glucose transporter, GLUT2. Within β-cells, glucose is further metabolized by the glycolysis and the citric acid cycle to generate NADH and FADH2, which donate electrons to the mitochondrial electron-transport chain. Protons are pumped out by complexes I, III, and IV of the electron-transport chain creating a proton electrochemical gradient. Protons reenter the mitochondria via ATP synthase to generate ATP. In pancreatic β cells, increasing ATP inhibits the K+ATP channel, causing plasma membrane depolarization, which opens voltage-gated Ca2+ channels. Increasing the intracellular Ca2+ concentration contributes to the release of insulin. UCP2 acts as a negative regulator of GSIS by decreasing ATP levels.
3.1. Influence of COX metabolites
Although PGE2 and PGF2α were identified in rat islets [49], PGE2 is the most predominant product of the COX pathway in β cells because PGE2 has been reproducibly shown to modulate β-cell function [45; 50; 51]. Thus, major research activities in this area have focused on the biochemistry and function of PGE2 in human and rodent islets [45; 51–54]. It is generally recognized that COX-1 is the constitutive isoform, while COX-2 is the inducible isoform in most tissues. In contrast, an earlier study demonstrated that COX-2 expression predominates over COX-1 in pancreatic islet cells under both basal and IL-1β-stimulated conditions [55]. Of note, the high basal expression of COX-2 in islets is probably due to the action of transcription factor, NF-IL6 [23]. Taken together, these results suggest that COX-2 is the major enzyme responsible for PGE2 production in β cells [23].
To understand the actions of PGE2 in the islets, islet expression of the mRNA of EP receptors has been studied by real-time RT-PCR. One interesting study showed that EP3 is the most abundant EP receptor type in rat islets [51]. Similarly, EP3 receptor is presents in human islets [53]. Although results regarding the effects of COX metabolites on insulin secretion have been contradictory, the consensus is that PGE2 has inhibitory effects on insulin secretion in islets [50; 52]. For example, Robertson and colleagues [54] have demonstrated that in rat islets, NS-398 and SC-236, COX-2 selective inhibitors, prevented IL-1β-induced inhibitory effects on insulin secretion, and exogenously adding PGE2 reestablished the inhibition of insulin secretion by IL-1 β. In addition, Fujita et al. [56] have demonstrated that COX-2 selective inhibitor (SC58236), but not COX-1 inhibitor (SC58560), inhibited PGE2 production and promoted GSIS in mouse islets. It is known that IL-1β activates COX-2 and EP3 expression via the NF-κB pathway and that EP3 has postreceptor activities resulting in a decrease in cAMP levels [51]. Thus, it has been postulated that during inflammation IL-1β promotes PGE2 levels via the induction of COX-2 in islets, after which increasing PGE2 binds to EP3 receptor, resulting in a decrease in cAMP levels and a sequential decrease in GSIS [51]. However, this notion has not been confirmed in human islets because PGE2 did not inhibit insulin secretion in perfusion experiments with these islets [52]. A recent review article has pointed out that the main reason for the action of COX-2 inhibitor on insulin secretion is the increase in ambient levels of AA in β cells [53]. Thus, the contribution of PGE2 to the control of insulin secretion remains unresolved.
3.2. Influence of LOX metabolites
To study the effects of the LOX pathway on pancreatic β-cell function, earlier studies have determined the effects of various LOX metabolites on GSIS in rat islets [57; 58]. Metz and colleagues [58] have shown that 5-HETE and 12-HETE did not alter insulin secretion, whereas 15-HETE, LTB4, and LTC4 inhibited insulin release. In addition, they showed that 12-HPETE acts as an important mediator that potentiates insulin secretion. Thus, it was postulated that LOX metabolites could provide an “on” signal for GSIS. However, during glucose metabolism, glutathione-derived metabolites convert this signal to an “off” response by a negative feedback loop [58]. This notion is supported by a recent publication [59] that siRNA to 5-LOX reduced GSIS and insulin gene expression in human islets. Although this notion was interesting, recent studies have not supported this hypothesis.
It has been demonstrated that 5-LOX products improve rather than decrease islet function in rodents. Also, there have been conflicting reports about whether or not 5-LOX is expressed in islets [15; 60]. Accumulating evidence indicates that 12-LOX is expressed in human islets and 12-HETE has been detected in human islets [15]. Thus, 12-HETE probably is the major LOX product in islets that regulates GSIS [49; 60].
The islet production of 12-HETE is elevated in Zucker rats, a model of T2DM. In diabetic Zucker rats, an inhibitor of 12-LOX activity inhibited AA-stimulated insulin secretion, implying that 12-LOX products are involved in the deficits in insulin secretion [61]. To study the effects of 12-LOX metabolites on β-cell function during basal and inflammatory conditions, Bleich et al. [62] determined GSIS in 12-LOX knockout (KO) and control islets in the absence or presence of cytokines. They showed that in the absence of cytokines, 12-LOX KO did not affect GSIS, whereas deletion of 12-LOX significantly improved GSIS in the presence of cytokines [62]. These results provided novel evidence that the deletion of 12-LOX is an effective approach improving β-cell function during inflammation. To investigate the importance of 12-LOX-derived lipid mediators in diabetes, a recent publication examined the effects of 12-LOX products on β-cell function in human islets [63]. In this report, Ma and colleagues [63] showed that at 1 nM, 12-HPETE and 12 (S)-HETE produced substantial reductions in insulin secretion. Taken together, these findings provide evidence that 12-LOX products are important negative mediators of insulin secretion in human islets. The importance of the LOX pathway on β-cell function has been reviewed previously [15; 60].
3.3. Influence of CYP metabolites
Less investigation has been devoted to the influence of CYP metabolites on pancreatic β-cell function. The relevance of EETs to pancreatic function is documented in a study by Falck and colleagues who demonstrated that EETs stimulate insulin secretion in isolated rat islets [64]. Regioselectively, only 5,6-EET (1 µM), but not 8,9-EET, 11,12-EET, or 14,15-EET, directly induce the release of insulin [64]. This report provided the first evidence that CYP metabolites are involved in regulating pancreatic β-cell function.
To advance this research area, Zeldin and colleagues [65] have investigated the expression profile of CYP epoxygenase, CYP2J, in human and rat pancreatic tissues. Using immunohistochemical analysis, they showed that CYP2J is highly expressed in the cells of islets, with minimal staining in pancreatic exocrine cells. They also showed that a significant amount of EETs are produced in human and rat pancreas [65], providing biochemical evidence that CYP epoxygenase products may regulate β-cell function. It is difficult to study the physiological function of EETs in vivo because these products are rapidly degraded by sEH to DHETs. Moreover, sEH inhibitors and KO are widely used to enhance the biological and cardiovascular actions of EETs [13; 66; 67].
Interestingly, using a hyperglycemic clamp approach, recent work by Luo et al. [68] has provided the first evidence that the genetic deletion and pharmacological inhibition of sEH promote insulin secretion in vivo, resulting in the prevention of hyperglycemia in diabetic mice. They showed that sEH KO and the selective inhibitor (trans-4-[4-(3-adamantan-1-ylureido)-cyclohexyloxy]-benzoic acid; t-AUCB) augmented GSIS in mouse islets [68]. Mechanistically, this report also demonstrates that the enhancement of GSIS by sEH KO in β cells is mediated through the amplifying pathway, which is an important target for the development of therapeutic insulin secretagogues [10]. Taken together, these findings support the notion that sEH KO or inhibition would increase pancreatic EET levels and contribute to the enhancement of GSIS. Nevertheless, it remains unclear which CYP epoxygenases are responsible for the production of EETs and whether EETs are able to promote insulin secretion in mouse and human islets.
4. Regulation of β-cell destruction by eicosanoids
T1DM, which occurs in 5%–10% of the diabetic patients, causes the loss of 70%–80% of pancreatic β-cell mass by the time of diagnosis [3]. There is no practical method of preventing or curing T1DM, which impairs the overall quality of life because patients require daily insulin administration. Pancreatic islet transplantation (PIT) has been used clinically in efforts to treat T1DM patients [69]. The average human pancreas has about 300,000 to 1.5 million pancreatic islets. It is estimated that about 60% of pancreatic islet cell mass is needed to maintain normal glucose metabolism [69]. Therefore, there is great demand for T1DM patients to obtain healthy islets from donors. However, significant numbers of islets undergo apoptosis, an important mechanism of β-cell destruction, after PIT procedures. It has been estimated that the rate of apoptosis following PIT is 10 times higher than the rate in native pancreatic islets [69]. Because transplanted encapsulated islets are encircled by reactive macrophages and T-lymphoctyes, which secrete cytokines into the internal core of the islet capsule, inflammatory cytokine-induced β-cell apoptosis appears to be the major mechanism that mediates β-cell damage [60]. Thus, approaches to inhibit inflammatory cytokine actions have become important to improve the function of islets after PIT [69].
In T2DM patients, it has been shown that about 25% to 50% of β-cell loss is found in initial pathological studies. As in T1DM, β-cell apoptosis is the main reason for β-cell loss [3]. Therefore, both T1DM and T2DM are associated with a significant loss of β cells [3]. In the following subsections, we will review the impact of COX-metabolites, LOX-metabolites, and CYP-metabolites on β-cell destruction.
4.1. The impact of COX metabolites
Since it is widely accepted that COX-2 is induced by inflammatory cytokines [21; 31], a lot of research effort has focused on the role of COX-2 in the pathogenesis of T1DM and T2DM. Studies have demonstrated that a high glucose level (25 mM) induces the expression of COX-2 in the human islets [70; 71]. Shanmugam and colleagues [71] have demonstrated increased expression of COX-2 in db/db mice, a mouse model for T2DM. Growing evidence demonstrates that COX-2 promoter contains NF-κB, IL-6, activated protein-1, and cAMP-response element [72].
Interleukin-1 (IL-1), an inflammatory cytokine, is released in islets by both T lymphocytes and activated macrophages. Treatment of islets with IL-1 causes transient induction of COX-2 expression; it peaks within 2–4 hr and decreases to basal levels after 24 hr of exposure [55]. Similarly, EP receptor promoter contains an NF-κB binding site [73]. The inhibition of NF-κB by sodium salicylate results in decreased expression of EP3 receptor [51]. Accordingly, it has been postulated that COX-derived PGE2 is the important lipid mediator for β-cell destruction.
Noteworthy, earlier work by Rabinovitch and colleagues [74] has demonstrated that indomethacin, a non-selective COX inhibitor, concentration-dependently protects rat islets from cytokine-induced destruction. More importantly, Oshima and colleagues [75] have used rat insulin-2 promoter to overexpress COX-2 and microsomal PGE synthase-1 in mouse β cells. They provided the first evidence that overexpressing COX-2 and PGE synthase in β cells causes increased levels of PGE2, which have deleterious effects on β-cell mass in the transgenic mice. Nevertheless, the reduction of β-cell mass is due to the inhibition of β-cell proliferation [75].
Further investigations of COX-2 in transplanted islets have tested whether selective COX-2 inhibitors improve the survival and function of transplanted islets in non-obese diabetic (NOD) and streptozotocin (STZ) models [76; 77]. These studies have shown that COX-2 inhibitor does not have a significant effect on initial graft loss, blood glucose levels, glucose tolerance, or insulin content of the grafts [76; 77]. Of note, acetylsalicylic acid, a non-selective COX inhibitor, significantly reduces initial graft loss and prolongs the survival of islet grafts in NOD mice [77]. However, the dose of acetylsalicylic acid used in these experiments was at least 10-fold higher than that required to inhibit PG synthesis. These results suggest that the effects of acetylsalicylic acid are due to COX- and PG-independent mechanisms [77]. Taken together, these previous reports [76; 77] have failed to show that COX-2 inhibitors have any beneficial effect in PIT.
4.2. The impact of LOX metabolites
Extensive research activities have focused on the effect of 12-LOX products on pancreatic β-cell function and destruction [15; 63]. There are three types of 12-LOX: platelet, leukocyte, and epidermal. Leukocyte 12-LOX is the major enzyme expressed in pancreatic islets [78]. 12(S)-HETE is the major product of leukocyte 12-LOX [78]. It is recognized that 12-LOX products, which are proinflammatory, are important in the pathogenesis of T1DM and T2DM [15; 63]. A study by Laybutt and colleagues [79] has demonstrated that 12-LOX expression is induced in islets of a partial pancreatectomy model of diabetes. Similarly, the production of 12-LOX products is induced in the islets of Zucker rats [61]. To determine the importance of 12-LOX in β-cell destruction, Chen and colleagues [78] showed that 12-LOX is expressed in human islets and the combined treatment with inflammatory cytokines induced the production of 12(S)-HETE in these islets. Similarly, 12(S)-HETE induces apoptosis in β-TC3 cells [78], providing direct evidence that leukocyte 12-LOX product has a deleterious effect on β cells.
Another aspect of LOX products acting as pro-apoptotic agents is indicated by Rabinovitch and colleagues who showed that nordihydroguaiaretic acid (NDGA), a lipoxygenase inhibitor, inhibits cytokine-induced β-cell destruction in rat islets [74]. To elucidate the protective role of LOX inhibitors in transplanted islets, Fu and colleagues [80] have tested whether NDGA protects the graft survival of neonatal pig pancreatic cell clusters in alloxan-diabetic mice. They showed that NDGA prevented β-cell toxicity and preserved function of xenografts early after transplantation [80]. Taken together, these findings suggest that LOX inhibitor exerts a beneficial effect in PIT.
4.3. Influence of CYP metabolites
Little research has been done on the role of CYP metabolites in pancreatic β-cell destruction. Several studies have indicated that EETs have anti-apoptotic effects in different cell types [81; 82]. For example, Yang and colleagues showed that overexpression of CYP epoxygenase significantly increased endothelial cell viability and inhibited TNF α-induced endothelial apoptosis. Simpkin et al. [82] have recently shown that the inhibition of sEH by 12-(3-adamantane-1-yl-ureido)dodecanoic acid decreased the expression of pro-apoptotic genes in neural tissues. Luo and colleagues [68] investigated the relevance of EETs to pancreatic β-cell destruction by determining the effects of sEH KO on islet cell apoptosis in STZ-induced diabetes. They showed that STZ-sEH (−/−) mice exhibited less islet cell apoptosis than did STZ-sEH (+/+) mice [68]. This was the first report to show that sEH is involved in β-cells apoptosis. Since sEH is expressed in human pancreas [83] and mouse β cells [68], it is possible that the blockade of sEH will be an important approach to increasing the bioavailability of EETs in β cells. Nevertheless, no study has directly addressed the role of EETs in protecting transplanted islets. Taken together, the preceding results suggest that EETs are important in protecting β-cells from apoptosis.
5. Eicosanoids and diabetes
Insulin resistance, which has large roles in both T2DM and T1DM [84–87], is characterized by impaired sensitivity to insulin in its main target organs. After insulin is produced and released from pancreatic β cells, it binds to its receptor and helps the liver, muscle, and fat to take up glucose from the blood, storing it as glycogen in the liver and muscle. Insulin also increases fatty acid synthesis and inhibits gluconeogenesis and lipolysis [87].
Early in the development of T2DM, compensatory insulin hypersecretion maintains normoglycemia, but progressive insulin resistance and/or β-cell dysfunction eventually lead to overt hyperglycemia. Chronic hyperglycemia, a major cause of the death in diabetic individuals, damages the kidneys, blood vessels, and other organs. In recent years, it has been postulated that low-grade inflammation contributes to insulin resistance [88]. Accumulating evidence from rodents to large-scale studies of populations has demonstrated that AA-derived eicosanoids regulate inflammatory cytokines and chemokines and contribute to the pathogenesis of insulin resistance and diabetes-related complications.
5.1. COX and diabetes
COXs, which are widely distributed enzymes, are activated by mechanical, chemical, and immunological stimuli. PG synthesis often accompanies the inflammatory response [89; 90]. Studies have shown that increased PGE2 is involved in insulin-regulated glycogen synthesis and gluconeogenesis in the liver [91; 92]. The expression of COX-2 is closely correlated with plasma leptin levels and Homeostasis Model of Assessment - Insulin Resistance (HOMA-IR), which is an index of whole-body insulin resistance [93]. In addition, hyperglycemia induces COX-2 expression [94–96] and activates the receptors for advanced glycation end products (RAGE), which therefore could be involved in vascular and renal diseases [94; 97].
Diabetes also causes chromatin remodeling and histone acetylation of the COX-2 gene promoter at NF-κB binding sites [98]. In diabetes, RAGE overexpression is associated with inflammation and COX-2 expression, which is significantly correlated with hemoglobin A1c [99]. A selective COX-2 inhibitor improves peripheral and hepatic insulin resistance and decreases plamsa cytokines [93; 100; 101]. Indomethacin, a non-selective COX inhibitor, prevents hyperglycemia-induced tachyarrhythmia [102]. Although early reports showed that PGs have a protective role during inflammation [103; 104], some COX-2 inhibitors, including nimesulide, endotoxin, and metformin, lead to intensified inflammation and impaired glucose metabolism [105; 106]. This discrepancy may be due to inhibitor selectivity and variability in the amount of AA that is available to LOXs, CYPs, or non-enzymatic oxidative modification. The association between COX genetic polymorphism and diabetes has been investigated. The COX rs20417 variant has 30% higher T2DM prevalence in Pima Indians [107]. COX-2 polymorphism also increases the risk of cardiovascular disease [108]. Patients with T1DM display a lower frequency of the protective CC genotype of the −765G>C COX-2 polymorphism [109].
5.2. LOX and diabetes
LOX enzymes catalyze the oxygenation of AA into lipid mediators [110]. LOX enzymes and their products regulate cytokine and chemokine expression in many tissues and organs, including pancreatic islets and adipose tissue, the respective sites where insulin is produced and acted on [15; 111].
Increasing evidence suggests that the 12/15-LOX pathway is involved in the pathogenesis of insulin resistance and adipose tissue inflammation [112–114]. High glucose levels enhance 12/15-LO pathway activation and expression in vascular smooth cells [115] and endothelial cells [116], as well as 15-HETE production in porcine aortic endothelial cells [117]. 5/12-LOX enzymes and products as well as 5-LO-activating protein (FLAP) are upregulated in adipocytes from diabetic animals [118; 119]. Increased production of 12/15-LOX eicosanoids accelerates monocyte or endothelial interactions in diabetes [120]. Moreover, 5-LOX knockout mice do not develop diabetic retinopathy [121]. Therefore, the LOX pathway could play a vital role in diabetic complications.
Direct evidence demonstrates that cells overexpressing 12/15-LOX secrete MCP-1 and osteopontin, which participate in the development of insulin resistance [112]. 12- and 15-HETEs induce inflammatory cytokine expression and impair insulin action in adipocytes [122–124]. Moreover, deletion of 12/15-LOX in mice improves high-fat-diet-induced inflammation and insulin resistance [112]. LOX inhibition decreases triglycerides and free fatty acids, and improves insulin action in both fructose-fed and fat-fed rat models of insulin resistance and T2DM [125; 126]. 12-LOX products also downregulate glucose transport in VSMC, which may lead to insulin resistance [127]. Moreover, deletion of 12-LOX resists T1DM induction in mice [62].
In obese subjects, different adipose tissues seem to express different LOX enzymes. For example, 15b–LOX and 12-LOX are expressed in both subcutaneous and omental fat, but 15a–LOX is expressed only in subcutaneous fat, predominantly in CD34-positive cells [128]. The increased mRNA expression of LOX5AP in subcutaneous adipose tissue observed in obese populations is associated with HOMA-IR [129]. In diabetic patients, urinary excretion of 12(S)-HETE is increased as compared with that in matched non-diabetic controls [130].
LOX5AP polymorphism is associated with increased risk of atherothrombotic cerebral infarction in Japanese people who have metabolic syndrome [131]. It has also been revealed that Japanese T2DM patients with the polymorphism have increased susceptibility to chronic kidney disease [132]. Similarly, 12-LOX is associated with diabetic nephropathy in European-American T2DM patients [133]. Further, 12-LOX, 5-LOX, and LOX5AP polymorphisms are associated with subclinical atherosclerosis in European-Americans with T2DM [134].
5.3. CYP and diabetes
As compared to COX and LOX pathways, less is known about the role of the CYP pathway in diabetes. AA is metabolized to 20-HETE by CYP4A families and to EETs by CYP2C and 2J families. EETs are further metabolized to DHETs by sEH. Diabetes and obesity are associated with reductions in the expression of CYP2C isoforms [135–137], but increases in the expression of CYP4A isoforms and sEH [136; 138; 139]. EETs are decreased in diabetic vitreous [121] and glomeruli [140]. It seems that insulin is involved in the regulation of theses enzymes. Direct evidence show that insulin reverse increased hepatic CYP4A isoforms in diabetic rats [139].
It has been demonstrated that the inhibition of CYP epoxygenase did not affect relaxation in insulin-resistant blood vessels [141; 142], suggesting that vasodilation induced by CYP-derived EETs is impaired in T2DM. Since EETs are produced by CYP2C/2J and degraded by sEH, CYP2C/2J induction or sEH inhibition is expected to enhance the beneficial properties of EETs. In-vivo CYP2J3 gene delivery has been shown to increase EET generation, reduce blood pressure, and reverse insulin resistance [143]. Furthermore, overexpression of CYP2J3 prevented fructose-induced decreases in insulin receptor signaling and phosphorylation of AMP-activated protein [143]. sEH genetic deletion or pharmacological inhibition decreases hyperglycemia in STZ and obese models [68; 144; 145]. Interestingly, Sodhi and colleagues have demonstrated that EETs agonist rescues the metabolic syndrome phenotype of heme oxygenase-2 knockout mice [146]. In addition, db/db obese mice lacking sEH have lower blood glucose than do db/db mice (M. H. Wang, unpublished data).
PPAR-α agonist, which induces CYP enzymes, attenuates hypertension and glomerular damage in diabetic rats [147]. Inhibition of 20-HETE production or reduction of EET degradation may have therapeutic potential to prevent erectile dysfunction associated with diabetes [148].
There are also reports of a close association between the sEH G860A (Arg287Gln) polymorphism and insulin resistance in T2DM patients [149]. CYP2C9 polymorphism displays a better response to anti-diabetic medicine in T2DM patients [150; 151], but carries a high risk of hypoglycemia, raising the possibility of a pharmacogenetic interaction [152]. Increased prevalence of the CYP2C8*4 mutation has been found in T2DM patients [153]. CYP2J2 G-50T polymorphism seems to contribute to the pathogenesis in patients with T2DM of early onset [154]. CYP2C9*2 and CYP2C9*3 alleles currently appear to have no clinical implications for dosing of sulfonylureas in patients with T2DM in the Netherlands [155]. There is also no association between CYP2C9, CYP2C19, or CYP2D6 genotype and diabetes susceptibility in Bosnian populations [156]. Moreover, CYP2C9 variant seems to have lower response to losartan in T1DM patients with nephropathy [157]. CYP2J2 has no association with T1DM or T2DM in Caucasians [158]. However, these results reflect the use of limited study populations. Large-scale studies are required to reveal potential relationships between CYP genetic variants and diabetic risk.
5.4. Omega 3-derived eicosanoids and diabetes
Besides generating from omega 6 (ω-6) polyunsaturated fatty acids (PUFAs), eicosanoids can also be produced from ω-3 PUFAs. Numerous studies demonstrate that lipid mediators generated from ω-3 PUFAs, which include eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3), possess anti-inflammatory and protective effects in diverse diseases including diabetes [159; 160].
To investigate the importance of ω-3 PUFAs in diabetes, investigators have studied the effects of overexpression of fat-1, a C. Elegans gene encoding an ω-3 fatty acid desaturase catalyzing the conversion of ω-6 to ω-3 PUFAs, in different diabetic models [161; 162]. Stable production of ω-3 PUFAs in transgenic mice carrying fat-1 gene enhances GSIS and protects β cells from cytokine-induced destruction [161]. Similarly, fat-1 transgenic mice have significantly higher levels of ω-3 PUFAs in the pancreases and are protected from STZ-induced diabetes [162]. These novel studies [161; 162] demonstrate that endogenously synthesized ω-3 PUFAs are β-cell protective. Similarly, an earlier study has demonstrated that ω-3 PUFA-enriched diet reduces hyperglycemia in STZ diabetes [163]. Moreover, ω-3 PUFAs intake alleviates obesity-induced insulin resistance and hepatic steatosis through upregulation of insulin sensitivity related genes, such as glucose transporter-2 and −4, insulin receptor substrate-1 and −2 [164] and intake of ω-3 PUFA-rich diet prevents obesity [165] and improves insulin sensitivity [166]. In T1DM patients, ω-3 PUFA treatment reduces cholesteryl ester transfer (CET) and increased concentrations of CET protein, which mediates lipid metabolism [167]. However, these studies are based on small size of populations and short following-up time, large-scale studies are necessary to establish the importance of ω-3 PUFAs in diabetes.
Conclusion
Pancreatic β-cell dysfunction and insulin resistance are important in causing the onset and progression of T1DM and T2DM. During diabetes, AA and its lipid metabolites, eicosanoids, have an important role in inflammation-induced β-cell dysfunction and insulin resistance. We have outlined the functions of COX-, LOX-, and CYP-derived eicosanoids that are produced in pancreatic islets under normal and disease conditions.
PGE2, the most important COX product found in islets, reduces insulin secretion and insulin sensitivity during cytokine-induced inflammation. The action of PGE2 on β-cell dysfunction during inflammation is probably mediated through the binding of PGE2 to EP3 receptor, causing a reduction in cAMP levels. Overproduction of PGE2 can also promote the loss of β-cell mass through the inhibition of β-cell proliferation. Nevertheless, it appears that COX-2 inhibitors do not exert any beneficial effects in pancreatic islet transplantation. Also, the effects of COX-2 inhibitors on insulin sensitivity need to be validated. Although it has been demonstrated that COX-2 inhibitor prevents hyperglycemia in the STZ-diabetic mouse model [168], more research is needed to establish the importance of COX-2 in human diabetes.
12-HETE is the most important LOX product in islets. Deletion of the 12-LOX gene increases insulin secretion in the presence of cytokines, suggesting that 12-LOX products are involved in β-cell dysfunction during inflammation. There is direct evidence that 12(S)-HETE, a 12-LOX product, promotes β-cell destruction. Animal and human studies have shown that the LOX pathway contributes to insulin resistance and diabetic complications. LOX inhibitors show the beneficial effects in pancreatic islet transplantation. Although selective 15-LOX inhibitors have been recently developed [169], no highly selective 12-LOX inhibitor is yet available for in vitro and in vivo studies. Selective 12-LOX inhibitors must be developed if we are to fully understand the importance of 12-LOX products in β-cell function and destruction.
EETs are the most important CYP products in islets. Although there is some evidence that EETs promote insulin secretion, there is still a deficit in current knowledge regarding the function of EETs with regard to β-cell destruction. A recent study has demonstrated that increasing EETs by inhibiting sEH promotes insulin secretion and reduces β-cell destruction, indicating EETs are a potential therapeutic target for the treatment of diabetes. Although these findings are interesting, we still need to prove the direct effects of EETs on β-cell function and destruction. Given the important effects of EETs on anti-inflammation, it is likely that EETs contribute to the control of insulin sensitivity. The development of novel pharmacological tools such as EET analogs for use in in vivo studies, as well as the development of molecular biology tools such as mouse models for β-cell-specific overexpression of CYP epoxygenases, is necessary to fully delineate the importance of the EETs pathway in diabetes.
Although growing evidence supports the existence of strong relations between eicosanoids and diabetes, the detailed mechanism whereby eicosanoids contribute to diabetes needs to be further explored. Identification of the putative EETs receptor will facilitate future studies in the CYP pathway. A recent study has clearly demonstrated that rats given dietary EPA/DHA supplementation promote the synthesis of pancreatic ω-3-derived epoxyeicosaquatraenoic acids (EEQs) [170]. Since EPA/DHA supplementation reduces hyperglycemia in STZ diabetes [163], it is possible that EEQs may have potential to regulate β-cell function and destruction.
Highlights.
-
➢
Cyclooxygenase (COX) products, specifically PGE2, inhibit insulin secretion and promote pancreatic β-cell destruction.
-
➢
Lipoxygenase (LOX) products, specifically 12-HETE, promotes β-cell dysfunction and β-cell destruction.
-
➢
Increasing the levels of cytochrome P450-derived EETs by suppressing soluble epoxide hydrolase (sEH) improves β-cell function and reduces β-cell destruction.
-
➢
ω-3 PUFAs are β-cell protective.
ACKNOWLEDGMENTS
Part of the work described in this review was supported by a National Heart, Lung, and Blood Institute Grant (R01 HL-70887) and PSRP and DODI grants from Georgia Health Sciences University to Dr. M. H. Wang. Dr. P. Luo’s research was supported by a National Natural Science Foundation of China (NSFCN81000341).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580–591. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 3.Cnop M, Welsh N, Jonas JC, et al. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 4.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 5.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011 doi: 10.1097/MED.0b013e3283444b09. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: Role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011 doi: 10.1007/s11154-011-9168-2. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 7.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia C, Feve B, Ferre P, et al. Diabetes and inflammation: fundamental aspects and clinical implications. Diabetes Metab. 2010;36:327–338. doi: 10.1016/j.diabet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–751. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 10.Henquin JC. Pathways in beta-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes. 2004;53:S48–S58. doi: 10.2337/diabetes.53.suppl_3.s48. [DOI] [PubMed] [Google Scholar]
- 11.Imig JD. Eicosanoid regulation of the renal vasculature. Am J Physiol. 2000;279:F965–F981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 12.Roman RJ. P450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 13.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao CM, Breyer MD. Roles of lipid mediators in kidney injury. Semin Nephrol. 2007;27:338–351. doi: 10.1016/j.semnephrol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Dobrian AD, Lieb DC, Cole BK, et al. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imig JD. Eicosanoids and renal vascular function in diseases. Clin Sci. 2006;111:21–34. doi: 10.1042/CS20050251. [DOI] [PubMed] [Google Scholar]
- 17.Harris RC. An update on cyclooxygenase-2 expression and metabolites in the kidney. Curr Opin Nephrol Hypertens. 2008;17:64–69. doi: 10.1097/MNH.0b013e3282f1bb7d. [DOI] [PubMed] [Google Scholar]
- 18.Feletou M, Huang Y, Vanhoutte PM. Vasoconstrictor prostanoids. Pflugers Arch. 2010;459:941–950. doi: 10.1007/s00424-010-0812-6. [DOI] [PubMed] [Google Scholar]
- 19.Wong MS, Vanhoutte PM. COX-mediated endothelium-dependent contractions: from the past to recent discoveries. Acta Pharmacol Sin. 2010;31:1095–1102. doi: 10.1038/aps.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 21.Harris RC. COX-2 and the kidney. J Cardiovasc Pharmacol. 2006;47:S37–S42. doi: 10.1097/00005344-200605001-00007. [DOI] [PubMed] [Google Scholar]
- 22.Cheng HF, Harris RC. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525–530. doi: 10.1161/01.HYP.0000116221.27079.ea. [DOI] [PubMed] [Google Scholar]
- 23.Robertson RP. Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes. 1998;47:1379–1383. doi: 10.2337/diabetes.47.9.1379. [DOI] [PubMed] [Google Scholar]
- 24.Vanhoutte PM. COX-1 and vascular disease. Clin Pharmacol Ther. 2009;86:212–215. doi: 10.1038/clpt.2009.108. [DOI] [PubMed] [Google Scholar]
- 25.Vanhoutte PM, Shimokawa H, Tang EH, et al. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 26.Kawabe J, Ushikubi F, Hasebe N. Prostacyclin in vascular diseases. - Recent insights and future perspectives. Circ J. 2010;74:836–843. doi: 10.1253/circj.cj-10-0195. [DOI] [PubMed] [Google Scholar]
- 27.Camara NO, Martins JO, Landgraf RG, et al. Emerging roles for eicosanoids in renal diseases. Curr Opin Nephrol Hypertens. 2009;18:21–27. doi: 10.1097/MNH.0b013e32831a9df7. [DOI] [PubMed] [Google Scholar]
- 28.Mochizuki N, Kwon YG. 15-lipoxygenase-1 in the vasculature: expanding roles in angiogenesis. Circ Res. 2008;102:143–145. doi: 10.1161/CIRCRESAHA.107.170191. [DOI] [PubMed] [Google Scholar]
- 29.Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. 2010;86:243–253. doi: 10.1093/cvr/cvq016. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn H, Chaitidis P, Roffeis J, et al. Arachidonic Acid metabolites in the cardiovascular system: the role of lipoxygenase isoforms in atherogenesis with particular emphasis on vascular remodeling. J Cardiovasc Pharmacol. 2007;50:609–620. doi: 10.1097/FJC.0b013e318159f177. [DOI] [PubMed] [Google Scholar]
- 31.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007;71:1105–1115. doi: 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- 32.Wang MH. Renal cytochrome P450-derived eicosanoids and hypertension. Curren. Hypertens Rev. 2006;2:227–236. [Google Scholar]
- 33.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 34.Imig JD, Navar LG, Roman RJ, et al. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 35.Campbell WB, Gebremedhin D, Pratt PF, et al. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Falck JR, Tuniki VR, et al. 20-125-Iodo-14,15-epoxyeicosa-5(Z)-enoic acid: a high-affinity radioligand used to characterize the epoxyeicosatrienoic acid antagonist binding site. J Pharmacol Exp Ther. 2008;331:1137–1145. doi: 10.1124/jpet.109.157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bukhari IA, Gauthier KM, Jagadeesh SG, et al. 14,15-Dihydroxy-eicosa-5(Z)-enoic acid selectively inhibits 14,15-epoxyeicosatrienoic acid-induced relaxations in bovine coronary arteries. J Pharmacol Exp Ther. 2011;336:47–55. doi: 10.1124/jpet.110.169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang W, Tuniki VR, Anjaiah S, et al. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125i-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther. 2008;324:1019–1027. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- 39.Pedrosa CM, Dubay GR, Falck JR, et al. Parathyroid hormone inhibits Na+-K+-ATPase through a cytochrome P450 pathway. Am J Physiol. 1994;266:F497–F505. doi: 10.1152/ajprenal.1994.266.3.F497. [DOI] [PubMed] [Google Scholar]
- 40.Nowicki S, Chen SL, Aizman O, et al. 20-Hydroxyeicosatetraenoic acid (20 HETE) activates protein kinase C: Role in regulation of rat renal Na+,K+-ATPase. J Clin Invest. 1997;99:1224–1230. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Escalante B, Falck JR, Yadagiri P, et al. 19(S)-hydroxyeicosatetraenoic acid is a potent stimulator of renal Na,K-ATPase. Biochem Biophys Res Commun. 1988;152:1259–1274. doi: 10.1016/s0006-291x(88)80422-4. [DOI] [PubMed] [Google Scholar]
- 42.Escalante B, Erlij D, Falck JR, et al. Cytochrome P450 arachidonate metabolites affect ion fluxes in rabbit medullary thick ascending limb. Am J Physiol. 1994;266:C1775–C1782. doi: 10.1152/ajpcell.1994.266.6.C1775. [DOI] [PubMed] [Google Scholar]
- 43.Wei Y, Lin DH, Kemp R, et al. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol. 2004;124:719–727. doi: 10.1085/jgp.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams JM, Murphy S, Burke M, et al. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDaniel ML, Kwon G, Hill JR, et al. Cytokines and nitric oxide in islet inflammation and diabetes. Proc Soc Exp Biol Med. 1996;211:24–32. doi: 10.3181/00379727-211-43950d. [DOI] [PubMed] [Google Scholar]
- 46.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 47.Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 48.Krauss S, Zhang CY, Scorrano L, et al. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metz S, VanRollins M, Strife R, et al. Lipoxygenase pathway in islet endocrine cells: Oxidative metabolism of arachidonic acid promotes insulin release. J Clin Invest. 1983;71:1191–1205. doi: 10.1172/JCI110868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson RP. Arachidonic acid metabolite regulation of insulin secretion. Diabetes Metab Rev. 1986;2:261–296. doi: 10.1002/dmr.5610020304. [DOI] [PubMed] [Google Scholar]
- 51.Tran PO, Gleason CE, Robertson RP. Inhibition of interleukin-1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes. 2002;51:1772–1778. doi: 10.2337/diabetes.51.6.1772. [DOI] [PubMed] [Google Scholar]
- 52.Persaud SJ, Muller D, Belin VD, et al. The role of arachidonic acid and its metabolites in insulin secretion from human islets of langerhans. Diabetes. 2007;56:197–203. doi: 10.2337/db06-0490. [DOI] [PubMed] [Google Scholar]
- 53.Persaud SJ, Muller D, Belin VD, et al. Expression and function of cyclooxygenase and lipoxygenase enzymes in human islets of Langerhans. Arch Physiol Biochem. 2007;113:104–109. doi: 10.1080/13813450701531177. [DOI] [PubMed] [Google Scholar]
- 54.Tran PO, Gleason CE, Poitout V, et al. Prostaglandin E(2) mediates inhibition of insulin secretion by interleukin-1beta. J Biol Chem. 1999;274:31245–31248. doi: 10.1074/jbc.274.44.31245. [DOI] [PubMed] [Google Scholar]
- 55.Sorli CH, Zhang HJ, Armstrong MB, et al. Basal expression of cyclooxygenase-2 and nuclear factor-interleukin 6 are dominant and coordinately regulated by interleukin 1 in the pancreatic islet. Proc Natl Acad Sci. 1998;95:1788–1793. doi: 10.1073/pnas.95.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujita H, Kakei M, Fujishima H, et al. Effect of selective cyclooxygenase-2 (COX-2) inhibitor treatment on glucose-stimulated insulin secretion in C57BL/6 mice. Biochem Biophys Res Commun. 2007;363:37–43. doi: 10.1016/j.bbrc.2007.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nathan MH, Pek SB. Lipoxygenase-generated eicosanoids inhibit glucose-induced insulin release from rat islets. Prostaglandins Leukot Essent Fatty Acids. 1990;40:21–25. doi: 10.1016/0952-3278(90)90111-w. [DOI] [PubMed] [Google Scholar]
- 58.Metz SA, Murphy RC, Fujimoto W. Effects on glucose-induced insulin secretion of lipoxygenase-derived metabolites of arachidonic acid. Diabetes. 1984;33:119–124. doi: 10.2337/diab.33.2.119. [DOI] [PubMed] [Google Scholar]
- 59.Mehrabian M, Schulthess FT, Nebohacova M, et al. Identification of ALOX5 as a gene regulating adiposity and pancreatic function. Diabetologia. 2008;51:978–988. doi: 10.1007/s00125-008-1002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bleich D, Chen S, Gu JL, et al. The role of 12-lipoxygenase in pancreatic beta-cells. Int J Mol Med. 1998;1:265–272. [PubMed] [Google Scholar]
- 61.Ahren B, Magrum LJ, Havel PJ, et al. Augmented insulinotropic action of arachidonic acid through the lipoxygenase pathway in the obese Zucker rat. Obes Res. 2000;8:475–480. doi: 10.1038/oby.2000.59. [DOI] [PubMed] [Google Scholar]
- 62.Bleich D, Chen S, Zipser B, et al. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103:1431–1436. doi: 10.1172/JCI5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma K, Nunemaker CS, Wu R, et al. 12-Lipoxygenase products reduce insulin secretion and {beta}-cell viability in human islets. J Clin Endocrinol Metab. 2010;95:887–893. doi: 10.1210/jc.2009-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falck JR, Manna S, Moltz J, et al. Epoxyeicosatrienoic acids stimulate glucagon and insulin release from isolated rat pancreatic islets. Biochem Biophys Res Commun. 1983;114:743–749. doi: 10.1016/0006-291x(83)90843-4. [DOI] [PubMed] [Google Scholar]
- 65.Zeldin DC, Foley J, Boyle JE, et al. Predominant expression of an arachidonate epoxygenase in islets of Langerhans cells in human and rat pancreas. Endocrinology. 1997;138:1338–1346. doi: 10.1210/endo.138.3.4970. [DOI] [PubMed] [Google Scholar]
- 66.Lee CR, Imig JD, Edin ML, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mustafa S, Sharma V, McNeill JH. Insulin resistance and endothelial dysfunction: Are epoxyeicosatrienoic acids the link? Exp Clin Cardiol. 2009;14:e41–e50. [PMC free article] [PubMed] [Google Scholar]
- 68.Luo P, Chang HH, Zhou Y, et al. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exp Ther. 2010;334:430–438. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 70.Persaud SJ, Burns CJ, Belin VD, et al. Glucose-induced regulation of COX-2 expression in human islets of Langerhans. Diabetes. 2004;53:S190–S192. doi: 10.2337/diabetes.53.2007.s190. [DOI] [PubMed] [Google Scholar]
- 71.Shanmugam N, Todorov IT, Nair I, et al. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (AGER), and in islets from diabetic mice. Diabetologia. 2006;4:100–107. doi: 10.1007/s00125-005-0065-7. [DOI] [PubMed] [Google Scholar]
- 72.Xie W, Merrill JR, Bradshaw WS, et al. Structural determination and promoter analysis of the chicken mitogen-inducible prostaglandin G/H synthase gene and genetic mapping of the murine homolog. Arch Biochem Biophys. 1993;300:247–252. doi: 10.1006/abbi.1993.1034. [DOI] [PubMed] [Google Scholar]
- 73.Arakawa T, Laneuville O, Miller CA, et al. Prostanoid receptors of murine NIH 3T3 and RAW 264.7 cells. Structure and expression of the murine prostaglandin EP4 receptor gene. J Biol Chem. 1996;271:29569–29575. doi: 10.1074/jbc.271.47.29569. [DOI] [PubMed] [Google Scholar]
- 74.Rabinovitch A, Baquerizo H, Sumoski W. Cytotoxic effects of cytokines on islet beta-cells: evidence for involvement of eicosanoids. Endocrinology. 1990;126:67–71. doi: 10.1210/endo-126-1-67. [DOI] [PubMed] [Google Scholar]
- 75.Oshima H, Taketo MM, Oshima M. Destruction of pancreatic beta-cells by transgenic induction of prostaglandin E2 in the islets. J Biol Chem. 2006;281:29330–29336. doi: 10.1074/jbc.M602424200. [DOI] [PubMed] [Google Scholar]
- 76.Juang JH, Kuo CH. Effects of cyclooxygenase-2 inhibitor and adenosine triphosphate-sensitive potassium channel opener in syngeneic mouse islet transplantation. Transplant Proc. 2010;42:4221–4224. doi: 10.1016/j.transproceed.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 77.Gysemans C, Stoffels K, Giulietti A, et al. Prevention of primary non-function of islet xenografts in autoimmune diabetic NOD mice by anti-inflammatory agents. Diabetologia. 2003;46:1115–1123. doi: 10.1007/s00125-003-1154-0. [DOI] [PubMed] [Google Scholar]
- 78.Chen M, Yang ZD, Smith KM, et al. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48:486–495. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- 79.Laybutt DR, Sharma A, Sgroi DC, et al. Genetic regulation of metabolic pathways in beta-cells disrupted by hyperglycemia. J Biol Chem. 2002;277:10912–10921. doi: 10.1074/jbc.M111751200. [DOI] [PubMed] [Google Scholar]
- 80.Fu SH, Chen YT, Chiang CH, et al. Enhancing engraftment of neonatal porcine xenoislet with CTLA4Ig and nordihydroguaiaretic acid. Transplant Proc. 2006;38:3283–3285. doi: 10.1016/j.transproceed.2006.10.089. [DOI] [PubMed] [Google Scholar]
- 81.Yang S, Lin L, Chen JX, et al. Cytochrome P450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol. 2007;293:H142–H151. doi: 10.1152/ajpheart.00783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simpkins AN, Rudic RD, Schreihofer DA, et al. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174:2086–2095. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Enayetallah AE, French RA, Barber M, et al. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J Histochem Cytochem. 2006;54:329–335. doi: 10.1369/jhc.5A6808.2005. [DOI] [PubMed] [Google Scholar]
- 84.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 85.McCarthy MI. Genomics, type 2 diabetes, and obesity. N.Engl.J Med. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 86.Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moller DE, Flier JS. Insulin resistance--mechanisms, syndromes, and implications. N Engl J Med. 1991;325:938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 88.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khanapure SP, Garvey DS, Janero DR, et al. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr Top Med Chem. 2007;7:311–340. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- 90.Williams KI, G.A. Higgs GA. Eicosanoids and inflammation. J Pathol. 1988;156:101–110. doi: 10.1002/path.1711560204. [DOI] [PubMed] [Google Scholar]
- 91.Henkel J, Neuschafer-Rube F, Pathe-Neuschafer-Rube A, et al. Aggravation by prostaglandin E2 of interleukin-6-dependent insulin resistance in hepatocytes. Hepatology. 2009;50:781–790. doi: 10.1002/hep.23064. [DOI] [PubMed] [Google Scholar]
- 92.Puschel GP, Kirchner C, Schroder A, et al. Glycogenolytic and antiglycogenolytic prostaglandin E2 actions in rat hepatocytes are mediated via different signalling pathways. Eur J Biochem. 1993;218:1083–1089. doi: 10.1111/j.1432-1033.1993.tb18468.x. [DOI] [PubMed] [Google Scholar]
- 93.Hsieh PS, Jin JS, Chiang CF, et al. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity. 2009;17:1150–1157. doi: 10.1038/oby.2008.674. [DOI] [PubMed] [Google Scholar]
- 94.Shanmugam N, Gaw G, Natarajan IR. Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes. 2004;53:795–802. doi: 10.2337/diabetes.53.3.795. [DOI] [PubMed] [Google Scholar]
- 95.Cosentino F, Eto M, De PP, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: Role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 96.Schambelan M, Blake S, Sraer J, et al. Increased prostaglandin production by glomeruli isolated from rats with streptozotocin-induced diabetes mellitus. J Clin Invest. 1985;75:404–412. doi: 10.1172/JCI111714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shanmugam N, Kim YS, Lanting L, et al. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem. 2003;278:34834–34844. doi: 10.1074/jbc.M302828200. [DOI] [PubMed] [Google Scholar]
- 98.Miao F, Gonzalo IG, Lanting L, et al. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279:18091–18097. doi: 10.1074/jbc.M311786200. [DOI] [PubMed] [Google Scholar]
- 99.Cipollone F, Iezzi A, Fazia M, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 100.Hsieh PS, Lu KC, Chiang CF, et al. Suppressive effect of COX2 inhibitor on the progression of adipose inflammation in high-fat-induced obese rats. Eur J Clin Invest. 2010;40:164–171. doi: 10.1111/j.1365-2362.2009.02239.x. [DOI] [PubMed] [Google Scholar]
- 101.Hundal RS, Petersen KF, Mayerson AB, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hasler WL, Soudah HC, Dulai G, et al. Mediation of hyperglycemia-evoked gastric slow-wave dysrhythmias by endogenous prostaglandins. Gastroenterology. 1995;108:727–736. doi: 10.1016/0016-5085(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 103.Trivedi SG, Newson J, Rajakariar R, et al. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc Natl Acad Sci. 2006;103:5179–5184. doi: 10.1073/pnas.0507175103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gilroy DW, Colville-Nash PR, Willis D, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 105.Coll T, Palomer X, Blanco-Vaca F, et al. Cyclooxygenase 2 inhibition exacerbates palmitate-induced inflammation and insulin resistance in skeletal muscle cells. Endocrinology. 2010;151:537–548. doi: 10.1210/en.2009-0874. [DOI] [PubMed] [Google Scholar]
- 106.Yapaci E, Uysal O, Demirbilek H, et al. Hypoglycaemia and hypothermia due to nimesulide overdose. Arch Dis Child. 2001;85:510. doi: 10.1136/adc.85.6.510a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Konheim YL, Wolford JK. Association of a promoter variant in the inducible cyclooxygenase-2 gene (PTGS2) with type 2 diabetes mellitus in Pima Indians. Hum Genet. 2003;113:377–381. doi: 10.1007/s00439-003-1000-y. [DOI] [PubMed] [Google Scholar]
- 108.Rudock ME, Liu Y, Ziegler JT, et al. Association of polymorphisms in cyclooxygenase (COX)-2 with coronary and carotid calcium in the Diabetes Heart Study. Atherosclerosis. 2009;203:459–465. doi: 10.1016/j.atherosclerosis.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hubacek JA, Pelikanova T, Lanska V, et al. A polymorphism in the cyclooxygenase 2 gene in type 1 diabetic patients with nephropathy. Physiol Res. 2010 doi: 10.33549/physiolres.932016. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 110.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 111.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 112.Sears DD, Miles PD, Chapman J, et al. 12/15-Lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nunemaker CS, Chen M, Pei H, et al. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol. 2008;295:E1065–E1075. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Law IK, Xu A, Lam KS, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Natarajan R, Gu JL, Rossi J, et al. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci. 1993;90:4947–4951. doi: 10.1073/pnas.90.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patricia MK, Natarajan R, Dooley AN, et al. Adenoviral delivery of a leukocyte-type 12 lipoxygenase ribozyme inhibits effects of glucose and platelet-derived growth factor in vascular endothelial and smooth muscle cells. Circ Res. 2001;88:659–665. doi: 10.1161/hh0701.088838. [DOI] [PubMed] [Google Scholar]
- 117.Brown ML, Jakubowski JA, Leventis LL, et al. Elevated glucose alters eicosanoid release from porcine aortic endothelial cells. J Clin Invest. 1988;82:2136–2141. doi: 10.1172/JCI113835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chakrabarti SK, Wen Y, Dobrian AD, et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am J Physiol. 2011;300:E175–E187. doi: 10.1152/ajpendo.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Horrillo R, Gonzalez-Periz A, Martinez-Clemente M, et al. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol. 2010;184:3978–3987. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- 120.Hatley ME, Srinivasan S, Reilly KB, et al. Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J Biol Chem. 2003;278:25369–25375. doi: 10.1074/jbc.M301175200. [DOI] [PubMed] [Google Scholar]
- 121.Schwartzman ML, Iserovich P, Gotlinger K, et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010;59:1780–1788. doi: 10.2337/db10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wen Y, Gu J, Chakrabarti SK, et al. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology. 2007;148:1313–1322. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- 123.Wen Y, Gu J, Vandenhoff GE, et al. Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am J Physiol. 2008;294:H1933–H1938. doi: 10.1152/ajpheart.00260.2007. [DOI] [PubMed] [Google Scholar]
- 124.Chakrabarti SK, Cole BK, Wen Y, et al. 12/15-Lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity. 2009;17:1657–1663. doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scribner KA, Gadbois TM, Gowri M, et al. Masoprocol decreases serum triglyceride concentrations in rats with fructose-induced hypertriglyceridemia. Metabolism. 2000;49:1106–1110. doi: 10.1053/meta.2000.8604. [DOI] [PubMed] [Google Scholar]
- 126.Reed MJ, Meszaros K, Entes LJ, et al. Effect of masoprocol on carbohydrate and lipid metabolism in a rat model of type 2 diabetes. Diabetologia. 1999;42:102–106. doi: 10.1007/s001250051121. [DOI] [PubMed] [Google Scholar]
- 127.Alpert E, Gruzman A, Totary H, et al. A natural protective mechanism against hyperglycaemia in vascular endothelial and smooth-muscle cells: Role of glucose and 12-hydroxyeicosatetraenoic acid. Biochem J. 2002;362:413–422. doi: 10.1042/0264-6021:3620413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dobrian AD, Lieb DC, Ma O, et al. Differential expression and localization of 12/15 lipoxygenases in adipose tissue in human obese subjects. Biochem Biophys Res Commun. 2010;403:485–490. doi: 10.1016/j.bbrc.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaaman M, Ryden M, Axelsson T, et al. ALOX5AP expression, but not gene haplotypes, is associated with obesity and insulin resistance. Int.J Obes. 2006;30:447–452. doi: 10.1038/sj.ijo.0803147. [DOI] [PubMed] [Google Scholar]
- 130.Antonipillai I, Nadler J, Vu EJ, et al. A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab. 1996;81:1940–1945. doi: 10.1210/jcem.81.5.8626861. [DOI] [PubMed] [Google Scholar]
- 131.Yamada Y, Kato K, Oguri M, et al. Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int J Mol Med. 2008;21:801–808. [PubMed] [Google Scholar]
- 132.Yoshida T, Kato K, Yokoi K, et al. Association of genetic variants with chronic kidney disease in Japanese individuals with type 2 diabetes mellitus. Int J Mol Med. 2009;23:529–537. doi: 10.3892/ijmm_00000161. [DOI] [PubMed] [Google Scholar]
- 133.Liu Y, Freedman BI, Burdon KP, et al. Association of arachidonate 12-lipoxygenase genotype variation and glycemic control with albuminuria in type 2 diabetes. Am J Kidney Dis. 2008;52:242–250. doi: 10.1053/j.ajkd.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Burdon KP, Rudock ME, Lehtinen AB, et al. Human lipoxygenase pathway gene variation and association with markers of subclinical atherosclerosis in the diabetes heart study. Mediators Inflamm. 2010 doi: 10.1155/2010/170153. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pass GJ, Becker W, Kluge R, et al. Effect of hyperinsulinemia and type 2 diabetes-like hyperglycemia on expression of hepatic cytochrome P450 and glutathione s-transferase isoforms in a New Zealand obese-derived mouse backcross population. J Pharmacol Exp Ther. 2002;302:442–450. doi: 10.1124/jpet.102.033553. [DOI] [PubMed] [Google Scholar]
- 136.Zhao X, Dey A, Romanko OP, et al. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol. 2005;288:R188–R196. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- 137.Lam JL, Jiang Y, Zhang T, et al. Expression and functional analysis of hepatic cytochromes P450, nuclear receptors, and membrane transporters in 10- and 25-week-old db/db mice. Drug Metab Dispos. 2010;38:2252–2258. doi: 10.1124/dmd.110.034223. [DOI] [PubMed] [Google Scholar]
- 138.Enriquez A, Leclercq I, Farrell GC, et al. Altered expression of hepatic CYP2E1 and CYP4A in obese, diabetic ob/ob mice, and fa/fa Zucker rats. Biochem Biophys Res Commun. 1999;255:300–306. doi: 10.1006/bbrc.1999.0202. [DOI] [PubMed] [Google Scholar]
- 139.Kroetz DL, Yook P, Costet P, et al. Peroxisome proliferator-activated receptor alpha controls the hepatic CYP4A induction adaptive response to starvation and diabetes. J Biol Chem. 1998;273:31581–31589. doi: 10.1074/jbc.273.47.31581. [DOI] [PubMed] [Google Scholar]
- 140.Luo P, Zhou Y, Chang HH, et al. Glomerular 20-HETE, EETs, and TGF-beta1 in diabetic nephropathy. Am J Physiol. 2009;296:F556–F563. doi: 10.1152/ajprenal.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Katakam PV, Hoenig M, Ujhelyi HR, et al. Cytochrome P450 activity and endothelial dysfunction in insulin resistance. J Vasc Res. 2000;37:426–434. doi: 10.1159/000025759. [DOI] [PubMed] [Google Scholar]
- 142.Park Y, Capobianco S, Gao X, et al. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. Am J Physiol. 2008;295:H1982–H1988. doi: 10.1152/ajpheart.01261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu X, Zhao CX, Wang L, et al. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 2010;59:997–1005. doi: 10.2337/db09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang LN, Vincelette J, Chen D, et al. Inhibition of soluble epoxide hydrolase attenuates endothelial dysfunction in animal models of diabetes, obesity and hypertension. Eur J Pharmacol. 2011;654:68–74. doi: 10.1016/j.ejphar.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 145.Do Carmo JM, da Silva AA, Morgan J, et al. Inhibition of soluble epoxide hydrolase reduces food intake and increases metabolic rate in obese mice. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.10.017. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sodhi K, Inoue K, Gotlinger KH, et al. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmcol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao X, Li LY. PPAR-alpha agonist fenofibrate induces renal CYP enzymes and reduces blood pressure and glomerular hypertrophy in Zucker diabetic fatty rats. Am J Nephrol. 2008;28:98–606. doi: 10.1159/000116885. [DOI] [PubMed] [Google Scholar]
- 148.Yousif MH, Benter IF. Role of cytochrome P450 metabolites of arachidonic acid in regulation of corporal smooth muscle tone in diabetic and older rats. Vascul Pharmacol. 2007;47:281–287. doi: 10.1016/j.vph.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 149.Ohtoshi K, Kaneto H, Node K, et al. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochem Biophys Res Commun. 2005;331:347–350. doi: 10.1016/j.bbrc.2005.03.171. [DOI] [PubMed] [Google Scholar]
- 150.Surendiran A, Pradhan SC, Agrawal A, et al. Influence of CYP2C9 gene polymorphisms on response to glibenclamide in type 2 diabetes mellitus patients. Eur J Clin Pharmacol. 2011 doi: 10.1007/s00228-011-1013-8. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 151.Zhou K, Donnelly L, Burch L, et al. Loss-of-function CYP2C9 variants improve therapeutic response to sulfonylureas in type 2 diabetes: a Go-DARTS study. Clin Pharmacol Ther. 2010;87:52–56. doi: 10.1038/clpt.2009.176. [DOI] [PubMed] [Google Scholar]
- 152.Ragia G, Petridis I, Tavridou A, et al. Presence of CYP2C9*3 allele increases risk for hypoglycemia in type 2 diabetic patients treated with sulfonylureas. Pharmacogenomics. 2009;10:1781–1787. doi: 10.2217/pgs.09.96. [DOI] [PubMed] [Google Scholar]
- 153.Weise A, Prause S, Eidens M, et al. Prevalence of CYP450 gene variations in patients with type 2 diabetes. Clin Lab. 2010;56:311–318. [PubMed] [Google Scholar]
- 154.Wang CP, Hung WC, Yu TH, et al. Genetic variation in the G-50T polymorphism of the cytochrome P450 epoxygenase CYP2J2 gene and the risk of younger onset type 2 diabetes among Chinese population: potential interaction with body mass index and family history. Exp Clin Endocrinol Diabetes. 2010;118:346–352. doi: 10.1055/s-0029-1243604. [DOI] [PubMed] [Google Scholar]
- 155.Swen JJ, Wessels JA, Krabben A, et al. Effect of CYP2C9 polymorphisms on prescribed dose and time-to-stable dose of sulfonylureas in primary care patients with type 2 diabetes mellitus. Pharmacogenomics. 2010;11:1517–1523. doi: 10.2217/pgs.10.121. [DOI] [PubMed] [Google Scholar]
- 156.Semiz S, Dujic T, Ostanek B, et al. Analysis of CYP2C9*2, CYP2C19*2, and CYP2D6*4 polymorphisms in patientswith type 2 diabetes mellitus. Bosn J Basic Med Sci. 2010;10:287–291. doi: 10.17305/bjbms.2010.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lajer M, Tarnow L, Andersen S, et al. CYP2C9 variant modifies blood pressure-lowering response to losartan in type 1 diabetic patients with nephropathy. Diabet Med. 2007;24:323–325. doi: 10.1111/j.1464-5491.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 158.Pucci L, Lucchesi D, Chirulli V, et al. Cytochrome P450 2J2 polymorphism in healthy Caucasians and those with diabetes mellitus. Am J Pharmacogenomics. 2003;3:355–358. doi: 10.2165/00129785-200303050-00006. [DOI] [PubMed] [Google Scholar]
- 159.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 160.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta. 2011;1814:210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 161.Wei D, Li J, Shen M, et al. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic beta-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes. 2010;59:471–478. doi: 10.2337/db09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bellenger J, Bellenger S, Bataille A, et al. High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: Inflammatory pathway inhibition. Diabetes. 2011;60:1090–1099. doi: 10.2337/db10-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Linn T, Noke M, Woehrle M, et al. Fish oil-enriched diet and reduction of low-dose streptozocin-induced hyperglycemia. Inhibition of macrophage activation. Diabetes. 1989;38:1402–1411. doi: 10.2337/diab.38.11.1402. [DOI] [PubMed] [Google Scholar]
- 164.Gonzalez-Periz A, Horrillo R, Ferre N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mori TA, Bao DQ, Burke V, et al. Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr. 1999;70:817–825. doi: 10.1093/ajcn/70.5.817. [DOI] [PubMed] [Google Scholar]
- 166.Lovejoy JC. The influence of dietary fat on insulin resistance. Curr Diab Rep. 2002;2:435–440. doi: 10.1007/s11892-002-0098-y. [DOI] [PubMed] [Google Scholar]
- 167.Bagdade JD, Ritter M, Subbaiah PV. Marine lipids normalize cholesteryl ester transfer in IDDM. Diabetologia. 1996;39:487–491. doi: 10.1007/BF00400682. [DOI] [PubMed] [Google Scholar]
- 168.Tabatabaie T, Waldon AM, Jacob JM, et al. COX-2 inhibition prevents insulin-dependent diabetes in low-dose streptozotocin-treated mice. Biochem Biophys Res Commun. 2000;273:699–704. doi: 10.1006/bbrc.2000.2959. [DOI] [PubMed] [Google Scholar]
- 169.Rai G, Kenyon V, Jadhav A, et al. Discovery of potent and selective inhibitors of human reticulocyte 15-lipoxygenase-1. J Med Chem. 2010;53:7392–7404. doi: 10.1021/jm1008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Arnold C, Markovic M, Blossey K, et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]


