Abstract
Contrast-enhanced CT coronary angiography (CTCA) has evolved as a reliable alternative imaging modality technique and may be the preferred initial diagnostic test in patients with stable angina with intermediate pre-test probability of CAD. However, because CTCA is moderately predictive for indicating the functional significance of a lesion, the combination of anatomic and functional imaging will become increasingly important. The technology will continue to improve with better spatial and temporal resolution at low radiation exposure, and CTCA may eventually replace invasive coronary angiography. The establishment of the precise role of CTCA in the diagnosis and management of patients with stable angina requires high-quality randomised study designs with clinical outcomes as a primary outcome.
Keywords: Coronary artery disease, Stable angina, Diagnosis, Multi-slice computed tomography, Computed tomography coronary angiography, Stress testing
Introduction
Stable angina is a common and disabling disease, and occurs when there is regional myocardial ischaemia caused by inadequate coronary perfusion due to chronic coronary obstruction. Despite the fact that William Heberden gave a clear description of stable angina as early as 1768, the optimal strategy for diagnosis in stable angina is still evolving and a variety of non-invasive and invasive tests are available.
Traditionally, ischaemic testing includes exercise ECG and stress myocardial perfusion imaging techniques for the non-invasive identification of inducible ischaemia, but non-invasive anatomic testing is emerging. Invasive coronary angiography (ICA) is generally considered the standard of reference for the detection of significant coronary artery stenosis.
Multi-slice CT (MSCT) has rapidly evolved as an alternative imaging test because of its non-invasive nature and high diagnostic performance. Cardiac CT has two modes: a) non-contrast enhanced CT to detect and quantify coronary calcium and b) contrast-enhanced CT to detect non-obstructive and obstructive coronary atherosclerosis. Coronary calcium is considered a proven marker of the presence of atherosclerosis and the prognostic value of coronary calcium scoring is independent and incremental to the predictive value of traditional risk factors [1]. Remarkable advances in MSCT technology have been achieved with successive CT scanner generations. The current state-of-the-art 64 slice CT scanners, necessary for contrast-enhanced CT coronary angiography (CTCA), provide high-definition images of coronary non-obstructive and obstructive atherosclerosis, with characterisation of coronary plaques into calcific and non-calcific components [2–4].
Despite the growing use of MSCT, its clinical utility in the hierarchy of coronary investigations remains to be established. There is an ongoing debate whether management of patients with stable angina should be primarily based on anatomical or functional testing. Notably, there is a well-known dissociation between the functional relevance of a coronary obstruction (ischaemia) and the severity of a coronary obstruction that is haemodynamically significant [5, 6]. This report provides a current perspective on the potential role of MSCT in patients presenting with stable angina. We propose an alternative diagnostic testing algorithm using MSCT for the management of stable angina, and discuss limitations and future directions of CTCA.
Current diagnosis and management of patients with stable angina
The current diagnostic work-up of patients with stable angina is based on the outcome of clinical evaluation, assessment of ischaemia and subsequent management taking into account prognosis and effectiveness of medical and revascularisation therapy [7, 8].
Clinical evaluation includes age, gender, history of chest pain, weight, blood pressure, ECG and laboratory tests of glucose and total cholesterol.
History of chest pain allows classification into A) typical angina that meets the following characteristics: 1) substernal chest discomfort; 2) provoked by exertion or emotional stress and 3) relieved by rest or nitroglycerine; B) Atypical angina that meets two of the above characteristics and C) non-anginal chest pain that meets only one or none of these characteristics.
The clinical evaluation is derived from simple easily obtainable variables and is used as the initial step in the diagnosis and management of patients with stable angina to categorise these patients into a low, intermediate or high pre-test probability group.
The pre-test probability categorisation is important because 1) it has a significant impact on the post-test probability of disease; 2) the prognosis and management of patients is different in each category; 3) the selection of a diagnostic test depends on the consequences this may have on the two above considerations.
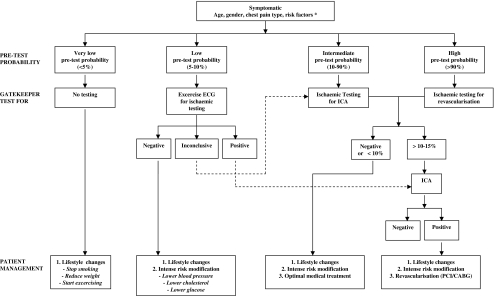
Based on the above considerations and in agreement with the guidelines, a diagnostic algorithm that is currently often used is presented (Fig. 1). This algorithm is an oversimplification of the sometimes complex clinical situation of patients presenting with stable angina and follows three steps: 1) pre-test risk assessment; 2) selection of a diagnostic test; and 3) subsequent patient management.
Fig. 1.
Established diagnostic algorithm. If risk factor diabetes or hypertension is present, patients are categorised to high pre-test probability category. CTCA computed tomography coronary angiography; ECG electrocardiography; ICA invasive coronary angiography. Ischaemic testing includes stress myocardial perfusion imaging; SPECT or single photon emission computed tomography [relative flow], PET or positron emission tomography [absolute flow], MRI or magnetic resonance imaging [flow reserve]; stress echocardiography [wall motion]
The pre-test probability of the presence of obstructive CAD can be estimated using prediction score algorithms devised by Diamond and Forrester or the Duke Clinical Score using the variables from the clinical evaluation [9, 10]. There is no consensus as to the exact range of pre-test likelihood classification into low, intermediate or high pre-test risk, and we have chosen for a very low (<5%), low (5–10%), intermediate (10–90%), and high (>90%) pre-test risk [11].
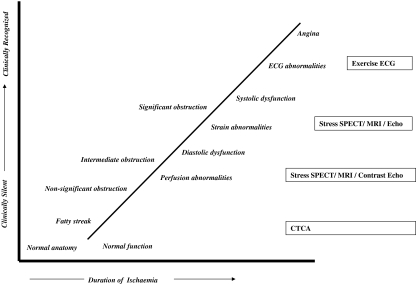
Cardiac diagnostic tests reveal the presence of myocardial ischaemia shown as presence of electrocardiographic ST-segment depression, myocardial perfusion defects or induced wall motion abnormalities. The ischaemic cascade demonstrates the sequence of abnormalities that occur during ischaemia (Fig. 2). Perfusion abnormalities are the earliest manifestation of coronary ischaemia that can be detected by highly sensitive tests such as SPECT/MRI/contrast echocardiography, followed by systolic dysfunction and associated stress-induced motion abnormalities, then ECG abnormalities as a later manifestation of ischaemia and finally angina [12]. The diagnostic accuracy of the various tests is summarised in Table 1.
Fig. 2.
The ischaemic cascade. Myocardial dysfunction occurs in a predictable sequence of events which is detectable prior to clinical symptoms
Table 1.
The diagnostic performance of non-invasive cardiac tests for the diagnosis of CAD [12]
| Sensitivity (%) | Specificity (%) | |
|---|---|---|
| Exercise ECG | 65–70 | 70–75 |
| Exercise stress echocardiography | 80–85 | 80–85 |
| Dobutamine stress echo | 80–85 | 85–90 |
| Exercise myocardial perfusion SPECT | 85–90 | 85–90 |
| Pharmacological myocardial perfusion SPECT | 80–90 | 80–90 |
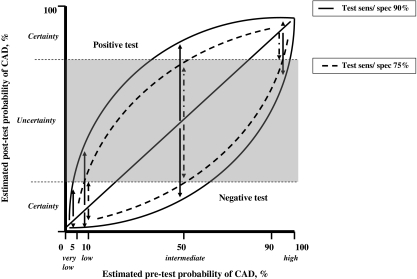
The post-test probability of CAD depends on the pre-test risk and the sensitivity and specificity of the test [9, 10]. The selection of a test depends predominately on the diagnostic accuracy of the test, but also other factors may play a role including safety, costs, availability, patient’s convenience and the use of radiation. No test is perfect and the goal of a test is to provide a level of certainty indicating the presence or absence of CAD (Fig. 3). The level of certainty is arbitrary and depends on the estimated prognosis of patients with a ‘missed’ diagnosis or whether additional testing is able to further improve the level of certainty allowing better patient decision management. Thus, a test may serve as a gatekeeper for additional testing, while a functional test may also be useful to make a decision regarding medical or revascularisation treatment.
Fig. 3.
Relation between pre- and post-test probability. Diagnostic accuracy improves with a test with a higher sensitivity and specificity. Bayesian theory has shown that the value of non-invasive testing is greatest in patients with an intermediate pre-test probability of having CAD. Assume certainty level. Very low pre-test probability (<5%): uncertainty will not be achieved. Low pre-test probability (5–10%): only certainty with a negative test result. Intermediate pre-test probability: (10–90%): certainty with a negative and positive test result. High pre-test probability: (>90%): only certainty with a positive test result
At the very low end of the likelihood spectrum no testing is required. Patients with low pre-test probability may undergo the inexpensive, widely available bicycle ECG stress test. Patients with intermediate pre-test probability are referred for functional testing to assess the presence and extent of myocardial ischaemia to guide the decision for medical treatment. Patients at high risk do not require testing to confirm the presence of the high likelihood of CAD but require testing to assess the extent of ischaemia to guide the decision for revascularisation.
Patient management following clinical evaluation, pre-test risk classification and outcome of selected testing is based on the presence of modifiable (smoking, obesity, exercise) or treatable risk factors (blood pressure, cholesterol, glucose), on the presence of mild to moderate extent of ischaemia requiring medical treatment or large extent of ischaemia where revascularisation has shown to reduce the extent of ischaemia and improve prognosis [13, 14].
Role of MSCT in diagnosis and management of patients with stable angina
So far, non-invasive tests, assessing the presence of coronary obstructions, were based upon the detection of myocardial ischaemia caused by coronary flow limiting stenosis. These tests were predominately used as ‘gatekeeper’ for invasive coronary angiography, the anatomical counterpart of myocardial perfusion abnormalities, because invasive coronary angiography is expensive, patient-unfriendly and in a few cases associated with complications [15].
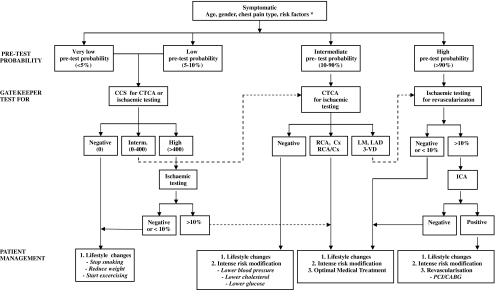
The introduction of CTCA may cause a shift in the current paradigm of the diagnostic work-up of patients with stable angina, because the non-invasive nature of this imaging modality may decrease the threshold to refer patients to establish the anatomical presence of coronary atherosclerotic obstruction and may provide three-dimensional information that might be of value to resolve complex coronary pathology [16] (Fig. 4). The presence of coronary calcium, as assessed by non-contrast enhanced CT, is a marker of the presence of coronary atherosclerosis [1], but its presence does not necessarily imply the presence of a significant coronary obstruction. There is a direct relation between the magnitude of the coronary calcium score and the presence of a coronary obstruction, which is located anywhere in the coronary tree and not necessarily at the calcific plaque site [17, 18]. The absence of coronary calcium does not completely exclude the presence of coronary atherosclerosis, and in a few cases non-calcified plaques are present, although these are mostly nonobstructive [19]. The absence of coronary calcium is associated with a very low risk of adverse coronary events [20].
Fig. 4.
Alternative diagnostic algorithm. * If risk factor diabetes or hypertension is present, patients are categorised to high pre-test probability category. CCS: coronary calcium score (expressed as Agatston Score), CTCA: computed tomography coronary angiography, ICA: invasive coronary angiography, RCA: right coronary artery, Cx: left circumflex artery, LM: left main, LAD: left anterior descending artery, 3-VD: three-vessel disease. Ischaemic testing includes stress myocardial perfusion imaging: SPECT or single photon emission computed tomography [relative flow], PET or positron emission tomography [absolute flow], MRI or magnetic resonance imaging [flow reserve]; stress echocardiography [wall motion]
Coronary calcium scoring may be useful as an initial, reliable gatekeeper for ischaemic testing in patients with a very low and low pre-test probability of CAD (Fig. 4). The use of the coronary calcium score is acceptable because it is patient-friendly, inexpensive and contrast is not necessary, but it is associated with, albeit, low radiation exposure (< 1 mSv).
The presence of extensive coronary calcium seriously limits the reliability of CTCA and consequently in patients with a high coronary calcium score (Agatston >400) an ischaemic test would be more effective than a CTCA (Fig. 4).
The presence of obstructive coronary atherosclerosis is assessed by contrast-enhanced CTCA. The diagnostic performance of CTCA has been extensively investigated in patients with intermediate and high pre-test probability by comparing the diagnostic accuracy of 64-slice CTCA to the gold standard invasive coronary angiography (Tables 2 and 3). The very high negative predictive value outperforms any other test and strongly supports the use of CTCA as reliable gatekeeper to invasive coronary angiography, in particular, in patients at intermediate risk [21].
Table 2.
Diagnostic performance of 64-slice CTCA and dual-source CTCA for the detection of significant CAD on a per-patient basis
| Scanner type | Sensitivity (range, %) | Specificity (range, %) | PPV (range, %) | NPV (range, %) | |
|---|---|---|---|---|---|
| Meta-analyses / systematic review ♦ | 64 | 93–99 | 88–98 | 93 | 96–100 |
| Multi-centre trials ◘ | 64 | 85–99 | 64–90 | 64–91 | 83–99 |
| Single-centre trials □ | DS | 92–100 | 73–98 | 68–96 | 92–100 |
Table 3.
Diagnostic performance of 64-slice CTCA and dual source CTCA for the detection of significant CAD on a per-segment basis
| Scanner type | Sensitivity (range, %) | Specificity (range, %) | PPV (range, %) | NPV (range, %) | |
|---|---|---|---|---|---|
| Meta-analyses / systematic review ♦ | 64 | 86–93 | 96–97 | 73–83 | 97–99 |
| Multi-centre trials ◘ | 64 | 88–90 | 90–97 | 47–76 | 99–99 |
| Single-centre trials □ | DS | 88–97 | 87–98 | 61–89 | 98–99 |
An alternative approach to the established diagnostic algorithm in patients with low to intermediate pre-test risk is the use of coronary CTCA as the initial test (Fig. 4). A negative CTCA is highly reliable to exclude disease and is associated with an excellent short to intermediate prognosis [22–25]. The positive predictive value of CTCA to detect a significant coronary obstruction is only moderate and it is recommended to perform an ischaemia test in patients who, according to CTCA, have left main and/or three-vessel disease, which is associated with an adverse prognosis that may be improved by revascularisation [26]. In patients with a high pre-test probability the question is one of prognosis and benefit from revascularisation rather than diagnosis and an initial functional test should assess the presence and extent of ischaemia to guide to medical treatment or referral to invasive coronary angiography and revascularisation.
Limitations of CTCA
The limitations of CTCA, despite remarkable technical developments, are fivefold: 1) calcification blooming artefacts; 2) limited spatial and temporal resolution; 3) unpredictability of haemodynamic significance of intermediate coronary lesions; 4) radiation exposure; and 5) difficulties to acquire motion-free, high-quality images in patients with arrhythmias.
Coronary calcifications cause blooming artefacts of coronary calcific lesions which either obscure adequate evaluation of the underlying coronary lumen or induce an overestimation of the severity of a coronary obstruction. Both problems result in limitations in the diagnostic performance of CTCA and even the introduction of newly developed CT technology with improved spatial resolution or use of dual-energy CT may not fully reduce this problem.
The spatial and temporal resolution of current CT technology falls short of the resolution obtained with invasive coronary angiography. This becomes particularly apparent in the diagnostic performance of smaller parts of the coronary tree, distal segments and side branches, where the sensitivity is approximately 79% as compared with over 90% in the proximal and mid coronary segments [27]. Motion artefacts, in particular in the RCA, are still present due to limited temporal resolution of CTCA.
The combination of calcifications and limited resolution results in a rather high number of false-positive outcomes, which become predominantly apparent when the diagnostic accuracy is calculated on a segment-based analysis (Table 3) and therefore as of yet cannot replace invasive coronary angiography, which requires precise anatomical delineation of coronary obstructions prior to PCI or CABG.
New CT technology with faster and more sensitive detectors using gemstone technology and introduction of iterative reconstruction algorithms may further improve spatial resolution. Dual energy CT may be helpful to more precisely characterise plaque components [28] and temporal resolution may be further improved by building CT configurations with more X-ray tubes.
The unpredictability of the haemodynamic significance of CT intermediate coronary lesions raises issues as to the referral for coronary revascularisation (PCI or CABG) which is deemed necessary if there is objective evidence of moderate to severe myocardial ischaemia [29, 30]. Therefore, it is recommended to perform a myocardial perfusion challenge following a CT scan with an intermediate lesion. Hybrid imaging with PET-CT and SPECT-CT integrating both functional and anatomic information has, in a few preliminary studies, shown that this yields a better diagnostic performance than stand-alone CT, SPECT or PET [31–34].
The radiation exposure, and the associated increased lifetime risk of cancer and mortality, in particular in younger individuals and women, is of concern [35, 36]. Increased awareness among radiologists, cardiologists and technicians should reduce the radiation exposure by using the newest CT technology which allows tailored CT protocols with use of prospective CT scanning [37, 38]. Unnecessary CT scanning must be avoided [39]. Institution of these measures has significantly reduced the effective dose to less than 4 mSv and with the use of the latest Flash CT scanner to approximately 1 mSv [40].
The use of 320-row CT scanner, allowing whole-heart imaging in one heart beat, may resolve arrhythmia issues, and result in motion-free coronary imaging [41, 42].
Future
The role of CTCA in the diagnosis and management of patients with stable angina is not firmly established. So far, numerous studies have evaluated the diagnostic performance of CTCA compared with invasive coronary angiography. The majority were single-centre studies performed by experienced investigators which probably resulted in better outcomes than may be expected from less experienced centres, which was already apparent in the lesser outcomes of the published three multi-centre studies [43–45].
In addition, CTCA studies were performed in selected patients referred for invasive coronary angiography thereby introducing a referral bias. The spectrum of patients with stable chest pain is much broader and also includes patients in whom invasive coronary angiography is not deemed necessary, but in whom CTCA as a non-invasive, patient-friendly imaging modality may play a diagnostic role. Investigating only patients with a positive CT scan for invasive coronary angiography, while not investigating patients with a negative CT scan in whom referral to invasive coronary angiography is deemed unethical, introduces a verification bias.
These dilemmas can be resolved by not using invasive coronary angiography as a surrogate comparison but instead a randomised trial of a strategy with CTCA and CT-derived clinical management decisions compared with a standard of care strategy with functional testing and its derived clinical management decisions using clinical endpoints as primary outcome and cost-effectiveness as secondary endpoints.
These randomised studies should provide adequate evidence, according to general accepted rigorous criteria, that CTCA as an alternative to existing functional tests may offer better patient outcome and/or may be cost-effective.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 2.Hausleiter J, Meyer T, Hadamitzky M, et al. Prevalence of noncalcified coronary plaques by 64-slice computed tomography in patients with an intermediate risk for significant coronary artery disease. J Am Coll Cardiol. 2006;48(2):312–8. doi: 10.1016/j.jacc.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 3.Husmann L, Gaemperli O, Schepis T, et al. Accuracy of quantitative coronary angiography with computed tomography and its dependency on plaque composition : Plaque composition and accuracy of cardiac CT. Int J Cardiovasc Imaging. 2008;24(8):895–904. [DOI] [PubMed]
- 4.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46(1):147–54. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 5.Scholte AJ, Schuijf JD, Kharagjitsingh AV, et al. Different manifestations of coronary artery disease by stress SPECT myocardial perfusion imaging, coronary calcium scoring, and multislice CT coronary angiography in asymptomatic patients with type 2 diabetes mellitus. J Nucl Cardiol. 2008;15(4):503–9. doi: 10.1016/j.nuclcard.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Meijboom WB, Van Mieghem CA, van Pelt N, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52(8):636–43. doi: 10.1016/j.jacc.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27(11):1341–81. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 8.Fraker TD, Jr, Fihn SD, Gibbons RJ, et al. 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation. 2007;116(23):2762–72. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
- 9.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350–8. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 10.Pryor DB, Harrell FE, Jr, Lee KL, et al. Estimating the likelihood of significant coronary artery disease. Am J Med. 1983;75(5):771–80. doi: 10.1016/0002-9343(83)90406-0. [DOI] [PubMed] [Google Scholar]
- 11.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine: endorsed by the American College of Emergency Physicians. Circulation. 2009;119(22):e561–87. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 12.Abrams J. Clinical practice. Chronic stable angina. N Engl J Med. 2005;352(24):2524–33. doi: 10.1056/NEJMcp042317. [DOI] [PubMed] [Google Scholar]
- 13.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 14.Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105–11. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 15.Zanzonico P, Rothenberg LN, Strauss HW. Radiation exposure of computed tomography and direct intracoronary angiography: risk has its reward. J Am Coll Cardiol. 2006;47(9):1846–9. doi: 10.1016/j.jacc.2005.10.075. [DOI] [PubMed] [Google Scholar]
- 16.van Mieghem CA, de Feyter PJ. Combining non-invasive anatomical imaging with invasive functional information: an unconventional but appropriate hybrid approach. Neth Heart J. 2009;17(7–8):292–4. doi: 10.1007/BF03086269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leschka S, Scheffel H, Desbiolles L, et al. Combining dual-source computed tomography coronary angiography and calcium scoring: added value for the assessment of coronary artery disease. Heart. 2008;94(9):1154–61. doi: 10.1136/hrt.2007.124800. [DOI] [PubMed] [Google Scholar]
- 18.Rozanski A, Gransar H, Wong ND, et al. Clinical outcomes after both coronary calcium scanning and exercise myocardial perfusion scintigraphy. J Am Coll Cardiol. 2007;49(12):1352–61. doi: 10.1016/j.jacc.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Henneman MM, Schuijf JD, Pundziute G, et al. Non-invasive evaluation with multislice computed tomography in suspected acute coronary syndrome: plaque morphology on multislice computed tomography versus coronary calcium score. J Am Coll Cardiol. 2008;52(3):216–22. doi: 10.1016/j.jacc.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158(4):554–61. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijboom WB, van Mieghem CA, Mollet NR, et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pre-test probability of significant coronary artery disease. J Am Coll Cardiol. 2007;50(15):1469–75. doi: 10.1016/j.jacc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Ostrom MP, Gopal A, Ahmadi N, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52(16):1335–43. doi: 10.1016/j.jacc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 24.Pundziute G, Schuijf JD, Jukema JW, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49(1):62–70. doi: 10.1016/j.jacc.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 25.Gilard M, Le Gal G, Cornily JC, et al. Midterm prognosis of patients with suspected coronary artery disease and normal multislice computed tomographic findings: a prospective management outcome study. Arch Intern Med. 2007;167(15):1686–9. doi: 10.1001/archinte.167.15.1686. [DOI] [PubMed] [Google Scholar]
- 26.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 27.Stein PD, Yaekoub AY, Matta F, et al. 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med. 2008;121(8):715–25. doi: 10.1016/j.amjmed.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 28.Johnson TR, Nikolaou K, Becker A, et al. Dual-source CT for chest pain assessment. Eur Radiol. 2008;18(4):773–80. doi: 10.1007/s00330-007-0803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention–summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113(1):156–75. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 30.Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization. A Report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology. Circulation. 2009;119(9):1330–52. [DOI] [PubMed]
- 31.Rispler S, Keidar Z, Ghersin E, et al. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49(10):1059–67. doi: 10.1016/j.jacc.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 32.Herzog BA, Husmann L, Landmesser U, et al. Low-dose computed tomography coronary angiography and myocardial perfusion imaging: cardiac hybrid imaging below 3 mSv. Eur Heart J. 2009;30(6):644. doi: 10.1093/eurheartj/ehn490. [DOI] [PubMed] [Google Scholar]
- 33.Di Carli MF, Hachamovitch R. Hybrid PET/CT is greater than the sum of its parts. J Nucl Cardiol. 2008;15(1):118–22. doi: 10.1016/j.nuclcard.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Di Carli MF, Dorbala S, Meserve J, et al. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48(5):783–93. doi: 10.2967/jnumed.106.032789. [DOI] [PubMed] [Google Scholar]
- 35.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 36.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. Jama. 2007;298(3):317–23. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 37.Scheffel H, Alkadhi H, Leschka S, et al. Low-dose CT coronary angiography in the step-and-shoot mode: diagnostic performance. Heart. 2008;94(9):1132–7. [DOI] [PubMed]
- 38.Herzog BA, Husmann L, Burkhard N, et al. Accuracy of low-dose computed tomography coronary angiography using prospective electrocardiogram-triggering: first clinical experience. Eur Heart J. 2008;29(24):3037–42. doi: 10.1093/eurheartj/ehn485. [DOI] [PubMed] [Google Scholar]
- 39.van der Wall. CT angiography, underuse, overuse, or appropriate use? Neth Heart J. 2009;17(6):223. [DOI] [PMC free article] [PubMed]
- 40.Achenbach S, Marwan M, Ropers D, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31(3):340–6. [DOI] [PubMed]
- 41.Dewey M, Zimmermann E, Deissenrieder F, et al. Non-invasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation. 2009;120(10):867–75. doi: 10.1161/CIRCULATIONAHA.109.859280. [DOI] [PubMed] [Google Scholar]
- 42.Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24(5):535–46. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 43.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 44.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 45.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 46.Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244(2):419–28. doi: 10.1148/radiol.2442061218. [DOI] [PubMed] [Google Scholar]
- 47.Abdulla J, Abildstrom SZ, Gotzsche O, et al. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28(24):3042–50. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 48.Mowatt G, Cook JA, Hillis GS, et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94(11):1386–93. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 49.Scheffel H, Alkadhi H, Plass A, et al. Accuracy of dual-source CT coronary angiography: First experience in a high pre-test probability population without heart rate control. Eur Radiol. 2006;16(12):2739–47. doi: 10.1007/s00330-006-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leber AW, Johnson T, Becker A, et al. Diagnostic accuracy of dual-source multi-slice CT-coronary angiography in patients with an intermediate pre-test likelihood for coronary artery disease. Eur Heart J. 2007;28(19):2354–60. doi: 10.1093/eurheartj/ehm294. [DOI] [PubMed] [Google Scholar]
- 51.Weustink AC, Meijboom WB, Mollet NR, et al. Reliable high-speed coronary computed tomography in symptomatic patients. J Am Coll Cardiol. 2007;50(8):786–94. doi: 10.1016/j.jacc.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 52.Johnson TR, Nikolaou K, Busch S, et al. Diagnostic accuracy of dual-source computed tomography in the diagnosis of coronary artery disease. Invest Radiol. 2007;42(10):684–91. doi: 10.1097/RLI.0b013e31806907d0. [DOI] [PubMed] [Google Scholar]
- 53.Brodoefel H, Reimann A, Burgstahler C, et al. Non-invasive coronary angiography using 64-slice spiral computed tomography in an unselected patient collective: effect of heart rate, heart rate variability and coronary calcifications on image quality and diagnostic accuracy. Eur J Radiol. 2008;66(1):134–41. doi: 10.1016/j.ejrad.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 54.Achenbach S, Ropers U, Kuettner A, et al. Randomized comparison of 64-slice single- and dual-source computed tomography coronary angiography for the detection of coronary artery disease. JACC Cardiovasc Imaging. 2008;1(2):177–86. doi: 10.1016/j.jcmg.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Tsiflikas I, Brodoefel H, Reimann AJ, et al. Coronary CT angiography with dual source computed tomography in 170 patients. Eur J Radiol. 2010;74(1):161–5. [DOI] [PubMed]
- 56.Heuschmid M, Burgstahler C, Reimann A, et al. Usefulness of non-invasive cardiac imaging using dual-source computed tomography in an unselected population with high prevalence of coronary artery disease. Am J Cardiol. 2007;100(4):587–92. doi: 10.1016/j.amjcard.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 57.Leschka S, Stolzmann P, Desbiolles L, et al. Diagnostic accuracy of high-pitch dual-source CT for the assessment of coronary stenoses: first experience. Eur Radiol. 2009;19(12):2896–903. [DOI] [PubMed]