Highlights
► HOCl–LDL promotes apoptosis and accumulation of ROS in T-cells. ► Apoptosis occurs via intrinsic routes confirmed by FADDdef or Casp-8def cells. ► Overexpression of Bcl-2 protects T-cells from apoptosis and ROS formation. ► HOCl-modified epitopes colocalize with MPO and T-cells in human placental tissue.
Keywords: MPO–H2O2–halide system, Placenta, Oxidized lipoproteins, Lymphocytes, Hypochlorite
Abstract
Myeloperoxidase is abundantly present in inflammatory diseases where activation of monocytes/macrophages and T-cell-mediated immune response occurs. The potent oxidant hypochlorous acid (HOCl), generated by the myeloperoxidase–H2O2–chloride system of activated phagocytes, converts low-density lipoprotein (LDL) into a proinflammatory lipoprotein particle. Here, we investigated the apoptotic effect of HOCl–LDL, an in vivo occurring LDL modification, on human T-cell lymphoblast-like Jurkat cells. Experiments revealed that HOCl–LDL, depending on the oxidant:lipoprotein molar ratio, induces apoptosis via activation of caspase-3, PARP cleavage and accumulation of reactive oxygen species. The absence of Fas-associated protein with death domain or caspase-8 in mutant cells did not prevent HOCl–LDL induced apoptosis. In contrast, overexpression of the anti-apoptotic Bcl-2 protein protects Jurkat cells against HOCl–LDL-induced apoptosis and prevents accumulation of reactive oxygen species. We conclude that HOCl–LDL-mediated apoptosis in Jurkat cells follows predominantly the intrinsic, mitochondrial pathway. Insitu experiments revealed that an antibody raised against HOCl–LDL recognized epitopes that colocalize both with myeloperoxidase and CD3-positive T-cells in human decidual tissue where local stimulation of the immune system occurs. We provide convincing evidence that formation of HOCl-modified (lipo)proteins generated by the myeloperoxidase–H2O2–chloride system contributes to apoptosis in T-cells.
1. Introduction
Apoptosis, a type of programmed cell death, is indispensable for cell growth control and immune defence. The extrinsic apoptotic pathway requires the binding of a ligand to a death receptor (Fas/CD95 or TNFR1), which recruits two signal-transducing molecules, e.g. TNFR1-associated death domain and Fas-associated protein with death domain (FADD). Binding of procaspase-8 to this complex results in activation by auto- and transproteolytic cleavage, which then initiates proteolytic cleavage of procaspase-3. Caspase-3 finally executes apoptosis e.g. through cleavage of the nuclear DNA repair enzyme poly(ADP-ribose)polymerase (PARP), induction of DNA fragmentation, chromatin condensation, cell shrinking, membrane blebbing, and formation of apoptotic bodies [1].
The intrinsic apoptotic pathway is due to swelling of mitochondria and release of a variety of apoptotic factors that may either directly or indirectly induce apoptosis via cytochrome C-mediated activation of procaspase-9 and subsequent recruitment of procaspase-3. Most importantly, the intrinsic apoptotic pathway is under tight control of members of the Bcl-2 family, which can either promote cell survival (e.g. Bcl-2 protein) or cell death (Bax) [2].
Apoptosis is a major component of normal development. However, apoptosis has been also recognized in a number of common and threatening vascular diseases e.g. atherosclerosis [3], where excessive accumulation of monocytes/macrophages, smooth muscle cells, and T-lymphocytes, and secretion of cytokines and growth factors is believed to be a major cause of disease progression. Most importantly, oxidation of low-density lipoprotein (ox-LDL) has been implicated in the pathogenesis of various inflammatory diseases [4]. For instance, ox-LDL modulates expression of growth factors, adhesion molecules, and tissue factor, stimulates smooth muscle cell proliferation, induces monocyte and T-cell recruitment and activation, and promotes foam cell and fatty streak formation [4]. Although multiple studies have shown that copper–ox-LDL, a convenient experimental model for oxidative LDL modification elicits apoptotic cell death in monocytes/macrophages (for review see: [5]), knowledge about the consequences of ox-LDL on lymphocyte apoptosis is limited [6–8]. Activated T-lymphocytes are present in human lesions [9] supporting evidence that T-cell-mediated immunity contributes to the pathogenesis of atherosclerosis and other inflammatory diseases [3].
The phagocytic enzyme myeloperoxidase (MPO) is abundantly present in various inflammatory diseases. Once activated MPO generates hypochlorous acid (HOCl) from H2O2 and physiological chloride concentrations [10]. HOCl, a potent oxidant and bactericidal and viricidal agent, reacts readily with biomolecules e.g. thiols and thioesters, Fe–S centers, nucleotides, unsaturated fatty acids and proteins to form reactive chloramines, which are in turn powerful oxidants. Most importantly, hypochlorite-modified LDL (HOCl–LDL), prone to elicit foam cell formation in vitro [11], is present in human lesion material [12]. Interactions of HOCl–LDL with cells of haematopoietic origin, as well as smooth muscle and endothelial cells (for review see: [13]) and epithelial cells [14] have been studied and a panel of atherogenic and proinflammatory features have been reported for this in vivo occurring oxidative LDL modification [13].
The present study aimed at investigating the interaction of HOCl–LDL with T-cells. We were interested whether HOCl–LDL acts as an initiator of the apoptotic machinery in wild-type lymphoblastic Jurkat cells. To reveal whether apoptosis occurs via extrinsic and/or intrinsic routes, Jurkat cell mutants deficient in FADD or caspase-8 or overexpressing Bcl-2 were used. MPO-dependent generation of HOCl-modified proteins has been detected in various inflammatory conditions e.g. atherosclerosis [15], glomerular and tubulointerstitial injury [16], and placental tissue [17], where immune cells are contributing. Thus, a specific aim of the present study was whether an antibody raised against HOCl–LDL might detect HOCl-modified epitopes that colocalize with T-lymphocytes in human tissues.
2. Materials and methods
2.1. Isolation and modification of LDL
LDL (d = 1.035 to 1.065 g/ml) was isolated by ultracentrifugation and desalted as described [18]. Modification of LDL (one mg LDL protein per ml PBS, pH 7.4) was performed with freshly prepared HOCl-solution (4 °C overnight, pH 7.4). Treatment with HOCl (0.4–1.6 mM) resulted in an oxidant:lipoprotein molar ratio in between 200:1 and 800:1 [19]. HOCl–LDL was passed over a PD10 column preequilibrated with RPMI to remove unreacted HOCl, filtered through a 0.22 μm membrane and used immediately for cell culture experiments.
2.2. Cells and cell culture experiments
The human T-leukemia Jurkat cell lines (wild-type: JA3 and J16) were from the American Type Culture Collection (Rockville, MD, USA). JA3 cells, deficient in FADD (FADDdef) or caspase-8 (Casp-8def) were provided by Drs. Blenis and Juo. J16 cells overexpressing Bcl-2 were provided by Dr. Korsmeyer. Cells were cultivated in RPMI 1640 with 10% FCS, supplemented with 10 mM Hepes, 0.2 M l-glutamine, 25 U/ml penicillin and 25 μg/ml streptomycin at 37 °C in a humidified 5% CO2 atmosphere [20]. Cells were seeded into cell culture flasks or 6-well plates and incubated with lipoproteins at indicated concentrations for indicated times.
2.3. Apoptosis assays
2.3.1. Detection of DNA strand breaks by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL assay)
Cells (1 × 106/ml) were incubated with monoclonal antibody (mAb) CH-11 (200 ng/ml) or lipoproteins. The cells were carefully resuspended by gently passing through a pipette tip, centrifuged and resuspended in Hanks balanced solution prior to cytospin preparation. One hundred μl of the cell suspension were used per cytospin spot. Cytospins were fixed (15 min, 25 °C) with 4% buffered paraformaldehyde and after rinsing in PBS immersed in a solution of 0.1% Triton X-100 (v/v) and 0.1% sodium citrate (w/v) for 2 min at 4 °C. After washing with PBS the TUNEL assay was performed (APOPTOSIS-I.S.-kit from YLEM, Rome, Italy) according to the manufacturers instructions; however, the streptavidin-peroxidase system was replaced by streptavidin-FITC (Dako) (dilution 1:15). Sections were mounted with Moviol (Calbiochem–Novabiochem, La Jolla, CA) and analyzed on a confocal laser-scanning microscope (Leica SP2, Leica Lasertechnik GmbH, Heidelberg, Germany), using the 488 nm laser line for the excitation of FITC (detection settings were 500–535 nm).
2.3.2. Detection of apoptotic morphological changes by 7-amino-actinomycin (7-AAD)
For the staining with the DNA specific dye 7-AAD (Immunotech, Vienna, Austria) cytospins were fixed with 3% paraformaldehyde (10 min, 25 °C), washed with PBS and incubated with 20 μl of a 7-AAD solution (10 min, 25 °C). All incubations were performed at 25 °C in a moist chamber. Sections were mounted as described above and analyzed by confocal laser-scanning microscopy using the 488 nm laser line for the excitation of 7-AAD. Detection settings were 535–675 nm.
2.3.3. Determination of DNA fragmentation
As a direct measurement of apoptotic cell death, DNA fragmentation was quantified as described [20]. Briefly, 2.5 × 105 cells were incubated in 24-well plates (Costar, Cambridge, MA) with or without apoptotic stimuli in 0.5 ml of medium at 37 °C. Cells were collected by centrifugation at 600g for 10 min at 4 °C, washed twice with PBS, and then resuspended in 100 μl of lysis solution containing 0.1% (v/v) Triton X-100, 0.1% (w/v) sodium citrate, and 50 μg/ml propidium iodide. Apoptosis was quantitatively determined by flow cytometry after incubation at 4 °C in the dark for at least 24 h as cells containing nuclei with subdiploid DNA content.
2.3.4. Caspase activity assays
Activity of caspase-3 and -8 was measured fluorimetrically using the Ac-DEVD-AMC and Ac-IETD-AMC substrates (Sigma). Cell pellets were lysed (25 mM HEPES, pH 7.5, 5 mM CHAPS, and 5 mM DTT) on ice (30 min), centrifuged (10.000g, 10 min) and lysates were incubated with 10 μM Ac-DEVD-AMC in assay buffer (25 mM HEPES, pH 7.5, 5 mM EDTA, 0.1% CHAPS and 5 mM DTT), in the presence of 10 mM DTT. Substrate cleavage and accumulation of AMC was followed in 30 min intervals for 2 h in a 96-well microtiter plate reader (excitation: 360 nm; emission: 460 nm). Caspase activity was calculated from the rate of AMC released per min normalized to cell protein content (BCA, Pierce).
2.4. Cell lysis, and Western Blot analysis
Cells (1 × 106), seeded in 6-well plates under serum-free conditions, were incubated in the absence or presence of lipoproteins. At indicated times, cells were harvested by centrifugation (5 min, 3500 rpm; 4 °C) and washed twice with cold PBS. Cell lysis was performed on ice in 36 μl lysis buffer (PBS with 1% Triton X-100; supplemented with protease inhibitor cocktail; Roche Diagnostics, Mannheim, Germany) for 30 min. Insoluble cell debris was removed by centrifugation (13,000 rpm; 4 °C). Protein lysates were diluted with 4 x reducing sample buffer (8% (w/v) SDS, 18% (v/v) glycerine, 114 mM Tris; pH 6.8) and boiled for 5 min (95 °C). Proteins were separated by PAGE (3.75% or 3.75–15%) under reducing conditions [19]. After transfer, PVDF membranes were incubated with mouse-anti-caspase-8 (1:1000, Alexis, clone C159), rabbit-anti-caspase-3 (1:1000, Cell Signaling, clone 8G10, #9665), or mouse anti-PARP (1:3000, Biomol, clone C-2-10) as primary antibodies. Immunoreactive bands were visualized by peroxidase-conjugated secondary antibodies (Sigma, Taufkirchen, Germany; diluted 1:5000) and subsequent ECL-development.
2.5. Analysis of intracellular ROS
Cells were seeded in 24-well plates and incubated with lipoproteins (500 μg/ml) for the indicated times and loaded with 10 μM 5-(and-6)-chloromethyl-2′,7′-dihydrofluorescence diacetate (CM-H2DCFHDA, Molecular Probes, dissolved in DMSO and pre-diluted in RPMI 1640) for 30 min at 37 °C and 5% CO2 in the dark [21]. Cells were washed twice with pre-warmed PBS, transferred to a 96-well microtiter plate and fluorescence was measured at 485 nm (excitation filters) and 545 nm (emission filters). Mean fluorescence intensity was normalized to cell protein content.
2.6. Immunofluorescence
The following primary mAb or polyclonal antibodies were used: CH-11 (murine mAb, IgM, anti-human Fas, apoptosis inducing, Coulter Instrumentation Lab.), CD3-phycoerythrin (PE)-labeled (murine mAb, IgG1, anti-human T-cells, Pharmingen), 2D10G9 (murine mAb, IgG2bk), MNF116 (murine mAb, IgG1, specific for cytokeratines present in all trophoblast populations, Dako), PE-labeled mouse IgG1 (Pharmingen), polyclonal anti-human MPO antiserum (Dako), non-immune mouse IgG (Dako), non immune rabbit IgG (Sigma). Goat anti-mouse cyanine-2 (Cy-2, Jackson Dianova) and goat anti-rabbit Cy-5 (Jackson Dianova) were used as secondary antibodies.
2.6.1. Tissues
Tissues used for this study were provided from the local Department of Obstetrics and Gynecology. Human first trimester decidual tissue was obtained after termination of pregnancy by curettage for psychosocial reasons (approved by the Ethical Committee of the Medical School, Karl-Franzens-University Graz). Term placental tissue was from placentas of normal uncomplicated pregnancies. Specimens were immediately frozen in a cryostat (Microm, Walldorf, Germany, Microm HM 500 OM), supported by tissue freezing medium (Tissue Tec OCT-compound, Miles, Elkhard, Ind., USA). Serial cryosections (5 μm) were collected on glass slides, air-dried for 2 h (25 °C), fixed in acetone for 5 min (25 °C) and stored at -40 °C until required. Decidual tissue was further subjected to immunohistochemistry using mAb MNF116 to differentiate between trophoblast-invaded decidua basalis and decidua parietalis.
2.6.2. Immunofluorescence and confocal laser scanning microscopy
For the detection of MPO, HOCl-modified epitopes, and T-cells, fluorescence double and triple labeling was performed. Sections of first trimester decidua were thawed, air dried, and fixed in acetone (5 min, 25 °C). After re-hydrating in phosphate buffered saline (PBS, pH 7.4), sections were blocked with UV ultra block (10 min) and for double labeling incubated (30 min) with mAb 2D10G9 raised against HOCl–LDL [19]. The samples were rinsed in PBS and incubated (30 min) with Cy-2-labeled goat anti-mouse antibody (dilution of 1:300). Then, the sections were incubated with anti-MPO antiserum, followed by a goat anti-rabbit Cy-5-labeled mAb (dilution 1:300) [17]. For triple labeling, the sections were additionally incubated (20 min) with a PE-labeled anti-human CD3 mAb. All incubation steps were performed in a dark moist chamber at 25 °C.
The samples were analyzed on a confocal laser scanning microscope in sequential mode (Leica SP2), using the 488 nm laser line for the excitation of Cy-2, the 543 nm line for PE, and the 647 nm line for Cy-5, respectively. Detection settings were: 500 to 535 nm (Cy-2), 555 nm to 620 nm (PE), and 665 nm to 750 nm (Cy-5). Negative controls were performed by replacing the primary antibody by either mouse IgG1, rabbit non-immune IgG or mouse IgG1 labeled with PE.
3. Results and discussion
Oxidative modification of LDL generates a panel of highly reactive, toxic compounds that elicit an inflammatory response and may promote apoptosis [5]. Activation of the extrinsic and intrinsic apoptotic pathways has been studied in cells of mesenchymal origin e.g. monocytes/macrophages, smooth muscle and endothelial cells, and T-lymphocytes. While oxidation of LDL by copper leads to fragmentation of both the protein and the lipid moiety, modification of LDL by HOCl, added as reagent or generated enzymatically by the MPO–H2O2–chloride system reacts primarily with Lys/His and the N-terminal α-amino groups giving rise to formation of chloramines [13].
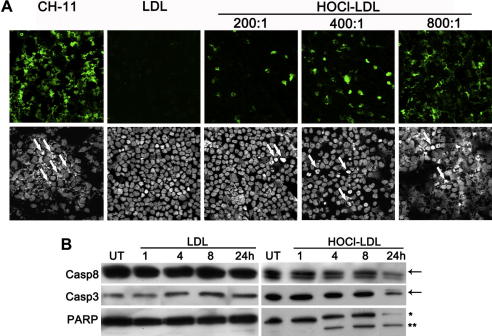
Using the TUNEL-assay we show that mAb CH-11 (acting as a Fas ligand), induced pronounced apoptotic fragmentation in wild-type Jurkat cells (Fig. 1A). When cells were treated with HOCl–LDL, fragmentation of nuclear chromatin, a hallmark of late-stage apoptosis, became apparent. Fig. 1A (upper panel) demonstrates that the degree of chromatin fragmentation dose-dependently increases with an increasing oxidant:lipoprotein molar ratio. Also staining of DNA with 7-AAD revealed that treatment of cells with HOCl–LDL (at increasing oxidant:lipoprotein molar ratio) is paralleled by an increasing number of morphologically changed nuclei with either condensed or already fragmented DNA (Fig. 1A, lower panel).
Fig. 1.
HOCl–LDL induces apoptosis wild-type Jurkat cells: (A) TUNEL assay (upper panel, green signal) and DNA 7-AAD staining (lower panel) of cells in the presence of mAb CH-11 or 500 μg/ml lipoprotein. The arrows point to morphologically changed nuclei, showing signs of apoptosis. (B) Cells were incubated with 500 μg/ml LDL or HOCl–LDL (oxidant:lipoprotein molar of 800:1) at indicated times. Cell lysates were subjected to SDS–PAGE and Western blotting. Membranes were incubated with anti-caspase-8, anti-caspase-3 or anti-PARP as primary antibodies. One representative experiment out of three is shown: (arrow) procaspase-8 or -3; (*) 116 kDa full length PARP; (**) 86 kDa cleaved PARP. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 1B demonstrates no change of procaspase-8 up to 8 h, while a 24 h treatment with HOCl–LDL led to an impaired immunoreactive procaspase-8 band, indicating a slight involvement of the extrinsic apoptotic pathway in wild-type cells. Next, activation of procaspase-3 as well as PARP-cleavage, two processes that are induced by extrinsic/intrinsic pathways, was followed. Western blot experiments demonstrated that HOCl–LDL led to activation of procaspases-3 (after 8 h) and PARP cleavage in a time-dependent manner starting 4 h after treatment (Fig. 1B).
In none of the experiments performed, native LDL showed any signs of apoptosis or nuclear fragmentation in Jurkat cells (Fig. 1).
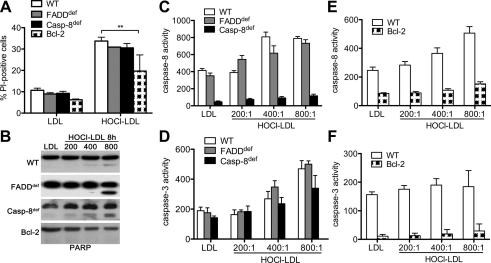
To get further insights in HOCl–LDL-mediated extrinsic and/or intrinsic- apoptotic pathway, mutant T-cells were used. HOCl–LDL treatment increased the number of propidium iodide-positive Jurkat cells with no significant difference between wild-type, FADDdef or Casp-8def cells (Fig. 2A). However, the number of propidium iodide-positive cells was significantly decreased in Bcl-2 cells. Again, native LDL (Fig. 2A) had no significant effect on formation of propidium iodide-positive cells when compared to untreated cells (data not shown). Incubation of Jurkat cell lines with HOCl–LDL (at increasing oxidant:lipoprotein molar ratios) revealed induced PARP cleavage in wild-type, FADDdef and Casp-8def cells. Although in Bcl-2 cells the 116 kDa full-length PARP tended to decrease, no evidence of the 86 kDa PARP cleavage product became apparent using HOCl–LDL. This set of experiments suggested preferential involvement of the intrinsic pathway in wild-type, FADDdef and Casp-8def cells. Again, native LDL had no impact on PARP cleavage in all the cell lines investigated (Fig. 2B).
Fig. 2.
HOCl–LDL induces apoptosis in mutant Jurkat cells: (A) Wild-type (WT), FADDdef, Casp-8def and Bcl-2- cells were incubated with 500 μg/ml lipoprotein for 24 h (A) or 8 h (B). Cells were harvested, stained with propidium iodide (PI) and subjected to FACS analysis (A). Cell lysates were subjected to SDS–PAGE, proteins were transferred, and membranes were incubated with anti-PARP (B). One representative experiment out of three is shown. (C and D) WT, FADDdef and Casp-8def or (E and F) WT and Bcl-2 cells were treated with 500 μg/ml lipoprotein for 4 h. Activation of caspase-8 (C, E) and caspase-3 (D, F) was measured using Ac-IETD-AMC/Ac-DEVD-AMC as substrates. (C–F) Caspase activity is given in nmoles AMC/min/μg protein.
Next, we assessed the activation of both initiator and executor caspase-8 and caspase-3. Fig. 2C shows that incubation of cells with HOCl–LDL led to a 2-fold increase of caspase-8 activity (compared to LDL), with no significant difference between wild-type and FADDdef cells. Only background caspase-8 activity was present in Casp-8def cells. As anticipated from PARP cleavage (Fig. 2B), HOCl–LDL activated caspase-3 in wild-type, FADDdef and Casp-8def cells (Fig. 2D). In contrast, overexpression of mitochondria-localized Bcl-2 prevented HOCl–LDL-induced activation of caspase-8 and caspase-3 (Fig. 2E and F). Together, these data suggest that the assembly of a FADD- and caspase-8-containing death-receptor complex is dispensable for HOCl–LDL-induced apoptosis, whereas Bcl-2 overexpression prevented HOCl–LDL-mediated PARP cleavage and activation of caspase-3 and caspase-8, indicating that the mitochondrial apoptotic cascade is involved.
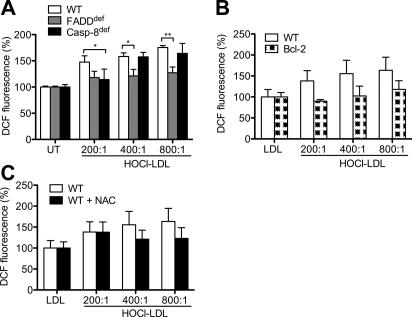
Although ROS play a significant role in modulating the intracellular signaling that leads to T-cell proliferation, recent data suggest that ROS also interfere with signaling events that affect T-cell survival and activation [22]. ROS appear to regulate apoptosis by affecting the extrinsic but preferentially the intrinsic pathway. Previous findings addressed that highly purified populations of primary T-cells produce ROS [22]. We now show that HOCl–LDL induced formation of ROS in wild-type, FADDdef and Casp-8def cells (Fig. 3A) and that ROS levels increased with an increasing oxidant:lipoprotein molar ratio. When compared to wild-type and Casp-8def cells, HOCl–LDL-mediated ROS formation was slightly lower in FADDdef cells, indicating that FADD could be involved in ROS production. However, overexpression of Bcl-2 in Jurkat cells totally blocked HOCl–LDL-induced ROS accumulation (Fig. 3B). Preincubation of wild-type Jurkat cells with the antioxidant N-acetylcysteine prior to treatment with HOCl–LDL decreased ROS formation (Fig. 3C) and apoptosis (data not shown) in further consequence. In summary, our data show that FADD is slightly involved in HOCl–LDL-induced ROS formation and that overexpression of Bcl-2 prevents ROS formation. Therefore, we conclude that HOCl–LDL preferentially activates the intrinsic, mitochondrial apoptotic pathway.
Fig. 3.
HOCl–LDL-mediated ROS formation in Jurkat cells: (A) Wild-type (WT), FADDdef and Casp-8def cells were incubated with 500 μg/ml HOCl–LDL for 3 h. (B) WT and Bcl-2 cells were incubated with 500 μg/ml lipoprotein for 3 h. (C) WT cells were pre-incubated with 25 mM N-acetylcysteine (NAC) for 30 min and treated with 500 μg/ml lipoprotein for 3 h. ROS levels were calculated by measuring DCF-fluorescence. Results are expressed as intensity (% of control); data represent means ± SD (n = 6) ∗p < 0.05 and ∗∗p < 0.01.
The capacity of HOCl–LDL to induce the apoptotic machinery has been investigated in epithelial cells [14], human monocytes and myelomonocytic cell lines [23–25]. HOCl–LDL has been reported to induce a marked caspase-dependent increase of apoptosis, loss of mitochondrial membrane potential and activation of caspase-3, -8, and -9 in U937 cells [24]. While HOCl–LDL was operative via extrinsic and intrinsic apoptotic pathways in U937 cells [24,25], the preferential pathway in T-cells involves the intrinsic route (Figs. 1–3). The fact that overexpression of Bcl-2 in Jurkat cells or the use of N-acetylcysteine interferes with ROS formation (Fig. 3B and C) and apoptosis in further consequence (data not shown) is indicative for mitochondrial involvement. Overexpression of Bcl-2 in U937 cells even prevented translocation of proapoptotic Bax but failed to prevent ROS generation [25]. This indicates that in U937 cells ROS is an upstream signal to promote mitochondrial damage [25]. The low activation of caspase-8 (but pronounced caspase-3 and PARP cleavage) in wild-type cells after 24 h (Fig. 1B) as well as caspase activation and PARP cleavage in FADDdef or Casp-8def cells and their inhibition by Bcl-2 is of further support for the intrinsic pathway in T-cells initiated by HOCl–LDL (Fig. 2). Most importantly, caspase-dependent activation in Jurkat cells induced by HOCl–LDL could be inhibited by the specific caspase-3 (7-DEVD.fmk) and caspase-9 inhibitor (Z-LEHD.fmk) (data not shown) even at lower (lipo)protein concentrations and a lower oxidant:lipoprotein molar ratio as reported for cells of monocytic origin [24,25].
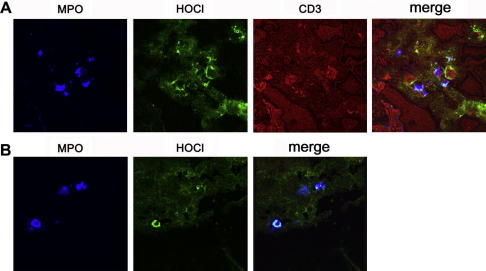
Using an antibody raised against HOCl–LDL [19], HOCl-modified epitopes were found in inflammatory conditions, where T-cells are involved [15–17]. We here aimed to identify colocalization of HOCl-modified proteins with T-cells. Therefore triple labeling experiments were performed. HOCl-modified proteins colocalize with MPO and CD3-positive T-cells in areas of beginning trophoblast invasion in first trimester decidua basalis (Fig. 4A), demonstrating involvement of the MPO–H2O2–halide system. Once heavily invaded, only colocalization of HOCl-modified proteins with MPO was detectable in first trimester tissue (Fig. 4B). The lack of CD3-positive T-cells (not shown) is apparently due to a defence mechanism of the invading trophoblast cells expressing the apoptosis inducing Fas-ligand to protect themselves against activated alloreactive maternal leukocytes [26].
Fig. 4.
Immunofluorescence for MPO, HOCl-epitopes and T-lymphocytes in human first trimester decidua basalis: (A) staining in an area with beginning trophoblast invasion. Colocalization of MPO (blue), CD3-positive T-cells (red), and HOCl-modified epitopes (green). (B) Cell-associated colocalization of MPO (blue) and HOCl-modified epitopes (green) in an area heavily invaded by trophoblast cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Apoptosis is a physiological process to maintain tissue homeostasis and to complement cell differentiation, migration, and proliferation. Even under pathological conditions, programmed cell death could represent a first line of defence initiated by immune dysregulation as a local response of T-cells. T-cells are abundantly present during early and later stages of inflammatory conditions where modification of (lipo)proteins by the MPO–H2O2–chloride system could amplify this process. We here show that HOCl–LDL, a physiologically occurring oxidative LDL modification, preferentially activates the intrinsic apoptotic pathway. The specific signaling cascades promoted by HOCl–LDL in wild-type and mutant Jurkat cell lines are currently under investigation.
Acknowledgments
We thank Cornelia Reinprecht and Monika Sundl for excellent technical assistance. This work was supported by the Austrian Science Fund (FWF, P19074-B05, F3007).
References
- 1.Nagata S., Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G., Reed J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 3.Kockx M.M. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler. Thromb. Vasc. Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- 4.Stocker R., Keaney J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 5.Salvayre R., Auge N., Benoist H. Oxidized low-density lipoprotein-induced apoptosis. Biochim. Biophys. Acta. 2002;1585:213–221. doi: 10.1016/s1388-1981(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 6.Alcouffe J., Caspar-Bauguil S., Garcia V. Oxidized low density lipoproteins induce apoptosis in PHA-activated peripheral blood mononuclear cells and in the Jurkat T-cell line. J. Lipid Res. 1999;40:1200–1210. [PubMed] [Google Scholar]
- 7.Alcouffe J., Therville N., Segui B. Expression of membrane-bound and soluble FasL in Fas- and FADD-dependent T lymphocyte apoptosis induced by mildly oxidized LDL. FASEB J. 2004;18:122–124. doi: 10.1096/fj.02-0808fje. [DOI] [PubMed] [Google Scholar]
- 8.Zurgil N., Solodeev I., Gilburd B. Monitoring the apoptotic process induced by oxidized low-density lipoprotein in Jurkat T-lymphoblast and U937 monocytic human cell lines. Cell. Biochem. Biophys. 2004;40:97–113. doi: 10.1385/CBB:40:2:097. [DOI] [PubMed] [Google Scholar]
- 9.Jonasson L., Holm J., Skalli O. Regional accumulations of T-cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 10.Klebanoff S.J. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 11.Hazell L.J., Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high uptake form for macrophages. Biochem. J. 1993;290:165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazell L.J., Arnold L., Flowers D. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malle E., Marsche G., Arnhold J. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim. Biophys. Acta. 2006;1761:392–415. doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Porubsky S., Schmid H., Bonrouhi M. Influence of native and hypochlorite-modified low-density lipoprotein on gene expression in human proximal tubular epithelium. Am. J. Pathol. 2004;164:2175–2187. doi: 10.1016/S0002-9440(10)63775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malle E., Waeg G., Schreiber R. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur. J. Biochem. 2000;267:4495–4503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 16.Grone H.J., Grone E.F., Malle E. Immunohistochemical detection of hypochlorite-modified proteins in glomeruli of human membranous glomerulonephritis. Lab. Invest. 2002;82:5–14. doi: 10.1038/labinvest.3780390. [DOI] [PubMed] [Google Scholar]
- 17.Hammer A., Desoye G., Dohr G. Myeloperoxidase-dependent generation of hypochlorite-modified proteins in human placental tissues during normal pregnancy. Lab. Invest. 2001;81:543–554. doi: 10.1038/labinvest.3780263. [DOI] [PubMed] [Google Scholar]
- 18.Malle E., Ibovnik A., Steinmetz A. Identification of glycoprotein IIb as the lipoprotein(a)-binding protein on platelets. Lipoprotein(a) binding is independent of an arginyl–glycyl–aspartate tripeptide located in apolipoprotein(a) Arterioscler. Thromb. 1994;14:345–352. doi: 10.1161/01.atv.14.3.345. [DOI] [PubMed] [Google Scholar]
- 19.Malle E., Hazell L., Stocker R. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler. Thromb. Vasc. Biol. 1995;15:982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- 20.Walczak H., Bouchon A., Stahl H. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2- or Bcl-xL-overexpressing chemotherapy-resistant tumor cells. Cancer Res. 2000;60:3051–3057. [PubMed] [Google Scholar]
- 21.Kitz K., Windischhofer W., Leis H.J. 15-Deoxy-Delta12,14-prostaglandin J2 induces Cox-2 expression in human osteosarcoma cells through MAPK and EGFR activation involving reactive oxygen species. Free Radic. Biol. Med. 2011;50:854–865. doi: 10.1016/j.freeradbiomed.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Hildeman D.A. Regulation of T-cell apoptosis by reactive oxygen species. Free Radic. Biol. Med. 2004;36:1496–1504. doi: 10.1016/j.freeradbiomed.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Vicca S., Hennequin C., Nguyen-Khoa T. Caspase-dependent apoptosis in THP-1 cells exposed to oxidized low-density lipoproteins. Biochem. Biophys. Res. Commun. 2000;273:948–954. doi: 10.1006/bbrc.2000.3017. [DOI] [PubMed] [Google Scholar]
- 24.Vicca S., Massy Z.A., Hennequin C. Apoptotic pathways involved in U937 cells exposed to LDL oxidized by hypochlorous acid. Free Radic. Biol. Med. 2003;35:603–615. doi: 10.1016/s0891-5849(03)00361-7. [DOI] [PubMed] [Google Scholar]
- 25.Ermak N., Lacour B., Drueke T.B. Role of reactive oxygen species and Bax in oxidized low density lipoprotein-induced apoptosis of human monocytes. Atherosclerosis. 2008;200:247–256. doi: 10.1016/j.atherosclerosis.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 26.Hammer A., Dohr G. Expression of Fas-ligand in first trimester and term human placental villi. J. Reprod. Immunol. 2000;46:83–90. doi: 10.1016/s0165-0378(99)00059-5. [DOI] [PubMed] [Google Scholar]