Summary
Adenosine triphosphate and its degradation product adenosine diphosphate excite sensory neurons via 2 different G protein-coupled receptors, P2Y1 and P2Y2, which mediate inhibition KV7 and sensitization of TRPV1 channels.
Keywords: P2Y receptors, KV7 channels, TRPV1 channels, Voltage-activated Ca2+ channels
Abstract
Nucleotides contribute to the sensation of acute and chronic pain, but it remained enigmatic which G protein-coupled nucleotide (P2Y) receptors and associated signaling cascades are involved. To resolve this issue, nucleotides were applied to dorsal root ganglion neurons under current- and voltage-clamp. Adenosine triphosphate (ATP), adenosine diphosphate (ADP), and uridine triphosphate (UTP), but not uridine diphosphate (UDP), depolarized the neurons and enhanced action potential firing in response to current injections. The P2Y2 receptor preferring agonist 2-thio-UTP was equipotent to UTP in eliciting these effects. The selective P2Y1 receptor antagonist MRS2179 largely attenuated the excitatory effects of ADP, but left those of 2-thio-UTP unaltered. Thus, the excitatory effects of the nucleotides were mediated by 2 different P2Y receptors, P2Y1 and P2Y2. Activation of each of these 2 receptors by either ADP or 2-thio-UTP inhibited currents through KV7 channels, on one hand, and facilitated currents through TRPV1 channels, on the other hand. Both effects were abolished by inhibitors of phospholipase C or Ca2+-ATPase and by chelation of intracellular Ca2+. The facilitation of TRPV1, but not the inhibition KV7 channels, was prevented by a protein kinase C inhibitor. Simultaneous blockage of KV7 channels and of TRPV1 channels prevented nucleotide-induced membrane depolarization and action potential firing. Thus, P2Y1 and P2Y2 receptors mediate an excitation of dorsal root ganglion neurons by nucleotides through the inhibition of KV7 channels and the facilitation of TRPV1 channels via a common bifurcated signaling pathway relying on an increase in intracellular Ca2+ and an activation of protein kinase C, respectively.
1. Introduction
Sensory dorsal root ganglion (DRG) neurons mediate the sensation of acute and chronic pain. Acute pain is elicited by thermal, mechanical, or chemical stimuli, whereas chronic pain results from the sensitization of sensory neurons most commonly due to inflammatory reactions. The sensitization is mediated by an “inflammatory soup” containing a plethora of signaling molecules, such as cytokines, neurotrophins, bradykinin, prostaglandins, and nucleotides. Interestingly, most analgesics target the synthesis, the release, or the receptors of these inflammatory mediators, as exemplified by inhibitors of cyclooxygenases, such as acetylsalicylic acid [4].
Adenosine triphosphate (ATP) and other nucleotides are released from all cells in response to a variety of stimuli and mediate their effects via a class of receptors termed P2 [25]: P2X receptors are ATP-gated cation channels composed of 3 of a repertoire of at least 7 different subunits (P2X1 to P2X7) that assemble in homomers or heteromers. P2Y receptors are G protein-coupled receptors, and 8 different subtypes have been identified (P2Y1,2,4,6,11,12,13,14) [1,13]. Sensory neurons express both P2X and P2Y receptors; in DRG neurons, the presence of all known P2X subunits but P2X7 has been reported, and the predominating receptors are P2X3 homomers and P2X2/3 heteromers [13,47]. Furthermore, the expression of P2X3 is altered under conditions of chronic pain [41,48]. Therefore, P2X3 receptors are considered promising targets for novel analgesics [12,44].
For P2Y receptors, expression of P2Y1,2,4,6,12,13,14 has been reported in DRG neurons [24,28,35]. Activation of P2Y2 receptors excites DRG neurons, but the underlying mechanisms remained obscure [30]. The respective agonists, ATP and uridine triphosphate [UTP], trigger increases in intracellular Ca2+ and the release of calcitonin gene-related peptide [36]. UTP has also been reported to activate cutaneous C-fibers [38]. Activation of P2Y1 receptors, in contrast, was suggested to mediate analgesic effects through an inhibition of voltage-activated Ca2+ channels (VACCs), which results in a reduction of transmitter release onto dorsal horn neurons [20]. However, more recent data suggest that inhibition of VACCs is rather mediated by P2Y12 instead of P2Y1 receptors [28]. Moreover, P2Y1 receptors were reported to mediate hyperalgesia [28], and this effect was proposed to be mediated by a sensitization of TRPV1 channels [40]. Later on, however, the P2Y receptor involved in the sensitization of TRPV1 channels by nucleotides was reported to be P2Y2 [27,31]. In addition, nucleotides were suggested to enhance nociception by modulating voltage-activated Na+ channels in DRG neurons [33]. Thus, it remained quite controversial which P2Y receptor subtypes control the functions of DRG neurons and whether the outcome is an increase or a decrease in nociception.
In addition to the modulation of voltage-activated Na+ and Ca2+ channels, P2Y receptors mediate the modulation of various neuronal K+ channels, including KV7 [49], Kir3.1/3.2 channels [19], and KCa2 channels [37]. Therefore, this study investigated which P2Y receptors control the excitability of DRG neurons, whether the modulation of K+ channels might contribute to this effect, and what the underlying signaling mechanisms might be.
2. Materials and methods
2.1. Materials
All the nucleotides (adenosine diphosphate [ADP], ATP, uridine diphosphate [UDP], UTP), capsaicin, and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis acetoxymethyl ester (BAPTA-AM), along with bulk chemicals, were obtained from Sigma–Aldrich (Vienna, Austria). Specific P2Y agonists and antagonists (2-thio-UTP and MRS 2179), iodoresiniferatoxin, U73122, and thapsigargin were obtained from Tocris (Bristol, UK); tetrodotoxin (TTX) was obtained from Latoxan (Rosans, France); cangrelor was a generous gift of AstraZeneca R&D Charnwood (Loughborough, UK).
2.2. Primary cultures of DRG neurons
Primary cultures of dissociated DRG neurons from young rats were prepared as described previously [32] with minor modifications. Briefly, ganglia were dissected from 10- to 12-day-old Sprague–Dawley rats that had been killed by decapitation in full accordance with all rules of the Austrian animal protection law (see http://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=Bundesnormen&Gesetzesnummer=20003541) and the Austrian animal experiment by-laws (see http://www.ris2.bka.gv.at/Dokumente/BgblPdf/2000_198_2/2000_198_2.pdf). Dissected ganglia were incubated in collagenase (1.5 mg/mL; Sigma) and dispase (3.0 mg/mL; Boehringer Mannheim, Vienna, Austria) for 30 minutes at 37°C. Then, they were incubated in trypsin (0.25% trypsin; Worthington, Lakewood, NJ) for 15 minutes at 37°C, dissociated by trituration, and resuspended in Dulbecco modified Eagle medium (InVitrogen, Lofer, Austria) containing 4.5 g/L glucose, 10 mg/L insulin, 25 000 IU/L penicillin, and 25 mg/L streptomycin (InVitrogen), 50 μg/L nerve growth factor (R&D Systems Inc., Minneapolis, MN), and 5% fetal calf serum (InVitrogen). Finally, dissociated cells were seeded onto 35-mm culture dishes coated with poly d-lysine (Sigma). The cultures were kept in a humidified 5% CO2 atmosphere at 37°C. The medium was exchanged at least once per week.
2.3. Electrophysiology
All currents or potentials were recorded at room temperature (20°C to 24°C) from small-diameter DRG neurons after 1 to 2 days in culture using an Axopatch 200B amplifier and the pCLAMP 8.1 hard- and software (Molecular Devices, Sunnyvale, CA). All of the neurons investigated displayed capsaicin-induced currents (Icaps) and were thus assumed to provide nociceptive functions. In a few experiments, capsaicin-insensitive neurons were used for comparison. Membrane excitability and currents through KV7 channels (IM) or TRPV1 channels (Icaps) of DRG neurons were determined in the perforated patch clamp mode, voltage-activated Ca2+ currents in the whole-cell configuration. Signals were low-pass filtered at 5 kHz, digitized at 10 to 50 kHz, and stored on an IBM-compatible computer. Traces were analyzed off-line by the Clampfit 8.2 program (Molecular Devices). Patch electrodes were pulled (Flaming-Brown puller, Sutter Instruments, Novato, CA) from borosilicate glass capillaries (Science Products, Frankfurt/Main, Germany). For perforated patch recordings, the internal solution contained (mM) K2SO4 (75), KCl (55), MgCl2 (8), and HEPES (10), adjusted to pH 7.3 with KOH; the recording electrodes were first front-filled with this internal solution and then back-filled with the same solution containing 200 μg/mL amphotericin B (in 0.8% dimethylsulfoxide [DMSO]). For whole-cell recordings, the pipette solution consisted of (mM) CsCl (130), tetraethylammonium chloride (20), CaCl2 (0.24), glucose (10), HEPES (10), EGTA (5), Mg-ATP (2), and Na-GTP (2), adjusted to pH 7.3 with CsOH. The external (bathing) solution routinely contained (mM) NaCl (140), KCl (6.0), CaCl2 (2.0), MgCl2 (2.0), glucose (20), HEPES (10), adjusted to pH 7.4 with NaOH. For the recordings of IM and Icaps, TTX (0.5 μM) was included to suppress voltage-activated Na+ currents. For Ca2+ current recordings, the external solution contained (mM) tetraethylammonium chloride (155), MgCl2 (0.8), CaCl2 (2.5), glucose (11), HEPES (10), and TTX (0.0005), adjusted to pH 7.3 with KOH. Tip resistances were between 1.5 and 3.5 MΩ. The resulting liquid junction potentials were below ±10 mV and were not taken into further consideration during experimentation or data evaluation. Drugs were applied via a DAD-12 drug application device (Adams & List, Westbury, NY), which permits a complete exchange of solutions surrounding the cells being investigated within less than 100 ms [5].
To test for the effects of different nucleotides on membrane excitability, DRG neurons were challenged by 5 increasing current injections (0.1 to 0.5 nA), each lasting for 2 seconds. Changes in excitability were evaluated by counting the number of action potentials fired during these 5 current injections in the absence and presence of nucleotides, respectively.
To quantify currents through KV7 channels (IM), noninactivating outward currents were activated by depolarizing the membrane to −30 mV. Once every 10 seconds, cells were hyperpolarized to −55 mV for 1-second periods to deactivate KV7 channels; the differences between current amplitudes 20 ms after the onset of hyperpolarisations and 20 ms before re-depolarization were taken as a measure for IM [6]. Currents through TRPV1 channels were evoked by 15-second applications of capsaicin (Icaps) to cells held at a potential of −70 mV once every 2 minutes, and the peak amplitudes of these currents were quantified. Ca2+ currents were elicited by 30-ms depolarizations from a holding potential of −80 to −10 mV at a frequency of 4 per minute. Leakage currents were corrected for by an online leak subtraction protocol that applies 4 hyperpolarizing pulses before the depolarization to −10 mV; currents were quantified by measuring peak current amplitudes.
Recordings obtained in the presence of nucleotides (b) were compared with those measured before (a) and after (c) the presence of the nucleotides; changes in current amplitudes were then calculated as percent of control = 200 * b/(a + c) or as percent inhibition = 100 − [200 * b/(a + c)]. For statistical comparison, such sequences were recorded, but nucleotide solutions were replaced by solvent.
2.4. Statistics
All data are given as arithmetic means ± SEM; n values give the numbers of single cells. Differences between 2 data points were evaluated by the nonparametric Mann–Whitney U test or the Wilcoxon signed rank test. Comparisons between multiple data points were evaluated by the nonparametric Kruskal–Wallis analysis of variance followed by a Dunn post-hoc test.
3. Results
3.1. Nucleotides increase the excitability of DRG neurons
The excitability of DRG neurons is well known to be enhanced in the presence of a variety of inflammatory mediators, such as bradykinin [26] and prostaglandins [18], which results in increased action potential firing frequencies. Moreover, changes in membrane excitability of DRG neurons correlate well with changes in nocifensive behavior [26]. Therefore, DRG neurons were investigated in perforated patch recordings (to maintain the integrity of cytosolic signaling components) in current clamp mode. To test for the excitability of the neurons, 2-second current injections were applied in increments of 100 pA as described, for instance, in investigations on the effects of prostaglandins [18].
A total of 64 cells had an average membrane potential of −65.1 ± 0.8 mV. In response to the injection of 100 pA for 2 seconds, none of the neurons fired any action potential; with increasing current injections, however, the number of elicited action potentials increased; with 500 pA injections, for instance, the neurons fired 1.4 ± 0.1 action potentials per 2 seconds (n = 64; Fig. 1A). Subsequently, the total number of action potentials fired in response to all 5 current injections will be evaluated. In the presence of 10 μM ATP, the neurons were depolarized by about 10 mV, and the number of action potentials elicited by the 5 consecutive periods of current injection increased to >40. After removal of ATP, the membrane potential and the number of elicited action potentials returned to the levels observed prior to the application of the nucleotide (Fig. 1A). Thus, ATP significantly and reversibly enhanced the excitability of DRG neurons.
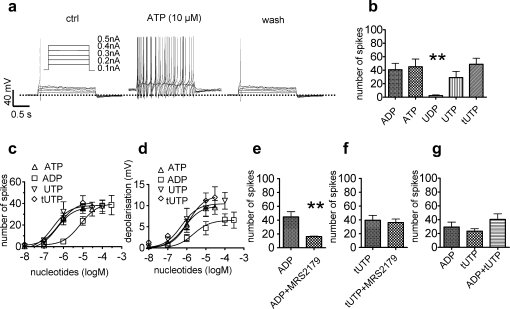
Fig. 1.
Increase in membrane excitability of rat sensory neurons caused by different nucleotides. Membrane potential was measured in perforated patch current clamp mode, and five 2-second current injections of increasing amplitudes (as shown in A) were applied. Nucleotides were present for 2 minutes before determining their effects. (A) Original traces obtained before (ctrl), during (ATP), and after (wash) the application of 10 μM ATP. (B) Total number of action potentials fired in response to the 5 current injections in the presence of ADP, ATP, UDP, UTP, and tUTP (all at 10 μM; n = 5 to 7). ∗∗Significant difference vs the effects of all other nucleotides at P < .01 (Kruskal–Wallis test). (C) Concentration response curves for the number of action potentials fired in the presence of nucleotides (n = 5 to 8). Calculated EC50 values were: 0.45 μM (ATP), 7.5 μM (ADP), 0.3 μM (UTP), and 0.63 μM (tUTP). (D) Concentration response curves for the membrane depolarization in the presence of nucleotides (n = 5 to 8). Calculated EC50 values were: 0.8 μM (ATP), 2.2 μM (ADP), 0.5 μM (UTP), 1.2 μM (tUTP). (E) Total number of action potentials fired in response to current injections in the presence of ADP (10 μM) either applied alone or together with MRS2179 (30 μM; n = 5). ∗∗Significant difference at P < .01 (Mann–Whitney U test). (F) Total number of action potentials fired in response to current injections in the presence of tUTP (10 μM) either applied alone or together with MRS2179 (30 μM; n = 5). (G) Total number of action potentials fired in response to current injections in the presence of ADP (10 μM), tUTP (10 μM), or ADP co-applied with tUTP (both at 10 μM; n = 5). ADP = adenosine diphosphate; ATP = adenosine triphosphate; tUTP = 2-thio-UTP; UDP = uridine diphosphate; UTP = uridine triphosphate.
For comparison, 10 μM of ADP, UDP, UTP, or thio-UTP were applied to DRG neurons instead of ATP; ADP, UTP, and 2-thio-UTP caused effects equivalent to those observed for ATP, whereas UDP hardly caused any change (Fig. 1B). The effects of the nucleotides on both the membrane potential and the number of elicited action potentials were concentration dependent, with ATP, UTP, and thio-UTP being more potent than ADP (Fig. 1C and D).
3.2. The effects of nucleotides are mediated by 2 separate receptors
None of the cloned P2Y receptors accept ADP and UTP as agonists acting at a similar range of concentrations [43]. Moreover, 2-thio-UTP is a P2Y2-preferring agonist [17], and ADP does not activate P2Y2 [43]. Therefore, we assumed that the actions of the 4 nucleotides, as described above, were mediated by at least 2 separate P2Y receptors. Because ADP is an agonist at P2Y1 receptors, and these receptors are expressed in a large number of different neurons [21], ADP was applied together with 30 μM of the specific P2Y1 antagonist MRS 2179 [43]. In fact, the number of action potentials triggered by current injections in the presence of 10 μM ADP was significantly reduced by MRS 2179 (Fig. 1E), whereas this antagonist had no effect on the number of action potentials triggered in the presence of 10 μM 2-thio-UTP (Fig. 1F). Thus, ADP and 2-thio-UTP acted via different receptors. Nevertheless, the excitatory effects mediated by these 2 receptors were not additive, as the combination of ADP and 2-thio-UTP raised the number of action potential to a similar extent as the separated application of these nucleotides (Fig. 1G).
3.3. Two separate receptors mediate an inhibition of KV7 channels by nucleotides
Knowing that ADP and 2-thio-UTP elicited excitatory effects via 2 separate P2Y receptors, ADP was used first to further investigate the underlying mechanisms. In current clamp experiments, ADP (10 μM) slowly reduced nondesensitizing outward currents at holding potentials more positive than −60 mV (not shown), as previously observed in sympathetic neurons [6]; this is indicative of an effect on KV7 channels. Therefore, the gating of these channels was assessed by applying hyperpolarizing voltage steps from a holding potential of −30 mV where KV7 channels are open to −55 mV in order to close the channels (Fig. 2A). ADP (10 μM) slowly reduced the holding currents at −30 mV as well as the slow deactivation of the current through KV7 channels (M current; IM) as observed at −55 mV (Fig. 2A). The reduction of outward currents at −30 mV by ADP reached a maximum after 30 seconds, and these currents returned to control values within 1 minute after removal of the nucleotide (Fig. 2B). This effect was also seen with 10 μM ATP and UTP, as well as with 10 μM of the selective P2Y2 agonist 2-thio-UTP (Fig. 2C). In concentration-response curves for the inhibition of IM, ATP turned out to be more potent than ADP, UTP, and 2-thio-UTP (Fig. 2E).
Fig. 2.
Inhibition of currents through KV7 channels by different nucleotides. Currents were determined in perforated patch voltage clamp mode; neurons were clamped to −30 mV and hyperpolarized to −55 mV for 1-second periods. (A) Original traces obtained before (control), during (ADP), and after (wash) the presence of 10 μM ADP. The dotted line indicates the zero current level. (B) Time course of the inhibition of the current measured at −30 mV by 10 μM ADP; current amplitudes were normalized to the first amplitude determined in the same neuron (n = 5). (C) Inhibition of currents through KV7 channels (IM; determined by the deactivation of currents at −55 mV) in the presence of ATP, ADP, UDP, UTP, and tUTP (all at 10 μM; n = 9). ∗∗Significant difference vs the effects of all other nucleotides at P < .01 (Kruskal–Wallis test). (D) Inhibition of currents through KV7 channels (IM) in capsaicin-insensitive neurons in the presence of ADP and tUTP (both at 10 μM; n = 5). (E) Concentration response curves for the inhibition of currents through KV7 channels by different nucleotides (n = 5 to 9). All values of inhibition were normalized to that evoked by 10 μM ADP in the very same cell. Calculated EC50 values were: 1.6 μM (ADP), 0.2 μM (ATP), 2.3 μM (UTP), and 1.1 μM (tUTP). (F) Inhibition of currents through KV7 channels (IM) by ADP (10 μM) either applied alone or together with MRS2179 (30 μM; n = 8). ∗∗Significant difference at P < .01 (Mann–Whitney U test). (G) Inhibition of currents through KV7 channels (IM) by tUTP (10 μM) either applied alone or together with MRS2179 (30 μM; n = 5). (H) Inhibition of currents through KV7 channels (IM) by ADP (10 μM), tUTP (10 μM), or ADP co-applied with tUTP (both at 10 μM; n = 5). ADP = adenosine diphosphate; ATP = adenosine triphosphate; tUTP = 2-thio-UTP; UDP = uridine diphosphate; UTP = uridine triphosphate.
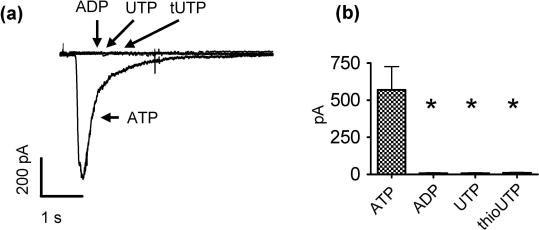
At least ATP might also induce inward currents in DRG neurons through an activation of P2X receptors. To reveal whether P2X might be involved in the slow induction of apparent inward currents as observed at −30 mV (Fig. 2B), the nucleotides were also applied to neurons clamped at −70 mV where KV7 channels are entirely inactivated [10]. Under these conditions, ATP, but not ADP, UTP, nor 2-thio-UTP (each at a concentration of 10 μM), caused rapidly activating inward currents that reached maximal amplitudes within ⩽100 ms and largely desensitized within the next 2 seconds (Supplementary Fig. 1A and B). Thus, the slow effects on KV7 channels described above can be easily discerned from actions mediated by P2X receptors. Moreover, ATP did reduce IM at concentrations (such as 0.1 μM; Fig. 2E) that were too low to activate P2X receptors (not shown).
To learn whether the inhibition of KV7 channels by nucleotides is specific for nociceptive neurons, ADP and 2-thio-UTP were also applied to capsaicin-insensitive neurons. There, the reduction of IM deactivation amplitudes was as pronounced as in capsaicin-sensitive neurons (compare Fig. 2C and D).
The inhibition of IM by ADP (Fig. 2F), but not that by 2-thio-UTP (Fig. 2G), was largely reduced by the P2Y1 antagonist MRS 2179. Thus, 2 separate P2Y receptors mediate an inhibition of KV7 channels in sensory neurons. Nevertheless, the effects of ADP and 2-thio-UTP on IM were not additive (Fig. 2H).
3.4. Phospholipase C and increase in intracellular Ca2+ mediate the inhibition of KV7 channels by nucleotides
To investigate the underlying signaling mechanisms, ADP and 2-thio-UTP were used as agonists for the 2 different receptors. All P2Y receptors with the exception of P2Y12,13,14 couple to Gq proteins and thereby to phospholipase C (PLC) [43]. When DRG neurons were treated with 1 μM of the PLC inhibitor U73122 [23] for 15 minutes, the inhibition by either ADP or 2-thio-UTP was significantly smaller than prior to this treatment. Furthermore, treatment of the neurons with 1 μM of the Ca2+-ATPase inhibitor thapsigargin [39] for 15 minutes or with 10 μM of the cell permeant Ca2+-chelator BAPTA-AM also reduced the inhibition of IM by the 2 nucleotides when compared with the pretreatment situation (Fig. 3B and C). Thus, inhibition of PLC, depletion of intracellular Ca2+ stores, and chelation of intracellular Ca2+ prevented the inhibition of KV7 channels by nucleotides.
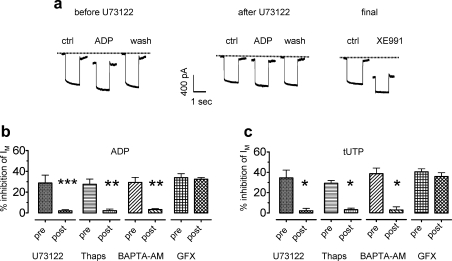
Fig. 3.
The inhibition of currents through KV7 channels by nucleotides involves phospholipase C and increases in intracellular Ca2+. Currents were determined in perforated patch voltage clamp mode; neurons were clamped to −30 mV and hyperpolarized to −55 mV for 1-second periods. (A) Original traces obtained before (control), during (ADP), and after (wash) the presence of 10 μM ADP; these measurements were performed before and at the end of a 15-minute application of U73122 (1 μM) followed by a final application of XE991 (10 μM). (B and C) Effects of U73122 (1 μM), thapsigargin (Thaps; 1 μM), BAPTA-AM (10 μM) and GF109203X (GFX; 1 μM) on the inhibition of currents through KV7 channels (IM) by either ADP (10 μM in B) or tUTP (10 μM in C); the inhibition of IM by the nucleotides was determined before (pre) and at the end (post) of 15-minute applications of U73122, thapsigargin, or GF109203X or of 30-minute applications of BAPTA-AM (n = 4 to 7). Significant differences were found between the results obtained before and after the application of U73122, thapsigargin, GF109203X, or BAPTA-AM at ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (Mann–Whitney U test). ADP = adenosine diphosphate; ATP = adenosine triphosphate; tUTP = 2-thio-UTP; UDP = uridine diphosphate; UTP = uridine triphosphate.
The PLC-mediated synthesis of inositol trisphosphate together with increases in intracellular Ca2+ can lead to the activation of Ca2+-dependent protein kinase C (PKC) isoforms that might be involved in the inhibition of KV7 channels. However, the specific PKC inhibitor GF 109203X [29] at a concentration of 1 μM did not alter the inhibition of KV7 channels by either ADP or 2-thio-UTP (Fig. 3B and C).
3.5. Two separate receptors mediate a sensitization of TRPV1 channels by nucleotides
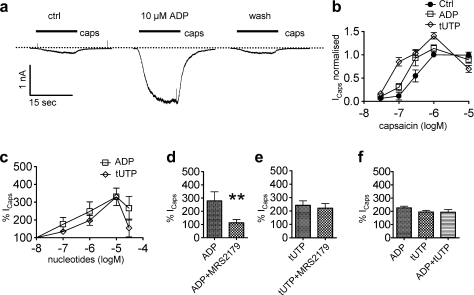
The sensitization of TRPV1 channels by inflammatory mediators is believed to contribute to the development of chronic pain [22]; nucleotides have been reported to cause sensitization of TRPV1 [40,31]. Hence, ADP and 2-thio-UTP as agonists for the 2 separate functional P2Y receptors in DRG neurons as identified above were used to reveal whether these 2 P2Y receptors might also be linked to TRPV1 channels. Indeed, currents evoked by 0.3 μM capsaicin were markedly enhanced in the presence of 10 μM ADP (Fig. 4A). This effect of ADP developed slowly and reached its maximum after 94.7 ± 19.5 seconds (n = 4). In concentration-response curves for capsaicin-induced currents, this enhancement was revealed to be due to a leftward shift in the presence of the nucleotide; 2-thio-UTP caused a leftward shift similar to that observed for ADP (Fig. 4B). ADP and 2-thio-UTP were about equipotent in potentiating capsaicin-evoked currents with half maximal effects at around 1 μM (Fig. 4C). The potentiation by ADP was attenuated in the presence of the P2Y1 antagonist MRS 2179 (Fig. 4D), whereas the effect of 2-thio-UTP remained unaltered (Fig. 4E). Thus, the 2 separate nucleotide receptors mediating the inhibition of KV7 channels also mediated a sensitization of TRPV1 channels. Moreover, the effects of ADP and 2-thio-UTP were not additive once again (Fig. 4F).
Fig. 4.
Potentiation of currents through TRPV1 channels by different nucleotides. Currents were determined in perforated patch voltage clamp mode; neurons were clamped to −70 mV, and capsaicin was applied for 15-second periods in the absence or presence of the indicated concentrations of nucleotides. (A) Original traces of currents evoked by 0.3 μM capsaicin (caps) before (ctrl), during (ADP), and after (wash) the presence of 10 μM ADP; capsaicin was applied as indicated by the bars. (B) Concentration response curves for the peak amplitudes of inward currents induced by the indicated concentrations of capsaicin in the absence (ctrl) or presence of 10 μM of ADP or tUTP (n = 5 to 6). All values were normalized to the current evoked by 1 μM capsaicin in the absence of nucleotides in the very same cell (Icaps normalized). (C) Concentration response curves for the potentiation of currents induced by capsaicin (0.3 μM) in the presence of the indicated concentrations of nucleotides (n = 4 to 6). Amplitudes in the presence of nucleotides were expressed as percentage of the amplitudes recorded in their absence (% Icaps). (D) Potentiation of capsaicin-induced currents (Icaps) by ADP (10 μM) either applied alone or together with MRS 2179 (30 μM; n = 6). ∗∗Significant difference at P < .01 (Mann–Whitney U test). (E) Potentiation of capsaicin-induced currents (Icaps) by tUTP (10 μM) either applied alone or together with MRS 2179 (30 μM; n = 5). (F) Potentiation of capsaicin-induced currents (Icaps) by ADP (10 μM), tUTP (10 μM), or ADP co-applied with tUTP (n = 5). ADP = adenosine diphosphate; tUTP = 2-thio-UTP.
3.6. Phospholipase C, increases in intracellular Ca2+, and protein kinase C mediate the sensitization of TRPV1 channels by nucleotides
TRPV1 channels are believed to be tonically inhibited by phosphatidylinositol 4,5-bisphosphate (PIP2), and a depletion of PIP2 in the membrane thus leads to sensitization of the receptors. Alternatively, the sensitization can occur via an activation of PKC [34]. To analyze the signaling cascades that link the 2 P2Y receptors to TRPV1 channels, the manipulations used to investigate the inhibition of KV7 channels were used again. As observed in those experiments, inhibition of PLC by 1 μM U73122 prevented the facilitation of capsaicin-evoked currents by either ADP or 2-thio-UTP (Fig. 5A–C).
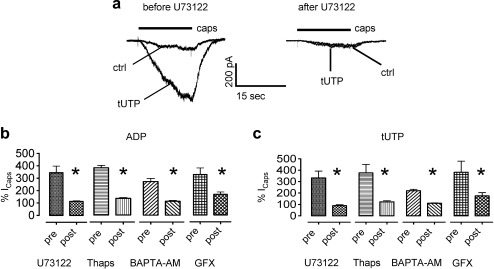
Fig. 5.
The potentiation of currents through TRPV1 channels by nucleotides involves phospholipase C, increases in intracellular Ca2+, and protein kinase C. Currents were determined in perforated patch voltage clamp mode; neurons were clamped to −70 mV. (A) Original traces obtained with 0.3 μM capsaicin (caps) in the absence (ctrl) or presence of tUTP; these recordings were performed before and at the end of a 15-minute application of U73122 (1 μM). (B and C) Effects of U73122 (1 μM), thapsigargin (1 μM), BAPTA-AM (10 μM), and GF109203X (1 μM) on the potentiation of currents through TRPV1 channels (Icaps) by either ADP (10 μM in B) or tUTP (10 μM in C); currents were determined before (pre) and at the end (post) of 15-minute applications of U73122, thapsigargin (Thaps), or GF109203X (GFX) or of 30-minute applications of BAPTA-AM (n = 4 to 7); ∗Significant differences between the results obtained before and after the application of U73122, thapsigargin, BAPTA-AM, or GF 109203X at P < .05 (Mann–Whitney U test). ADP = adenosine diphosphate; tUTP = 2-thio-UTP.
The depletion of intracellular Ca2+ stores by thapsigargin and the chelation of intracellular Ca2+ by BAPTA-AM also abolished the sensitization of TRPV1 channels by the nucleotides. Likewise, when PKC was inhibited by GF 109203X, the facilitation of capsaicin-evoked currents by either ADP or 2-thio-UTP was largely reduced (Fig. 5B and C).
3.7. The increase in the excitability of DRG neurons involves KV7 and TRPV1 channels
To test whether the increase in the excitability of DRG neurons observed in the presence of nucleotides is brought about by the inhibition of KV7 channels and/or by the sensitization of TRPV1 channels, the nucleotides were applied together with either flupirtine, an activator of KV7 channels [10], XE991, an inhibitor of KV7 channels [10], and/or iodoresiniferatoxin, an antagonist at TRPV1 channels [2].
Flupirtine 100 μM hyperpolarized DRG neurons by about 5 mV (Fig. 6C), but failed to significantly reduce the small number of action potentials fired in response to the injection of depolarizing currents with increasing amplitudes (0.1 to 0.5 nA) in the absence of nucleotides (Fig. 6A and B). However, flupirtine entirely prevented action potential firing in the presence of either 100 μM ADP (Fig. 6A and B) or 10 μM 2-thio-UTP (Fig. 6B). In addition, flupirtine abolished the depolarizations caused by either of these 2 nucleotides (Fig. 6C).
Fig. 6.
Role of KV7 channels in the increase of membrane excitability caused by nucleotides. Membrane potential was measured in perforated patch current clamp mode, and 2-second current injections of increasing amplitudes (as shown in A) were applied. Nucleotides and other drugs were applied for 2 minutes before determining their effects. (A) Original traces obtained in the absence or presence of ADP (100 μM) applied either alone or together with flupirtine (both at 100 μM). (B) Total number of action potentials fired in response to current injections in the absence (ctrl) or presence of flupirtine (100 μM), ADP (100 μM), tUTP (10 μM), or the nucleotides together with 100 μM flupirtine (Flup; n = 6). ∗∗Significant difference vs the effects of ADP or tUTP applied alone at P < .01 (Mann–Whitney U test). (C) Changes in membrane potential in the presence of flupirtine (100 μM), ADP (100 μM), tUTP (10 μM), or the nucleotides together with 100 μM flupirtine (Flup; n = 6). ∗Significant difference vs the effects of ADP or tUTP applied alone at P < .05 (Mann–Whitney U test). (D) Total number of action potentials fired in response to current injections in the absence (ctrl) or presence of XE991 (10 μM), ADP (100 μM), tUTP (10 μM), or the nucleotides together with XE991 (n = 6). ∗Significant difference vs the effect of ADP or tUTP applied alone at P < .05 (Mann–Whitney U test). (E) Changes in membrane potential in the presence of XE991 (10 μM), ADP (100 μM), tUTP (10 μM), or the nucleotides together with XE991 (n = 6). ∗Significant difference vs the effects of ADP or tUTP applied alone at P < .05 (Mann–Whitney U test). ADP = adenosine diphosphate; tUTP = 2-thio-UTP.
XE991 at 10 μM, a concentration that fully blocks currents through KV7 channels in DRG neurons [26], enhanced the action potential firing and caused depolarization of DRG neurons to about the same extent as ADP or 2-thio-UTP (Fig. 6D and E). Nevertheless, in the presence of 10 μM XE991, the 2 nucleotides were still able to further depolarize the neurons and to facilitate action potential firing (Fig. 6D and E).
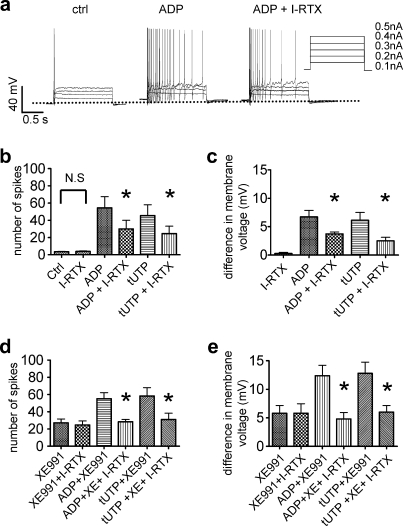
Iodoresiniferatoxin (0.3 μM), in contrast, did not alter action potential firing in the absence of nucleotides, but significantly reduced the number of action potentials fired in response to current injections in the presence of either 100 μM ADP (Fig. 7A and B) or 10 μM 2-thio-UTP (Fig. 7B). Moreover, iodoresiniferatoxin per se did not alter the membrane potential, but significantly reduced the depolarizing effect of either ADP or 2-thio-UTP (Fig. 7C). Finally, when applied in the continuing presence of XE991 (10 μM), iodoresiniferatoxin prevented any depolarization and any enhancement of action potential firing by either ADP or 2-thio-UTP (Fig. 7D and E). Thus, the blockade of KV7 channels and of TRPV1 channels is sufficient to abolish the excitation of DRG neurons by nucleotides.
Fig. 7.
Role of TRPV1 channels in the increase of membrane excitability caused by nucleotides. Membrane potential was measured in perforated patch current clamp mode and 2-second current injections of increasing amplitudes (as shown in A) were applied. Nucleotides and other drugs were applied for 2 minutes before determining their effects. (A) Original traces obtained in the absence (ctrl) or presence of ADP (100 μM) applied either alone or together with iodoresiniferatoxin (I-RTX; 0.3 μM). (B) Total number of action potentials fired in response to current injections in the absence (ctrl) or presence of iodoresiniferatoxin (I-RTX; 0.3 μM), ADP (100 μM), tUTP (10 μM), or the nucleotides together with iodoresiniferatoxin (I-RTX). ∗Significant difference vs the effect of 100 μM ADP or 10 μM tUTP applied alone at P < .05 (Mann–Whitney U test; n = 5 to 7). (C) Changes in membrane potential in the presence of iodoresiniferatoxin (0.3 μM), ADP (100 μM), tUTP (10 μM), or the nucleotides together with iodoresiniferatoxin (I-RTX). ∗Significant difference vs the effect of 100 μM ADP or 10 μM tUTP applied alone at P < .05 (Mann–Whitney U test; n = 5 to 7). (D) Total number of action potentials fired in response to current injections in the presence of 10 μM XE991 (XE), 0.3 μM iodoresiniferatoxin (I-RTX)), 100 μM ADP, or 10 μM tUTP applied either alone or in combination. ∗Significant difference vs the effect of ADP or tUTP when co-applied with XE991 at P < .05 (Mann–Whitney U test; n = 6 to 8). (E) Changes in membrane potential in the presence of 10 μM XE991 (XE), 0.3 μM iodoresiniferatoxin (I-RTX)), 100 μM ADP, or 10 μM tUTP applied either alone or in combination. ∗Significant difference vs the effect of ADP or tUTP when co-applied with XE991 at P < .05 (Mann–Whitney U test; n = 6 to 8). ADP = adenosine diphosphate; tUTP = 2-thio-UTP.
3.8. P2Y12 receptors mediate an inhibition of voltage-gated Ca2+ channels in DRG neurons
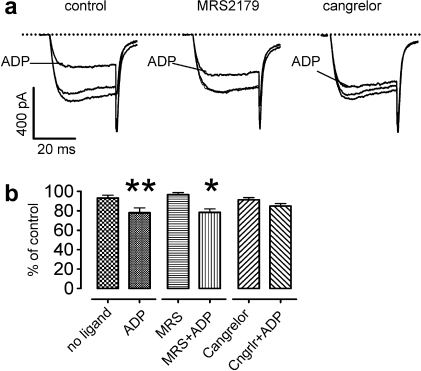
Nucleotides have been suggested to reduce neurotransmission from DRG neurons onto dorsal horn neurons by an inhibition of voltage-activated Ca2+ channels, but it remained controversial whether this effect was mediated by P2Y1 [20] or P2Y12 [28] receptors. To clarify this issue, voltage-activated Ca2+ currents were recorded in the absence and presence of ADP. The nucleotide caused a significant decrease in Ca2+ current amplitudes (Fig. 8A and B), and this inhibitory effect was abolished by the P2Y12 receptor antagonist cangrelor (1 μM), but not by the P2Y1 antagonist MRS2179 (30 μM; Fig. 8A and B).
Fig. 8.
Inhibition of voltage-activated Ca2+ currents in sensory neurons by ADP. Currents were determined in whole-cell voltage clamp mode; neurons were clamped to −80 mV and depolarized to −10 mV for 30 ms at a frequency of 4 min−1. (A) Original traces obtained before, during (ADP), and after the application of 10 μM ADP in either the absence (control) or presence of MRS2179 (30 μM) or cangrelor (1 μM). (B) Effects of ADP on peak amplitudes of Ca2+ currents in either the absence (no ligand) or presence of MRS2179 (30 μM) or cangrelor (1 μM; n = 8). ∗, ∗∗Significant difference vs the currents determined in the absence of ADP at P < .05 and P < .01, respectively (Wilcoxon signed rank test). ADP = adenosine diphosphate; tUTP = 2-thio-UTP.
4. Discussion
Although it is firmly established that ionotropic ATP receptors containing P2X3 subunits do contribute to the well-known algetic effects of this nucleotide, the role of metabotropic P2Y receptors has remained at least controversial, if not unclear [44]. Here, we demonstrate that activation of both P2Y1 and P2Y2 receptors leads to a marked increase in the excitability of DRG neurons; such an increase in membrane excitability in DRG neurons is a cellular correlate of enhanced nociceptive behavior [11,26]. Furthermore, the results reveal that both an inhibition of KV7 channels and a potentiation of TRPV1 channels are involved in the excitation of DRG neurons via these 2 nucleotide receptors. The same signaling mechanisms are involved in the excitatory actions of bradykinin in DRG neurons [11,26,46].
ADP, ATP, UTP, and 2-thio-UTP enhanced action potential firing in response to current injections with the following order of agonist potency: ATP = UTP = 2-thio-UTP > ADP. ADP is an agonist at P2Y1,12,13 receptors [43], and the effects of ADP were largely attenuated by the selective P2Y1 receptor antagonist MRS 2179 [14], which demonstrates the participation of this latter receptor. The other nucleotides used are either poor or not at all agonists at P2Y1 receptors [43]. Hence, another nucleotide receptor must have contributed, and this was P2Y2 for the following reasons: (1) UDP did not mimic the effects of UTP; thus, a role of P2Y6 can be excluded [43]. (2) ATP and UTP were about equipotent as observed for rat P2Y2 and P2Y4 receptors [43]. (3) UTP and 2-thio-UTP were equipotent; this only holds true for P2Y2, but not for P2Y4 receptors [17]. Thus, the nucleotides enhanced the excitability of DRG neurons via both P2Y1 and P2Y2 receptors.
Recombinant P2Y1,2,4,6 receptors mediate an inhibition of KV7 channels [7], and DRG neurons express KV7.2, KV7.3, and KV7.5 [10]. Most recently, acute nociception induced by bradykinin was shown to involve inhibition of KV7 channels and activation of a Ca2+-dependent chloride current [26]. Here, all the nucleotides mentioned above reduced currents through KV7 channels, and the action of ADP, but not that of 2-thio-UTP, was attenuated by the P2Y1 antagonist MRS 2179. Thus, the activation of both P2Y1 and P2Y2 receptors contributed to the closure of KV7 channels. However, we found no evidence for an induction of chloride currents by any of the nucleotides tested (not shown). The inhibition of KV7 channels by both P2Y1 and P2Y2 has also been observed with receptors heterologously expressed in sympathetic neurons [9].
TRPV1 channels mediate acute and chronic pain [22], and ADP as well as 2-thio-UTP enhanced currents through these receptors; this effect of ADP, but not that of 2-thio-UTP, was again antagonized by MRS 2179. Hence, in contrast to what has been indicated by previous experiments, it is not P2Y1 [40] or P2Y2 [31,27], but both receptors that contribute to the sensitization of TRPV1 channels by nucleotides. The most likely reason for the conflicting interpretations of previous results is the fact that a full range of selective agonists and antagonists for these 2 receptors as employed here had not been used. Alternatively, the reason might be species differences between rats and mice.
Both P2Y1 and P2Y2 receptors are linked to Gq proteins and thereby to PLC [43]. In accordance with this, the PLC inhibitor U73122 [23] prevented the effects of both ADP and 2-thio-UTP, on KV7 channels as well as on TRPV1 channels. The actions of these nucleotides on KV7 and TRPV1 channels were also abolished when Ca2+-ATPases had been inhibited by thapsigargin [39] or when intracellular Ca2+ had been chelated by BAPTA-AM; thus, increases in intracellular Ca2+ were involved in both of these effects. Downstream of Ca2+, however, the signaling cascades linking the 2 P2Y receptors to either KV7 channels or TRPV1 channels differed from each other: the sensitization of TRPV1 channels was mediated by an activation of protein kinase C, as indicated by the inhibitory effect of GF 109203X, whereas the inhibition of KV7 channels was not altered by this PKC inhibitor. This bifurcated signaling pathway was shared by both receptors, P2Y1 and P2Y2. As a consequence, the simultaneous activation of both receptors by ADP and 2-thio-UTP did not achieve larger effects than the activation of only one of these receptors.
These results lead to the question of how P2Y receptor activation could modulate KV7 channels, on one hand, and TRPV1 channels, on the other hand. The inhibition of neuronal KV7 channels by G-protein coupled receptors involves either depletion of membrane PIP2, phosphorylation via PKC, or binding of intracellular Ca2+ to channel-bound calmodulin. In sympathetic neurons, the first 2 mechanisms were shown to mediate KV7 channel inhibition via M1 muscarinic receptors [16]; the latter mechanism has been found to mediate the inhibition via bradykinin and P2Y receptors [8,49]. The present results support this latter idea, as manipulations that prevented increases in intracellular Ca2+ abolished the effects of nucleotides on KV7 channels, but inhibition of PKC failed to alter the effect of P2Y receptor activation.
Obviously, the facilitation of currents through TRPV1 channels via P2Y receptors cannot involve Ca2+/calmodulin, as this signaling entity, if anything, mediates the desensitization of TRPV1, but not a sensitization [34]. However, PLC-mediated depletion of membrane PIP2 [15] as well as activation of Ca2+-dependent PKC [42] are both known to potentiate currents evoked by low, but not those evoked by high, capsaicin concentrations. In accordance with both mechanisms, the nucleotides caused a leftward shift in the concentration-response curves of capsaicin-evoked currents. However, the fact that prevention of increases in intracellular Ca2+ as well as inhibition of PKC abolished the sensitization of TRPV1 channels by ADP or 2-thio-UTP demonstrate a role of Ca2+-dependent PKC.
Because this latter result proves that activation of either P2Y1 or P2Y2 leads to the activation of Ca2+-dependent PKC, which then phosphorylates TRPV1, one has to ask why activated PKC did not contribute to the inhibition of KV7 channels as previously reported for the signaling cascade linking M1 muscarinic receptors to this ion channel family [16]. The most likely explanation is offered by the finding that Ca2+/calmodulin disrupts the association of KV7 channels and AKAP79/150, which is a prerequisite for the phosphorylation of KV7 channels by PKC [3].
The increase in action potential firing in the presence of ADP or 2-thio-UTP was completely abolished in the presence of the nonopioid analgesic flupirtine, which is known to activate KV7 channels [45]. This appears to indicate that the entire increase in excitability was mediated by the inhibition of the K+ channels. However, the hyperpolarization of the neurons caused by flupirtine might also counteract the excitatory effects of the nucleotides independently of the interaction at KV7 channels. Hence, this set of data does not unequivocally demonstrate that KV7 channels were involved in the effects of the nucleotides on excitability and membrane potential. However, blockage of KV7 channels by XE991 depolarized DRG neurons and enhanced their excitability to a similar extent as the activation of the 2 nucleotide receptors; this verifies that the closure of KV7 channels is sufficient to depolarize DRG neurons and to enhance their excitability.
Nevertheless, the excitatory actions of ADP or 2-thio-UTP were additive to that of XE991, which hints to a role of an additional mechanism. In line with this conclusion, the TRPV1 antagonist iodoresiniferatoxin also attenuated the nucleotide-induced increase in membrane excitability. Moreover, the effects of the nucleotides on action potential firing and membrane potential in the presence of XE991 were entirely abolished by iodoresiniferatoxin. Hence, the inhibition of KV7 channels as well as the sensitization of TRPV1 channels both contributed to the increase in excitability triggered by the activation of either P2Y1 or P2Y2 receptors.
In contrast to the excitatory effects shown above, ADP has been reported to elicit analgesic rather than proalgesic effects, and an inhibition of transmembrane Ca2+ entry in DRG neurons was implied as underlying mechanism [20]. The inhibition of VACCs was first suggested to be mediated by P2Y1 [20], whereas a later report demonstrated a role of P2Y12 and/or P2Y13 receptors [28]. In accordance with that latter result, we found that the inhibition of VACCs by ADP was attenuated by the P2Y12 antagonist cangrelor, but not by MRS2179. This confirms that P2Y1 contributes to the sensation of pain in concert with P2Y2, whereas analgesic effects are mediated by P2Y receptors coupled to inhibitory G proteins [28].
Taken together, our results reveal that ATP may cause pain not only by the activation of ionotropic P2X receptors, but also via P2Y2 receptors. In addition, the ATP degradation product ADP also contributes to the sensation of pain as it activates P2Y1 receptors. These 2 metabotropic nucleotide receptors share a bifurcated signaling cascade to enhance the excitability of DRG neurons through a twofold mechanism: an inhibition of KV7 channels through Ca2+/calmodulin and a sensitization of TRPV1 channels via Ca2+-dependent PKC. Because this bifurcated mechanism is also associated with bradykinin B2 receptors, it may well be a general signal cascade employed by painful mediators [11].
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
This study was supported grants from the Austrian Science Fund (FWF), by the doctoral programme CCHD (supported by the FWF, the Medical University of Vienna, and the Research Center for Molecular Medicine, CeMM), and by the Higher Education Commission of Pakistan.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.pain.2011.04.016.
Appendix A. Supplementary data
Supplementary Fig. 1.
Induction of currents through P2X receptors of rat sensory neurons by nucleotides. Currents were determined in perforated patch voltage clamp mode; neurons were clamped to −70 mV, and nucleotides were applied for 2 seconds. (A) Original traces of currents evoked by ADP, ATP, UTP, and tUTP at concentrations of 10 μM. (B) Comparison of inward currents induced by the different nucleotides (all at 10 μM; n = 5). ∗Significant difference vs the effects of 10 μM ATP at P < .05 (Kruskal–Wallis test). ADP = adenosine diphosphate; tUTP = 2-thio-UTP.
References
- 1.Abbracchio M.P., Burnstock G., Boeynaems J.M., Barnard E.A., Boyer J.L., Kennedy C., Knight G.E., Fumagalli M., Gachet C., Jacobson K.A., Weisman G.A. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appendino G., Daddario N., Minassi A., Moriello A.S., De Petrocellis L., Di Marzo V. The taming of capsaicin. Reversal of the vanilloid activity of N-acylvanillamines by aromatic iodination. J Med Chem. 2005;48:4663–4669. doi: 10.1021/jm050139q. [DOI] [PubMed] [Google Scholar]
- 3.Bal M., Zhang J., Hernandez C.C., Zaika O., Shapiro M.S. Ca2+/calmodulin disrupts AKAP79/150 interactions with KCNQ (M-Type) K+ channels. J Neurosci. 2010;30:2311–2323. doi: 10.1523/JNEUROSCI.5175-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehm S., Betz H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J Neurosci. 1997;17:4066–4075. doi: 10.1523/JNEUROSCI.17-11-04066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm S. Selective inhibition of M-type potassium channels in rat sympathetic neurons by uridine nucleotide preferring receptors. Br J Pharmacol. 1998;124:1261–1269. doi: 10.1038/sj.bjp.0701956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehm S. P2Ys go neuronal: modulation of Ca2+ and K+ channels by recombinant receptors. Br J Pharmacol. 2003;138:1–3. doi: 10.1038/sj.bjp.0705044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bofill-Cardona E., Vartian N., Nanoff C., Freissmuth M., Boehm S. Two different signaling mechanisms involved in the excitation of rat sympathetic neurons by uridine nucleotides. Mol Pharmacol. 2000;57:1165–1172. [PubMed] [Google Scholar]
- 9.Brown D.A., Filippov A.K., Barnard E.A. Inhibition of potassium and calcium currents in neurones by molecularly-defined P2Y receptors. J Auton Nerv Syst. 2000;81:31–36. doi: 10.1016/s0165-1838(00)00150-8. [DOI] [PubMed] [Google Scholar]
- 10.Brown D.A., Passmore G.M. Neural KCNQ (KV7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D.A., Passmore G.M. Some new insights into the molecular mechanisms of pain perception. J Clin Invest. 2010;120:1380–1383. doi: 10.1172/JCI42143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 14.Chhatriwala M., Ravi R.G., Patel R.I., Boyer J.L., Jacobson K.A., Harden T.K. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang H.H., Prescott E.D., Kong H., Shields S., Jordt S.E., Basbaum A.I., Chao M.V., Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 16.Delmas P., Brown D.A. Pathways modulating neural KCNQ/M (KV7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 17.El-Tayeb A., Qi A., Muller C.E. Synthesis and structure–activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem. 2006;49:7076–7087. doi: 10.1021/jm060848j. [DOI] [PubMed] [Google Scholar]
- 18.England S., Bevan S., Docherty R.J. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippov A.K., Fernandez-Fernandez J.M., Marsh S.J., Simon J., Barnard E.A., Brown D.A. Activation and inhibition of neuronal G protein-gated inwardly rectifying K(+) channels by P2Y nucleotide receptors. Mol Pharmacol. 2004;66:468–477. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 20.Gerevich Z., Borvendeg S.J., Schroder W., Franke H., Wirkner K., Norenberg W., Furst S., Gillen C., Illes P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussl S., Boehm S. Functions of neuronal P2Y receptors. Pflugers Arch. 2006;452:538–551. doi: 10.1007/s00424-006-0063-8. [DOI] [PubMed] [Google Scholar]
- 22.Immke D.C., Gavva N.R. The TRPV1 receptor and nociception. Semin Cell Dev Biol. 2006;17:582–591. doi: 10.1016/j.semcdb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Jin W., Lo T.M., Loh H.H., Thayer S.A. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994;642:237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K., Fukuoka T., Yamanaka H., Dai Y., Obata K., Tokunaga A., Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- 25.Lazarowski E.R., Boucher R.C., Harden T.K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 26.Liu B., Linley J.E., Du X., Zhang X., Ooi L., Zhang H., Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malin S.A., Davis B.M., Koerber H.R., Reynolds I.J., Albers K.M., Molliver D.C. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138:484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malin S.A., Molliver D.C. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21. doi: 10.1186/1744-8069-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martiny-Baron G., Kazanietz M.G., Mischak H., Blumberg P.M., Kochs G., Hug H., Marme D., Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 30.Molliver D.C., Cook S.P., Carlsten J.A., Wright D.E., McCleskey E.W. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci. 2002;16:1850–1860. doi: 10.1046/j.1460-9568.2002.02253.x. [DOI] [PubMed] [Google Scholar]
- 31.Moriyama T., Iida T., Kobayashi K., Higashi T., Fukuoka T., Tsumura H., Leon C., Suzuki N., Inoue K., Gachet C., Noguchi K., Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanoff C., Boehm S., Hohenegger M., Schutz W., Freissmuth M. 2′,3′-Dialdehyde GTP as an irreversible G protein antagonist. Disruption and reconstitution of G protein-mediated signal transduction in cells and cell membranes. J Biol Chem. 1994;269:31999–32007. [PubMed] [Google Scholar]
- 33.Park S.Y., Kim H.I., Shin Y.K., Lee C.S., Park M., Song J.H. Modulation of sodium currents in rat sensory neurons by nucleotides. Brain Res. 2004;1006:168–176. doi: 10.1016/j.brainres.2004.01.061. [DOI] [PubMed] [Google Scholar]
- 34.Rohacs T., Thyagarajan B., Lukacs V. Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol. 2008;37:153–163. doi: 10.1007/s12035-008-8027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan H.Z., Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- 36.Sanada M., Yasuda H., Omatsu-Kanbe M., Sango K., Isono T., Matsuura H., Kikkawa R. Increase in intracellular Ca(2+) and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;111:413–422. doi: 10.1016/s0306-4522(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 37.Schicker K.W., Chandaka G.K., Geier P., Kubista H., Boehm S. P2Y1 receptors mediate an activation of neuronal calcium-dependent K+ channels. J Physiol. 2010;588:3713–3725. doi: 10.1113/jphysiol.2010.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stucky C.L., Medler K.A., Molliver D.C. The P2Y agonist UTP activates cutaneous afferent fibers. Pain. 2004;109:36–44. doi: 10.1016/j.pain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990;29:8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- 40.Tominaga M., Wada M., Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuzuki K., Kondo E., Fukuoka T., Yi D., Tsujino H., Sakagami M., Noguchi K. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91:351–360. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- 42.Vellani V., Mapplebeck S., Moriondo A., Davis J.B., McNaughton P.A. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Wirkner K., Sperlagh B., Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol. 2007;36:165–183. doi: 10.1007/s12035-007-0033-y. [DOI] [PubMed] [Google Scholar]
- 45.Wladyka C.L., Kunze D.L. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol. 2006;575:175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Z.Z., Pan H.L. Role of TRPV1 and intracellular Ca2+ in excitation of cardiac sensory neurons by bradykinin. Am J Physiol. 2007;293:R276–R283. doi: 10.1152/ajpregu.00094.2007. [DOI] [PubMed] [Google Scholar]
- 47.Xiang Z., Bo X., Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett. 1998;256:105–108. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- 48.Xu G.Y., Huang L.Y. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaika O., Tolstykh G.P., Jaffe D.B., Shapiro M.S. Inositol triphosphate-mediated Ca2+ signals direct purinergic P2Y receptor regulation of neuronal ion channels. J Neurosci. 2007;27:8914–8926. doi: 10.1523/JNEUROSCI.1739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]