Highlights
► Pre-stimulus alpha phase is not randomly distributed in time across trials. ► Ongoing oscillatory activity is not random noise. ► Pre-stimulus oscillatory activity contributes to the appearance of event-related potentials.
Abbreviations: EEG, electroencephalogram; ERPR, event-related phase reorganization; ERP, event-related potential; PLI, phase-locking index; PPC, point of phase concentration; EOI, electrode of interest
Keywords: ERP, P1, Alpha, Phase
Abstract
Since years there is a hotly discussed dispute whether event-related potentials are either generated by an evoked component or by resetting of ongoing phase. We argue that phase-reset must not be proven in order to accept the general involvement of phase in ERP-generation as it is only one of several possible mechanisms influencing or generating certain ERP-components. Supporting data are presented showing that positive peaks of ongoing pre-stimulus alpha activity are not randomly distributed in time across trials. Most importantly, we found that a certain kind of pre-stimulus phase concentration that represents a continuous development of an alpha wave up to the time window where the P1 is generated is associated with an enlarged event-related component. We conclude that ongoing oscillations cannot be considered random background noise (even before stimulus onset) and that there are probably more phase-mechanisms that can contribute to the ERP-generation.
1. Introduction
Event-related potentials (ERPs) are one of the most frequently used methods in EEG research and they have been proven useful in psychological, clinical and physiological research. It is generally believed that different ERP-components (such as the P1, N1 or P3) reflect different aspects of information processing in the brain [19] ranging from ‘sensory’ to ‘cognitive’ components. One important aspect thereby is that they represent comparatively constant brain responses related to the processing of external stimuli and/or events [31].
The ERP is usually understood as a signal that appears superimposed and without interaction with the ongoing (‘background’) EEG, which is considered random noise. The most common method to measure the ERP is to average the EEG response for each time point over a number of single trials. Thereby, the traditional approach – which may be termed ‘evoked model’ – assumes that the event-related EEG response consists solely of the ERP, understood as a constant evoked response with constant latency and polarity. As a consequence, the ERP is considered to be independent of the ongoing EEG, which also means that it does not interact with the EEG.
In contrast to the evoked model, several researchers have suggested that the ERP may be generated at least in part by a reset of the phase of ongoing oscillations [4–6,23,28,36]. More recently, the event-related phase reorganization (ERPR) model was proposed [22], which states that the ERP is the manifestation of an active reorganizing of ongoing oscillations. According to this theory the highly debated phase-reset is only one – highly specific – mechanism that may underlie the generation of ERPs. There are other oscillatory mechanisms that may contribute to (or influence) the generation of the ERP, such as (phase-aligned) evoked oscillations (an oscillation is transiently elicited by a stimulus and/or event) or pre-stimulus phase concentration (the timing of ongoing oscillations is influenced by attention in a way that e.g., the excitatory phase can coincide with the processing of the stimulus/event). This extension is of crucial importance because several studies failed to prove or to reject the phase-reset model, mainly because of methodological problems (for reviews see e.g. [35,45]. As a consequence, the general idea that the ongoing phase influences (or even generates) the ERP was rejected. The ERPR-model goes beyond phase-reset in the sense that other mechanisms may be even more important for the generation of the ERP. Although there is much evidence that the phase of ongoing oscillations is crucial for cognitive processing there is, to our knowledge, no unambiguous evidence showing that oscillatory phase influences the generation of ERP-components in a specific way.
Pre-stimulus phase concentration (or alignment) could be an alternative to phase-reset that allows for a proper timing of oscillatory phase which reflects continuous changes between high vs. low cortical excitability [15]. It must be mentioned that theoretically, all oscillations reflect the ongoing tendency between maximal excitation and inhibition but with respect to the visual ‘sensory’ evoked potentials alpha is assumed to play a major role [21]. This is not a new idea as alpha has always been related to early visual processing since the early days of EEG research but recent evidence suggests a clear dependency between ongoing alpha and P1-appearance: (a) alpha phase-locking is strongest during the P1-time window [24], (b) there are functional similarities between induced alpha-power and P1-amplitude [14], (c) the magnitude of pre-stimulus alpha-power influences P1-amplitude [7,33], (d) the topographic distribution of P1-latencies behave like a travelling alpha wave [20], (e) the P1 is mainly generated by phase-aligned, evoked alpha-oscillations [16], (f) there is a negative correlation between resting individual-alpha-frequency (IAF) and P1 latency [23], (g) the appearance of early visual potentials is dominated by evoked alpha-oscillations [18].
According to the general idea that evoked components are not independent of organized ongoing oscillatory activity it can be assumed that the polarity of the evoked component should match in time and polarity with the respective ongoing phase in order to produce an enlarged ERP. Such a mechanism would not depend on instantaneous phase reset after stimulus presentation but rather it would operate on a proper timing of ongoing phase, most likely based on temporal expectancy. The only presumptions are that the oscillation is functionally (processing) relevant and that the occurrence of the stimulus is predictable in time. In the present study we try to show that (i) a systematic phase concentration can be observed in a pre-stimulus time-period and that (ii) the exact timing of phase concentration has an influence on the ERP-amplitude in a way that pre-stimulus (positive) peak-concentration compatibly timed according to an alignment to the P1 results in an enhanced P1-amplitude.
A proof of pre-stimulus phase concentration allows us to document an influence of ongoing oscillations on the generation of ERPs by avoiding many of the technical problems that are associated with an investigation of the phase reset model (particular because of filter-problems applied to a post-stimulus period). If these notions hold true, pre-stimulus phase-activity cannot be seen as random noise and evoked activity would be influenced by pre-stimulus phase alignment in a way that optimal alignment increases P1-amplitude and leads overall to a strong alpha-characteristic of the early ERP-components.
2. Experimental procedures
2.1. Subjects
After written informed consent, a sample of 16 subjects participated in the present experiment. The sample consisted of 11 females and 5 males (mean age 21.13; range 18–26 years). All subjects were right handed and reported no psychological or neurological disorders.
2.2. Experimental design
The data for the present study were taken from a picture recognition experiment in which a set of 240 pictures (120 pictures of real objects and 120 pictures of distorted, meaningless objects) were presented as short video clip. One trial consisted of a fixed pre-stimulus period of 2500 ms and the video clip which lasted for 4000 ms. At onset of the video clip, subjects viewed a completely blurred image that gradually became less distorted until the undistorted picture appeared 4000 ms later. Pre-testing ensured that objects could be reliably recognized already around 2000 ms. The aim of the experimental design was to induce a cognitive processing mode that is characterized by continuous, attentive expectancy about the appearance of an object. Subjects had to decide by a button press, whether the contours of an object, appearing gradually in the video clip, represented a real and meaningful or a meaningless object.
2.3. EEG recording and data analysis
EEG-signals were recorded using a 64-channel BrainAmp system (BrainProducts) and data were sampled at 1000 Hz with a bandpass filter set 0.5–100 Hz. Signals were referenced to a nose electrode and offline re-referenced to the average of two earlobe electrodes. To prevent for influences of net current a notch filter was set at 50 Hz. A set of 64 Ag–AgCl-electrodes were mounted according to the extended international 10-20 system using an EasyCap. Impedances were kept below 8 kΩ. To control for vertical eye artefacts a bipolar EOG-channel was used. Epochs containing eye or muscle artefacts were rejected. On the average, a set of 216 trials remained for each subject (range: 175–235 trials). For the present study the following 17 parietal, parieto-occipital and occipital electrodes were used: P7, P5, P3, P1, Pz, P2, P4, P6, P8, PO7, PO3, POz, PO4, PO8, O1, Oz and O2. These sites were those that exhibited the largest P1 amplitudes. We extracted segments with a length of 1000 ms ranging from 600 ms pre-stimulus to 400 ms post-stimulus. For pre-processing BrainVision Analyzer (BrainProducts) software was used, all other procedures were implemented by custom-made algorithms programmed in Matlab R2009b (Mathworks Inc.).
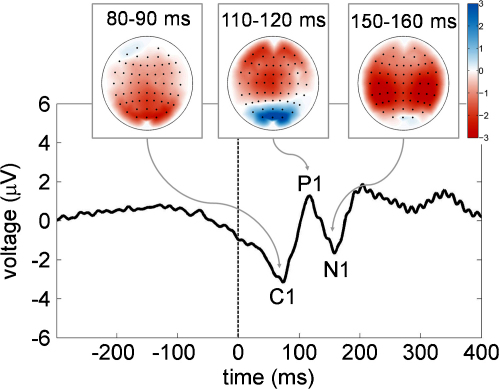
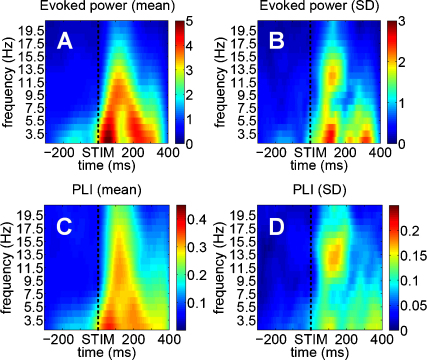
For the frequency-based analyses (power and phase estimates) we used a Gabor wavelet analysis and filtered the data between 2.5 and 20.5 Hz. For the main analyses related to phase-concentration we used a band ranging from 11.5 and 13.5 Hz. In other words, we focus on a narrow alpha band with mean frequency of 12.5 Hz. Filter parameter ‘gamma’ (which determines the time-frequency resolution) was set to a comparatively high value of 2π (instead of 1 for standard analyses). With this value, time resolution is comparatively high, but frequency resolution is low. A high time resolution is important to avoid a distorting influence on pre-stimulus phase stemming from an influence of post-stimulus ERP components. The loss in frequency resolution is not of importance for our analysis, because we focus on the upper alpha band only. The rational for choosing a fixed upper-alpha frequency band was based on the following observations: (a) the posterior grand-average ERP had a clear frequency characteristic in the upper alpha range with an 82 ms peak-to-peak latency between the C1 and N1 component (see Fig. 1; C1: 75 ms, −3.16 μV; P1: 117 ms, +1.27 μV; N1: 157 ms, −1.68 μV) and (b) across subjects the highest variation in evoked power and phase-locking was evidently prominent in this frequency-range (cfg. Fig. 2B and D).
Fig. 1.
Grand-average ERP (broad-band filter 1–70 Hz) for 17 posterior electrodes (dashed line = video onset). The tiny topographic plots depict the spatial distribution for the three ERP-components (C1, P1, N1).
Fig. 2.
Evoked and phase-locked activity for all subjects (all 17 posterior electrodes included). (A) Mean evoked activity, (B) standard deviation of the evoked activity for every time/frequency-point across subjects, (C) and (D) show the same as (A) and (B) but here for the PLI. Notice the high variation in the upper alpha-range between 11.5 and 13.5 Hz.
2.3.1. Calculation ERPs, whole power, evoked power and phase-locking (PLI)
Individual ERPs were obtained by averaging broad-band filtered trials (1–70 Hz) across time for every channel. Evoked power was calculated on the basis of individual ERPs, and reflects amplitude estimates of evoked alpha oscillations respectively. In order to calculate whole power, single trial power estimates were obtained, and then averaged over trials. PLI is calculated by first averaging individual phases over trials and then estimating the magnitude of the average – therefore PLI reflects the normalized degree of phase variation between trials (with PLI = 0 reflecting minimal and PLI = 1 maximal phase concentration; see [37]).
2.3.2. Pre-stimulus alpha phase concentration
The aim of the present analysis is to test, whether in a pre-stimulus period immediately preceding the video clip onset, a phase concentration in the alpha band can be observed that is phase locked with the later appearing P1 amplitude of the ERP elicited by the onset of the video clip.
In order to test, whether, an alpha phase concentration can be observed in the pre-stimulus interval, we focused on a time period between −200 ms to −50 ms (relative to the onset of the video clip) in which we determined the exact time point of the appearance of positive alpha peaks. The focus on positive peaks is important because we want to test a possible pre-stimulus phase locking with the first positive peak of the ERP which is the P1. Before this analysis was carried out the 10% of trials with lowest power and highest power were excluded – thus, 20% of the trials (for each recording site and subject) were excluded. This was done, on the one hand to reduce noise (trials with very small power usually exhibit a very irregular phase development) and on the other hand to allow for a more conservative testing (trials with very large power tend to exhibit a very regular phase development).
2.3.3. Detection of positive peaks
Based on the Gabor analysis, phase values were extracted for each sample point (=time point) and electrode site. For a sine wave, these data exhibit an exact, ramp-like form with a sharp drop at 360° down to 0°, which represents the positive peak. EEG waves are irregular and do not always exhibit this exact ramp-like form – they sometimes show an irregular increase and early drop of the ramp. In an attempt to detect the regular positive peaks, we determined the time points where the continuously calculated first derivation reached a maximum (maxima found before 350° were ignored). Based on this procedure, we received a matrix (rows representing trials and columns representing time points) with binary values (1 indicates a positive peak and 0 the absence of a positive peak). From this matrix we calculated the frequency (=arithmetic sum) of all positive peaks for each time point (in ms) over all trials, separately for each electrode site and subject. The obtained values (frequencies for each time point) were normalized (the maximal value = 100%) and then smoothed (by filtering). To determine time periods with phase concentration, values exceeding the 95th percentile were selected. If more than a single value was found, those were selected representing the longest contiguous chain in time (with a cut of at 5 ms). The mean value of the corresponding time period (e.g., if the time period extends from −100 ms to −108 ms, the evaluated time point is 104 ms) was termed point of phase concentration (PPC).
2.3.4. Testing for a significant concentration of positive peaks
So far, we have described how positive peaks were determined and time periods with a high accumulation of positive peaks were selected. In order to test the significance of this finding, we used the following reasoning. Our hypothesis is that already in the pre-stimulus period, upper alpha oscillations align their phase in a way that one of the positive post-stimulus peaks coincides with the P1 amplitude. Thus, we expect that only around the time of PPC positive peaks accumulate. Because we focus on alpha oscillations with 12.5 Hz (i.e. an oscillation with a period of 80 ms), the positive peak is centred in the midpoint of a half period of 40 ms. Thus, we used 5 intervals with a length of 8 ms (40/5 = 8 ms), where the middle interval of 8 ms represents the PPC. Or in other words, the middle interval extends ±4 ms from PPC, the adjacent intervals from ±12 ms, and from ±20 ms. Using chi2 tests, we tested whether the number of peaks exhibits non-uniformity between intervals. In addition we calculated paired-samples t-tests for every interval combination to ensure that the middle interval (centred to PPC) features the highest peak accumulation.
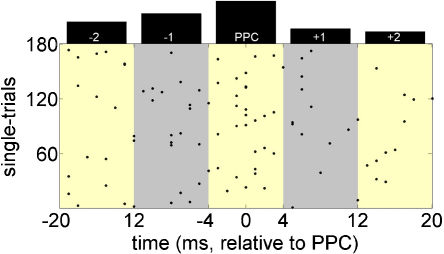
Except for 2 subjects, for all remaining subjects at least one electrode site yielded significant effects (chi2 test at or beyond p < .05). For the final analysis, those electrode was selected for each subject which showed the largest positive deviance in the PPC period in relation to the adjacent 4 intervals. In this way, we obtained 11 different posterior electrodes (‘electrodes of interest’, EOIs; for detailed listing see Table 1), where a significant concentration of positive peaks could be observed. For illustrative purpose a concrete example (single subject) is shown in Fig. 3.
Table 1.
In 14 out of 16 subjects, we observed a significant pre-stimulus phase concentration of positive peaks (PPC). EOI stands for ‘electrode of interest’ and marks the selected electrode where the PPC was found. The time point of PPC varies between −173 and −62 ms pre-stimulus. Subjects are listed and coloured according to their group assignment due to their individual phase-lags. Note the differences between the mean phase-lag values.
| Subject | PPC | EOI | Phase-lag (°) |
|---|---|---|---|
| In-phase | |||
| A | −119 | PO4 | 9 |
| C | −120 | O2 | 5 |
| D | −126 | P1 | 14 |
| F | −117 | P6 | 18 |
| H | −195 | P3 | 27 |
| I | −116 | POz | 23 |
| N | −136 | P1 | 59 |
| Mean | 22.14 | ||
| Out-of-phase | |||
| E | −79 | PS | 162 |
| G | −172 | P7 | 131 |
| J | −173 | PO3 | 126 |
| K | −147 | O1 | 108 |
| L | −169 | Oz | 144 |
| M | −62 | PO3 | 86 |
| P | −74 | PO3 | 140 |
| Mean | 128.14 | ||
Fig. 3.
Illustration of the main procedure dedicated to check for systematic phase-concentration in a single-subject. The scatter-plot shows the peak-matrix (single-trials × time) centred to PPC (time = 0) with a length of 40 ms (half of alpha-period). Each dot represents a positive peak. By dividing this period into five equally sized bins we were able to check for differences in total peak-sum across these bins. As shown by the above histogram, the middle interval (surrounding PPC) exhibits clearly most of the positive peaks and overall a non-uniform distribution is present.
2.3.5. Testing for a significant relationship between PPC and P1
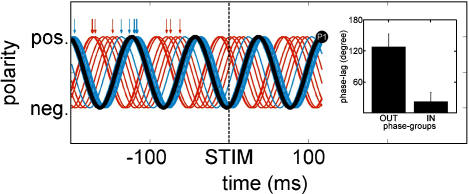
For each subject, we have an individual PPC that marks the time-point of highest peak-accumulation in the pre-stimulus interval. Now, a ‘normative’ 12.5 Hz oscillation was defined, with a positive peak centred exactly at the latency of the P1 component (which was at 117 ms). This normative oscillation was the same for all subjects. Then, a second ‘individual’ 12.5 Hz oscillation was defined with a positive peak centred at PPC. Finally, for each subject, the phase lag was determined by calculating cross-correlation between the two oscillations – therefore this phase-lag quantifies the deviations between the individual and the theoretical best timed oscillation. The result of this procedure is shown in Fig. 4 and indicates that two groups of subjects can be distinguished. For one group, the individual oscillations follow the normative oscillation quite well, for the other group they are out-of-phase (yet more or less counter-phase). These two groups were built by a median split based on individual phase-lags and were termed ‘in-phase’ and ‘out-of-phase’-group.
Fig. 4.
Oscillatory timing tested against a normative oscillation. In order to evaluate proper timing of peak-concentration we constructed a normative oscillation (black line) peaking at 117 ms (average P1-latency) and individual oscillations on the basis of PPCs (represented by arrows). By calculating phase-lags between the signals we were able to split the subjects into two groups according to their PPC-timing. The red lines represent subjects in the ‘out-of-phase’ group, blue lines subjects in the ‘in-phase’-group. The tiny graphic depicts the mean phase-lag values for the two phase-groups. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
In order to compare the groups for differences in ERP-waveforms the following procedure was implemented: At first, individual average ERPs including the 12 chosen electrodes were calculated. Afterwards, two-sample t-tests were conducted for every time-point (comparing the mean amplitude-values between groups) ranging from −300 to +400 ms and the obtained t-values were checked for significance against a bootstrapped reference distribution. In detail the reference distributions were obtained as follows: each subject was randomly assigned to one of the two groups and once again t-tests were computed for every time-point (producing null effects). This was done 10,000 times and therefore this procedure resulted in 700 t-value distributions (one for every time-point). This enabled us to quantify the probability that randomly chosen group assignment accidentally produced an equal or even stronger effect than obtained by the actual assignment (based on individual alpha phase-lag relative to a normative oscillation). Real-data t-values below the 2.5 or above 97.5 percentile (in bootstrapped distribution) were regarded as significant.
Finally average values for whole-power, evoked power and phase-locking were compared between the groups for three time intervals (t1: −200 to 0 ms, t2: 0 to +200 ms, t3: 200 to 400 ms). In order to stress the importance of timing as opposed to the amount of peak-alignment we also compared the relative number of peaks in relation to overall singe-trial number (peak-sum divided by single-trial number). Statistical testing was accomplished by two-sample t-test between groups and t-values were tested once again tested against bootstrapped reference distributions.
3. Results
3.1. Major results
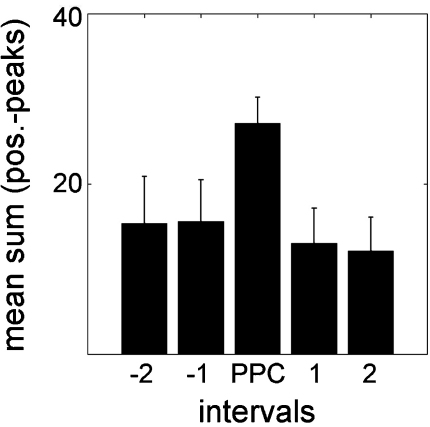
As shown in Table 1, 14 out of 16 subjects exhibited a significant period of phase concentration (PPC) for positive peaks (chi2 tests p < .05). This indicates that there were preferred time periods in the pre-stimulus interval, where positive peaks concentrated. Overall most of positive peaks could be found in the interval centred to PPC as compared to the adjacent ones (all paired-samples t-tests were significant at p < .01; see Fig. 5).
Fig. 5.
The sum of positive peaks was highest in the intervals surrounding PPC across subjects. All other adjacent intervals exhibit lower values and therefore PPC really mark time-points with highest peak accumulation. The bars represent mean-values, the whiskers the respective standard deviations.
The individual phase-lags can also be seen in Table 1 and are illustrated in Fig. 4. The two phase-groups (built by median split) logically differed in their respective mean phase-lag (‘in-phase’-group m = 22.14°; ‘out-of-phase’-group m = 128.14°).
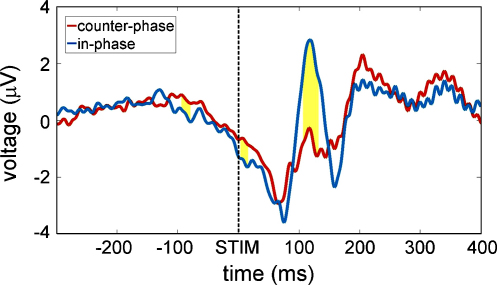
Probably the most interesting finding is the relationship between pre-stimulus phase concentration and P1 amplitude (cf. Fig. 6). Bootstrapping-statistics revealed significant effects between the two groups for three consecutive time-periods (period 1: −94 to −79 ms, period 2 = 3 to 16 ms, and period 3 = 106 to 132 ms). This means that for the respective time-points the possibility that a random chosen combination of group assignment produces a higher difference than obtained by our data analysing procedure was below 95%. In consequence we found that the ‘in-phase’-group exhibited a larger mean P1-component (period 3) than the ‘out-of-phase’-group (‘in-phase’-group mean = 2.29 μV, ‘out-of-phase’-group mean = −0.74 μV). We consider this an important finding, documenting that a particular kind of pre-stimulus phase alignment of alpha that either coincides with the P1, or not, exerted an influence on the amplitude of the P1.
Fig. 6.
Separate ERPs for the two phase groups. The yellow areas mark the significant time-periods when amplitude differed significantly (revealed by bootstrapping statistics). The three periods correspond to the predicted discrepancies (for both groups relative to the normative oscillations) whereas the most prominent effect is the difference in P1-amplitude. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
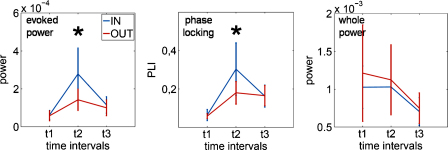
When comparing the two groups with respect to whole power, evoked power and phase locking (as measured by the PLI), differences were observed only for the post-stimulus interval ranging from 0 to 200 ms (within the broad latency range of the P1) for evoked power and PLI but not for whole power (cf. Fig. 7). Therefore the ‘in-phase’-group showed higher evoked activity and a larger PLI (phase-locking). The lacking effects in whole power prior and after stimulus onset emphasize the influence of phase and rule out main-effects caused by oscillatory amplitude. Interestingly, in addition to the absent pre-stimulus PLI-effect no differences in peak-number relative to single-trial number were observed. Thus, the two groups did not differ in the strength of pre-stimulus phase concentration, but in the timing of phase concentration which either is in or out of phase with respect to the normative oscillation.
Fig. 7.
Mean values for evoked power, phase-locking and whole power for both groups respectively. The only significant effects are present at t2 (0–200 ms) for evoked power and phase-locking – no difference in whole power were observable. Asterisks mark the significant differences and whiskers once again the corresponding standard deviations.
3.2. Control analysis
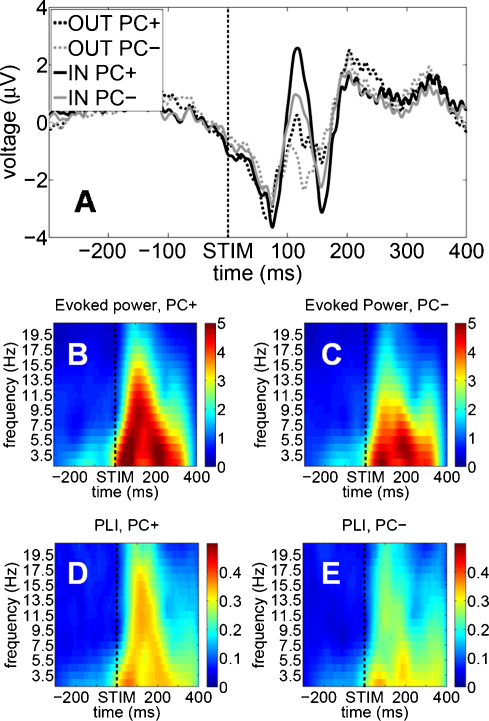
So far, we showed that (i) periods of significant pre-stimulus phase concentrations can be observed and that (ii) two types of phase concentrations (in- vs. out of phase) can be observed between subjects. Furthermore, we did not analyze data separately for that electrode (for each of the subjects) which exhibited phase concentration. Instead we averaged over all electrodes of interest (EOIs). What we did not yet show is, whether an electrode with a significant phase-concentration (denoted PC+ in the following) showed a larger P1, evoked alpha activity and/or phase-locking as compared to electrodes with no concentration (denoted PC−). The motivation behind this argument is the assumption that electrodes showing systematic phase-effects in a pre-stimulus interval should show higher event-related phase-reactivity too indicating an overall strong phase-modulation. As control electrodes we selected those which exhibited a clear uniform distribution for positive peaks over all analyzed pre-stimulus intervals. In other words, we selected electrodes with highest p-levels. We then averaged ERPs for both phase-groups and did a manual peak detection for detecting the individual P1-amplitudes which was defined as the most positive peak between +80 and +120 ms. In addition we calculated evoked-power and phase-locking for three frequency-ranges (freq1: 9.5–11.5 Hz, freq2: 11.5–13.5 Hz, freq3: 13.5–15.5 Hz) during a post-stimulus time-window from +50 to +200 ms. Statistical testing for effects on the P1-amplitudes was done by using an ANOVA with the factors CONCENTRATION (PC+ vs. PC−) and GROUP (in-group vs. out-group). Concerning evoked power and phase-locking we focused on the differences between PC+ and PC− by performing an ANOVA with the factors CONCENTRATION and FREQUENCY.
The findings related to the P1-amplitude are summarized in Fig. 8A and show that PC+ electrodes were characterized by a larger P1 than PC− electrodes as indicated by the significant main effect CONCENTRATION (F(1/12) = 6.07, p < .05). In addition, the in-phase group exhibited a larger P1 than the out-of phase group (significant main effect GROUP (F(1/12) = 6.54, p < .05). Overall these results speak in favour for a general effect between the two groups but also for a specific effect of the phase-concentration on P1-amplitude. For evoked power (cf. Fig. 8B and C), the results of the 2-way ANOVA revealed a significant main effect for phase CONCENTRATION (F(1/13) = 4.72, p < .05) and FREQUENCY (F(2/26) = 22.31, p = .000). Regarding the PLI the main effect CONCENTRATION revealed a significant difference between the two subsets of electrodes too (F(1/13) = 5.47, p = .036; cfg. Fig. 8D and E). These results confirm that we were able to detect those electrodes that showed overall a high phase modulation (in a pre-stimulus as well in a post-stimulus interval).
Fig. 8.
Control analysis related to the differences between PC+ vs. PC−. (A) ERPs for the two phase-groups selectively averaged for electrodes with high phase-concentration (PC+) and with no concentration at all (PC−), (B) mean evoked activity for PC+, (C) mean evoked activity for PC−, (D) and (E) the same as (B) and (C) but here for the PLI.
4. Discussion
Our main findings show that a systematic appearance of pre-stimulus phase in time can be observed. In almost all subjects we observed a pre-stimulus time-period where positive peaks were more prominent than in adjacent intervals across trials. In addition, the timing of those pre-stimulus peak-alignments was related to the P1-amplitude and the strength of alpha-characteristic of the early ERP-components. On the basis of these results it must be assumed that pre-stimulus ongoing oscillations cannot be considered random background noise and that phase-dynamics have potential influences on the generation of ERPs. Nonetheless the results also suggest that the absolute P1-amplitude is based on a complex interaction between pre- and post-stimulus phase-modulation at least.
The possible involvement of phase in ERP-generation was extensively investigated by studies focussing on a proof of phase-reset [1,2,26,30]. Although it is plausible that phase-reset is the major mechanism for ERP-generation a general question must be answered: Do we want to proof phase-reset or the general involvement of ongoing oscillatory dynamics in ERP-generation?
With respect to the pre-stimulus period both theories, the ‘traditional’ evoked model on the one hand and the phase-reset model on the other hand, assume that phases are always distributed randomly across trials before stimulus onset – this presumption is often expressed in the use of words like ‘spontaneous’ or ‘fluctuating’ activity for ongoing activity before stimulus onset. Nonetheless it was already shown that these apparent, ‘stochastic fluctuations’ have a significant impact on speed, accuracy and even perception quality [8,9,12,17,29,41,42]. Although phase sometimes is considered functionally relevant, no relationship between pre- and post-stimulus phase was proposed so far. In contrast, the independence between pre-stimulus and post-stimulus phase was even postulated as a clear premise for the validity of phase-reset theory [30]. Therefore, cognitive processing was associated with a reset of phase after stimulus-onset that is accomplished by an instantaneous or non-instantaneous shift in absolute phase [34].
What are the general assumptions about brain functions that can be postulated on the basis of our findings? The two major theories, evoked and phase-reset theory, seem to be strongly influenced by the classical, computational theory that suggests that the brain works like a serial mental computer [13,32]. In this view the brain is seen as a passive, stimulus-driven device that reacts to sensory inputs and accomplishes serial ‘bottom-up’ processing in a hierarchically organized way by manipulating static representations [11]. Simplified, the brain takes incoming data structures, performs complex, algorithm-like computations, passes the information to other ‘brain modules’ and solves the problem in a linear processing routine from low-level to high-level representations. This is also expressed in the presumption that early ERP components reflect exogenous whereas later components are associated with ‘endogenous’, cognitive processes [25].
The observed results suggest that the phase-reset model represents just a narrow view about the brain dynamics associated with sensory processing. We suggest a more dynamic approach that implies the ability of the brain to couple its rhythmic, neuronal activity adaptively to an external stream of events and to time its oscillatory phases according to temporal expectancies. This ability is probably the expression of an induced top-down mechanism based on previous experience and knowledge that operates on incoming stimuli and which had already been related to coherent oscillatory activity [11,43,44].
The hypothesis that the phase of ongoing oscillations has an impact on the generation of ERP-components is not new. It already was suggested by Basar and co-workers (for an overview see [3]) who observed systematically timed patterns in the EEG without direct external sensory stimulation but in relation to internal states such as expectancy. In a number of single-subject studies they described so called ‘internal event-related oscillations’ that emerged when subjects successfully predicted upcoming stimuli in time. Those patterns were characterized by rhythmic, pre-stimulus EEG oscillations in the alpha-range that were time-locked to a presumed target. These effects were already interpreted as evidences for the brains ability to time its neuronal oscillatory activity prior to the onset of a sensory event.
Interestingly, almost the same pre-stimulus mechanism as proposed by Basar was recently discussed by Schroeder and colleagues when they argued that low frequency oscillations can be used as instruments for sensory selection [38]. The authors presented results from animal research where internal oscillations tended to become entrained to rhythms embedded in a stream of stimuli [27]. They claimed that in natural environments stimuli often occur in a predictable manner and that sensory processing can track this by working in a ‘rhythmic mode’ whereas high excitatory phases are timed to stimulus onset respectively. Although they discussed mainly frequencies in the theta-, delta- and gamma-range, it is plausible that functional relevant oscillations that are crucial for actual cognitive demands and probably are engaged in ERP-generation enable this type of processing. Such an adaptive ability contradicts the notion that the brain is passively waiting for sensory input and proposes the idea of ‘active sensing’ through ‘adaptive coupling’ to the actual situation [10,39,40].
By combining the assumptions that pre-stimulus phase is involved in active stimulus processing and that the ongoing phase is involved in the generation of evoked components the observed results are a logic consequence. Pre-stimulus phase-alignment would be an elegant solution for tuning the processing of incoming sensory percepts in order to embed them in ongoing cognitive processing dedicated to goal-directed behaviour.
Acknowledgement
This research was supported by project P 21503-B18 of the Austrian Science Fund.
References
- 1.Barry R.J., De Pascalis V., Hodder D., Clarke A.R., Johnstone S.J. Preferred EEG brain states at stimulus onset in a fixed interstimulus interval auditory oddball task, and their effects on ERP components. International Journal of Psychophysiology. 2003;47:187–198. doi: 10.1016/s0167-8760(02)00151-4. [DOI] [PubMed] [Google Scholar]
- 2.Barry R.J, Rushby J.A., Johnstone S.J., Clarke A.R., Croft R.J., Lawrence C.A. Event-related potentials in the auditory oddball as a function of EEG alpha phase at stimulus onset. Clinical Neurophysiology. 2004;115:2593–2601. doi: 10.1016/j.clinph.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Basar E. Dynamic memory manifrested by induced alpha. In: Basar E., editor. Brain Function and Oscillations. II. Integrative Brain Function. Neurophysiology and Cognitive Processes. Springer; Berlin: 1999. pp. 302–321. [Google Scholar]
- 4.Başar E., Başar-Eroglu C., Karakaş S., Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neuroscience Letters. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- 5.Basar E, Demiralp T., Schürmann M., Basar-Eroglu C., Ademoglu A. Oscillatory brain dynamics, wavelet analysis, and cognition. Brain and Language. 1999;66:146–183. doi: 10.1006/brln.1998.2029. [DOI] [PubMed] [Google Scholar]
- 6.Basar E., Gonder A., Ungan P. Comparative frequency analysis of single EEG-evoked potential records. Journal of Biomedical Engineering. 1980;2:9–14. doi: 10.1016/0141-5425(80)90086-2. [DOI] [PubMed] [Google Scholar]
- 7.Brandt M.E., Jansen B.H. The relationship between prestimulus-alpha amplitude and visual evoked potential amplitude. International Journal of Neuroscience. 1991;61:261–268. doi: 10.3109/00207459108990744. [DOI] [PubMed] [Google Scholar]
- 8.Britz J., Landis T., Michel C.M. Right parietal brain activity precedes perceptual alternation of bistable stimuli. Cerebral Cortex. 2009;19:55–65. doi: 10.1093/cercor/bhn056. [DOI] [PubMed] [Google Scholar]
- 9.Busch N.A., Dubois J., VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. Journal of Neuroscience. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark A. An embodied cognitive science? Trends in Cognitive Sciences. 1999;3:345–351. doi: 10.1016/s1364-6613(99)01361-3. [DOI] [PubMed] [Google Scholar]
- 11.Engel A.K., Fries P., Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nature Reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 12.Ergenoglu T., Demiralp T., Bayraktaroglu Z., Ergen M., Beydagi H., Uresin Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Cognitive Brain Research. 2004;20:376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Fodor J.A., Pylyshyn Z.W. Connectionism and cognitive architecture: a critical analysis. Cognition. 1988;28:3–71. doi: 10.1016/0010-0277(88)90031-5. [DOI] [PubMed] [Google Scholar]
- 14.Freunberger R., Höller Y., Griesmayr B., Gruber W., Sauseng P., Klimesch W. Functional similarities between the P1 component and alpha oscillations. European Journal of Neuroscience. 2008;27:2330–2340. doi: 10.1111/j.1460-9568.2008.06190.x. [DOI] [PubMed] [Google Scholar]
- 15.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Gruber W.R., Klimesch W., Sauseng P., Doppelmayr M. Alpha phase synchronization predicts P1 end N1 latency and amplitude size. Cerebral Cortex. 2005;15:371–377. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- 17.Hanslmayr S., Aslan A., Staudigl T., Klimesch W., Herrmann C.S., Bäuml K.H. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Hanslmayr S., Klimesch W., Sauseng P., Gruber W., Doppelmayr M., Freunberger R., Pecherstorfer T., Birbaumer N. Alpha phase reset contributes to the generation of ERPs. Cerebral Cortex. 2007;17:1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- 19.Heinze H.-J., Münte T.F., Mangun G.R. Birkhäuser; Boston: 1994. Cognitive Electrophysiology. [Google Scholar]
- 20.Klimesch W., Hanslmayr S., Sauseng P., Gruber W.R., Doppelmayr M. P1 and traveling alpha waves: evidence for evoked oscillations. Journal of Neurophysiology. 2007;97:1311–1318. doi: 10.1152/jn.00876.2006. [DOI] [PubMed] [Google Scholar]
- 21.Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Research Reviews. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Klimesch W., Sauseng P., Hanslmayr S., Gruber W., Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neuroscience and Biobehavioral Reviews. 2007;31:1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Klimesch W., Schabus M., Doppelmayr M., Gruber W., Sauseng P. Evoked oscillations and early components of event-related potentials: an analysis. International Journal of Bifurcation and Chaos in Applied Sciences and Engineering. 2004;14:705–718. [Google Scholar]
- 24.Klimesch W., Schack B., Schabus M., Doppelmayr M., Gruber W., Sauseng P. Phase-locked alpha and theta oscillations generate the P1–N1 complex and are related to memory performance. Cognitive Brain Research. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Koivisto M., Revonsuo A. Event-related brain potential correlates of visual awareness. Neuroscience and Biobehavioral Reviews. 2010;34:922–934. doi: 10.1016/j.neubiorev.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Kruglikov S.Y., Schiff S.J. Interplay of electroencephalogram phase and auditory-evoked neural activity. Journal of Neuroscience. 2003;23:10122–10127. doi: 10.1523/JNEUROSCI.23-31-10122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakatos P., Karmos G., Mehta A.D., Ulbert I., Schroeder C.E. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 28.Makeig S., Westerfield M., Jung T.P., Enghoff S., Townsend J., Courchesne E., Sejnowski T.J. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- 29.Mathewson K.E., Gratton G., Fabiani M., Beck D.M., Ro T. To see or not to see: prestimulus α phase predicts visual awareness. Journal of Neuroscience. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazaheri A., Jensen O. Posterior α activity is not phase-reset by visual stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2948–2952. doi: 10.1073/pnas.0505785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouraux A., Iannetti G.D. Across-trial averaging of event-related EEG responses and beyond. Magnetic Resonance Imaging. 2008;26:1041–1054. doi: 10.1016/j.mri.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Newell A. Physical symbol systems. Cognitive Science. 1980;4:135–183. [Google Scholar]
- 33.Rajagovindan R., Ding M. From prestimulus alpha oscillation to visual-evoked response: an inverted-U function and its attentional modulation. Journal of Cognitive Neuroscience. 2010:1–16. doi: 10.1162/jocn.2010.21478. [DOI] [PubMed] [Google Scholar]
- 34.Ritter P., Becker R. Detecting alpha rhythm phase reset by phase sorting: caveats to consider. Neuroimage. 2009;47:1–4. doi: 10.1016/j.neuroimage.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 35.Sauseng P., Klimesch W., Gruber W.R., Hanslmayr S., Freunberger R., Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Sayers B.M., Beagley H.A., Henshall W.R. The mechanism of auditory evoked EEG responses. Nature. 1974;247:481–483. doi: 10.1038/247481a0. [DOI] [PubMed] [Google Scholar]
- 37.Schack B., Weiss S. Quantification of phase synchronization phenomena and their importance for verbal memory processes. Biological Cybernetics. 2005;92:275–287. doi: 10.1007/s00422-005-0555-1. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder C.E., Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends in Neurosciences. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder C.E., Wilson D.A., Radman T., Scharfman H., Lakatos P. Dynamics of active sensing and perceptual selection. Current Opinion in Neurobiology. 2010;20:172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson E., Varela F.J. Radical embodiment: neural dynamics and consciousness. Trends in Cognitive Sciences. 2001;5:418–425. doi: 10.1016/s1364-6613(00)01750-2. [DOI] [PubMed] [Google Scholar]
- 41.Thut G., Nietzel A., Brandt S.A., Pascual-Leone A. α-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. Journal of Neuroscience. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Dijk H., Schoffelen J.M., Oostenveld R., Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. Journal of Neuroscience. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varela F., Lachaux J.P., Rodriguez E., Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 44.Von Stein A., Chiang C., König P. Top-down processing mediated by interareal synchronization. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeung N., Bogacz R., Holroyd C.B., Cohen J.D. Detection of synchronized oscillations in the electroencephalogram: an evaluation of methods. Psychophysiology. 2004;41:822–832. doi: 10.1111/j.1469-8986.2004.00239.x. [DOI] [PubMed] [Google Scholar]