Abstract
OBJECTIVES
To determine whether depression status is associated with an increased risk of coronary heart disease (CHD) events, defined as CHD death or nonfatal acute myocardial infarction (MI).
DESIGN
Prospective cohort study.
SETTING
An urban primary care practice.
PARTICIPANTS
2728 adults (71.4% women, 65.5% Black) aged 60 years and older who were screened for depression between 1991 and 1993.
MEASUREMENTS
Depressive symptom severity at baseline was assessed by the Center for Epidemiological Studies Depression Scale (CES-D). Data regarding baseline demographic and clinical variables, as well as laboratory evidence of acute MI, were obtained from an electronic medical record system. All-cause mortality and CHD death were determined from the National Death Index through 2006.
RESULTS
A total of 423 (15.5%) participants reported elevated symptoms of depression (CES-D ≥ 16). During the 13 to 16 years of follow-up, 1646 (60.3%) individuals died from any cause, and 727 (26.6%) died from CHD or suffered an acute MI. Cox proportional hazards models revealed that individuals with elevated depressive symptoms were more likely to experience a CHD event, even after adjustment for demographics and comorbid health conditions (Relative Risk [RR] = 1.46, 95% CI: 1.20–1.77). Depression status was also a significant predictor of all-cause mortality in adjusted models.
CONCLUSIONS
We report the longest prospective study to date to examine depression status as an independent risk factor for CHD among a cohort of older adults including large numbers of women and underrepresented minorities. The present findings underscore the need to consider depression as a common and modifiable risk factor for CHD events among older adults.
Keywords: cardiovascular disease, coronary heart disease, myocardial infarction mortality, depression, elderly, primary care, prospective study
INTRODUCTION
Both coronary heart disease (CHD) and depression are common and expensive health conditions among older adults.(1) Despite major public health efforts over the past 50 years, CHD remains the single leading cause of death among men and women in the U.S, and 82% of all CHD deaths occur in adults over the age of 65.(1) The cornerstone of public health interventions to reduce CHD mortality has been treatment of traditional cardiovascular risk factors, such as hypertension, hyperlipidemia, and diabetes.(2) Interestingly, accumulating epidemiologic evidence suggests that depression may also be an independent risk factor for CHD.(3–9) To date, three systematic reviews of prospective observational studies examining the association between depression and CHD have been published.(10–12) Each of these meta-analyses concluded that depression predicts the onset of CHD in individuals free of cardiovascular disease at baseline. Importantly, the reported effect size of depression is comparable to that of many traditional cardiovascular risk factors.(13)
Unfortunately, no prior studies have been published demonstrating experimental evidence that treatment of depression reduces the risk of CHD. In addition, as revealed by the prior meta-analyses, the extant observational literature is limited by a number of key methodological concerns. First, only two prior studies have enrolled a cohort of older adults larger than 1,000 individuals and achieved a follow-up period approaching 10 or more years, despite the fact that most CHD deaths occur among older adults.(3, 8) Second, most prior studies have enrolled limited numbers of women or minorities. For example, in the most recent meta analysis,(11) three of 28 studies included men only(14–16) and only a single study focused on women.(17) Notably, CHD is the leading cause of death among women and African Americans yet they are frequently under-represented in the literature.(18–19) Among the two largest prior studies with the longest follow-up periods, neither included under-represented minorities.(3, 8) Third, to identify nonfatal CHD events and comorbid medical conditions, most past studies have relied on self-reports or hospital claims data alone which have limited sensitivity, particularly for myocardial infarction.(20)
In this article, we report the results of a cohort study that addresses the aforementioned methodological concerns. The present cohort consists of the largest numbers reported to date of African-Americans and women as well as a follow-up period ranging from 13–16 years. For presentation purposes, we refer to this follow-up period as 15 years. In addition, our primary outcomes are both incident nonfatal acute myocardial infarction (MI), as determined by cardiac enzyme testing, and CHD death, as determined by National Death Index data. Rather than relying on patient reports, we also had access to physician diagnoses and reports of traditional cardiovascular risk factors. Our hypothesis was that elderly adults with elevated depressive symptoms at baseline would be more likely to experience a CHD event during the follow-up interval than those who reported minimal or no depressive symptoms.
METHODS
Sample
The cohort was composed of 2728 elderly primary care patients who were screened for depression during regularly scheduled primary care appointments in an urban public health system. The full details of the screening program have previously been reported.(21–24) Briefly, from 1991 to 1993, all patients attending the primary care practice aged 60 years and older were screened for depression using the Center for Epidemiological Studies Depression Scale (CES-D)(25). Research assistants administered the CES-D face-to-face during office visits. A total of 4413 primary care patients were contacted, of which 115 refused screening, 57 were not able to complete the testing due to severe cognitive impairment, and 284 were not eligible because they did not speak English, were in prison or a nursing home, or had a hearing impairment. Of the remaining 3957, 190 were unable to answer more than four of the CES-D items, which left a baseline cohort of 3,767 individuals. For this study, we excluded the 1,039 participants who had an existing diagnosis of CHD, cerebrovascular accident, congestive heart failure, or arteriosclerotic vascular disease at baseline, leaving a final sample of 2728 individuals. Patients were then followed through December 2006, which constituted 13–16 years of follow-up.(26)
Measurements
The CES-D is a 20-item self-report scale commonly used in epidemiological studies to assess depressive symptom severity; however, it does not provide a clinical diagnosis.(25) The CES-D has been reported to have an internal consistency of .90 in patient samples and .85 in community samples, an average test-retest reliability of .54, a high correlation with other self-report depression scales, and strong construct validity.(25) Respondents are asked how often (rarely, sometimes, occasionally, or most of the time) they have experienced the listed symptom during the past week.(23) Total scores range from 0–60. Consistent with past epidemiologic studies,(6, 23, 25, 27) participants with a CES-D total score ≥ 16 were classified as “depressed” in this report; however, this classification is not equivalent to a clinical diagnosis of major depressive disorder or dysthymic disorder. Those with a CES-D total score < 16 were classified as “non-depressed.”
Data regarding baseline demographic variables and traditional cardiovascular risk factors – i.e., age, gender, race, a history of smoking, cholesterol level, body weight, and history of diabetes or hypertension – were extracted from the Regenstrief Medical Record System (RMRS)(28) at the time of screening (see Table 1). At all of the sites of care in the targeted health system, providers electronically record all diagnoses, laboratory test results, procedures, and prescribed medications. This information is routinely collected and stored in a searchable database (i.e., the RMRS) that has been used extensively for epidemiologic studies of the process and outcomes of care.(22) Hyperlipidemia was defined as a cholesterol level > 200 mg/dl, and we defined obesity as those subjects with an ideal body weight in the highest quartile of our subject population. For this population, this equated to any subject with an ideal body weight greater than 137%. We also determined the presence or absence of the following six chronic conditions as diagnosed by the patient’s physician: diabetes, hypertension, atherosclerotic vascular disease, CHD, congestive heart failure, and cerebrovascular disease.(22) If patients were ever reported by their physician to have a history of smoking, they were considered to have a positive history of smoking.
Table 1.
Baseline Characteristics (N=2728).
| Characteristic | Depression Status† | ||
|---|---|---|---|
| Non-Depressed (n=2305) | Depressed (n=423) | P value‡ | |
| Demographics | |||
| Age, mean (SD) (range=60 to 102) | 67.7 (7.0) | 66.1 (6.5) | <.0001 |
| Female, % | 70.4 | 77.1 | .0051 |
| Black, % | 68.1 | 51.5 | <.0001 |
| Cardiovascular Risk Factors | |||
| Diabetes, % | 20.3 | 22.5 | .3041 |
| Hypertension, % | 61.5 | 55.6 | .0220 |
| History of smoking, % | 27.6 | 32.2 | .0582 |
| Cholesterol level > 200, % | 48.5 | 50.6 | .4202 |
| Ideal body weight in highest quartile, % | 25.5 | 23.7 | .4377 |
Individuals with a CES-D score of ≥16 were classified as depressed, and those with a CES-D score <16 were classified as non-depressed.
An independent samples t test with 2726 degrees of freedom was used to compare the depressed and non-depressed groups on age. All other comparisons were based upon a chi-square test with 1 degree of freedom.
All-cause mortality and CHD death were determined using data from the National Death Index (NDI). After identifying all of the deaths in the cohort, we classified those deaths for which ischemic heart disease (ICD-9 codes 410–414 and ICD-10 codes 120–125) was listed as the cause of death on the death certificate as CHD deaths. Nonfatal acute MI was defined as a serum creatinine kinase myocardial band isoenzyme (CK-MB) test value of > 3.0 ng/ml or a serum troponin test value > 0.3 mcg/L as recorded in the electronic medical record. Survival time was calculated as the number of months from the baseline assessment to the event. If an event did not occur, patients were censored at the end of the observation period, which was December 31, 2006. For CHD-related events, patients were also censored at the time of death if it occurred before the end of observation period. Because screening began in January 1991 and ended in May 1993, patients had approximately 13 to 16 years of follow-up. Our primary outcome was time to first CHD event, defined as acute MI or CHD death. The secondary outcomes were CHD death alone, acute MI alone, and all-cause mortality.
Analysis
Chi-square tests (for categorical variables) and independent samples t tests (for continuous variable) were performed to compare the depression groups on the baseline characteristics. To examine the relationship between baseline depression status and the four outcomes of interest (i.e., acute MI or CHD death, CHD death alone, acute MI alone, and all-cause mortality), we first constructed a series of Kaplan-Meier survival curves to illustrate the time to events of depressed and non-depressed individuals over the follow-up period. We then used Cox proportional hazards regression models to assess the independent risk of depressed status after adjustment for demographic variables and traditional cardiovascular risk factors. Because depressive symptoms were measured only at baseline, we investigated the violation of the proportional hazards assumption by including an interaction term between the depression indicator (CES-D score >=16 vs. <16) and log (time) in each final Cox proportional hazard model. Results for each interaction term are as follows: (1) acute MI or CHD death, Wald chi-square = 0.63, df = 1, p = .4289; (2) CHD death, Wald chi-square = 0.13, df = 1, p = .7170;(3) acute MI, Wald chi-square = 1.27, df = 1, p = .2602; (4) all-cause mortality, Wald chi-square = 2.21, df = 1, p = .1368. Given that no interaction term was significant, we could not find evidence of violation of the proportional hazards assumption. Because our sample consisted of large numbers of women and minorities, we also explored whether the relationship between baseline depression status and time to CHD event was moderated by race or gender.
RESULTS
The baseline characteristics of participants with and without depressive symptoms can be found in Table 1. Among the 2,728 patients who were screened at baseline and who did not have a current diagnosis of CHD, 423 (15.5%) were identified as having significant symptoms of depression (CES-D ≥ 16). As was expected, the mean CES-D total score was higher among elderly adults in the depressed group (24.1 vs. 5.2, t = 52.2, df = 476, p < .0001). Depressed individuals also were younger and were more likely to be female. Participants with low levels of baseline depressive symptoms were more likely to be Black and to have a diagnosis of hypertension at baseline. During this follow-up interval, 1,646 (60.3%) participants died. The mean time to death was 125.0 months ± 59.3 months.
Figure 1 shows the Kaplan-Meier survival curves for time to events of depressed and non-depressed individuals over the follow-up period for the four outcomes of interest: acute MI or CHD death, CHD death, acute MI, and all-cause mortality. Our primary endpoint of either an acute MI or CHD death, which occurred in 26.6% of the cohort (n = 727). Depressed individuals were more likely to experience the primary endpoint than those without depressive symptoms (31.9% vs. 25.7%, log-rank chi-square = 10.3, df = 1, p value = 0.0014). There were 269 (9.9%) CHD deaths; the rate of CHD mortality was not significantly different between the two groups (12.1% vs. 9.5%, log rank chi-square = 3.09, df = 1, p value = 0.0788). A total of 517 (19.0%) participants experienced an acute MI diagnosed by cardiac enzyme testing. Those in the depressed group were more likely to have had an acute MI (24.3% vs. 18.0%, log rank chi-square = 13.18, df = 1, p value = 0.0003). The depressed group and non-depressed group did not differ with respect to all-cause mortality (62.9% vs. 59.9%, log rank chi-square = 1.40, df = 1, p value = 0.2365).
Figure 1.
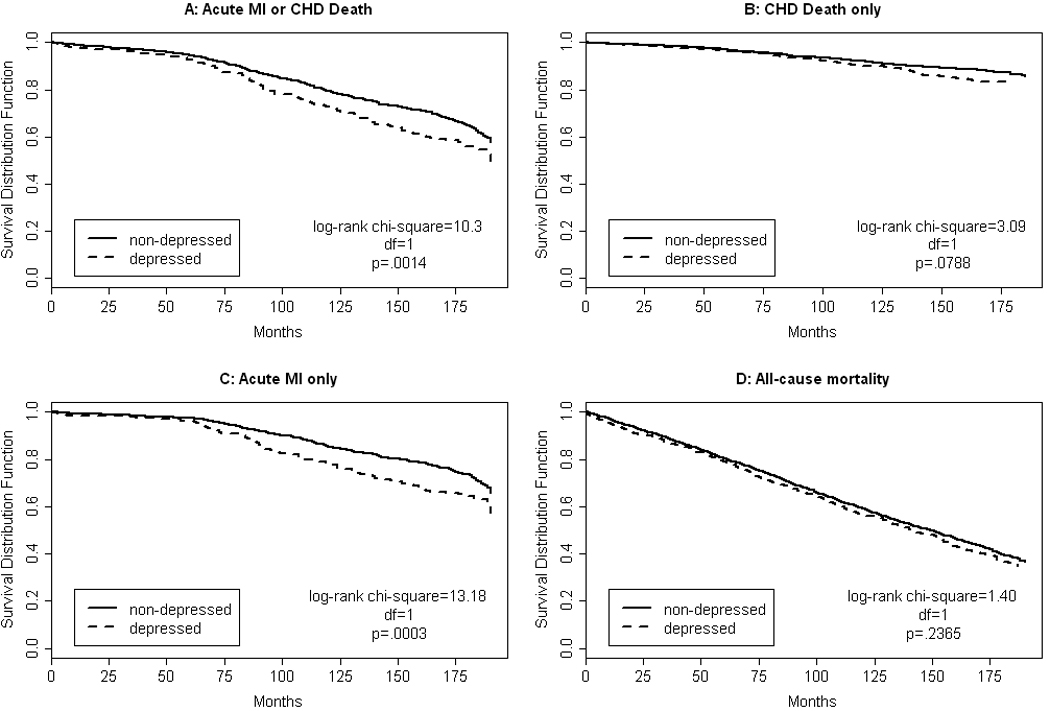
Kaplan Meier survival curves (unadjusted) for time to acute myocardial infarction (MI) or coronary heart disease (CHD) death (panel A), CHD death (panel B), acute MI (panel C), and all-cause mortality (panel D) of depressed (dashed line) and non-depressed (solid line) individuals over the 15-year follow-up period.
Table 2 presents the unadjusted and adjusted relative risk ratios obtained from Cox proportional hazards models. As can be seen, depression status remained a predictor of time to CHD event, defined as acute MI or CHD death, after adjustment for demographics only (RR = 1.50) and after adjustment for demographics and traditional cardiovascular risk factors (RR = 1.46). Cox proportional hazards models that examined CHD death and acute MI as separate outcomes revealed that depressed individuals were not more likely to die from CHD in an unadjusted model (RR = 1.31); however, after controlling for demographics (RR = 1.51) and both demographics and cardiovascular risk factors (RR = 1.48), the risk ratio was significant. Those in the depressed group remained more likely to experience an acute MI after adjustment for demographics only (RR = 1.59) and for demographics and cardiovascular risk factors (RR = 1.55). With respect to all-cause mortality, depression status was also a significant predictor in the demographics adjusted model (RR = 1.20) and in the model adjusting for both demographics and cardiovascular risk factors (RR = 1.16).
Table 2.
Unadjusted and Adjusted Cox Proportional Hazard Relative Risksa
| Outcome | No. of events |
Unadjusted | Demographics adjustedb |
Cardiovascular Risk Factor adjustedc |
|||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | p value | RR (95% CI) | p value | RR (95% CI) | p value | ||
| Acute MI/CHD Death | 727 | 1.36 (1.13–1.64) | 0.0014 | 1.50 (1.24–1.81) | < 0.0001 | 1.46 (1.20–1.77) | 0.0001 |
| CHD Death | 269 | 1.31 (0.97–1.78) | 0.0796 | 1.51 (1.11–2.06) | 0.0092 | 1.48 (1.09–2.02) | 0.0128 |
| Acute MI | 517 | 1.49 (1.20–1.85) | 0.0003 | 1.59 (1.28–1.98) | <0.0001 | 1.55 (1.24–1.93) | 0.0001 |
| All-Cause Mortality | 1646 | 1.08 (0.95–1.23) | 0.2378 | 1.20 (1.05–1.37) | 0.0071 | 1.16 (1.01–1.33) | 0.0320 |
Note: RR=relative risk, CI=confidence interval, MI=myocardial infarction, CHD= coronary heart disease
each p-value was based upon a Wald chi-square test with 1 degree of freedom
adjusted for age, gender, and race
adjusted for age, gender, race, diabetes, hypertension, history of smoking, cholesterol, and ideal body weight
In the models described above, the baseline CES-D score is treated as a dichotomous variable using the common cut-off score of ≥ 16 to indicate those subjects with significant symptoms of depression. To explore the possibility of a dose-response relationship between CES-D score and CHD risk, we repeated the Cox proportional hazard models using the following cut-points: CESD score < 16, CESD score 16–23, and CES-D score ≥ 24. In this model, we did find evidence of a dose-response relationship. After adjustment for demographics and cardiovascular risk factors, participants with CES-D score ≥ 24 (RR = 1.61, 95% CI: 1.22–2.11) and those with CES-D score between 16–23 (RR = 1.36, 95% CI: 1.06–1.74) had a higher risk ratio than participants with a CES-D score < 16.
Because our sample consisted of large numbers of women and minorities, it provided an opportunity to examine whether the increased CHD risk associated with depression (CES-D ≥ 16) is comparable across race-gender groups. Thus, we conducted a series of exploratory analyses in which we entered depression status × gender (Wald chi-square = 1.68, df = 1, p = .1953), depression status × race (Wald chi-square = 0.81, df = 1, p = .3692), and depression status × gender × race (Wald chi-square = 0.12, df = 1, p = .7267) interactions into the adjusted Cox proportional hazards model predicting time to acute MI or CHD death. None of these interaction terms was significant, indicating that the excess CHD risk conferred by depression was similar across race-gender groups. To illustrate, the depression status risk ratio was 1.36 for women versus 1.80 for men and was 1.35 for black adults versus 1.61 for white adults.
CONCLUSION
Elderly adults with significant depressive symptoms at baseline and without a current diagnosis of CHD at baseline were more likely to experience a CHD event over a 15-year follow-up period. We found that individuals in the depressed group were approximately 1.5 times more likely to suffer a CHD event (i.e., acute MI or CHD death), even after controlling for demographics and known cardiovascular risk factors. The effect was also observed in the all-cause mortality models adjusted for cardiovascular risk factors. Our exploratory analyses revealed that the excess CHD risk conferred by depression is comparable across race-gender groups, suggesting that elevated depressive symptom severity is a predictor of CHD events among older women and men as well as older white and black adults.
The present study contributes to the growing body of literature indicating that depression is an independent risk factor for CHD. To our knowledge, this investigation is the longest prospective cohort study to date examining the depression-CHD relationship in a large sample of older adults (see Table 3). In addition, our study is the first to include large numbers of both women and minorities. Although Ahto et al.(3) also observed that more severe depressive symptoms were predictive of an increased risk of CHD death over a 12-year period for both men and women, that study did not include minority participants. The findings that we report are generally consistent with those of existing studies of older adults, which have found that depression is associated with an increased risk of CHD death and nonfatal CHD events.(3, 8) Our results, however, are not completely congruent with those of studies in which separate risk ratios for men and women were calculated. For example, Mendes de Leon and colleagues (8) reported that depressive symptoms were positively associated with risk of CHD events in elderly women but not in elderly men. In contrast, Ferketich et al.(6) found that elevated depressive symptom severity was associated with an increased risk of CHD death only among men. In addition, our findings are generally consistent with prior studies that report an association between depression and all-cause mortality although the present study is again among the longest in terms of follow-up.(29–30)
Table 3.
Long Term Prospective Studies of Large Adult Populations Examining the Depression-CHD Relationship*
| Study | Follow- up Period (years) |
Cohort Size (baseline free of CHD) |
Mean Age (years) |
Women (%) |
Minorities (%) |
Nonfatal CHD Measure |
CHD Death Measure |
|---|---|---|---|---|---|---|---|
| Brown 2010 | 15 | 3767 (2717) | 67 | 68.8 | 63.4 | serum CK-MB > 0.3 ng/ml or serum troponin >0.3 mcg/L | NDI Data |
| Ahto 2007 | 12 | 1196 (715) | 71 | 59 | 0 | N/A | Official Death Registry |
| Mendes De Leon 1998 | 9 | 2488 (2391) | 72 | 60.5 | 0 | discharge diagnosis, serum CK with increase > 4% in MB fraction | Death Certificates |
CHD=coronary heart disease, CK-MB=creatine kinase myocardial band isoenzyme, NDI=National Death Index.
Follow-up period approaching 10 years, sample size ≥ 1,000 participants, and mean age ≥ 60 years.
Given the size and composition of our sample and the quality of our outcome measures, the risk ratios that we report for depression status may be considered to be a reasonably stable estimate of the population risk ratios. Notably, it is likely that the observed risk ratio is clinically meaningful, given that its magnitude is comparable to that of traditional cardiovascular risk factors. To illustrate, in our fully adjusted model examining predictors of time to CHD event, only the risk ratio for diabetes (RR = 1.94) – but not those for hypertension (RR = 1.17), hypercholesterolemia (RR = 1.01), and smoking (RR = 1.40) – exceeded that for depression status (RR = 1.46). In addition, the risk ratio for depression status is similar in size to those observed for traditional risk factors in the Framingham Heart Study. (31)
Although the evidence linking depression to future CHD is substantial, the mechanisms underlying this association remain poorly understood.(32) In most past studies, depression remained a predictor of incident CHD after adjustment for traditional risk factors(10–12), which suggests that other mechanisms are responsible. Among the leading candidate mediators of the depression-CHD relationship are poor health behaviors associated with depression, including smoking,(33) physical inactivity,(34) and non-adherence to medical regimens.(35) Other plausible mechanisms are depression-related physiological changes that are thought to contribute to CHD, such as autonomic nervous system dysfunction,(36) hypothalamic-pituitary-adrenal axis dysregulation,(37) augmented inflammation,(38) and altered platelet function.(39) It is also possible that a third factor – e.g., genetic influences(40) or subclinical cerebrovascular disease(41)– could cause an elevation in depressive symptoms and an increase in the risk of subsequent CHD events. Using data from the National Diet and Nutrition Survey, investigators recently explored possible mediating variables in the association between baseline depressive symptoms and mortality for up to 9 years.(42) While these potential mediators, many of which were also associated with cardiovascular risk, explained 54% of the association between late life depression and mortality, depression remained an independent risk factor for mortality. Declining physical function has also been reported to mediate the association between depression and mortality. However, even controlling for physical function and a host of other comorbid conditions, the association between depression and mortality remain.(43–44)
There are several limitations of our study. First, because the CES-D does not provide a diagnosis of major depressive disorder or dysthymic disorder, we cannot equate elevated depressive symptom severity with a clinical diagnosis of depression. Second, the present analysis relies on depression status measured at a single point in time. A patient’s level of depressive symptoms is known to vary over time but our study, like many in the field(45) [e.g. Lesperance 2002] assumes that the effect of depressive symptoms at baseline will be constant over the many years of follow-up. This assumption is inherent in the choice of a Cox proportional hazard model as the analytic method. Model diagnostics supported the validity of this assumption but reliance on a depression status measure at a single point in time is a limitation of this study. Measuring symptoms at a single point in time rather than relying on formal diagnostic criteria at multiple points in time would tend to result in misspecification, which should reduce the likelihood of observing differences between study groups. Nonetheless, obtaining only a single assessment of depressive symptom severity did limit the analytic options available to us. Third, we did not have access to data regarding non-fatal MI’s that occurred outside the targeted health care system. For this shortcoming to influence the study findings, however, one would have to hypothesize that non-depressed individuals were systematically more likely to seek care outside of this system.
In summary, our investigation makes a significant contribution to the literature indicating that depression is an independent risk factor for CHD, given that it is the longest prospective study of older adults and is the first to include large numbers of both women and minorities. It is the lack of experimental evidence, however, that prevents one from reaching a definitive causal inference. Specifically, past clinical trials, although few in number, have failed to demonstrate that the treatment for depression reduces the likelihood of subsequent CHD events.(46–49) Despite these disappointing results, there is suggestive evidence that depression treatment has the potential to improve cardiovascular outcomes, as pharmacologic and psychological interventions for depression have been associated with improved endothelial(50–51) and autonomic function (52) and decreased systemic inflammation(53) and smoking rates.(54) Furthermore, because all the aforementioned negative trials involved patients who had already experienced a cardiovascular event, it is possible that the depression interventions were delivered too late in the natural history of CHD. For traditional cardiovascular risk factors, public health efforts stress primary prevention in addition to secondary prevention (2). In a primary prevention approach, clinicians would screen for and treat depression in much the same manner as is currently recommended for hypertension, hyperlipidemia, or diabetes. Because evidence suggests that atherosclerosis may be more reversible(55) and that some treatment effects may be larger(56) before the development of advanced atherosclerotic lesions, future research should explore whether treatment of depression, delivered prior to the onset of clinical CHD, reduces cardiovascular risk.
Acknowledgments
Ms. Brown was supported by a Medical Student Training in Aging Research (MSTAR) Award from the American Federation of Aging Research. This work also supported by K24 AG026770, R24 MH080827, and R21HS017630.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
“No Disclosures to Report”
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Linton MF, Fazio S. A practical approach to risk assessment to prevent coronary artery disease and its complications. The American journal of cardiology. 2003;92:19i–26i. doi: 10.1016/s0002-9149(03)00505-8. [DOI] [PubMed] [Google Scholar]
- 3.Ahto M, Isoaho R, Puolijoki H, et al. Stronger symptoms of depression predict high coronary heart disease mortality in older men and women. Int J Geriatr Psychiatry. 2007;22:757–763. doi: 10.1002/gps.1735. [DOI] [PubMed] [Google Scholar]
- 4.Ariyo AA, Haan M, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–1779. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 5.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–1980. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 6.Ferketich AK, Schwartzbaum JA, Frid DJ, et al. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch Intern Med. 2000;160:1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 7.Luukinen H, Laippala P, Huikuri HV. Depressive symptoms and the risk of sudden cardiac death among the elderly. Eur Heart J. 2003;24:2021–2026. doi: 10.1016/j.ehj.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Mendes de Leon CF, Krumholz HM, Seeman TS, et al. Depression and risk of coronary heart disease in elderly men and women: New Haven EPESE, 1982–1991. Established Populations for the Epidemiologic Studies of the Elderly. Arch Intern Med. 1998;158:2341–2348. doi: 10.1001/archinte.158.21.2341. [DOI] [PubMed] [Google Scholar]
- 9.Penninx BW, Beekman AT, Honig A, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 10.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 11.Van der Kooy K, van Hout H, Marwijk H, et al. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 12.Rugulies R. Depression as a predictor for coronary heart disease. a review and meta-analysis. American journal of preventive medicine. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 13.Rozanski A, Blumenthal JA, Davidson KW, et al. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Ford DE, Mead LA, Chang PP, et al. Depression is a risk factor for coronary artery disease in men: the precursors study. Arch Intern Med. 1998;158:1422–1426. doi: 10.1001/archinte.158.13.1422. [DOI] [PubMed] [Google Scholar]
- 15.Gump BB, Matthews KA, Eberly LE, et al. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- 16.Sesso HD, Kawachi I, Vokonas PS, et al. Depression and the risk of coronary heart disease in the Normative Aging Study. The American journal of cardiology. 1998;82:851–856. doi: 10.1016/s0002-9149(98)00491-3. [DOI] [PubMed] [Google Scholar]
- 17.Whooley MA, Browner WS. Association between depressive symptoms and mortality in older women Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1998;158:2129–2135. doi: 10.1001/archinte.158.19.2129. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld AG. State of the heart: building science to improve women's cardiovascular health. Am J Crit Care. 2006;15:556–566. quiz 567. [PubMed] [Google Scholar]
- 19.Clark LT. Issues in minority health: atherosclerosis and coronary heart disease in African Americans. Med Clin North Am. 2005;89:977–1001. doi: 10.1016/j.mcna.2005.05.001. 1994. [DOI] [PubMed] [Google Scholar]
- 20.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. American journal of epidemiology. 2004;160:1152–1158. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 21.Callahan CM, Wolinsky FD, Stump TE, et al. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med. 1998;13:746–752. doi: 10.1046/j.1525-1497.1998.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stump TE, Callahan CM, Hendrie HC. Cognitive impairment and mortality in older primary care patients. Journal of the American Geriatrics Society. 2001;49:934–940. doi: 10.1046/j.1532-5415.2001.49184.x. [DOI] [PubMed] [Google Scholar]
- 23.Callahan CM, Hui SL, Nienaber NA, et al. Longitudinal study of depression and health services use among elderly primary care patients. Journal of the American Geriatrics Society. 1994;42:833–838. doi: 10.1111/j.1532-5415.1994.tb06554.x. [DOI] [PubMed] [Google Scholar]
- 24.Callahan CM, Hendrie HC, Dittus RS, et al. Improving treatment of late life depression in primary care: a randomized clinical trial. Journal of the American Geriatrics Society. 1994;42:839–846. doi: 10.1111/j.1532-5415.1994.tb06555.x. [DOI] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 26.McDonald CJ, Overhage JM, Tierney WM, et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54:225–253. doi: 10.1016/s1386-5056(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 27.Bremmer MA, Hoogendijk WJ, Deeg DJ, et al. Depression in older age is a risk factor for first ischemic cardiac events. Am J Geriatr Psychiatry. 2006;14:523–530. doi: 10.1097/01.JGP.0000216172.31735.d5. [DOI] [PubMed] [Google Scholar]
- 28.McDonald CJ, Tierney WM, Overhage JM, et al. The Regenstrief Medical Record System: 20 years of experience in hospitals, clinics, and neighborhood health centers. MD Comput. 1992;9:206–217. [PubMed] [Google Scholar]
- 29.Rapp MA, Gerstorf D, Helmchen H, et al. Depression predicts mortality in the young old, but not in the oldest old: results from the Berlin Aging Study. Am J Geriatr Psychiatry. 2008;16:844–852. doi: 10.1097/JGP.0b013e31818254eb. [DOI] [PubMed] [Google Scholar]
- 30.Yaffe K, Edwards ER, Covinsky KE, et al. Depressive symptoms and risk of mortality in frail, community-living elderly persons. Am J Geriatr Psychiatry. 2003;11:561–567. [PubMed] [Google Scholar]
- 31.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 32.Nemeroff CB. The curiously strong relationship between cardiovascular disease and depression in the elderly. Am J Geriatr Psychiatry. 2008;16:857–860. doi: 10.1097/JGP.0b013e318189806a. [DOI] [PubMed] [Google Scholar]
- 33.Black DW, Zimmerman M, Coryell WH. Cigarette smoking and psychiatric disorder in a community sample. Ann Clin Psychiatry. 1999;11:129–136. doi: 10.1023/a:1022355826450. [DOI] [PubMed] [Google Scholar]
- 34.Kritz-Silverstein D, Barrett-Connor E, Corbeau C. Cross-sectional and prospective study of exercise and depressed mood in the elderly : the Rancho Bernardo study. American journal of epidemiology. 2001;153:596–603. doi: 10.1093/aje/153.6.596. [DOI] [PubMed] [Google Scholar]
- 35.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 36.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67 Suppl 1:S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 37.Grippo AJ, Johnson AK. Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev. 2002;26:941–962. doi: 10.1016/s0149-7634(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 38.Kop WJ, Gottdiener JS. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom Med. 2005;67 Suppl 1:S37–S41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- 39.Bruce EC, Musselman DL. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med. 2005;67 Suppl 1:S34–S36. doi: 10.1097/01.psy.0000164227.63647.d9. [DOI] [PubMed] [Google Scholar]
- 40.McCaffery JM, Frasure-Smith N, Dube MP, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulos GS, Meyers BS, Young RC, et al. 'Vascular depression' hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 42.Hamer M, Bates CJ, Mishra GD. Depression, physical function, and risk of mortality: national diet and nurtrition survey in adults older than 65 years. American Journal of Geriatric Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181df465e. [published ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Reynolds SL, Haley WE, Kozlenko N. The impact of depressive symptoms and chronic diseases on active life expectancy in older Americans. Am J Geriatr Psychiatry. 2008;16:425–432. doi: 10.1097/JGP.0b013e31816ff32e. [DOI] [PubMed] [Google Scholar]
- 44.Turvey CL, Schultz SK, Beglinger L, et al. A longitudinal community-based study of chronic illness, cognitive and physical function, and depression. Am J Geriatr Psychiatry. 2009;17:632–641. doi: 10.1097/jgp.0b013e31819c498c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lesperance F, Frasure-Smith N, Talajic M, et al. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 46.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 47.Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 48.Rollman BL, Belnap BH, LeMenager MS, et al. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA. 2009;302:2095–2103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 50.Pizzi C, Mancini S, Angeloni L, et al. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;86:527–532. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- 51.van Zyl LT, Lesperance F, Frasure-Smith N, et al. Platelet and endothelial activity in comorbid major depression and coronary artery disease patients treated with citalopram: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy Trial (CREATE) biomarker sub-study. J Thromb Thrombolysis. 2009;27:48–56. doi: 10.1007/s11239-007-0189-3. [DOI] [PubMed] [Google Scholar]
- 52.Carney RM, Freedland KE, Stein PK, et al. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000;62:639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Doering LV, Cross R, Vredevoe D, et al. Infection, depression, and immunity in women after coronary artery bypass: a pilot study of cognitive behavioral therapy. Altern Ther Health Med. 2007;13:18–21. [PubMed] [Google Scholar]
- 54.Trockel M, Burg M, Jaffe A, et al. Smoking behavior postmyocardial infarction among ENRICHD trial participants: cognitive behavior therapy intervention for depression and low perceived social support compared with care as usual. Psychosom Med. 2008;70:875–882. doi: 10.1097/PSY.0b013e3181842897. [DOI] [PubMed] [Google Scholar]
- 55.Ikeda N, Torii R. When does atherosclerosis become irreversible? Chronological change from an early to an advanced atherosclerotic lesion observed by angioscopy. Angiology. 2005;56:361–370. doi: 10.1177/000331970505600401. [DOI] [PubMed] [Google Scholar]
- 56.Sutton-Tyrrell K, Wildman R, Newman A, et al. Extent of cardiovascular risk reduction associated with treatment of isolated systolic hypertension. Arch Intern Med. 2003;163:2728–2731. doi: 10.1001/archinte.163.22.2728. [DOI] [PubMed] [Google Scholar]


