Abstract
Background
The 1918 influenza pandemic was associated with an unusual age pattern of mortality, with most deaths occurring among young adults. Few studies have addressed changes in the age distribution for influenza-related mortality in the pre-pandemic and post-pandemic period, which has implications for pandemic preparedness. In the present paper, we analyse the age patterns of influenza-related excess mortality in the decades before and after the 1918 pandemic, using detailed historic surveillance data from Copenhagen.
Methods
Weekly age-specific rates of respiratory mortality and influenza-like-illnesses were compiled for 1904 to 1937. Seasonal excess rates of morbidity and mortality attributable to influenza were calculated using a seasonal regression approach. To characterize the age patterns of influenza-related deaths in individual seasons, we used two rate ratio (RR) measures representing ratios of excess mortality rates between age groups and influenza seasons.
Results
Individuals aged 15-64 years experienced sharply elevated excess respiratory mortality rates in the 1918-19 and 1919-20 pandemic periods, compared to pre-pandemic seasons (RR for excess mortality in the fall of 1918 = 67 relative to inter-pandemic seasons). Of all excess respiratory deaths occurring during 1918-19, 84% were reported in individuals 15-64 yrs. By contrast, seniors over 65 years of age experienced no measurable excess mortality during 1918-19 and moderate excess mortality in the recrudescent pandemic wave of 1919-20. The first post-pandemic season associated with high excess mortality rates in individuals over 65 yrs was 1928-29, with 73% of excess deaths occurring among seniors. We estimate that the age patterns of influenza-related mortality returned to pre-pandemic levels after 1925, based on trends in the rate ratio of excess respiratory mortality in people under and over 65 years.
Conclusions
The unusual elevation of excess respiratory mortality rates in young and middle-aged adults was confined to the first three years of A/H1N1 virus circulation 1918-20; the rapid return to “epidemic” mortality pattern in this age group was probably due to high attack rates and build-up of immunity. In contrast, seniors were completely spared from pandemic mortality during 1918-19, likely due to childhood exposure to an A/H1-like influenza virus. The rise in excess mortality rates in seniors in the recrudescent pandemic wave of 1919-20 may suggest the emergence of an early influenza A/H1N1 drift variant. Subsequent drift events may have been associated with the particularly severe 1928-29 epidemic in Denmark and elsewhere.
Keywords: 1918 influenza pandemic, excess mortality, age shift, antigenic drift, Copenhagen
Introduction
Despite renewed interest in the analysis of archival data to characterize the epidemiology of the 1918 A/H1N1 influenza pandemic, changes in the age mortality patterns in the years immediately following the pandemic have yet to be quantified. In particular, a better understanding of the population groups at high risk of influenza-related mortality is essential to define priority groups for vaccination and treatment in pandemic and post-pandemic seasons, and of particular interest since the emergence of the H1N1pdm virus in April 2009 [1]-[4]. In addition, epidemiological analyses describing changes in the age patterns of deaths during the post-pandemic period may help shed light on the genetic evolution of novel viruses a few years after emergence into human populations.
Four influenza pandemics occurred in recent history following the emergence of novel influenza A variants; the A/H1N1 ‘Spanish Influenza’ in 1918, the A/H2N2 ‘Asian Influenza’ in 1957, the A/H3N2 ‘Hong Kong Influenza’ in 1968, and A/H1N1pdm influenza in 2009. In contrast to inter-pandemic seasons, where the majority of influenza-related deaths occur in the elderly, pandemics have been associated with a signature mortality age shift towards younger population [5]. The most extreme historic pandemic age shift occurred with the 1918 A/H1N1 pandemic, where the majority of deaths occurred among persons under 45 years of age, sparing people over the age of 45 [6],[7]. As A/H1N1 re-emerged in 1977 after 20 years of disappearance, disease was concentrated among children and young adults [8],[9]. A more moderate shift of mortality occurred during the 1968 pandemic, where people over the age of 77 years had attenuated mortality, relative to inter-pandemic seasons [10]. One hypothesis for the pandemic mortality shift to younger age groups is “antigen recycling”, a phenomenon by which exposure to an influenza antigen in childhood yields lifelong protection and results in mortality sparing in seniors when a similar antigen re-emerges many decades later [11]. Of note, the age mortality patterns of the most recent H1N1-pdm pandemic 2009 appear consistent with the antigen recycling phenomenon, with a mean age of influenza-related deaths of ∼30-42 years, compared to ∼77 years in inter-pandemic seasons [4],[12],[13].
Comprehensive historical morbidity and mortality data are not available from most locations; but Copenhagen had a unique weekly disease surveillance system in place since the 1870s. All physicians contributed weekly reports on the number of influenza cases and respiratory deaths with age information, to the “Ugelisterne” system [7],[14]. On the basis of these uniquely detailed age-stratified surveillance records in the years before, during, and after the 1918-pandemic, we investigate changes in the age patterns of illnesses and deaths from pandemic to post-pandemic periods.
Methods
Data
We compiled weekly respiratory deaths and medically-attended influenza-like-illnesses from the Copenhagen “Ugelisterne” surveillance system from 1904 to 1937 [7],[14]. (see [14] for a detailed description). Respiratory deaths included influenza, pneumonia and bronchitis, as most influenza-related deaths during the 1918 pandemic were ascribed to bronchitis in Copenhagen [7]. Data were collected from physicians by patrolling police officers until 1920, at which point reporting became mandatory and data collection was handled by mail. The number of participating physicians was nearly complete throughout the study period and did not significantly increase after 1920 [14]. Mortality and morbidity data were available for 5 age groups: under 1 years, 1-4 years, 5-14 years, 15-64 years, and 65 years and older. Although there might be trends in case reporting rates in our study period, we concentrate here on age patterns, and do not expect reporting rates to change differently between age groups. Population estimates were compiled from the Copenhagen census data collected approximately every five years between 1901 and 1940. Age-specific annual population estimates were computed for intermediate years by linear interpolation.
Excess mortality and morbidity approach
Since weekly age-specific counts of respiratory deaths were low and prone to stochastic variations, we stabilised the time-series by aggregating the data by 4-week periods [7] (Figure 1). We fitted a spline-Serfling baseline model to these 4-weekly time series to determine the expected level of morbidity and mortality in the absence of influenza activity [7],[15]. Briefly, the time series for each age class were first de-trended with a spline function fitted to summer months June-August. Next, a seasonal regression model including harmonic, linear, and quadratic trends was fitted to data for April-November, excluding winter months when influenza may potentially circulate. We also excluded all of 1918 due to extended pandemic activity. The seasonal model allowed estimation of an expected baseline of mortality in the absence of influenza virus activity [16],[17] (Figure 2).
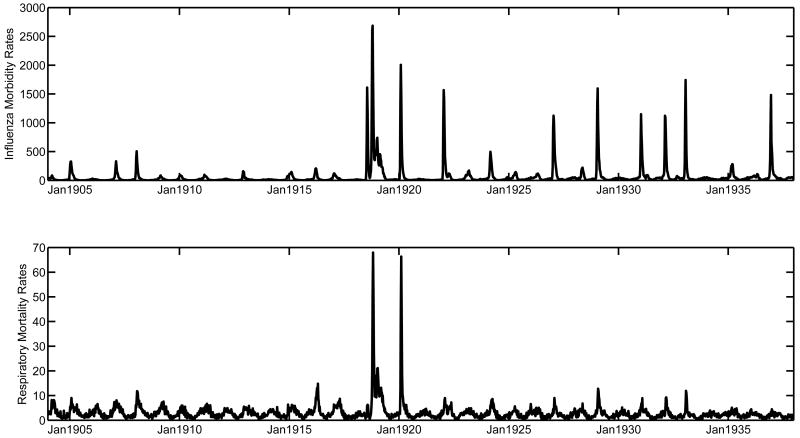
Figure 1.
Weekly rates of medically-attended influenza illnesses (top) and respiratory mortality (bottom) per 100,000 for all ages, Copenhagen, 1904-1937. The abrupt rise in influenza illness rates post-1918 might be due to a change in reporting rates or an increased circulation of the influenza virus in the A/H1N1 era.
Figure 2.
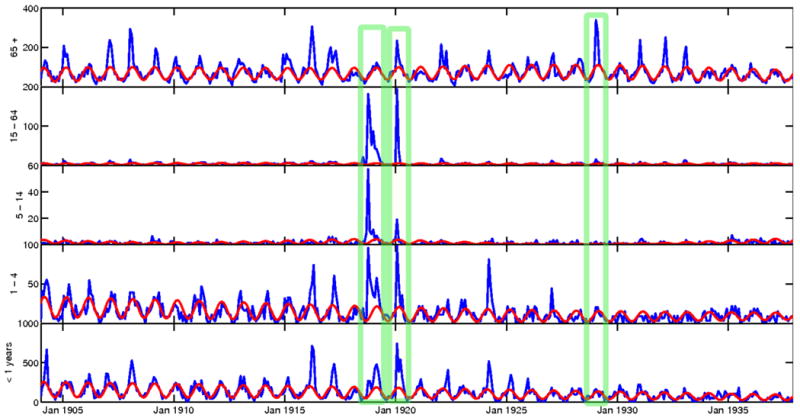
4-weekly respiratory mortality rates per 100,000 by age group, Copenhagen, 1904-1937. The red line represents the modelled baseline respiratory mortality in absence of influenza. The marked seasons are 1918-19, 1919-20, and 1928-29.
Influenza epidemic periods were determined by applying the same seasonal regression approach to influenza-specific death counts for all ages combined (R2=0.44), and identifying 4-week periods where observed mortality was above the 95% confidence interval of the model [16]. Hence influenza epidemic periods were identical for all age groups.
For each 4-week epidemic period and age group, we calculated the excess respiratory mortality rate as the difference between the observed mortality rate and the baseline mortality rate. Seasonal excess mortality rates were calculated as the cumulative sum of excess mortality rates for all 4-week epidemic periods within a season. We obtained excess mortality rates for each influenza season from 1904 to 1937 (n=35 seasons), including the three waves of the 1918 pandemic in summer 1918 (June - August), fall 1918 (September - December) and winter 1919 (January - May). We used a similar modelling approach to calculate seasonal excess rates of medically-attended influenza-like-illnesses.
To characterize the age distribution of influenza-related excess mortality in the pandemic and post-pandemic seasons, we computed two rate ratio (RR) measures. The first measure was the ratio of excess mortality rate in persons under 65 years of age relative to the excess mortality rate in persons aged 65 years and older, adapted from [5],[6] (which we refer to as RR<>65). Mild influenza seasons with fewer than 10 excess respiratory deaths and seasons where one age group had 0 excess mortality were excluded from the RR<>65 analysis. The second RR measure was used to characterize changes in the risk of death in the post-pandemic period, relative to pre-pandemic seasons (RRpre-post). The RRpre-post measure was defined as the excess respiratory mortality rate in any season during 1918-37 divided by the average excess respiratory mortality rate for the pre-pandemic period 1904-17, separately for each of the 5 age groups. For instance, a RRpre-post of 1.0 for age group “A” in fall 1918 indicates that an individual in age group A had the same probability of dying from influenza-related causes in fall 1918 as in earlier years. A similar RRpre-pos measure has been used in the past to facilitate comparisons across age groups or countries that have different background risk of death [6],[18].
Results
Overall mortality and morbidity patterns
Figure 1 shows weekly rates of influenza visits and respiratory mortality in Copenhagen during 1904-1937. Weekly mortality peak rates during the pandemic period 1918-20 were 7-fold higher than in surrounding seasons (Figure 1, upper graph), while weekly morbidity peak rates increased only 2-foldhigher during the pandemic (Figure 1, lower graph). We applied Serfling seasonal regression to these data aggregated by 4-week periods to estimate excess mortality attributable to influenza. Excess respiratory mortality rates were 7- to 18-fold higher during fall 1918, the most lethal pandemic wave, than in the most severe pre- and post-pandemic season (Supplementary Table 1). By contrast, excess influenza morbidity rates in fall 1918 were only 3- to 4.5- fold higher than the most severe surrounding seasons.
Seasons associated with high excess respiratory mortality rates during the post-pandemic period 1918-37 coincided with high excess influenza morbidity rates (correlation coefficient between seasonal excess rates of respiratory mortality and medically-attended influenza illnesses rho=0.72, P< 0.0001). One season however, 1923-24, appeared as an outlier, with relatively high excess mortality and low excess morbidity. In addition, excess mortality and excess morbidity time series had similar peak timing (excess mortality peaked on average 3.5 wks later than excess morbidity, range = -2 to 15 wks).
Age-specific excess mortality rates
Figure 2 shows the 4-weekly respiratory time series with the spline-Serfling baseline models for each of the five age groups. The age-specific seasonal models explained 6-60% of the variance in respiratory mortality outside of influenza periods, with the poorest fit found in the 5-14 yrs age group data. Table 1 provides estimates of excess mortality rates by season and broad age groups (< and >65 years) for the period from 1904 to 1937, while Supplementary Table 2 provides estimates for detailed age groups. Of the 35 seasons included in this study, eleven had no or low excess mortality (<8 excess respiratory deaths throughout the season, Table 1). Seniors over 65 years old and infants under 1 year consistently experienced the highest excess respiratory mortality rates, except for the pandemic period 1918-20. The 5-14 year old age group had the lowest excess mortality rates of all age groups
Table 1.
Age-specific excess respiratory mortality rate and rate ratio by season, Copenhagen, 1904-1937. Rate ratio (RR<>65) was calculated as the ratio of excess respiratory mortality rate in people < 65 years of age to excess respiratory mortality rate in those > 65 years of age. The RR<>65 was not calculated for seasons with fewer than 10 excess deaths total (shown in bold numerals) and seasons where one of the age groups had 0 excess deaths (shown in bold, italic numerals).
| Season | Total excess respiratory rates per 100,000 and (total numbers) | Excess respiratory mortality rate, <65 years | Excess respiratory mortality rate, 65+ years | Rate ratio, <65/65+ |
|---|---|---|---|---|
| 1904-05 | 17.5 (73) | 8 | 239.1 | 0.033 |
|
|
||||
| 1905-06 | 0 (0) | 0 | 0 | |
|
|
||||
| 1906-07 | 25.9 (111) | 7.6 | 351.5 | 0.022 |
|
|
||||
| 1907-08 | 44.1 (192) | 25.1 | 382 | 0.066 |
|
|
||||
| 1908-09 | 0 (0) | 0 | 0 | |
|
|
||||
| 1909-10 | 4.5 (21) | 0 | 83.2 | |
|
|
||||
| 1910-11 | 7.6 (35) | 5.5 | 97.5 | 0.056 |
|
|
||||
| 1911-12 | 0 (0) | 0 | 0 | |
|
|
||||
| 1912-13 | 13.5 (64) | 7.9 | 132.8 | 0.059 |
|
|
||||
| 1913-14 | 0 (0) | 0 | 0 | |
|
|
||||
| 1914-15 | 16.7 (82) | 5.1 | 275.6 | 0.019 |
|
|
||||
| 1915-16 | 59.2 (295) | 38 | 450.2 | 0.084 |
|
|
||||
| 1916-17 | 13.2 (67) | 3.9 | 189.5 | 0.020 |
|
|
||||
| 1917-18 | 0 (0) | 0 | 0 | |
|
|
||||
| 1918-19 summer | 13.8 (72) | 15.2 | 0 | |
|
|
||||
| 1918-19 fall | 336.6 (1779) | 356.9 | 55.3 | 6.45 |
|
|
||||
| 1918-19 winter | 85.1 (450) | 91.3 | 36.4 | 2.51 |
|
|
||||
| 1919-20 | 193.6 (1045) | 196.2 | 202.4 | 0.97 |
|
|
||||
| 1920-21 | 0 (0) | 0 | 0 | |
|
|
||||
| 1921-22 | 34.4 (194) | 24.9 | 238.9 | 0.10 |
|
|
||||
| 1922-23 | 0.1 (1) | 0.6 | 1 | |
|
|
||||
| 1923-24 | 28.4 (164) | 23.9 | 103.5 | 0.23 |
|
|
||||
| 1924-25 | 4.5 (26) | 3.2 | 22.2 | 0.14 |
|
|
||||
| 1925-26 | 5.5 (33) | 1.9 | 50 | 0.039 |
|
|
||||
| 1926-27 | 15.4 (92) | 8.8 | 95.2 | 0.092 |
|
|
||||
| 1927-28 | 7.2 (43) | 3.4 | 55.2 | 0.062 |
|
|
||||
| 1928-29 | 38.8 (235) | 10.5 | 410.7 | 0.026 |
|
|
||||
| 1929-30 | 0 (0) | 0 | 0 | |
|
|
||||
| 1930-31 | 14.4 (90) | 4.9 | 137.9 | 0.036 |
|
|
||||
| 1931-32 | 16.9 (107) | 2.5 | 212.8 | 0.012 |
|
|
||||
| 1932-33 | 22.8 (145) | 12.1 | 167.4 | 0.072 |
|
|
||||
| 1933-34 | 1.2 (8) | 1.7 | 17.7 | |
|
|
||||
| 1934-35 | 7.3 (49) | 5.6 | 56.8 | 0.098 |
|
|
||||
| 1935-36 | 0 (0) | 0.4 | 0 | |
|
|
||||
| 1936-37 | 0 (0) | 0 | 0 | |
To obtain a summary indicator of the age distribution of excess respiratory mortality in the pandemic period and surrounding seasons, we calculated the ratio of excess mortality rates in people <65 year old to excess mortality rates in those over 65 years in each season (RR<>65). In pre-pandemic years 1904-17, RR<>65 was less than 0.1 indicating that the older population had a 10-fold higher risk of influenza-related death per capita than the younger population (Figure 3, Table 1). We could not estimate RR<>65 for the summer wave of the 1918 pandemic, as seniors had no measurable excess mortality. During fall 1918 and winter 1919, RR<>65 was estimated at 6.5 and 2.5, respectively,— indicating a dramatic shift of influenza-related deaths towards young age groups. Overall, of all excess respiratory deaths occurring in the 1918-19 pandemic period, 84% were accounted for by the 15-64 year old (Supplementary Table 3).
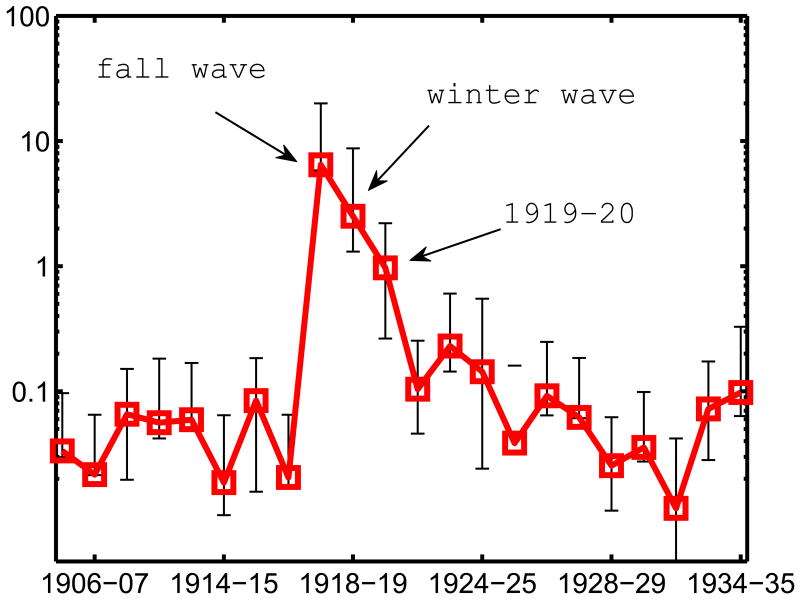
Figure 3.
Broad characterization of the age patterns of influenza-related mortality rates by season, Copenhagen, 1904-1937, based on the Rate Ratio measure (RR<>65). The RR<>65 is calculated as the excess respiratory mortality rates in the younger population (<65 year old) relative to excess respiratory mortality rates in the senior population (65+ year old).
An intermediate age pattern was observed in the next winter, 1919-20, with RR<>65 slightly above 1.0, suggesting a similar risk of influenza-related death in people under and over 65. In the following years, excess mortality gradually shifted back towards older people, and RR<>65 decreased. By 1925-26, seven years after the emergence of the pandemic H1N1 virus, the RR<>65 was back to its pre-pandemic level at 0.1 or below, and remained at this low level until 1937, the end of our study.
To explore post-pandemic adaptation in each age group in more detail, we calculated a second rate ratio measures for each age group and severe influenza seasons during 1918-37, using the average excess respiratory mortality rate in pre-pandemic seasons as denominator (RRpre-post; Figure 4 and supplementary Table 1). RRpre-post was highest for the 5-14 and 15-64 year old in the fall of 1918 (RRpre-post = 124 and 67, respectively) and remained substantially above 1.0 during the 1919-20 season (RRpre-post = 25 and 34, respectively). RRpre-post decreased substantially by the next season associated with influenza activity in 1921-22 and remained between 0.1 and 10 in this age group thereafter.
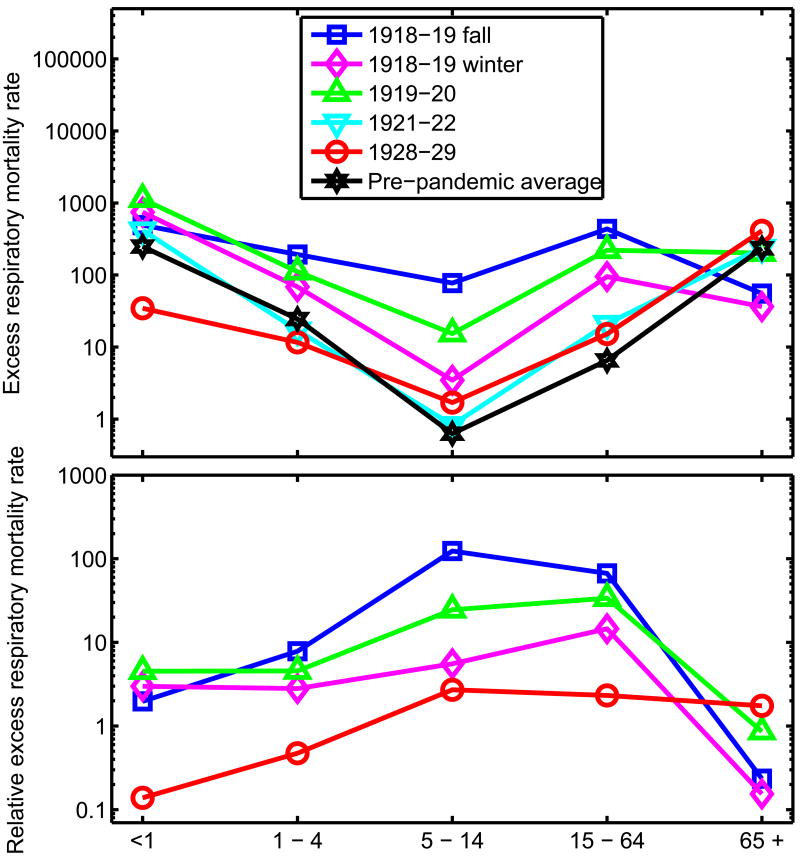
Figure 4.
Characterization of the age patterns of influenza-related mortality in the pandemic and post-pandemic periods, Copenhagen, 1918-1937. Excess respiratory mortality rates per 100,000 by age groups and season (top) and Rate Ratio of mortality relative to baseline, for selected seasons (bottom, RRpre-post). Specifically, RRpre-post is calculated as the ratio of excess mortality rate in any season during 1918-37 to the average excess mortality rate in pre-pandemic seasons 1904-17, separately for each age group.
Infants and children aged 1-4 years experienced a moderately elevated RRpre-post during the pandemic (RRpre-post = 5-8), as compared with young and middle-aged adults, and the RRpre-post returned to ∼1.0 by 1922-23. In people 65 and over, RRpre-post remained under 1.0 during the 1918-20 pandemic period and reached its highest level of 1.7 in 1928-29. In particular, 73% of all excess respiratory deaths occurring in the 1928-29 season were accounted for by seniors (Supplementary Table 3).
Discussion
To our knowledge, our study is the first to quantify detailed changes in the age patterns of influenza-related deaths in the decades following the 1918-20 pandemic, using a consistent approach throughout time to estimate influenza mortality burden. Based on comprehensive data from Copenhagen, Denmark, we have shown evidence for a shift in the age pattern of influenza-related mortality in fall 1918 and winter 1919, where the population under 65 years of age experienced nearly 7 times higher excess mortality rates than older individuals. An intermediate age pattern of deaths was found in the following winter in 1919-20, with nearly equal excess mortality rates in people under and over 65 years. While young and middle-aged adults stopped experiencing increased respiratory mortality by 1921-22, the age distribution of influenza-related mortality did not fully return to inter-pandemic patterns until 1925-26, and the first noticeably severe epidemic in older people occurred in 1928-29. Overall, these results suggest a relatively rapid post-pandemic adaptation to the 1918 A/H1N1 pandemic in the Copenhagen population, within 3-10 years of emergence of the novel virus.
There are several strengths and limitations of our study. We took advantage of the high quality Copenhagen surveillance system and its thorough records, which provide mortality and morbidity information by week and age group, resulting in a unique dataset covering the early part of the 20th century. However, these data are limited to a relatively small city of ∼500,000 inhabitants in 1918, introducing demographic noise. Aggregating the data by 4-week periods stabilized excess mortality estimates, while maintaining adequate temporal resolution. In addition, by using respiratory mortality rather than total mortality, we produced more specific and robust estimates of influenza mortality burden, especially in age groups with high background rates of deaths (infants and seniors), and during moderate influenza seasons. Excess mortality models fit reasonably well in all age groups, except in 5-14 year olds, a group that did not experience much seasonality or high death rates in normal years. Reassuringly, we found evidence of excess mortality in this age group in the 1918-20 pandemic period, suggesting that our estimates for 5-14 yr old are relatively accurate.
An important caveat in our study is the poor age resolution in the Copenhagen data, especially in the adult population (15-64 yrs, and 65 yrs and over), limiting our ability to finely characterize the age distribution of influenza-related deaths. Another caveat is related to variations in socio-economic conditions and healthcare, which have been shown to affect influenza-related mortality rates [19]. Long-term declines in background mortality rates during the study period, due to improvements in socio-economic conditions and healthcare, are expected to drive a decline in excess mortality rates attributed to influenza In particular, baseline respiratory mortality declined in the early 20th century in children under 5 years in Copenhagen. However, the mortality patterns evidenced in this study were so different across age groups, and post-pandemic changes occurred so rapidly and dramatically, that slow trends in background mortality would not affect overall conclusions.
Another important caveat is related to reporting rates of medically-attended influenza-like-illnesses, which we suspect may have increased in the post-pandemic periods with increasing influenza awareness. Alternatively, the high influenza morbidity rates observed during 1918-37 may truly reflect high influenza transmission rates in the post-1918 H1N1 era, compared to those of viruses circulating before 1918. Given the potential reporting bias in morbidity data, and the lack of an appropriate pre-pandemic baseline, we were unable to quantify post-pandemic changes in the age distribution of cases. Nevertheless we were able to show a relatively good agreement between high excess mortality and excess morbidity seasons in the post-pandemic period 1918-37 (correlation=0.72, P<0.001).
One limitation with the applied statistical approach is that we can not disentangle competing causes of respiratory excess mortality, e.g. effects from co-circulating virus or bacteria and effects of extreme weather conditions. This is not likely to be a limitation for the assessment of excess mortality in the period of 1918-20 where the evidence of the effect of influenza A/H1N1 is overwhelming, in Copenhagen but also in Europe, Asia and the Americas [6],[18]-[20]. However, knowledge of the etiologic agent associated with the respiratory epidemics in 1921-22, 1923-24 and 1928-29 would be important to shed light on this issue.
Our study confirms that seniors over 65 years of age had very low mortality during the pandemic period 1918-19 relative to surrounding seasons in Copenhagen (see also, [7]) -- a pattern of mortality sparing consistent with previous studies of the pandemic impact in New York City, USA, and Japan [6],[18], but inconsistent with historical Mexican data [20]. It is intriguing that seniors in Europe, Asia, and the US experienced higher death rates in epidemic years like 1915-16 (pre-H1N1 era) or 1928-29 (H1N1 era), than during the 1918 pandemic. This finding suggests that seniors might have had protection from previous exposure to similar antigens during childhood [1],[6], and that the level of protection may have varied between geographical locations depending on the circulation of historical influenza viruses.
If one assumes that seniors were spared from mortality during the 1918-19 pandemic waves because of earlier exposure to antigenically similar viruses, then the disappearance of such protection over time may provide indirect evidence of drift in H1N1 viruses circulating in the 1920s and 1930s. Seniors over 65 experienced moderately high influenza mortality burden during the 4th wave of the pandemic in the winter of 1919-20, with excess respiratory mortality rates nearly equal to those in younger individuals. This could be taken as evidence of an early antigenic drift in the pandemic virus, albeit a minor one. Using the pre-pandemics seasons of 1904-1917 to establish a baseline of excess mortality rates during seasonal epidemics suggests that the first markedly severe post-pandemic season in seniors occurred in 1928-29. This increase in excess mortality could be evidence of a large antigenic drift in influenza A/H1N1 viruses around 1928 in Copenhagen. Given that the 1928-29 season was also particularly severe in the US [21], it is possible that an antigenically-novel influenza H1N1 variant could have spread globally around that time. At this stage, our inferences about putative years associated with antigenic drift remain relatively hypothetical given the paucity of studies formally matching epidemiologic and antigenic data [22],[23], especially following the emergence of a pandemic virus. A key area for future research is to systematically test age-related mortality and morbidity indicators that may help identify antigenic drift in influenza viruses in inter-pandemic periods [5],[24],[25]. Such indicators could be developed by combining age-specific epidemiological data with measures of inter-annual variation in antigenic characteristics of circulating strains [16],[26],[27], as in recent efforts [23],[24]. Once identified, such indicators could then be applied retrospectively to historical time periods where mortality statistics have been collected but no virus specimen is available.
It has now become established that the 1918 pandemic was associated with mortality sparing in people over 65 years of age in Europe and the US, and a similar phenomenon may have occurred during the 1968-70 pandemic in people over 77 years of age in the US [10]. In the case of the 1968-70 pandemic, a return to normal baseline age mortality patterns occurred within 4-5 years, based on the ratio of excess mortality rates in people under and over 65 years [5]. The first virological evidence of antigenic shift occurred 7 years after pandemic virus emergence, in 1975-76, a season also associated with a severe outbreak in seniors [5],[26]. By contrast, the 1957 pandemic was not conclusively associated with mortality sparing in seniors, although there was a shift towards younger age of death, with people under 65 years accounting for 36% of all excess influenza-related deaths [5]. Over the next decade, this proportion was reduced 28-fold [5]. Overall, based on all three pandemics in the 20th century, we conclude that the period of “adaptation” to a new pandemic virus may take between 3-10 years. This is important to consider in light of the current influenza A (H1N1)pdm 2009 pandemic, as younger populations may be at increased risk of severe influenza-related disease for a few years, relative to seasonal epidemics occurring before 2009.
In terms of pandemic preparedness, the rich historical data from Copenhagen indicate that intervention strategies should be focused on young adults and children during the early pandemic waves. When the age pattern returns to normal, with highest mortality rates experienced among senior populations, the focus should return to mitigating disease burden in older age groups. The upcoming seasons of H1N1-pdm virus circulation may see a similar change from the current pattern of most deaths in people younger than 60 years to one of most deaths occurring in seniors. It will be important to keep monitoring the age pattern of influenza-related deaths in coming years to adjust vaccination and mitigation strategies accordingly.
Supplementary Material
Acknowledgments
This work was conducted in the context of the Multinational Influenza Seasonal Mortality Study (MISMS), an on-going international collaborative effort to understand influenza epidemiological and evolutionary patterns (http://www.origem.info/misms/index.php). This effort is supported in part by Fogarty International Center, National Institutes of Health and the Office of Global Health Affairs' International Influenza Unit, Office of the Secretary, Department of Health and Human Services. NS was partly sponsored by The Danish Council for Independent Research – Medical Sciences. LS and VA acknowledge support from the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security.
LS reports to have consulted for SDI, a health data business, and received support from Pfizer for pneumococcal vaccine research. No other potential conflict of interest was reported.
The authors would like to thank the four anonymous reviewers for their time in reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonsen L, Olson DR, Viboud C, Heiman E, Miller MA, Reichert TA. Pandemic influenza and mortality: past evidence and projections for the future. In: Knobler S, Oberholtzer K, editors. Forum on Microbial Threats. Pandemic influenza: Assessing capabilities for prevention and response. Washington, DC: Institute of Medicine, The National Academy of Sciences; 2005. pp. 89–114. [Google Scholar]
- 2.Miller MA, Viboud C, Olson DR, Grais RF, Rabaa MA, Simonsen L. Prioritization of Influenza Pandemic Vaccination to Minimize Years of Life Lost. J Infect Dis. 2008;198:305–11. doi: 10.1086/589716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowell G, Viboud C, Wang X, Bertozzi S, Miller M. Adaptive vaccination strategies to mitigate pandemic influenza: Mexico as a case study. PLoS Currents:Influenza. 2009 August 17;1:RRN1004. doi: 10.1371/currents.RRN1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary Estimates of Mortality and Years of Life Lost Associated with the 2009 A/H1N1 Pandemic in the US and Comparison with Past Influenza Seasons. PLoS Currents:Influenza. 2010 March 20;2:RRN1153. doi: 10.1371/currents.RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus Epidemic Influenza Mortality: A Pattern of Changing Age Distribution. J Infect Dis. 1998;178:53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 6.Olson DR, Simonsen L, Edelson PJ, Morse SS. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci U S A. 2005;102(31):11059–63. doi: 10.1073/pnas.0408290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreasen V, Viboud C, Simonsen L. Epidemiologic Characterization of the 1918 Influenza Pandemic Summer Wave in Copenhagen: Implications for Pandemic Control Strategies. J Infect Dis. 2008;197(2):270–8. doi: 10.1086/524065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glezen WP, Keitel WA, Taber LH, Piedra PA, Clover RD, Couch RB. Age distribution of patients with medically-attended illnesses caused by sequential variants of influenza A/H1N1: comparison to age-specific infection rates, 1978-1989. Am J Epidemiol. 1991;133(3):296–304. doi: 10.1093/oxfordjournals.aje.a115874. [DOI] [PubMed] [Google Scholar]
- 9.Zimmer SM, Burke DS. Historical Perspective — Emergence of Influenza A (H1N1) Viruses. N Engl J Med. 2009;361:279–85. doi: 10.1056/NEJMra0904322. [DOI] [PubMed] [Google Scholar]
- 10.Simonsen L, Reichert TA, Miller M. In: Options for the Control of Influenza V, International Congress Series 1263. Kawaoka Y, editor. Okinawa, Japan: Elsevier; 2004. pp. 791–4. [Google Scholar]
- 11.Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104:572–8. [Google Scholar]
- 12.Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, Miller MA. Severe Respiratory Disease Concurrent with the Circulation of H1N1 Influenza. N Engl J Med. 2009;361:674–9. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 13.Vaillant L, La Ruche G, Tarantola A, Barboza A epidemic intelligence team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009 Aug 20;14(33):pii: 19309. doi: 10.2807/ese.14.33.19309-en. PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Metcalf CJE, Bjørnstad ON, Grenfell BT, Andreasen V. Seasonality and comparative dynamics of six childhood infections in pre-vaccination Copenhagen. Proc Biol Sci. 2009;276:4111–8. doi: 10.1098/rspb.2009.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viboud C, Grais RF, Lafont BA, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis. 2005;192:233–48. doi: 10.1086/431150. [DOI] [PubMed] [Google Scholar]
- 16.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of Influenza Vaccination on Seasonal Mortality in the US Elderly Population. Arch Intern Med. 2005;165:265–72. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- 17.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78(6):494–506. [PMC free article] [PubMed] [Google Scholar]
- 18.Richard SA, Sugaya N, Simonsen L, Miller MA, Viboud C. A comparative study of the 1918-1920 influenza pandemic in Japan, USA and UK: mortality impact and implications for pandemic planning. Epidemiol Infect. 2009;137(8):1062–72. doi: 10.1017/S0950268809002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918-20 pandemic: a quantitative analysis. Lancet. 2006;368(9554):2211–8. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 20.Chowell G, Viboud C, Simonsen L, Miller MA, Acuna-Soto R. Mortality Patterns Associated with the 1918 Influenza Pandemic in Mexico: Evidence for a Spring Herald Wave and Lack of Preexisting Immunity in Older Populations. J Infect Dis. 2010;202(4):567–75. doi: 10.1086/654897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble GR. Epidemiological and clinical aspects of influenza. In: Beare AS, editor. Basic and applied influenza research. Boca Raton: CRC Press, Inc.; 1982. pp. 11–50. [Google Scholar]
- 22.Wolf Y, Nikolskaya A, Cherry J, Viboud C, Koonin E, Lipman D. Prediction of seasonal influenza severity from sequence and serological data. PLoS Currents:Influenza. 2010 doi: 10.1371/currents.RRN1200. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu A, Peng Y, Du X, Shu Y, Jiang T. Correlation of Influenza Virus Excess Mortality with Antigenic Variation: Application to Rapid Estimation of Influenza Mortality Burden. PLoS Comput Biol. 2010;6(8):e1000882. doi: 10.1371/journal.pcbi.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MA, Viboud C, Balinska M, Simonsen L. The Signature Features of Influenza Pandemics — Implications for Policy. N Engl J Med. 2009;360:2596–8. doi: 10.1056/NEJMp0903906. [DOI] [PubMed] [Google Scholar]
- 25.Viboud C, Tam T, Fleming D, Miller MA, Simonsen L. 1951 Influenza Epidemic, England and Wales, Canada, and the United States. Emerg Infect Dis. 2006;12(4):661–8. doi: 10.3201/eid1204.050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus ADME, Fouchier RAM. Mapping the Antigenic and Genetic Evolution of Influenza Virus. Science. 2004;305(5682):371–6. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- 27.Viboud C, Bjørnstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. Synchrony, Waves, and Spatial Hierarchies in the Spread of Influenza. Science. 2006;312:447–51. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.