ICAP-1 prevents recruitment of kindlin-2 to β1 integrin to control dynamics of fibrillar adhesion sites, fibronectin deposition, and osteoblast mineralization during bone formation.
Abstract
The morphogenetic and differentiation events required for bone formation are orchestrated by diffusible and insoluble factors that are localized within the extracellular matrix. In mice, the deletion of ICAP-1, a modulator of β1 integrin activation, leads to severe defects in osteoblast proliferation, differentiation, and mineralization and to a delay in bone formation. Deposition of fibronectin and maturation of fibrillar adhesions, adhesive structures that accompany fibronectin deposition, are impaired upon ICAP-1 loss, as are type I collagen deposition and mineralization. Expression of β1 integrin with a mutated binding site for ICAP-1 recapitulates the ICAP-1–null phenotype. Follow-up experiments demonstrated that ICAP-1 negatively regulates kindlin-2 recruitment onto the β1 integrin cytoplasmic domain, whereas an excess of kindlin-2 binding has a deleterious effect on fibrillar adhesion formation. These results suggest that ICAP-1 works in concert with kindlin-2 to control the dynamics of β1 integrin–containing fibrillar adhesions and, thereby, regulates fibronectin deposition and osteoblast mineralization.
Introduction
The extracellular matrix controls tissue integrity, function, and differentiation (Rozario and DeSimone, 2010). The proteins and proteoglycans in the extracellular matrix depend largely on the tissue (Manabe et al., 2008). Several matrix proteins, such as fibronectin, laminins, or collagens, mediate cell adhesion and support cell differentiation. In addition to the role of its various components in interacting with cells, the physical properties of the extracellular matrix are of paramount importance in defining cell fate and behavior. For instance, human mesenchymal stem cells (hMSCs) cultured on the matrix of various degrees of stiffness undergo different cell fates so that a compliant matrix drives cells to become neuronal-like, whereas stiffer surfaces trigger differentiation of the hMSC into osteoblasts (Engler et al., 2006). Finally, the extracellular matrix acts as a reservoir for signaling molecules (Hynes, 2009); this function appears to be particularly important for bone tissue (Ramirez and Rifkin, 2009). Thus, signaling proteins, such as the bone morphogenetic proteins (BMPs) or FGFs, are sequestered by the extracellular matrix in active conformations (Dallas et al., 2005; Fontana et al., 2005). Such sequestration appears to be crucial not only during development but also to coordinate bone resorption and deposition (Matsuo, 2009).
Integrins are the main class of receptors implicated in cell–extracellular matrix interactions (Hynes, 1992). These receptors trigger cell adhesion and transmit outside-in and inside-out signals and, thereby, are involved in numerous cellular functions, such as proliferation, apoptosis, cell fate decision, and extracellular matrix organization (Giancotti and Ruoslahti, 1999). One of the most obvious functions of the extracellular matrix and of cell adhesion receptors is to control developmental processes. Indeed, the importance of various integrin family members for tissue-specific development or function has been unraveled by the use of genetically modified mice in which specific integrins have been targeted (Bouvard et al., 2001).
Bones are formed by the close interplay between osteoblasts, which are bone matrix–depositing cells, and osteoclasts, which are bone-resorbing cells. The precise function of the different integrins in bone homeostasis is rather puzzling, inasmuch as data reported on osteoblasts are contradictory. Although some in vitro data strongly suggest that β1 integrins are critical for osteoblast differentiation and function, the role of β1 integrins in vivo is less clear (Moursi et al., 1996; Xiao et al., 1998; Wang et al., 2006; Hamidouche et al., 2009). Cell type–specific Cre-mediated deletion of β1 integrin in the osteoblast lineage directed by the 2.3-kb type I collagen promoter leads to minor developmental and functional defects resulting from a defect in mechanotransduction in the osteocytes (Phillips et al., 2008). The minor phenotype suggests either an important compensatory effect from other integrins, such as αv forming heterodimers with other β subunits, or/and an early role of β1 integrins that was not revealed because of its late deletion. Similarly, the expression of a dominant-negative form of β1 integrin in mature osteoblasts shows only mild effects on bone formation (Zimmerman et al., 2000).
The mild effects of targeting β1 integrin in late osteoblast lineage contrast with the phenotypic analysis of Icap-1 (Itgb1bp1tm1Ref)–deficient mice. ICAP-1 is a small protein that interacts in a specific manner with the β1A integrin cytoplasmic domain (Chang et al., 1997; Zhang and Hemler, 1999). It negatively regulates talin binding onto β1 integrin and, thereby, would be expected to limit integrin activation (Bouvard et al., 2003, 2006, 2007; Millon-Frémillon et al., 2008). Germline deletion of Icap-1 in a mouse impairs osteoblast differentiation and proliferation in vitro and in vivo. Icap-1–deficient osteoblasts display defects of adhesion, compaction, and migration (Bouvard et al., 2007; Millon-Frémillon et al., 2008), which explains, at least partly, the bone phenotype observed in vivo.
In this paper, we provide a molecular explanation of how ICAP-1, likely by direct binding onto β1 integrin, affects osteoblast function. We show that fibronectin assembly is controlled by the binding of ICAP-1 to the β1 integrin tail and that such binding is required for bone mineralization. Our results reveal the critical role of ICAP-1 in modulating the dynamics of fibrillar adhesions, which are adhesive structures responsible for fibronectin deposition. We demonstrate that the control of matrix assembly by ICAP-1–β1 integrin interaction plays an important role in governing essential developmental events, such as osteoblast mineralization. We also provide evidence that ICAP-1 negatively regulates recruitment of kindlin-2 onto the β1 integrin cytoplasmic domain and that an excess of kindlin-2 binding has a deleterious effect on fibrillar adhesion formation.
Results
Osteoblast cell compaction depends on fibronectin organization
We previously demonstrated that in vitro bone nodule formation is defective in the absence of the ICAP-1 protein (Bouvard et al., 2007). Because ICAP-1 interacts with β1 integrin (Chang et al., 1997; Zhang and Hemler, 1999; Bouvard et al., 2003) and despite the contradictory data concerning β1 integrins and bone formation described in the Introduction, we examined the roles of β1 integrins and a major ligand, fibronectin, in osteoblast function. Primary osteoblasts from β1fl/fl or Fnfl/fl mice were immortalized, and the gene of interest was deleted by viral transduction with Cre recombinase. Deletion was confirmed by immunostaining and FACS analysis for β1 integrin (Fig. S1) and by Western blotting for fibronectin (Fig. S2 C). The resulting cell lines retained their ability to differentiate into osteoblasts, and the following results were confirmed for at least two separate lines of each (Chiba et al., 1993; Bouvard et al., 2007).
Inasmuch as osteoblast condensation occurs during early differentiation, we asked whether β1 integrins were required in this process (Hall and Miyake, 2000), especially because ICAP-1 loss leads to abnormal compaction at 24 h (Fig. 1 A; Bouvard et al., 2007). β1−/− cells were unable to form spheroids, in contrast to wild-type or rescue cells (Fig. 1 B), and instead, they formed only small aggregates, presumably because of the presence of cadherins that mediated cell–cell adhesions (Stains and Civitelli, 2005). Because α5β1 integrin has been shown to be critical for fibronectin deposition and organization (Hynes et al., 1992), we therefore examined whether the defect in osteoblast compaction could result from a defect in fibronectin deposition. For this purpose, we analyzed osteoblasts lacking fibronectin. These fibronectin-null cells were unable to form spheroids, in contrast to the wild type (Fig. 1 C). Thus, cell compaction requires both β1 integrins and their extracellular ligand fibronectin. Fibronectin might either activate specific signals or provide an extracellular scaffold that allows cell compaction. To distinguish these two possibilities, we used a 49-residue peptide called functional upstream domain (FUD) that has been shown to bind to multiple N-terminal type I modules of fibronectin and, thereby, inhibit assembly of fibronectin into an insoluble matrix (Ensenberger et al., 2001, 2004; Tomasini-Johansson et al., 2001; Zhou et al., 2008; Maurer et al., 2010). Treating the osteoblasts with FUD resulted in abnormal compaction, suggesting that fibronectin deposition is required for compaction to proceed normally (Fig. 1 C). FUD treatment neither alters cell shape nor proliferation, and therefore, adhesion mediated by β1 integrins was presumably not affected (Fig. S2). To confirm the key role of fibronectin fibrillogenesis in mediating β1 integrin effects on osteoblast compaction, we inhibited fibrillogenesis by using a Rho-associated kinase (ROCK) inhibitor. RhoA/ROCK act downstream of α5β1 and mediate cell contractility required during compaction and for fibronectin fibrillogenesis (Zhong et al., 1998; Yoneda et al., 2007). Confirming previous data, the inhibition of ROCK reduced the insoluble fraction of fibronectin and hence the deposition of fibronectin in the extracellular matrix (Fig. 1 D; Schwarzbauer, 1991; Zhong et al., 1998). ROCK inhibition also reduced osteoblast compaction (Fig. 1 D). It has been proposed that ICAP-1 might be involved in ROCK membrane targeting in myoblasts (Stroeken et al., 2006). We therefore wondered whether the effect of ICAP-1 on osteoblast compaction could be caused by inefficient ROCK signaling. Icap-1–deficient cells were treated with the ROCK inhibitor Y27632, and both fibronectin deposition and cell compaction were analyzed. As shown in Fig. 1 D, inhibition of ROCK in Icap-1–deficient cells further blocked fibronectin assembly relative to ROCK inhibition alone or to ICAP-1–deficient cells, which shows an additive effect. This finding suggests that ROCK and ICAP-1 do not belong to the same linear signaling pathway but rather to separate pathways. In summary, our data show that β1 integrin, ICAP-1, and fibronectin are required for osteoblast compaction and suggest that β1 integrin effects on compaction are mediated by its ability to modulate fibronectin assembly.
Figure 1.
Cell matrix interaction and contractility are required for osteoblast compaction. (A) Cellular compaction of Icap-1+/+ (wild type), Icap-1WT (rescue), and Icap-1−/− osteoblasts after 24 or 48 h. (B) Cellular compaction of β1fl/fl (wild type), β1WT (rescue), and β1−/− osteoblasts after 24 h. (C) Cellular compaction after 24 h of Fn−/− osteoblasts and Fnfl/fl (wild type) treated or not treated with 100 ng/ml FUD. (D) ROCK and ICAP-1 additive control of cell compaction and fibronectin deposition. (top) Fibronectin deposition was monitored in Icap-1+/+ (wild type) and Icap-1−/− osteoblasts treated with DMSO (control) or ROCK inhibitor (Y27632). (left) Fibronectin amounts (Fn) were estimated by Western blotting, and the protein load was normalized using actin (Act). (right) Quantification of fibronectin deposition shown as the means and SDs from three independent experiments using ImageJ software. (bottom) Cell compaction of Icap-1+/+ (wild type) and Icap-1−/− in presence of DMSO (control) or ROCK inhibitor (Y27632) were imaged after 24 h. Sol, soluble; Insol, insoluble. Bars, 1 mm.
Icap-1 loss reduces fibronectin deposition
The nodule formation assays for osteoblast function were performed in 3D cultures. To determine whether a fibronectin defect could be extended to 2D cultures that would be suited for multiprobe fluorescence microscopy, the experiments were repeated using cells seeded on plates. We investigated whether matrix-associated fibronectin deposition could be perturbed by the loss of ICAP-1. Indeed, both wild-type and rescue cells displayed a larger fraction of matrix-associated insoluble fibronectin than Icap-1–null osteoblasts (insoluble to soluble ratio in control cells: 1.3 ± 0.2 vs. 0.7 ± 0.2 in Icap-1–deficient cells; P < 0.001; Fig. 2, A and B). Immunolabeling of fibronectin on cultured cells showed that most of the cells in Icap-1–null cultures were associated with punctate deposits of fibronectin, whereas most of the ICAP-1–expressing cells were associated with dense deposits of fibronectin (Fig. 2 C). Similar results were obtained on spheroids cultures, showing that the fibronectin fibrillogenesis defect was not restricted to 2D culture conditions (Fig. S3, A–C). Importantly, there was no reduction in fibronectin expression and secretion in Icap-1–deficient cells as measured by quantitative PCR and Western blotting. Indeed, fibronectin mRNA expression and fibronectin secreted to medium were increased when ICAP-1 was lost (Fig. S3 D). Thus, the defect in fibronectin assembly observed in Icap-1–null cells was not caused by a decrease in fibronectin expression or secretion.
Figure 2.
Icap-1−/− osteoblasts in 2D culture exhibit a defect in fibronectin deposition. (A) Icap-1+/+ (wild type), Icap-1WT (rescue), and Icap-1−/− cells were cultured for 3 d and then lysed in a buffer containing deoxycholate to separate the insoluble matrix-bound fibronectin (Insol) from the soluble fibronectin (Sol). Fibronectin (Fn) amounts were estimated by Western blotting, and the protein load was normalized using actin (Act). (B) Quantification of 10 independent experiments using ImageJ software. Quantifications are shown as the means and SDs of the ratio of insoluble/soluble fibronectin fraction (a single and a double asterisk show a significant difference with P = 0.001 and 0.0004, respectively; NS, no significant difference with P = 0.3). (C) Fibronectin deposition of Icap-1−/−, Icap-1WT (rescue), and Icap-1+/+ (wild type) cells in 2D culture. Cells were fixed and immunostained for fibronectin and counterstained with DAPI. Bar, 20 µm.
Direct β1 integrin–Icap-1 interaction controls fibronectin assembly by osteoblasts
We previously reported that ICAP-1 regulates β1 integrin function by reducing its affinity, likely by impairing talin recruitment (Bouvard et al., 2003; Millon-Frémillon et al., 2008). To determine whether ICAP-1–mediated down-regulation of β1 integrin affinity is involved in fibronectin fibrillogenesis, we generated various point mutations in the human β1 integrin cytoplasmic domain that have been reported to interfere with specific functions. One of those is the mutation at valine 787, which is important for ICAP-1 binding on β1 integrin (Chang et al., 2002). To minimize a potential side effect of this mutation on the recruitment of other molecules, such as kindlins, we generated a mutated β1 in which valine 787 was replaced by a threonine. This point mutation mimics the membrane-distal part of the β2 integrin cytoplasmic tail that does not bind ICAP-1 despite its high similarity with β1 integrin while still binding other proteins, such as kindlins (Chang et al., 2002; Moser et al., 2009). The V787T mutation resulted in decreased ICAP-1 binding to β1 integrin without interfering with the binding of kindlin-2 and talin head (Fig. S4). The β1V787T integrin mutant was introduced into β1-null osteoblasts, and positive cells were selected by FACS based on human β1 expression (Fig. S1 A). Compared with control cells, osteoblasts expressing the β1V787T integrin showed a significant decrease in insoluble matrix-bound fibronectin (Fig. 3, A and B). In line with this observation, fibronectin immunofluorescent staining in confluent cultures of β1V787T cells revealed less fibronectin deposition than in control cells (Fig. 3 C). Altogether, the defect in fibronectin fibrillogenesis observed in both Icap-1–null cells and β1V787T cells strongly suggests that efficient fibronectin fibrillogenesis requires the direct binding of ICAP-1 onto the β1 integrin cytoplasmic tail.
Figure 3.
Fibronectin fibrillogenesis requires β1 integrin in an ICAP-1–dependent manner. (A) β1fl/fl (wild type), β1WT (rescue), β1D759A, β1−/−, and β1V787T cells were cultured for 3 d and then lysed in a buffer containing deoxycholate to separate the insoluble matrix-bound fibronectin (Insol) from the soluble fibronectin (Sol). Fibronectin (Fn) amounts were estimated by Western blotting, and the protein load was normalized using actin (Act). (B) Quantification of four independent experiments using ImageJ software. Quantifications are shown as means and SDs of ratio of insoluble/soluble fibronectin fraction (a double and single asterisk shows a significant difference with P = 0.001 and 0.015, respectively; NS, no significant difference with a P = 0.18). (C) β1WT (rescue), β1fl/fl (wild type), β1D759A, β1−/−, and β1V787T cells were fixed and immunostained for fibronectin and counterstained with DAPI. Bar, 10 µm.
The introduction of D759A point mutation into β1 integrin (known to trigger a preactivation state; Hughes et al., 1996; Sakai et al., 1998) reproduced the effect of the lack of ICAP-1 on focal adhesion dynamics (Millon-Frémillon et al., 2008). We therefore asked whether this mutation also alters fibronectin fibrillogenesis. In line with previous results with fibroblasts (Sakai et al., 1998), β1D759A osteoblasts did not reduce fibronectin deposition significantly (Fig. 3). Next, we wondered whether the fibronectin fibrillogenesis defect observed in Icap-1–null cells was associated with altered fibronectin reorganization. Wild-type, rescue, and Icap-1–null osteoblasts were seeded on a fibronectin coat, cultured for 3 h, fixed, and double stained for fibronectin and β1 integrin to visualize the capability of the cells to reorganize the surrounding extracellular matrix (Fig. 4). Whereas wild-type and rescue cells reorganized the fibronectin coating into fibrils that partially co-distributed with fibrillar arrays of β1 integrins, only minimal redistribution of fibronectin or fibrillar arrays of integrins were observed with Icap-1–null osteoblasts (Fig. 4 A). Treatment of wild-type cells with the FUD peptide also blocked fibronectin redistribution in this assay (Fig. 4 B). These findings suggest that ICAP-1 controls fibrillar adhesion dynamics, which in turn, leads to fibronectin matrix reorganization.
Figure 4.
Fibronectin reorganization depends on Icap-1 and its ability to self-assemble. (A) Icap-1+/+ (wild type), Icap-1WT (rescue), and Icap-1−/− cells were seeded on fibronectin-coated coverslips. After 4 h of incubation, cells were fixed and immunostained for β1 integrin (green) and for total fibronectin (red). (B) Wild-type cells were seeded on fibronectin in the absence or presence of FUD (Icap-1+/+ +FUD). After 4 h of incubation, cells were fixed and immunostained for β1 integrin (green) and for total fibronectin (red). Bars, 10 µm.
Fibrillar adhesion dynamics are impaired in Icap-1–null cells
Based on the evidence that ICAP-1 is likely required for fibronectin fibrillogenesis via its direct binding onto β1 integrin and modulation of movement of β1 integrins into fibrillar adhesions, we further analyzed the molecular organization of adhesive structures. Thus, we immunostained cultured cells for fibronectin, talin, and β1 (Fig. 5). As shown in previous experiments, Icap-1–deficient osteoblasts exhibited reduced staining for fibronectin (Fig. 5 A). Talin staining in wild-type and Icap-1–null cells was located at the periphery of both cells but in thinner and more elongated streaks in mutant cells than in control cells (Fig. 5 B). Thus, in wild-type cells, talin appears to preferentially remain within focal adhesions rather than following fibrillar adhesions. When talin and β1 integrin were costained in wild-type cells, talin was concentrated at the cell periphery, whereas β1 integrin displayed a different distribution pattern with extended streaks originating from the cell edge and pointing to the cell center. Costaining of Icap-1–null cells demonstrated colocalization of talin and β1 integrin throughout the length of the streaks. Image analysis corroborated that talin and β1 integrin distribution patterns were different in Icap-1–null and wild-type cells (Fig. 5 C). Thus, in Icap-1–deficient cells, talin and β1 integrin colocalized in adhesive structures, suggesting that β1 integrins are not translocated normally into fibrillar adhesions, or fibrillar adhesion formation is somehow otherwise impaired.
Figure 5.
Fibrillar adhesion formation is defective in Icap-1−/− osteoblasts. (A) Icap-1+/+ (wild type) or Icap-1−/− osteoblasts were seeded on fibronectin-coated coverslips in complete medium. After overnight incubation, the cells were fixed and immunostained for talin and fibronectin (Fn) and counterstained with DAPI (blue). (B) Immunostaining of talin (red) and β1 integrin (green) counterstained with DAPI (blue) of Icap-1+/+ (wild type) or Icap-1−/− osteoblasts. A typical area used for pixel plot analysis is boxed. (C) Pixel intensity profile along focal adhesion for talin and β1 integrin is represented from cell edge to cell center. These plots are representative of ≥10 different plots analyzed. Bars, 10 µm.
To analyze fibrillar adhesion dynamics further, we generated Icap-1–deficient and wild-type cells expressing monomeric RFP (mRFP)–tagged tensin, a marker of fibrillar adhesions (Zamir et al., 1999). We took advantage of the dual localization of tensin to focal and fibrillar adhesions to follow its translocation from one structure to another. Both control and Icap-1–deficient cells were seeded on fibronectin-coated glass coverslips in the absence of serum, resulting in tensin localization at peripheral focal adhesions (Fig. 6 A). After 1 h of adhesion, the serum-free medium was replaced by serum-containing complete medium to increase cell contractility and enable fibronectin fibrillogenesis (Zhang et al., 1994). After 4 h, the cells were fixed, and mRFP fluorescence was analyzed to localize tensin. As shown in Fig. 6 A, at time 0, tensin was concentrated at the cell periphery in all genotypes. After addition of complete medium to wild-type and rescue cells, tensin moved centrally, conversely to Icap-1–null cells in which tensin remained at the cell edges (Fig. 6 A). The apparent perturbation of tensin dynamics upon ICAP-1 loss was confirmed using time-lapse video microscopy of wild-type and Icap-1–null cells expressing mRFP-tensin that were seeded on glass coverslips in complete medium (Fig. 6 B). As expected, in wild-type cells, time course analysis of mRFP-tensin localization showed translocation from the cell edge to cell center (Zamir et al., 1999). In contrast, in Icap-1–null osteoblasts, tensin translocation was not directionally oriented toward the cell center, but rather the protein kept a static localization (Fig. 6 B). This defect in fibrillar adhesion formation was further confirmed using a β1 integrin antibody in the pulse-chase experiment (Fig. S5; Pankov et al., 2000). Although control cells displayed clear β1 integrin translocation from peripheral focal adhesions sites to fibrillar adhesions, Icap-1–deficient cells displayed only faint β1 staining close to the cell edge, suggesting a profound perturbation of β1 dynamics. These results all indicate that ICAP-1 has an important role in the dynamics of fibrillar adhesions and provide a reasonable explanation for the fibronectin deposition defect observed in Icap-1–deficient cells.
Figure 6.
Tensin dynamics are impaired in Icap-1−/− osteoblasts. (A) Localization of mRFP-tensin in Icap-1+/+ (wild type), Icap-1WT (rescue), and Icap-1−/− cells in the absence (T = 0 h) or presence (T = 4 h) of serum. Arrows indicate peripheral focal adhesions; the arrowhead indicates dorsal fibrillar adhesions. (B) Time-lapse video microscopy of mRFP-tensin in Icap-1+/+ (wild type) and Icap-1−/− osteoblasts seeded on glass coverslips. Frames at time 0, 30, and 300 min were extracted from a representative video and arbitrarily colored in green, red, and blue. The boxed areas in the top images are shown at higher magnifications below. Bars, 10 µm.
Icap-1 regulates recruitment of kindlin-2 on the β1 integrin cytoplasmic domain
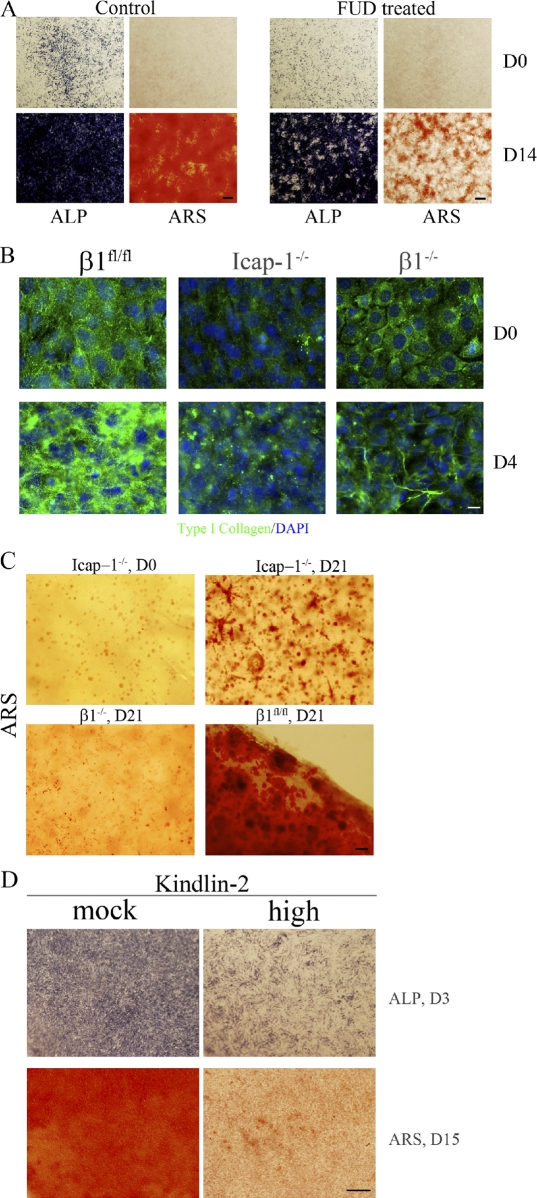
ICAP-1 and kindlins interact with an overlapping binding site on the cytoplasmic domain of β1 integrin (Chang et al., 1997; Zhang and Hemler, 1999; Larjava et al., 2008; Ma et al., 2008; Montanez et al., 2008; Meves et al., 2009). Therefore, we addressed the potential effect of ICAP-1 in the regulation of kindlin-2 binding on β1 integrin. First, we made use of Icap-1–deficient cells and the β1V787T integrin mutant to analyze whether loss of ICAP-1 binding on β1 integrin could affect kindlin-2 localization. β1fl/fl, Icap-1−/−, and β1V787T were transduced with EGFP–kindlin-2 retrovirus to generate cell lines. Based on EGFP expression, clones were selected for their expression level (Fig. S4 and not depicted). Interestingly, high expression of EGFP–kindlin-2 was achieved readily in control cells but always low in Icap-1–deficient cells as well as in β1V787T mutant cells, already suggesting a molecular interaction. Kindlin-2 localization in focal adhesions was easily detectable in β1V787T and Icap-1–deficient cells, whereas control cells displayed faint staining, mainly at the cell edge (Fig. 7 A). Increasing the expression of kindlin-2 in control cells was associated with a greater localization at focal adhesion sites (unpublished data). This observation suggested that ICAP-1 negatively regulates kindlin-2 localization within focal adhesions. To address the role of ICAP-1 in regulating kindlin-2 binding on β1 integrin cytoplasmic domain more directly, we expressed ICAP-1 in HEK 293 cells and analyzed whether ICAP-1 modulates the interaction of kindlin-2 with the GST-β1 fusion protein in a pull-down assay. ICAP-1 overexpression significantly reduced the amount of kindlin-2 in GST-β1 pull-down assays, again arguing that ICAP-1 negatively regulates kindlin-2 binding on β1 integrin (Fig. 7 B).
Figure 7.
ICAP-1 regulates fibrillogenesis by negatively regulating kindlin-2 binding on β1 integrin. (A) EGFP–kindlin-2 localization in wild-type, β1V787T, or Icap-1−/− cells seeded overnight on glass coverslips. (B) Kindlin-2 binding on β1 integrin in the presence of a normal (mock) or high level of ICAP-1 (Icap-1) was analyzed using pull-down assays. Kindlin-2 binding on GST alone, GST-β1, and GST-β3 as well as ICAP-1 expression was visualized by Western blotting, and kindlin-2 bindings to GST-β1 (blue) and GST-β3 (green) were quantified using ImageJ software and shown as the means and SDs of six independent experiments (asterisk indicates a significant difference with P = 0.05). (C) Visualization of fibronectin (Fn) deposition in cells expressing different levels of EGFP–kindlin-2 (from nontransfected cells [mock], moderate [medium], and high level [high]). Fibronectin deposition was visualized by immunofluorescence (top) or after biochemical fractionation to determine the relative quantity of matrix-bound fibronectin (insol) and the nonorganized counterparts (sol; bottom). Data are the means and SDs representatives of three different experiments performed with two different clones (the asterisk indicates a significant difference with P = 0.0009). Bars, 10 µm.
To explore whether part of the Icap-1–null phenotype is caused by an excess of kindlin-2 binding onto β1 integrin, we selected an osteoblast cell line in which kindlin-2 expression was maximal (Fig. S4) and used it to see whether fibronectin fibrillogenesis proceeded correctly. Such overexpression of kindlin-2 dramatically reduced fibronectin deposition, relative to nontransfected cells (Fig. 7 C).
Matrix-associated fibronectin controls osteoblast mineralization
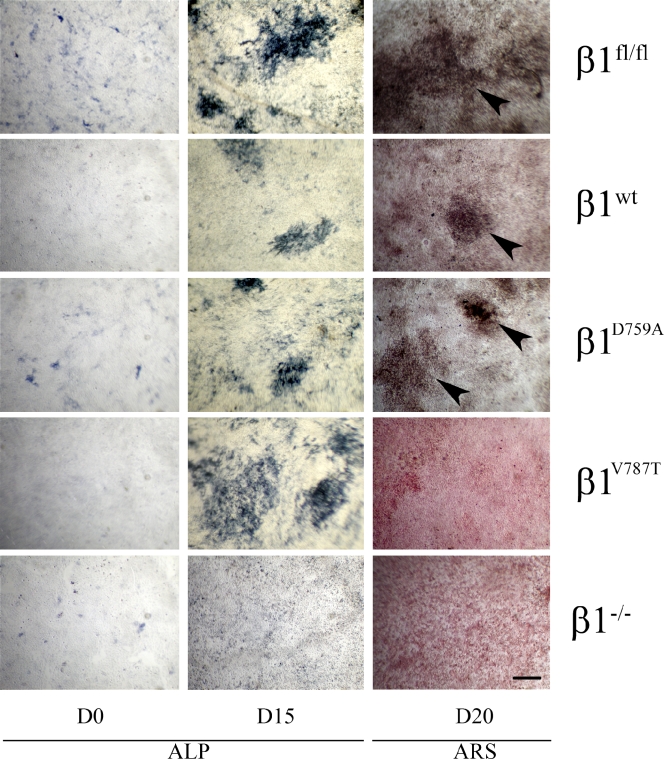
We previously reported that Icap-1–null mice exhibit decreased osteoblast proliferation, differentiation, and mineralization, resulting in a distinct bone phenotype (Bouvard et al., 2007). On the other hand, fibronectin has been shown to be crucial for osteoblast differentiation and survival in vitro and in vivo (Moursi et al., 1996, 1997; Bentmann et al., 2010). We therefore examined whether the mineralization defect of Icap-1–null osteoblasts might be caused by the aforementioned defect in fibronectin fibrillogenesis. For this, we monitored the in vitro mineralization capabilities of osteoblasts expressing β1fl/fl (wild type), β1−/−, β1WT (rescue), β1D759A, and β1V787T. As expected, the induction of differentiation of wild-type and rescue β1 osteoblasts resulted in the appearance of mineralized bone nodules, revealed by Alizarin red S staining at day 20 (Fig. 8, arrowheads). We also observed mineralization with the β1D759A mutant (Fig. 8, arrowheads), which agrees with the fact that this point mutation does not impair fibronectin deposition (Fig. 3). Although the color uptake varied depending on the speed of mineralization, we constantly observed bone nodules when osteoblasts expressing β1fl/fl (rescue), β1WT, and β1D759A were used, in sharp contrast to osteoblasts expressing β1−/− and β1V787T, which were unable to form mineralized bone nodules despite their ability to express alkaline phosphatase, an early marker of osteoblast commitment (Fig. 8). Interestingly, osteoblasts expressing β1−/− and β1V787T also displayed a fibronectin deposition defect, similar to that of Icap-1–null osteoblasts (Figs. 2 and 3). These observations suggest that fibronectin organization is crucial for osteoblast mineralization. To test by a second approach whether fibronectin organization is required for proper mineralization, we blocked fibronectin assembly in wild-type cells by FUD and followed mineralization. In contrast to untreated wild-type cells, which displayed extensive mineralization, FUD-treated cells showed a significant reduction in mineralization (Fig. 9 A). Again, as we observed for cells with altered β1 integrin, the expression of alkaline phosphatase was still detectable, showing that treated cells have retained their capacity to commit to osteoblasts. Together, these data indicate that fibronectin organization is crucial for osteoblast mineralization.
Figure 8.
β1 integrin regulates in vitro mineralization in an Icap-1–dependent manner. β1fl/fl (wild type), β1WT (rescue), β1D759A, β1V787T, and β1−/− cells were induced to differentiate into osteoblasts. Expression of alkaline phosphatase (ALP) was used to follow the early commitment of cells to the osteoblast lineage at day 0 (D0) and day 15 (D15). Mineralization was visualized by Alizarin red S staining (ARS) at day 20 (D20). Arrowheads indicate mineralized bone nodules. Bar, 1 mm.
Figure 9.
Blocking fibronectin fibrillogenesis impairs mineralization. (A) Wild-type cells were induced to differentiate into osteoblasts in the presence (FUD treated) or absence (control) of FUD, and the expressions of alkaline phosphatase (ALP) and mineralization (Alizarin red S [ARS]) were monitored at day 0 (D0) and day 14 (D14). (B) Wild-type (β1fl/fl), Icap-1−/−, and β1−/− cells were cultured as described for in vitro mineralization assay. At day 0, the medium was changed to induce differentiation. Cells were fixed either at day 0 or day 4, and type I collagen deposition was analyzed by immunofluorescence staining. (C) Wild-type (β1fl/fl), Icap-1−/−, and β1−/− cells were embedded in highly concentrated type I collagen gel (5 mg/ml). After 1 wk in normal medium to allow cell proliferation, the medium was changed for the osteogenic medium, and the culture was continued for an additional 21 d. Gels were then stained with Alizarin red S to detect mineralized foci. (D) Mineralization of cells expressing high levels of kindlin-2 (high) was analyzed after their culture in osteoblast differentiation media. Expression of alkaline phosphatase was used to follow the early commitment of cells to the osteoblast lineage at day 3 (D3), and mineralization was visualized by Alizarin red S staining at day 15 (D15). Bars: (A, C, and D) 1 mm; (B) 20 µm.
We next considered how fibronectin influences mineralization and hypothesized that fibronectin directs deposition of other molecules to support mineralization. Because fibronectin directly binds to type I collagen and is important for its deposition, we immunostained for type I collagen control (wild type), Icap-1−/−, or β1−/− osteoblasts during their differentiation (Fig. 9 B). In contrast to controls that clearly showed a significant and reproducible increase in collagen immunoreactivity after differentiation, Icap-1– as well as β1-deficient cells did not increase their amount of type I collagen. Very similar results were obtained when control cells were treated with FUD (unpublished data). These results show that fibronectin is an important regulator of type I collagen deposition by osteoblasts. To relate the lack of mineralization in cultures of mutant cells with the absence of a “mineralizable” matrix, we seeded control, Icap-1–, and β1-deficient cells in gels containing a high concentration of collagen. Induction of differentiation led to mineralization in both control and Icap-1–deficient osteoblasts, although to a much lower extent in Icap-1–null osteoblasts (Fig. 9 C). β1 integrin–deficient osteoblasts also displayed mineralization but to a much lower extent than control cells, likely reflecting their profound defect in proliferation (Fig. 9 C). Therefore, by providing an appropriate matrix, Icap-1−/− cells were able to mineralize, demonstrating that in vitro, the absence of mineralization is primarily caused by an altered matrix.
Because the effect of a lack of ICAP-1 on fibronectin deposition could be reproduced by kindlin-2 overexpression, we asked whether this phenocopy could be extended to the mineralization defect. Indeed, overexpression of kindlin-2 strongly repressed mineralization, supporting our previous findings (Fig. 9 D). Altogether, these data highlight a novel important function of ICAP-1 in regulating kindlin-2 recruitment on β1 integrin and the subsequent extracellular matrix organization.
Discussion
Icap-1 regulates fibronectin assembly in a β1 integrin–dependent manner
The experiments described herein define a new role for ICAP-1 in facilitating fibronectin fibrillogenesis. Our investigations explain why germline deletion of Icap-1 in mice impairs osteoblast differentiation and proliferation in vitro and in vivo and why Icap-1–deficient osteoblasts display defects of adhesion, compaction, and migration.
Building on our previous study demonstrating increased assembly of focal adhesions in the absence of ICAP-1 (Millon-Frémillon et al., 2008), we show here that loss of ICAP-1 perturbs the maturation of focal adhesions into fibrillar adhesions. Interestingly, expressing preactivated integrin bearing mutation D759A reproduced the altered dynamics of focal adhesions seen in Icap-1–null cells but not the reduced fibronectin fibrillogenesis. Reconciling this apparent discrepancy calls for more extensive work, but it is likely that the transition of focal adhesions to fibrillar adhesions requires cycling and/or recruitment of critical proteins. Supporting this view is the distribution of talin, which is more concentrated in focal adhesions than in fibrillar adhesions. Conversely, tensin is almost absent from focal adhesions (for cells cultured in complete medium) but enriched in fibrillar adhesions. Thus, one can easily envision that focal and fibrillar adhesion dynamics, formation, or initiation might be differentially regulated. In such a model, the importance of the salt bridge of α and β1 integrin cytoplasmic tails might be more important in one context than the other. Talin, which has been reported to disrupt the salt bridge (Anthis et al., 2009), is more concentrated in focal adhesions. The salt bridge disruption may be less important for the dynamics of fibrillar adhesions, which contain little talin, and may be, instead, controlled by other tail–effector interactions. In any case, our findings are consistent with the absence of an obvious phenotype in a knockin mouse model expressing the D759A mutation (Czuchra et al., 2006).
The effect of ICAP-1 on the cell ability to assemble fibronectin fibers was likely dependent on the direct interaction between ICAP-1 and the β1 integrin chain, as ascertained by the finding that expression of mutated β1 integrin with reduced ICAP-1 affinity recapitulates both defects: i.e., the lack of fibronectin assembly and mineralization defect. Furthermore, we provide evidence that ICAP-1 plays an important role in regulating the recruitment of β1 integrins to fibrillar adhesions and, thereby, the dynamics of fibrillar adhesions.
These results support the view that fibrillar adhesions and focal adhesions are distinct structures with specific composition and dynamics (Cukierman et al., 2001; Green et al., 2009). In addition, the two adhesion types support different functions of β1 integrins: focal adhesions for cell adhesion and fibrillar adhesions for deposition and organization of the extracellular matrix. How these structures are related is an open question. Locking integrin affinity would be expected to cause defects in spreading and migration mediated by focal adhesions or in extracellular matrix organization mediated by fibrillar adhesions. One interesting observation is the segregation of β1 integrins in either focal adhesions or fibrillar adhesions depending on the cellular context. We always observed formation of fibrillar adhesion sites when cells were cultured on an uncoated substrate. Conversely, forcing β1 integrin into focal adhesions by seeding cells on concentrated fibronectin-coated surfaces or blocking fibronectin assembly was associated with reduced fibrillar adhesions but increased focal adhesion formation. Our hypothesis is that either ICAP-1 loss or increase in kindlin-2 expression favors β1 localization at focal adhesion sites and disfavors its recruitment at fibrillar adhesions. However, this view does not rule out that β1 integrin could be required at an early stage in focal adhesion assembly before being engaged in fibrillar adhesions. This would explain why cells need β1 integrin activation for fibrillar adhesions to be formed (Green et al., 2009). Loss of ICAP-1 would interfere with the release of integrin-associated proteins, such as kindlin and talin in focal adhesions and, thereby, would reduce the formation or maturation of fibrillar adhesion sites. Additional work should be performed to decipher at the molecular level how β1 integrin participates in focal to fibrillar adhesion assembly. But, for the first time, our present work points out the importance of integrin cellular adaptors in this process.
Integrin-linked kinase (ILK), PINCH, parvins, and kindlins belong to a protein complex that is involved in fibrillar adhesion maturation (Vouret-Craviari et al., 2004; Stanchi et al., 2009). Loss of kindlins in mice leads to severe phenotypes associated with integrin dysfunction in cells (Moser et al., 2008, 2009; Ussar et al., 2008). Kindlins bind to the most distal NPxY motif on the β1 integrin cytoplasmic tail (Meves et al., 2009), where the ICAP-1 binding site has also been mapped (Chang et al., 1997). Thus, the two proteins would be expected to compete for the same overlapping site. This hypothesis is supported by our experiments demonstrating that the loss of ICAP-1 or the expression of a β1 integrin mutation within the ICAP-1 binding site increases kindlin-2 recruitment on the β1 integrin cytoplasmic domain and within focal adhesion sites. ILK, which is recruited at focal adhesion via kindlin-2 in worm and C2C12 cells (Mackinnon et al., 2002; Dowling et al., 2008), is also involved in fibronectin deposition (Stanchi et al., 2009). Although the interplay among ILK, ICAP-1, and kindlin-2 remains to be unraveled, one may now place ILK downstream of ICAP-1 as well as kindlin-2.
Fibronectin fibrillogenesis is required for osteoblast mineralization
Both the composition and the physical state of the extracellular matrix play an important role in controlling osteoblast differentiation and mineralization. For instance, hMSCs cultured on a stiff matrix preferentially commit to the osteoblast lineage (McBeath et al., 2004; Engler et al., 2006). The extracellular matrix can affect osteoblast differentiation both by providing specific integrin binding sites and by acting as a reservoir for small signaling molecules, such as BMPs or FGFs (Margosio et al., 2003; Grünert et al., 2007). Previous studies have established the involvement of the extracellular matrix in osteoblast differentiation and mineralization (Moursi et al., 1996, 1997). Indeed, fibronectin has been shown to be important for osteoblast differentiation and survival (Moursi et al., 1996, 1997). Our present study not only provides important molecular mechanisms explaining these data but also shows the first direct experimental evidence that fibronectin assembly in itself is crucial for mineralization. By modulating β1 integrin translocation into fibrillar adhesions, ICAP-1 regulates the amount, the structure, and the assembly of matrix-associated fibronectin, which is important for the formation of a competent extracellular matrix allowing proper mineralization. Our attempts to identify the specific integrin receptors involved in this process by using blocking antibodies raised against specific α subunits have failed, possibly because of quick endocytosis of the antibodies during the course of the experiment (unpublished data). However, considering the predominant role of the α5β1 integrin for fibronectin fibrillogenesis, it is likely that this integrin is also crucial for mineralization. It has been reported that fibronectin serves as a scaffolding matrix for additional extracellular proteins, such as collagens and TGF-β, but also for sequestering and presenting diffusible factors, such as BMPs and FGFs (Sottile and Hocking, 2002; Huang et al., 2009; Hynes, 2009). Therefore, interfering with fibronectin assembly will affect the overall matrix environment, making it less permissive for proper mineralization. In line with our present data is the observation that the maintenance of an extracellular matrix of fibronectin as well as collagen requires continuous fibronectin assembly (Sottile and Hocking, 2002; Shi et al., 2010).
Fibronectin is important for osteoblast compaction
Osteoblast compaction is an important early step during their differentiation (Lecanda et al., 2000). In the absence of efficient fibronectin assembly, osteoblast compaction was severely reduced. Therefore, Icap-1–deficient osteoblasts that displayed reduced fibronectin deposition were unable to properly compact. Similarly, osteoblasts deficient in β1 integrin expression had a severe defect in cell compaction. Consistent with our findings, fibronectin is important for cell compaction of mesenchymal cells, showing that a proper extracellular matrix also supports cell compaction in the mesenchymal cell lineage (Robinson et al., 2004; Salmenperä et al., 2008). Surprisingly, cadherins expressed on osteoblasts (Stains and Civitelli, 2005) are not sufficient to support efficient cell compaction in the absence of β1 integrin even though small cell aggregates were observed in β1-null osteoblasts, suggesting that cadherins could be involved at earlier stages. More investigations will be necessary to address the exact function of cadherins during this process.
ROCK has been shown to interact with ICAP-1 (Stroeken et al., 2006). In our present work, we did not evidence any linear connection between ICAP-1 and ROCK in the regulation of cell compaction. Indeed, inhibition of ROCK as well as loss of ICAP-1 expression led to cell compaction and the fibronectin deposition defect. However, ROCK inhibition in Icap-1–deficient cells further reduced cell compaction and fibronectin fibrillogenesis, suggesting that both proteins may act through distinct signaling pathways. Such ROCK-dependent pathways could be activated via the fibronectin receptor syndecan as recently proposed (Wang et al., 2010).
In conclusion, we report a molecular mechanism for the osteoblast differentiation defect that is present in Icap-1–deficient mice. ICAP-1, likely by interacting directly with β1 integrin, is important for translocation of β1 integrins into fibrillar adhesions, which are required for proper fibronectin self-assembly into fibrils. Moreover, we show that fibronectin assembly, in turn, allows mineralization. Thus, for the first time, we provide the mechanism by which ICAP-1 affects bone mineralization at a late stage of osteoblast differentiation.
Materials and methods
Mice and antibodies
Mice with a targeted mutation on the Icap-1 locus (Itgb1bp1tm1Ref) were genotyped as previously reported (Bouvard et al., 2007). Mouse strains with floxed alleles of the genes encoding β1 integrin (Itgb1tm1Ref) and fibronectin (Fn1tm1Ref) have been described previously (Brakebusch et al., 2000; Potocnik et al., 2000; Sakai et al., 2001).
Polyclonal anti–ICAP-1 antibodies were described previously (1:1,500; Bouvard et al., 1998). Monoclonal antibodies against actin (A2066; 1:1,000), vinculin (clone hVIN1; 1:2,000), and talin (clone 8d4; 1:200) as well as the polyclonal antibodies against fibronectin (F3648; 1:1,000) and kindlin-2 (K3269; 1:1,000) were obtained from Sigma-Aldrich. The polyclonal anti–β1 integrin serum was obtained from Millipore (1:1,500). The polyclonal anti–β1 integrin cytoplasmic domain antibody was described previously (Martel et al., 2001). The monoclonal anti–β1 integrin antibodies 9EG7 and MB1.2 were purchased from BD (1:100) and Millipore (1:100), respectively. Antiphosphotyrosine monoclonal antibody 4G10 used as hybridoma supernatant was produced in our laboratory. The monoclonal anti-EGFP antibody (b-2; 1:1,000) was purchased from Santa Cruz Biotechnology, Inc.
Plasmids
The β1-expressing construct was based on the pCLMFG retroviral vector, in which the wild-type human β1 integrin had been directionally inserted using EcoR1 and Not1 sites. D759A and V787T mutations were introduced in β1 integrin by a mutation kit QuikChange; QIAGEN and verified by sequencing. Expression of mRFP-tensin was performed using the pCLMFG-mRFP-tensin plasmid as previously described (Stanchi et al., 2009). FUD arises from the first fibronectin binding motif of the Streptococcus pyogenes adhesin protein F1. It encompasses the 43 residues of the upstream nonrepetitive domain plus the first six residues of the first 37-residue repeat of the RD5 region (Tomasini-Johansson et al., 2001). FUD was produced recombinantly as previously described (Ensenberger et al., 2004). pCLMFG-EGFP–kindlin-2 was obtained from R. Fässler (Max Planck Institute of Biochemistry, Martinsried, Germany). cDNA encoding the talin head domain was extracted from pBlueScript(SK−)-talin (aa 1–1,445; gift from R.O. Hynes, Massachusetts Institute of Technology, Cambridge, MA) using Spe1 and EcoRV sites and inserted in the pEGFP-N1 plasmid by SalI restriction after refilling.
Isolation, immortalization, infection, and in vitro Cre-mediated deletion of osteoblasts
A primary mouse osteoblast-enriched cell population was isolated from newborn calvaria by using a mixture of 0.3 mg/ml collagenase type I (Sigma-Aldrich) and 0.25% trypsin (Invitrogen) as described previously (Bellows et al., 1986; Bouvard et al., 2007). Cells were grown in α-MEM medium containing 10% FCS. Primary osteoblasts (passage 2) were immortalized by transduction with a retrovirus expressing the large SV40 T antigen (Fässler et al., 1995), cloned, and tested for their ability to induce alkaline phosphatase upon differentiation (Mansukhani et al., 2000) as previously described (Bouvard et al., 2007). At least five clones from wild-type or floxed mice were isolated. Rescue of ICAP-1 or β1 integrin expression in null cells was performed via retroviral infection using the pCLMFG-Icap-1-IRES-EGFP and the pCLMFG-β1 vectors, respectively, as previously described (Bouvard et al., 2007; Millon-Frémillon et al., 2008). β1- and fibronectin-floxed immortalized osteoblasts were infected with an adenoviral supernatant encoding the Cre recombinase (provided by R. Meuwissen, Institut Albert Bonniot, Grenoble, France) for 1 h in PBS supplemented with 2% FCS and 1 mM MgCl2.
Solid-phase assay and pull-down assay
ICAP-1 binding onto the cytoplasmic tail of β1WT or β1V787T integrin was performed using an enzyme-linked immunosorbent assay. A 96-well tray (MaxiSorp; Thermo Fisher Scientific) was coated overnight at 4°C with various concentrations of His–ICAP-1 (0, 1, and 5 µg/ml) and blocked for 1 h at room temperature with a 3% BSA/PBS solution. 5 µg/well GST, 3% BSA alone, or 10 µg/well GST-tagged cytoplasmic β1WT and cytoplasmic β1V787T were incubated for 1 h at 37°C. After three washes with 3% BSA/0.01% Tween 20/PBS, the cytoplasmic β1WT and cytoplasmic β1V787T peptides were detected using a polyclonal antibody against the β1 cytoplasmic tail for 45 min at 37°C and an HRP-conjugated secondary antibody (Bio-Rad Laboratories) for an additional 45 min at 37°C. Peroxidase activity was visualized using ABTS reagent at 405 nm. The efficiency of ICAP-1 binding onto cytoplasmic β1WT or cytoplasmic β1V787T was expressed after subtraction of GST and BSA signals.
Pull-down assays for talin and kindlin-2 were performed as previously described (Lad et al., 2007). In brief, either HEK 293 or ICAP-1–transfected HEK 293 cells were washed with cold PBS and lysed by scraping in 0.5 ml cell lysis buffer (50 mM NaCl, 10 mM Pipes, 150 mM sucrose, 50 mM NaF, 40 mM Na4P2O7.10H2O, 1 mM Na3VO4, pH 6.8, 0.5% Triton X-100, 0.1% sodium deoxycholate, and EDTA-free protease inhibitor tablet) on ice. The cell lysate was cleared by centrifugation at 15,000 g for 30 min at 4°C. 500 µg lysate was incubated with 10 µg GST-β1–, GST-β3–, or GST-coated beads for 2 h at 4°C. After three washes in lysis buffer, beads were resuspended in 2× Laemmli buffer, and samples were used in Western blotting to visualize talin and kindlin-2.
Compaction assay in hanging drops
Immortalized cells were harvested by trypsin digestion and washed twice in DME medium. Drops of 10 µl DME-FCS (10%) medium containing 25,000 cells were spotted onto the coverlid of 10-cm Petri dishes, inverted, and placed on a Petri dish containing 8 ml PBS. Spheroid compaction was then followed over a 72 h period, and images were taken with a binocular microscope (SMZ-2T; Nikon) equipped with a digital camera (DP70; Olympus). When ROCK inhibitor Y27632 (EMD) was used, cells were resuspended into DME-FCS supplemented with 10 µM Y27632 and then spotted on the coverlid as described previously in this paper.
Osteoblast differentiation
In vitro differentiation of isolated osteoblasts was performed essentially as previously described (Globus et al., 1998). In brief, 60,000 cells per well were plated in a 24-well tray. After 3 d of culture, when cells were confluent, the medium was switched to differentiation medium (α-MEM, 10% FCS, 50 µg/ml ascorbic acid, and 10 mM β-glycerophosphate) and changed every other day. The differentiation process was visualized by alkaline phosphatase staining for osteoblast activity and by Alizarin red S staining for calcium deposition as previously described (Bouvard et al., 2007). For collagen gel mineralization, a highly concentrated type I collagen solution was used (9.3 mg/ml; BD). A total of 300 µl type I collagen (5 mg/ml final concentration) containing 8 × 105 cells per gel was loaded in a 24-well plate. Gels were grown for 1 wk and then placed in differentiation medium for 3 wk. Gels were stained directly with Alizarin red S dye or cryosectioned before staining.
Visualization and quantification of fibronectin deposition and secretion
104 cells were seeded into a 24-well tray and cultured for 3 d in complete medium. Matrix-associated fibronectin was extracted after cell lysis in deoxycholate-containing buffer and centrifugation (15,000 rpm for 30 min at 4°C) as previously described (Schwarzbauer, 1991). The pellet fraction containing the pool of fibronectin associated within the matrix is referred to as insoluble fibronectin, whereas supernatant fibronectin is referred to as soluble fibronectin. Western blotting was performed as described previously (Bouvard et al., 1998). Quantification of fibronectin in soluble and insoluble fractions was performed using ImageJ (National Institutes of Health). Samples were also blotted for vinculin (1:1,500) or actin (1:1,500) to ensure that the same amounts of protein were loaded. ROCK inhibitor Y27632 (EMD) was used at the final concentration of 10 µM and added to cells seeded into a 24-well tray.
For fibronectin secretion, cells were incubated overnight in serum-free condition. Both culture supernatant and cells were used to visualize by Western blotting the amount of secreted and cellular fibronectin. Band intensity was quantified using ImageJ software.
For cellular fibronectin, cells were resuspended in trypsin/EDTA. Trypsin was then blocked with soybean trypsin inhibitor, and cells were washed twice in PBS (this treatment leads to an undetectable amount of cell surface–associated fibronectin as measured by FACS). Then, cells were lysed in radioimmunoprecipitation assay buffer, and equal amounts of protein were loaded on a gel for Western blotting to quantify fibronectin expression.
RNA isolation and real-time quantitative PCR
Total RNA was harvested from wild-type and Icap-1–null cell cultures by a minispin kit (NucleoSpin RNA II; Macherey-Nagel) according to the manufacturer’s instructions. Then, 1.5 µg total RNA was reverse transcribed using a cDNA synthesis kit (SuperScript VILO; Invitrogen) and 0.4 µl of the resulting cDNA reaction mix was subjected to quantitative PCR using quantitative PCR mix (GoTaq qPCR Master Mix; Promega) in a real-time PCR system (Mx3005P; Agilent Technologies). Real-time data were collected for 40 cycles at 95°C for 30 s, 55°C for 1 min, and 72°C for 30 s. Mouse primers for fibronectin and collagen I were the following: forward, 5′-ATGTGGACCCCTCCTGATAGT-3′, and reverse, 5′-GCCCAGTGATTTCAGCAAAGG-3′; and forward, 5′-CCTGGTAAAGATGGGCC-3′, and reverse, 5′-CACCAGGTTCACCTTTCGCACC-3′, respectively. The level of RNA for Icap-1–null cells compared with wild-type cells and normalized to Ranbp1 was calculated using the comparative cycle threshold method of quantification.
Time-lapse video microscopy
mRFP-tensin–expressing osteoblasts were seeded in complete medium on uncoated chambers (Labtek; Thermo Fisher Scientific) and imaged as previously described (Millon-Frémillon et al., 2008). In brief, after overnight spreading, cells were subjected to time-lapse video microscopy using a microscope (Axiovert 200M; Carl Zeiss) equipped with a thermostatic chamber. Images were acquired every 5 min over a 6-h period. Out of the stack, three images corresponding to three different time points were then selected and overlapped using MetaMorph software (Molecular Devices) after subtracting cell displacement. The centripetal translocation of fibrillar adhesions was shown by arbitrarily coloring each time-point image.
FACS, immunohistology, and immunofluorescence
FACS analysis and immunohistology were performed as previously described (Bouvard et al., 2007). For immunofluorescence, cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 (this step was omitted in the case of fibronectin staining), and incubated with appropriate primary antibodies. After being rinsed, coverslips were incubated with an appropriate Alexa Fluor–conjugated secondary antibody. The cells were mounted in Mowiol/DAPI solution and imaged on an inverted confocal microscope (LSM510; Carl Zeiss).
Online supplemental material
Fig. S1 shows expression and localization of β1 integrins and its mutated forms in β1-null osteoblasts. Fig. S2 shows that FUD treatment alters neither cell shape nor cell proliferation and survival. Fig. S3 shows that Icap-1−/− spheroids exhibit a defect in fibronectin deposition that is not caused by a defect in fibronectin and type I collagen expression or fibronectin secretion. Fig. S4 shows that β1V787T integrin mutation interferes with ICAP-1 binding but not with kindlin-2 recruitment. Fig. S5 shows a defect in the translocation of β1-containing fibrillar adhesion. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201007108/DC1.
Supplementary Material
Acknowledgments
This work is dedicated to Rachel.
We are grateful to Dr. Reinhard Fässler for providing us with β1 integrin– and fibronectin-floxed mice and the EGFP–kindlin-2 construct and for critical reading of the manuscript. We also thank Drs. Martin Humphries and M. Billaud for their critical reading and suggestions. We thank Dr. Alexei Grichine for microscopy and Dr. Juliana Ribeyron and Dr. Sandrine De Seranno for FACS sorting.
M. Brunner is supported by a Ministère de la Recherche et Technologie fellowship, and A. Millon-Frémillon was supported by the Association pour la Recherche sur le Cancer. This research was supported by a Pro-A Institut National de la Santé et de la Recherche Médicale grant and Ligue Contre le Cancer (to D. Bouvard), National Institutes of Health grant HL21644 (to D. Mosher), the Max Planck Society and the University of Heidelberg (to I. Nakchbandi), and the Association pour la Recherche sur le Cancer and Région Rhône-Alpes (to C. Albigès-Rizo). The team is supported by the Ligue Nationale Contre le Cancer as Equipe Labellisée Ligue 2010.
Footnotes
Abbreviations used in this paper:
- BMP
- bone morphogenetic protein
- FUD
- functional upstream domain
- hMSC
- human mesenchymal stem cells
- ILK
- integrin-linked kinase
- mRFP
- monomeric RFP
- ROCK
- Rho-associated kinase
References
- Anthis N.J., Wegener K.L., Ye F., Kim C., Goult B.T., Lowe E.D., Vakonakis I., Bate N., Critchley D.R., Ginsberg M.H., Campbell I.D. 2009. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 28:3623–3632 10.1038/emboj.2009.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows C.G., Sodek J., Yao K.L., Aubin J.E. 1986. Phenotypic differences in subclones and long-term cultures of clonally derived rat bone cell lines. J. Cell. Biochem. 31:153–169 10.1002/jcb.240310207 [DOI] [PubMed] [Google Scholar]
- Bentmann A., Kawelke N., Moss D., Zentgraf H., Bala Y., Berger I., Gasser J.A., Nakchbandi I.A. 2010. Circulating fibronectin affects bone matrix, whereas osteoblast fibronectin modulates osteoblast function. J. Bone Miner. Res. 25:706–715 [DOI] [PubMed] [Google Scholar]
- Bouvard D., Molla A., Block M.R. 1998. Calcium/calmodulin-dependent protein kinase II controls alpha5beta1 integrin-mediated inside-out signaling. J. Cell Sci. 111:657–665 [DOI] [PubMed] [Google Scholar]
- Bouvard D., Brakebusch C., Gustafsson E., Aszódi A., Bengtsson T., Berna A., Fässler R. 2001. Functional consequences of integrin gene mutations in mice. Circ. Res. 89:211–223 10.1161/hh1501.094874 [DOI] [PubMed] [Google Scholar]
- Bouvard D., Vignoud L., Dupé-Manet S., Abed N., Fournier H.N., Vincent-Monegat C., Retta S.F., Fässler R., Block M.R. 2003. Disruption of focal adhesions by integrin cytoplasmic domain-associated protein-1 alpha. J. Biol. Chem. 278:6567–6574 10.1074/jbc.M211258200 [DOI] [PubMed] [Google Scholar]
- Bouvard D., Millon-Fremillon A., Dupe-Manet S., Block M.R., Albigès-Rizo C. 2006. Unraveling ICAP-1 function: toward a new direction? Eur. J. Cell Biol. 85:275–282 10.1016/j.ejcb.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Bouvard D., Aszodi A., Kostka G., Block M.R., Albigès-Rizo C., Fässler R. 2007. Defective osteoblast function in ICAP-1-deficient mice. Development. 134:2615–2625 10.1242/dev.000877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C., Grose R., Quondamatteo F., Ramirez A., Jorcano J.L., Pirro A., Svensson M., Herken R., Sasaki T., Timpl R., et al. 2000. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 19:3990–4003 10.1093/emboj/19.15.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.D., Wong C., Smith H., Liu J. 1997. ICAP-1, a novel β1 integrin cytoplasmic domain–associated protein, binds to a conserved and functionally important NPXY sequence motif of β1 integrin. J. Cell Biol. 138:1149–1157 10.1083/jcb.138.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.D., Hoang B.Q., Liu J., Springer T.A. 2002. Molecular basis for interaction between Icap1 alpha PTB domain and beta 1 integrin. J. Biol. Chem. 277:8140–8145 10.1074/jbc.M109031200 [DOI] [PubMed] [Google Scholar]
- Chiba H., Sawada N., Ono T., Ishii S., Mori M. 1993. Establishment and characterization of a simian virus 40-immortalized osteoblastic cell line from normal human bone. Jpn. J. Cancer Res. 84:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E., Pankov R., Stevens D.R., Yamada K.M. 2001. Taking cell-matrix adhesions to the third dimension. Science. 294:1708–1712 10.1126/science.1064829 [DOI] [PubMed] [Google Scholar]
- Czuchra A., Meyer H., Legate K.R., Brakebusch C., Fässler R. 2006. Genetic analysis of β1 integrin “activation motifs” in mice. J. Cell Biol. 174:889–899 10.1083/jcb.200604060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas S.L., Sivakumar P., Jones C.J., Chen Q., Peters D.M., Mosher D.F., Humphries M.J., Kielty C.M. 2005. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 280:18871–18880 10.1074/jbc.M410762200 [DOI] [PubMed] [Google Scholar]
- Dowling J.J., Vreede A.P., Kim S., Golden J., Feldman E.L. 2008. Kindlin-2 is required for myocyte elongation and is essential for myogenesis. BMC Cell Biol. 9:36 10.1186/1471-2121-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A.J., Sen S., Sweeney H.L., Discher D.E. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126:677–689 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Ensenberger M.G., Tomasini-Johansson B.R., Sottile J., Ozeri V., Hanski E., Mosher D.F. 2001. Specific interactions between F1 adhesin of Streptococcus pyogenes and N-terminal modules of fibronectin. J. Biol. Chem. 276:35606–35613 10.1074/jbc.M105417200 [DOI] [PubMed] [Google Scholar]
- Ensenberger M.G., Annis D.S., Mosher D.F. 2004. Actions of the functional upstream domain of protein F1 of Streptococcus pyogenes on the conformation of fibronectin. Biophys. Chem. 112:201–207 10.1016/j.bpc.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Fässler R., Pfaff M., Murphy J., Noegel A.A., Johansson S., Timpl R., Albrecht R. 1995. Lack of β1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J. Cell Biol. 128:979–988 10.1083/jcb.128.5.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Chen Y., Prijatelj P., Sakai T., Fässler R., Sakai L.Y., Rifkin D.B. 2005. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 19:1798–1808 10.1096/fj.05-4134com [DOI] [PubMed] [Google Scholar]
- Giancotti F.G., Ruoslahti E. 1999. Integrin signaling. Science. 285:1028–1032 10.1126/science.285.5430.1028 [DOI] [PubMed] [Google Scholar]
- Globus R.K., Doty S.B., Lull J.C., Holmuhamedov E., Humphries M.J., Damsky C.H. 1998. Fibronectin is a survival factor for differentiated osteoblasts. J. Cell Sci. 111:1385–1393 [DOI] [PubMed] [Google Scholar]
- Green J.A., Berrier A.L., Pankov R., Yamada K.M. 2009. beta1 integrin cytoplasmic domain residues selectively modulate fibronectin matrix assembly and cell spreading through talin and Akt-1. J. Biol. Chem. 284:8148–8159 10.1074/jbc.M805934200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünert M., Dombrowski C., Sadasivam M., Manton K., Cool S.M., Nurcombe V. 2007. Isolation of a native osteoblast matrix with a specific affinity for BMP2. J. Mol. Histol. 38:393–404 10.1007/s10735-007-9119-0 [DOI] [PubMed] [Google Scholar]
- Hall B.K., Miyake T. 2000. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 22:138–147 [DOI] [PubMed] [Google Scholar]
- Hamidouche Z., Fromigué O., Ringe J., Häupl T., Vaudin P., Pagès J.C., Srouji S., Livne E., Marie P.J. 2009. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. USA. 106:18587–18591 10.1073/pnas.0812334106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Zhang Y., Kim B., Ge G., Annis D.S., Mosher D.F., Greenspan D.S. 2009. Fibronectin binds and enhances the activity of bone morphogenetic protein 1. J. Biol. Chem. 284:25879–25888 10.1074/jbc.M109.024125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P.E., Diaz-Gonzalez F., Leong L., Wu C., McDonald J.A., Shattil S.J., Ginsberg M.H. 1996. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J. Biol. Chem. 271:6571–6574 10.1074/jbc.271.12.6571 [DOI] [PubMed] [Google Scholar]
- Hynes R.O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 69:11–25 10.1016/0092-8674(92)90115-S [DOI] [PubMed] [Google Scholar]
- Hynes R.O. 2009. The extracellular matrix: not just pretty fibrils. Science. 326:1216–1219 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O., George E.L., Georges E.N., Guan J.L., Rayburn H., Yang J.T. 1992. Toward a genetic analysis of cell-matrix adhesion. Cold Spring Harb. Symp. Quant. Biol. 57:249–258 [DOI] [PubMed] [Google Scholar]
- Lad Y., Harburger D.S., Calderwood D.A. 2007. Integrin cytoskeletal interactions. Methods Enzymol. 426:69–84 10.1016/S0076-6879(07)26004-5 [DOI] [PubMed] [Google Scholar]
- Larjava H., Plow E.F., Wu C. 2008. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9:1203–1208 10.1038/embor.2008.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda F., Warlow P.M., Sheikh S., Furlan F., Steinberg T.H., Civitelli R. 2000. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 151:931–944 10.1083/jcb.151.4.931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.Q., Qin J., Wu C., Plow E.F. 2008. Kindlin-2 (Mig-2): a co-activator of β3 integrins. J. Cell Biol. 181:439–446 10.1083/jcb.200710196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon A.C., Qadota H., Norman K.R., Moerman D.G., Williams B.D. 2002. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 12:787–797 10.1016/S0960-9822(02)00810-2 [DOI] [PubMed] [Google Scholar]
- Manabe R., Tsutsui K., Yamada T., Kimura M., Nakano I., Shimono C., Sanzen N., Furutani Y., Fukuda T., Oguri Y., et al. 2008. Transcriptome-based systematic identification of extracellular matrix proteins. Proc. Natl. Acad. Sci. USA. 105:12849–12854 10.1073/pnas.0803640105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A., Bellosta P., Sahni M., Basilico C. 2000. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J. Cell Biol. 149:1297–1308 10.1083/jcb.149.6.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margosio B., Marchetti D., Vergani V., Giavazzi R., Rusnati M., Presta M., Taraboletti G. 2003. Thrombospondin 1 as a scavenger for matrix-associated fibroblast growth factor 2. Blood. 102:4399–4406 10.1182/blood-2003-03-0893 [DOI] [PubMed] [Google Scholar]
- Martel V., Racaud-Sultan C., Dupe S., Marie C., Paulhe F., Galmiche A., Block M.R., Albiges-Rizo C. 2001. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem. 276:21217–21227 10.1074/jbc.M102373200 [DOI] [PubMed] [Google Scholar]
- Matsuo K. 2009. Cross-talk among bone cells. Curr. Opin. Nephrol. Hypertens. 18:292–297 10.1097/MNH.0b013e32832b75f1 [DOI] [PubMed] [Google Scholar]
- Maurer L.M., Tomasini-Johansson B.R., Ma W., Annis D.S., Eickstaedt N.L., Ensenberger M.G., Satyshur K.A., Mosher D.F. 2010. Extended binding site on fibronectin for the functional upstream domain of protein F1 of Streptococcus pyogenes. J. Biol. Chem. 285:41087–41099 10.1074/jbc.M110.153692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., Chen C.S. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 6:483–495 10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- Meves A., Stremmel C., Gottschalk K., Fässler R. 2009. The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 19:504–513 10.1016/j.tcb.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Millon-Frémillon A., Bouvard D., Grichine A., Manet-Dupé S., Block M.R., Albigès-Rizo C. 2008. Cell adaptive response to extracellular matrix density is controlled by ICAP-1–dependent β1-integrin affinity. J. Cell Biol. 180:427–441 10.1083/jcb.200707142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez E., Ussar S., Schifferer M., Bösl M., Zent R., Moser M., Fässler R. 2008. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22:1325–1330 10.1101/gad.469408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Nieswandt B., Ussar S., Pozgajova M., Fässler R. 2008. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14:325–330 10.1038/nm1722 [DOI] [PubMed] [Google Scholar]
- Moser M., Bauer M., Schmid S., Ruppert R., Schmidt S., Sixt M., Wang H.V., Sperandio M., Fässler R. 2009. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med. 15:300–305 10.1038/nm.1921 [DOI] [PubMed] [Google Scholar]
- Moursi A.M., Damsky C.H., Lull J., Zimmerman D., Doty S.B., Aota S., Globus R.K. 1996. Fibronectin regulates calvarial osteoblast differentiation. J. Cell Sci. 109:1369–1380 [DOI] [PubMed] [Google Scholar]
- Moursi A.M., Globus R.K., Damsky C.H. 1997. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J. Cell Sci. 110:2187–2196 [DOI] [PubMed] [Google Scholar]
- Pankov R., Cukierman E., Katz B.Z., Matsumoto K., Lin D.C., Lin S., Hahn C., Yamada K.M. 2000. Integrin dynamics and matrix assembly: tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 148:1075–1090 10.1083/jcb.148.5.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.A., Almeida E.A., Hill E.L., Aguirre J.I., Rivera M.F., Nachbandi I., Wronski T.J., van der Meulen M.C., Globus R.K. 2008. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol. 27:609–618 10.1016/j.matbio.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Potocnik A.J., Brakebusch C., Fässler R. 2000. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 12:653–663 10.1016/S1074-7613(00)80216-2 [DOI] [PubMed] [Google Scholar]
- Ramirez F., Rifkin D.B. 2009. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr. Opin. Cell Biol. 21:616–622 10.1016/j.ceb.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E.E., Foty R.A., Corbett S.A. 2004. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol. Biol. Cell. 15:973–981 10.1091/mbc.E03-07-0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T., DeSimone D.W. 2010. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341:126–140 10.1016/j.ydbio.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Zhang Q., Fässler R., Mosher D.F. 1998. Modulation of β1A integrin functions by tyrosine residues in the β1 cytoplasmic domain. J. Cell Biol. 141:527–538 10.1083/jcb.141.2.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Johnson K.J., Murozono M., Sakai K., Magnuson M.A., Wieloch T., Cronberg T., Isshiki A., Erickson H.P., Fässler R. 2001. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat. Med. 7:324–330 10.1038/85471 [DOI] [PubMed] [Google Scholar]
- Salmenperä P., Kankuri E., Bizik J., Sirén V., Virtanen I., Takahashi S., Leiss M., Fässler R., Vaheri A. 2008. Formation and activation of fibroblast spheroids depend on fibronectin-integrin interaction. Exp. Cell Res. 314:3444–3452 10.1016/j.yexcr.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J.E. 1991. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J. Cell Biol. 113:1463–1473 10.1083/jcb.113.6.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Harman J., Fujiwara K., Sottile J. 2010. Collagen I matrix turnover is regulated by fibronectin polymerization. Am. J. Physiol. Cell Physiol. 298:C1265–C1275 10.1152/ajpcell.00341.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J., Hocking D.C. 2002. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell. 13:3546–3559 10.1091/mbc.E02-01-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stains J.P., Civitelli R. 2005. Cell-cell interactions in regulating osteogenesis and osteoblast function. Birth Defects Res. C Embryo Today. 75:72–80 10.1002/bdrc.20034 [DOI] [PubMed] [Google Scholar]
- Stanchi F., Grashoff C., Nguemeni Yonga C.F., Grall D., Fässler R., Van Obberghen-Schilling E. 2009. Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation. J. Cell Sci. 122:1800–1811 10.1242/jcs.044602 [DOI] [PubMed] [Google Scholar]
- Stroeken P.J., Alvarez B., Van Rheenen J., Wijnands Y.M., Geerts D., Jalink K., Roos E. 2006. Integrin cytoplasmic domain-associated protein-1 (ICAP-1) interacts with the ROCK-I kinase at the plasma membrane. J. Cell. Physiol. 208:620–628 10.1002/jcp.20699 [DOI] [PubMed] [Google Scholar]
- Tomasini-Johansson B.R., Kaufman N.R., Ensenberger M.G., Ozeri V., Hanski E., Mosher D.F. 2001. A 49-residue peptide from adhesin F1 of Streptococcus pyogenes inhibits fibronectin matrix assembly. J. Biol. Chem. 276:23430–23439 10.1074/jbc.M103467200 [DOI] [PubMed] [Google Scholar]
- Ussar S., Moser M., Widmaier M., Rognoni E., Harrer C., Genzel-Boroviczeny O., Fässler R. 2008. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 4:e1000289 10.1371/journal.pgen.1000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouret-Craviari V., Boulter E., Grall D., Matthews C., Van Obberghen-Schilling E. 2004. ILK is required for the assembly of matrix-forming adhesions and capillary morphogenesis in endothelial cells. J. Cell Sci. 117:4559–4569 10.1242/jcs.01331 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhao G., Olivares-Navarrete R., Bell B.F., Wieland M., Cochran D.L., Schwartz Z., Boyan B.D. 2006. Integrin beta1 silencing in osteoblasts alters substrate-dependent responses to 1,25-dihydroxy vitamin D3. Biomaterials. 27:3716–3725 10.1016/j.biomaterials.2006.02.022 [DOI] [PubMed] [Google Scholar]
- Wang Z., Collighan R.J., Gross S.R., Danen E.H., Orend G., Telci D., Griffin M. 2010. RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 and alpha5beta1 integrin co-signaling. J. Biol. Chem. 285:40212–40229 10.1074/jbc.M110.123703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G., Wang D., Benson M.D., Karsenty G., Franceschi R.T. 1998. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J. Biol. Chem. 273:32988–32994 10.1074/jbc.273.49.32988 [DOI] [PubMed] [Google Scholar]
- Yoneda A., Ushakov D., Multhaupt H.A., Couchman J.R. 2007. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol. Biol. Cell. 18:66–75 10.1091/mbc.E06-08-0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E., Katz B.Z., Aota S., Yamada K.M., Geiger B., Kam Z. 1999. Molecular diversity of cell-matrix adhesions. J. Cell Sci. 112:1655–1669 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Checovich W.J., Peters D.M., Albrecht R.M., Mosher D.F. 1994. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J. Cell Biol. 127:1447–1459 10.1083/jcb.127.5.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.A., Hemler M.E. 1999. Interaction of the integrin beta1 cytoplasmic domain with ICAP-1 protein. J. Biol. Chem. 274:11–19 10.1074/jbc.274.1.11 [DOI] [PubMed] [Google Scholar]
- Zhong C., Chrzanowska-Wodnicka M., Brown J., Shaub A., Belkin A.M., Burridge K. 1998. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141:539–551 10.1083/jcb.141.2.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Rowe R.G., Hiraoka N., George J.P., Wirtz D., Mosher D.F., Virtanen I., Chernousov M.A., Weiss S.J. 2008. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev. 22:1231–1243 10.1101/gad.1643308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D., Jin F., Leboy P., Hardy S., Damsky C. 2000. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev. Biol. 220:2–15 10.1006/dbio.2000.9633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.