nSMase2, which cleaves sphingomyelin to generate bioactive lipids, is required for chondrocyte apoptosis and, cell autonomously, for bone mineralization.
Abstract
A deletion mutation called fro (fragilitas ossium) in the murine Smpd3 (sphingomyelin phosphodiesterase 3) gene leads to a severe skeletal dysplasia. Smpd3 encodes a neutral sphingomyelinase (nSMase2), which cleaves sphingomyelin to generate bioactive lipid metabolites. We examined endochondral ossification in embryonic day 15.5 fro/fro mouse embryos and observed impaired apoptosis of hypertrophic chondrocytes and severely undermineralized cortical bones in the developing skeleton. In a recent study, it was suggested that nSMase2 activity in the brain regulates skeletal development through endocrine factors. However, we detected Smpd3 expression in both embryonic and postnatal skeletal tissues in wild-type mice. To investigate whether nSMase2 plays a cell-autonomous role in these tissues, we examined the in vitro mineralization properties of fro/fro osteoblast cultures. fro/fro cultures mineralized less than the control osteoblast cultures. We next generated fro/fro;Col1a1-Smpd3 mice, in which osteoblast-specific expression of Smpd3 corrected the bone abnormalities observed in fro/fro embryos without affecting the cartilage phenotype. Our data suggest tissue-specific roles for nSMase2 in skeletal tissues.
Introduction
ECM mineralization in bones and teeth is a genetically regulated process. In humans, genetic mutations may lead to a variety of diseases affecting ECM mineralization in skeletal and dental tissues, which include X-linked hypophosphatemia, hypophosphatasia, rickets, and some forms of osteogenesis/dentinogenesis imperfecta (Tanaka and Deluca, 1974; Whyte, 1994; Nesbitt et al., 1999; Glorieux et al., 2002; Goldberg et al., 2008). Although considered as a critical physiological process, the molecular mechanism of ECM mineralization is still not fully understood. Identification of novel genetic regulators of this process and elucidation of their modes of action may lead to effective interventions for genetic diseases associated with abnormal skeletal mineralization.
Our current understanding suggests that skeletal and dental ECM mineralization can be attributed to a large extent to the unique promineralization environment of these hard tissues. Two mineral ions, Pi and calcium, when present at physiological concentrations, will promote apatitic mineral crystal growth within and between newly synthesized collagen fibrils in the skeletal ECM (Murshed et al., 2005). Apart from the mineral ions themselves, extracellular levels of mineralization inhibitors can also affect ECM mineralization (Luo et al., 1997; Okawa et al., 1998; Ho et al., 2000; Murshed et al., 2004). For example, >40 yr ago, it was shown that PPi, a chemical derivative of Pi, can potently inhibit the mineralization process (Fleisch and Bisaz, 1962; Terkeltaub, 2001). More recently, it has been shown that matrix gla protein (MGP), a small extracellular protein, prevents ECM mineralization in the cartilage and vascular tissues (Luo et al., 1997; Murshed et al., 2004).
Type 1 collagen, a scaffolding ECM protein, and tissue-nonspecific alkaline phosphatase (ALPL [alkaline phosphatase, liver/bone/kidney]), an enzyme required for the cleavage of PPi in the bone matrix, are both necessary for normal bone mineralization (Waymire et al., 1995; Hessle et al., 2002; Murshed et al., 2005). We recently demonstrated the importance of these key determinants of ECM mineralization in an in vivo mouse model, in which Alpl forced expression in the dermis, a fibrillar, collagen-rich soft connective tissue, resulted in ectopic mineralization of the skin (Murshed et al., 2005). Although these findings established the concurrent requirements of a mineral-scaffolding protein matrix and phosphatase activities in skeletal ECM mineralization, they did not rule out the existence of other mechanisms working in concert to regulate this process. A recently identified mutation in a mouse model, which displays altered sphingolipid metabolism and poorly mineralized skeletal tissues, further enforces the likelihood that multiple mechanisms are involved in skeletal mineralization (Guenet et al., 1981; Aubin et al., 2005).
Although initially considered as inert structural molecules, sphingolipids are now recognized as important mediators for signal transduction pathways affecting various cell functions (Merrill et al., 1997; Milhas et al., 2010; Wu et al., 2010). Bone deformities in mouse models lacking a functional Smpd3 (sphingomyelin phosphodiesterase 3) gene underscore the importance of sphingolipid metabolism in skeletal tissues (Aubin et al., 2005; Stoffel et al., 2005). Smpd3 encodes neutral sphingomyelinase 2 (nSMase2), a membrane-bound enzyme, which cleaves sphingomyelin to generate the lipid second messenger ceramide. Ceramide generated by sphingomyelinases or by a de novo pathway affects a wide range of cellular processes, including cell death, proliferation, and differentiation (Obeid et al., 1993; Bose et al., 1995; Richard et al., 1996; Sanchez-Alavez et al., 2006).
In recent years, studies have provided useful perspectives on novel physiological roles for nSMase2 (Kolak et al., 2007; Rutkute et al., 2007; Tellier et al., 2007). Further insight into the functions of this enzyme came with the development of animal models lacking nSMase2 activity. Currently, there are two nSMase2-deficient mouse models available: one was generated by gene targeting (Smpd3−/−), whereas the other carries a chemically induced deletion of 1,758 bp encompassing part of intron 8 and the adjacent exon 9 of the Smpd3 gene (Aubin et al., 2005; Stoffel et al., 2005, 2007). The latter mutation known as fragilitas ossium or fro replaces the last 33 amino acids of nSMase2, resulting in a significant reduction of total neutral sphingomyelinase activities in the tissues of the fro/fro mice (Aubin et al., 2005; Stoffel et al., 2005, 2007). In their recent studies, Stoffel et al. (2005, 2007) characterized the skeletal phenotypes of the Smpd3−/− mice as a chondrodysplasia and speculated a systemic role for neuronal Smpd3 in the regulation of the skeletal development. Although both the Smpd3−/− and fro/fro mutants show similar gross skeletal abnormalities, some phenotypic differences exist between these two models. For example, the skeletal phenotype appears to be milder in Smpd3−/− mice. Also, no bone or tooth mineralization defects were reported in this gene-targeted model. These differences raise the possibility that additional, as yet unknown, mutations in the chemically mutagenized fro/fro model may cause the severe skeletal abnormalities.
The goals of our current study were to characterize the skeletal phenotype of fro/fro mice and to investigate the local role of Smpd3 in osteoblasts. Toward these goals, we performed a detailed characterization of the skeletal tissues in fro/fro embryos and adult mice using skeletal preparations, microcomputed tomography (micro-CT), and histology/histomorphometric analysis. We demonstrate here that the fro mutation affects bone ECM mineralization in both embryos and in adult mice and that there is a delay of apoptosis in the hypertrophic chondrocytes in the developing fro/fro skeleton. We also show that osteoblast-specific expression of the Smpd3 transgene in fro/fro;Col1a1-Smpd3 mice completely rescues the bone mineralization defects, whereas the cartilage phenotype that appears during early skeletal development remains unaffected. Our work establishes the fro mutation as the sole cause of skeletal abnormalities in the fro/fro mice and suggests a cell-autonomous, tissue-specific role for nSMase2 in the developing skeleton.
Results
Impaired bone mineralization in fro/fro mice
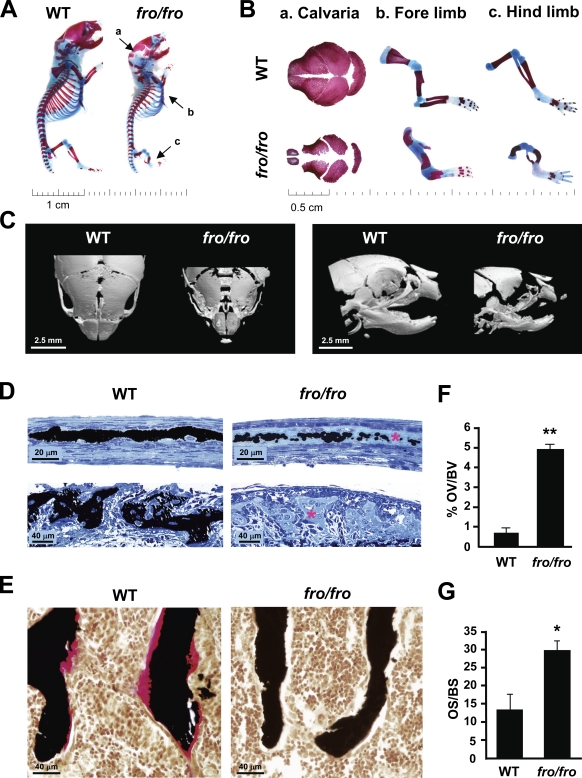
fro/fro neonates are characterized by a shortened body stature with skeletal abnormalities (Aubin et al., 2005). As shown by the skeletal preparations of the newborn mice, both flat (intramembranous) and long (endochondral) bones are affected—the parietal suture was poorly mineralized, whereas both fore- and hind limbs were severely bent in the fro/fro mutants (Fig. 1, A and B). Further analysis by micro-CT revealed a reduction of mineralized tissue in the flat bones of the skull and alveolar bones in the jaw (Fig. 1 C).
Figure 1.
Skeletal abnormalities in fro/fro mice. (A and B) Alizarin red (stains mineralized tissues)– and Alcian blue (stains cartilage matrix)–stained skeletal preparations of 2-d-old wild-type (WT) and fro/fro mice showing hypomineralization of the calvaria (a) and short (b) and bent (c) fore- and hind limbs in the latter genotype. (C) Micro-CT analysis of 2-d-old WT and fro/fro heads confirming severe hypomineralization of various head skeletal elements as seen from the dorsal (left) and the lateral (right) views. (D) Light micrographs of von Kossa–stained mineral (black) in parietal (top) and alveolar bone (bottom) sections of a 2-d-old WT mouse and its fro/fro littermate. There is a marked decrease in mineralization, revealed by extensive areas of unmineralized osteoid (asterisks) in the fro/fro bones. (E) Von Kossa and van Gieson staining of vertebral bones from 1-mo-old WT and fro/fro littermates demonstrating a marked increase of unmineralized bone volume (pink staining) in the latter genotype (n = 5). (F and G) Comparison of the percentage of osteoid volume over total bone volume (OV/BV) and osteoid surface over bone surface (OS/BS) in WT and fro/fro mice (n = 5). Error bars represent standard deviations. *, P < 0.05; **, P < 0.01.
To further confirm that the observed decrease in mineralized tissue was attributable purely to a mineralization defect, we examined wild-type (WT) and mutant mice for the presence of excess osteoid, the proteinaceous matrix secreted by osteoblasts, which subsequently becomes mineralized. Histological analysis of the parietal bones in the skullcap of 2-d-old fro/fro mice revealed a severely hypomineralized matrix at the suture (Fig. 1 D, top). Also, there was a marked reduction of mineralization in the alveolar bones from these mice (Fig. 1 D, bottom). By 1 mo of age, the trabecular bones of fro/fro mice showed an increase of ∼5% in osteoid volume over total bone volume as measured by histomorphometry (Fig. 1, E and F). Similarly, there was a significant increase in the osteoid surface in the fro/fro bones (Fig. 1 G). These results indicate that osteoid ECM is indeed deposited but is not efficiently mineralized. None of these skeletal abnormalities were seen in +/fro mice; therefore, we used these mice as controls for our subsequent in vivo analyses.
The fro mutation abolishes nSMase2 activity but does not affect its membrane localization
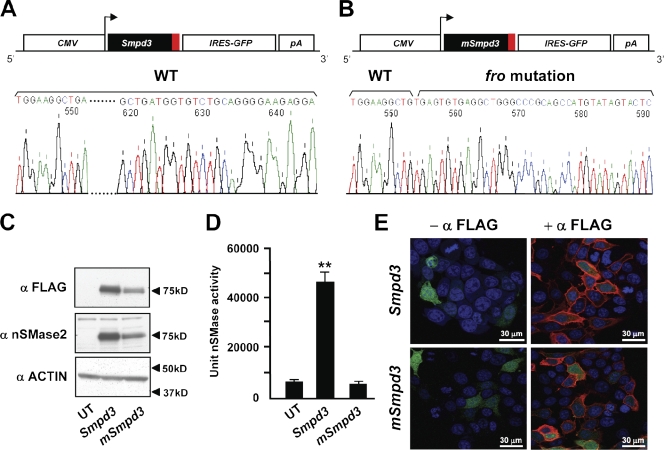
The fro mutation causes a deletion in Smpd3, resulting in a replacement of the last 33 amino acids of nSMase2. In a previous study, reduced nSMase activity was reported in skin samples collected from fro/fro mice (Aubin et al., 2005). This reduced enzymatic activity can be caused by a loss of the catalytic site and/or by impaired membrane targeting of the enzyme. To investigate this, we generated two FLAG-tagged expression constructs CMV-Smpd3 and CMV-mSmpd3 encoding the WT and a mutated nSMase2 that carries the fro mutation, respectively (Fig. 2, A and B). The constructs were used to transfect MCF-7 cells, and the expression of both WT and mutated proteins was confirmed by Western blotting (Fig. 2 C). As shown in Fig. 2 D, there was a marked increase in nSMase activity in cells transfected with CMV-Smpd3 but not with the CMV-mSmpd3 construct. We next examined the membrane localization of the WT and mutated nSMase2 proteins by indirect immunofluorescence and confocal microscopy using an antibody raised against the FLAG tag. Importantly, there was no alteration in the membrane localization pattern of mutant nSMase2 when compared with the WT protein (Fig. 2 E). These data confirm that the mutation of the predicted active site is the sole reason for the loss of catalytic activity of this enzyme in fro/fro mice.
Figure 2.
Effects of fro mutation on nSMase2 activity and localization. (A and B) Schematic depiction of WT (Smpd3; A) and mutant Smpd3 (mSmpd3; carries fro mutation; B) expression constructs. The red boxes represent the FLAG coding sequence (CMV, cytomegalovirus promoter; IRES, internal ribosome entry site; and pA, SV40 polyadenylation signal). (C) Western blots showing expression of WT and mutant nSMase2 in transfected MCF-7 cells. FLAG-tagged proteins from the transfected cells were detected using an anti-FLAG (top) or an anti–mouse nSMase2 (middle) antibody. UT, untransfected. (D) A mixed micelle assay using 14C-labeled methyl-sphingomyelin shows that mutated nSMase2 does not have any nSMase activity. Error bars represent standard deviations. (E) Indirect immunofluorescence microscopy analyses showing comparable cell membrane localization of WT and mutated nSMase2 (shown in red) in transfected MCF-7 cells. The green and blue stains represent GFP localization and the nucleus, respectively. **, P < 0.01.
The fro mutation affects skeletal development
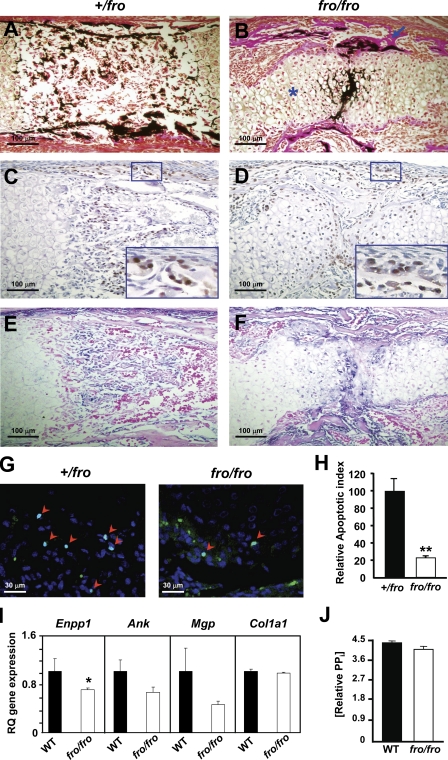
To investigate the effects of the fro mutation on the developing skeleton, we performed histological analysis of the long bones from embryonic day 15.5 (E15.5) +/fro and fro/fro embryos. There was an increased presence of unmineralized collagenous matrix in the cortical bones of the humerus (a representative long bone) of the fro/fro embryos in comparison to the cortical bones of their +/fro littermates (Fig. 3, A and B). This poor mineralization was not attributable to impaired osteoblast differentiation, as osterix (SP7) immunopositive cells were present in the cortical bones of the fro/fro mice (Fig. 3, C and D). Also, alkaline phosphatase activity was detected within the unmineralized matrix of the fro/fro bones, further indicating that osteoblast differentiation was not affected (Fig. 3, E and F). We next prepared total RNAs from the bones of newborn WT and fro/fro mice and examined the effects of the fro mutation on the expression of Runx2 and Atf4, encoding two key transcription factors involved in early and late osteogenic differentiation, respectively. In agreement with our histological analysis of the embryonic mice, quantitative real-time PCR (qRT-PCR) analysis showed that the expression of these osteoblast marker genes were not altered in fro/fro bones (Fig. S1).
Figure 3.
Effects of fro mutation on the developing skeleton. (A and B) Von Kossa and van Gieson staining of the humerus from E15.5 +/fro and fro/fro embryos. Note the unmineralized cortical bones (arrow) and the expanded hypertrophic zone (asterisk) in the fro/fro mice. (C and D) Immunostaining of humerus sections from E15.5 +/fro and fro/fro embryos using an antiosterix antibody shows osteoblast differentiation is unaffected in the latter genotype. For each panel, a magnified view of the marked area has been shown in the insets. (E and F) Incubation with a chromogenic substrate solution demonstrates the comparable presence of alkaline phosphatase activities in the +/fro and fro/fro bone sections. (G and H) TUNEL assay showing impaired apoptosis of hypertrophic chondrocytes in developing fro/fro endochondral bones (n = 4). Arrowheads indicate the TUNEL-positive cell nuclei. (I) qRT-PCR showing a mild down-regulation of Enpp1 expression in the parietal bones from fro/fro mice. Note that there is no significant alteration of Ank, Mgp, and Col1a1 expression in fro/fro bones. RQ, relative quantification. (J) PPi levels are comparable in both WT and fro/fro bone samples. Error bars represent standard deviations. *, P < 0.05; **, P < 0.01.
Although osteoblast differentiation was not affected in fro/fro mice, we did not observe any osterix-positive cells in the marrow space in the long bones of fro/fro embryos, whereas infiltration of osteoblast progenitors into the marrow space was normal in their +/fro littermates. Instead, we observed an unusual persistence of hypertrophic chondrocyte-like cells in the midshaft regions of the fro/fro long bones (Fig. 3, C and D). A possible explanation for this observation might be that the hypertrophic chondrocytes in these mutant bones were not undergoing apoptosis at a rate comparable with that of +/fro hypertrophic chondrocytes. To investigate this possibility, we performed TUNEL assay on the humerus sections from both +/fro and fro/fro embryos at E15.5. A decreased presence of TUNEL-positive nuclei in the fro/fro hypertrophic zones in comparison to the corresponding areas of the +/fro bones confirmed that there was indeed a reduction of the apoptosis of fro/fro hypertrophic chondrocytes (Fig. 3, G and H).
The presence of ALPL activity in the developing fro/fro bones suggests normal PPi hydrolysis in the ECM. However, the PPi levels in the ECM may increase because of an up-regulation of Enpp1 and Ank, which encode two proteins critical for the maintenance of tissue homeostasis of this mineralization inhibitor (Ho et al., 2000; Hessle et al., 2002). We analyzed the expression of these two genes by qRT-PCR and found that there was in fact a mild down-regulation of Enpp1 expression in the bones of newborn fro/fro mice, whereas Ank expression was not altered (Fig. 3 I). Also, we did not observe any up-regulation of Mgp and Col1a1 expression (Fig. 3 I). We then used a fluorogenic sensor to measure PPi levels in the bones of adult WT and fro/fro mice. We observed that PPi was present at comparable levels in the bone samples from both genotypes (Fig. 3 J). Collectively, these data suggest that the hypomineralization defect seen in fro/fro mice was not caused by the increase of MGP or PPi in the bone ECM.
Loss of nSMase2 in osteoblasts affects mineralization in vitro
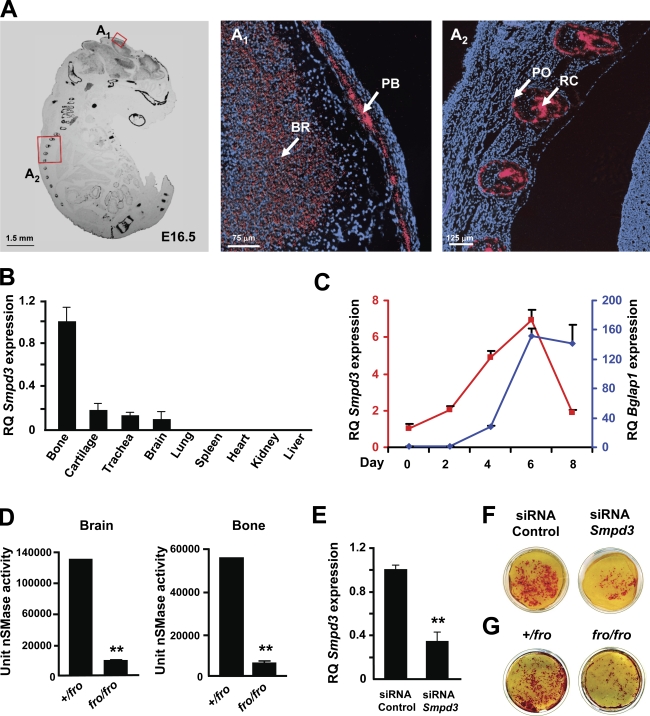
We next examined Smpd3 expression in late-stage mouse embryos and also in tissues collected from WT mice at the preweaning age. The sense and antisense probes generated from the Smpd3 cDNA were hybridized separately on fixed whole-embryo paraffin sections prepared from E16.5 WT embryos. The in situ hybridization analysis performed with the antisense probe showed a high level of Smpd3 expression in all bone types, cartilage, and in the brain (Fig. 4 A). A similar Smpd3 expression pattern was also observed in 2-wk-old mice (Fig. 4 B). Next, we examined Smpd3 expression during the differentiation of MC3T3-E1 preosteoblasts cultured in the presence of ascorbic acid and β-glycerol phosphate. Under these culture conditions, we observed a progressive induction of Smpd3 expression, which was down-regulated in fully mature osteoblasts (Fig. 4 C).
Figure 4.
Smpd3 expression and function in osteoblasts. (A) In situ hybridization showing Smpd3 expression in different skeletal elements in an E16.5 WT mouse embryo. The magnified views of the areas in red boxes are shown in A1 and A2 (PB, parietal bone of the skull cap; BR, brain; PO, periosteum in the rib; and RC, rib cartilage). Red stain represents the localization of the Smpd3 transcript, whereas the blue stain represents the nucleus. (B) qRT-PCR showing high levels of Smpd3 expression in bone, brain, and cartilage. Tissues were collected from a 2-wk-old WT mouse. All expression analyses were performed using hypoxanthine guanine phosphoribosyl transferase (Hprt) expression in the tissue as an internal control and Smpd3 expression in the bone as a calibrator (RQ, relative quantification). (C) Smpd3 and Bglap1 (red and blue lines, respectively) gene expression analysis in differentiating MC3T3-E1 preosteoblasts at five different time points. Smpd3 expression reaches its peak by day 6. Late osteoblast marker Bglap1 expression was used to monitor terminal differentiation of the MC3T3-E1 cells. (D) A mixed micelle assay using 14C-labled methyl-sphingomyelin shows a significant decrease in nSMase activity in both brain and bone tissues collected from the fro/fro mice. (E) Smpd3 knockdown by using an siRNA technique in MC3T3-E1 preosteoblasts. (F) Alizarin red staining shows reduced in vitro mineral deposition in cultures of MC3T3-E1 cells transfected by Smpd3 siRNAs in comparison with the control group. (G) Alizarin red staining shows reduced mineral deposition in cultures of differentiated fro/fro osteoblasts in comparison with the +/fro osteoblasts. The cultures were grown for 10 d in an osteogenic medium containing ascorbic acid and β-glycerol phosphate. Error bars represent standard deviations. **, P < 0.01.
A high level of expression of Smpd3 in embryonic and postnatal bones and in a differentiating osteoblastic cell line suggests a local role for this enzyme in bone. To examine whether nSMase2 deficiency in fro/fro bones causes a reduction of total nSMase activity, we prepared calvarial bone extracts from both +/fro and fro/fro mice and performed an in vitro enzymatic assay using 14C-labeled sphingomyelin (Marchesini et al., 2003). As a control experiment, we performed the same analysis on the extracts prepared from both +/fro and fro/fro brain tissues. As shown in Fig. 4 D, there was a comparable decrease of nSMase activities in both brain and bone extracts from fro/fro mice.
We next investigated whether a loss of nSMase2 activity affects the in vitro mineralization capacities of cultured osteoblasts. First, we transfected MC3T3-E1 preosteoblasts with siRNA oligonucleotides to knock down Smpd3 gene expression. Gene expression analysis by qRT-PCR revealed that there was an ∼60% reduction of Smpd3 expression in the siRNA-transfected cells (Fig. 4 E). We cultured both control and Smpd3 siRNA-transfected cells in the presence of ascorbic acid and β-glycerol phosphate to induce differentiation and mineralization. Upon culturing for 10 d in the aforementioned medium, cells were stained with Alizarin red, a calcium-binding dye. We observed reduced mineralization in the cultures with Smpd3 siRNA-transfected cells in comparison to the cultures with control siRNA-transfected cells (Fig. 4 F). This observation was further confirmed in experiments performed with primary osteoblasts isolated from the newborn +/fro and fro/fro mice. When cultured in the presence of ascorbic acid and β-glycerol phosphate, fro/fro osteoblast cultures showed reduced mineralization in comparison to the +/fro cultures (Fig. 4 G).
Osteoblast-specific expression of Smpd3 in fro/fro mice increases bone nSMase activity
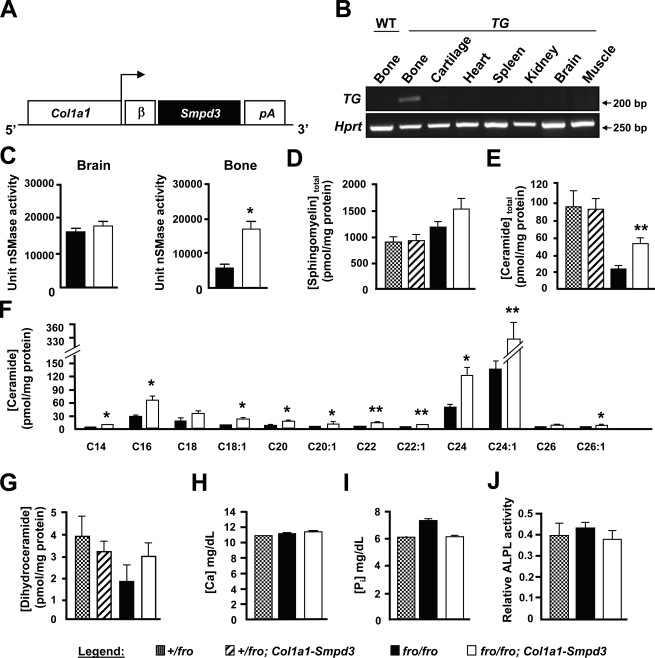
Collectively, a decline of total nSMase activity in fro/fro bones and reduced in vitro mineralization by fro/fro osteoblasts (Fig. 4, D and G) strongly suggest a local and specific role for nSMase2 in bone. To investigate this in vivo, we overexpressed Smpd3 specifically in the bones of fro/fro mice. For this purpose, we generated a Col1a1-Smpd3 transgene construct using a 2.3-kb Col1a1 promoter fragment, earlier shown to be specifically expressed in osteoblasts (Fig. 5 A). Pronuclear injection of this construct into fertilized mouse eggs resulted in four founders, of which two showed bone-specific expression of the transgene. No transgene expression was detected in any other tissue in these founders (Fig. 5 B). These founders were then mated with +/fro mice to first generate +/fro;Col1a1-Smpd3 mice, which were mated again with +/fro mice to obtain fro/fro;Col1a1-Smpd3 mice.
Figure 5.
Biochemical analysis of tissue and serum samples from fro/fro;Col1a1-Smpd3 mice. (A) Schematic representation of the Col1a1-Smpd3 transgene construct. (B, top) Semiquantitative PCR analysis confirming bone-specific expression of the transgene (TG). (bottom) Hprt expression analysis has been shown as a control for the cDNA amount. Amplicon sizes in base pairs are indicated on the left. (C) Enzymatic assays using 14C-labled methyl-sphingomyelin shows no change in nSMase activity in fro/fro;Col1a1-Smpd3 brain samples. As expected, in fro/fro;Col1a1-Smpd3 bone samples, the nSMase activity is increased in comparison with the fro/fro bone samples. (D–G) Lipid analysis using liquid chromatography/mass spectrometry of sphingomyelin (D), total ceramide (E), individual ceramide species (F), and dihydroceramide (G). A significant increase of total ceramide levels is caused by the increase of several long-chain ceramide species in fro/fro bones (n = 4). (H–J) Serum calcium (H), Pi (I), and alkaline phosphatase (J) activities are comparable in +/fro, fro/fro, and fro/fro;Col1a1-Smpd3 mice (n = 5). Error bars represent standard deviations. *, P < 0.05; **, P < 0.01.
By visual examination, there was no gross skeletal abnormalities in the fro/fro;Col1a1-Smpd3 mice. Also, these mice survived the perinatal death routinely seen in fro/fro mice (described in Fig. 6). In agreement with our transgene expression data, we observed a threefold increase of total nSMase activities in the bones of newborn fro/fro;Col1a1-Smpd3 mice in comparison to the bones of fro/fro mice, whereas brain nSMase activities remained indistinguishable between these two genotypes (Fig. 5 C). Interestingly, despite a significant increase of bone nSMase activities in fro/fro;Col1a1-Smpd3 mice, there was no detectable decrease of total bone sphingomyelin levels (Fig. 5 D). However, an increase of total ceramide levels in the bones of fro/fro;Col1a1-Smpd3 mice was observed when compared with the fro/fro bones (Fig. 5 E). Interestingly, we found a significant increase of several long-chain ceramide species (e.g., C16, C24, and C24:1) in the bones of the former genotype (Fig. 5 F). No significant alterations were observed in total dihydroceramide levels in the bone extracts prepared from any of the mouse models analyzed (Fig. 5 G). Also, several known serum parameters affecting ECM mineralization (e.g., calcium, Pi, and alkaline phosphatase levels) were unaltered in the fro/fro;Col1a1-Smpd3 mice (Fig. 5, H–J).
Figure 6.
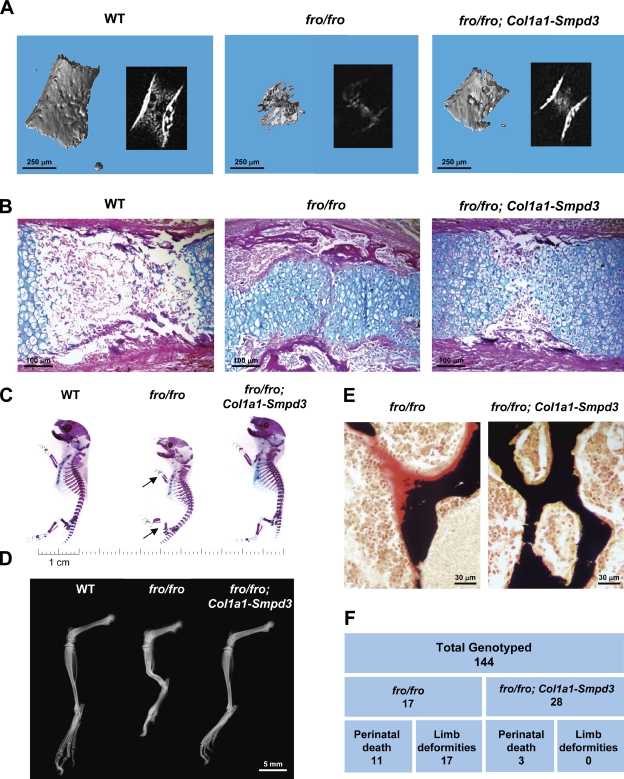
Analysis of fro/fro;Col1a1-Smpd3 bones. (A) Micro-CT analysis of E15.5 fro/fro humerus showing a poorly mineralized bone collar and cartilage matrix. Although bone collar mineralization defects are completely rescued in fro/fro;Col1a1-Smpd3 embryos, mineralization defects in the cartilage matrix are still present. Insets show the cross-sectional x-ray images of the analyzed bones. (B) Alcian blue and van Gieson staining of humerus sections from E15.5 fro/fro embryos confirm the micro-CT findings. Note the abnormal presence of the hypertrophic chondrocyte-like cells in the shaft region of the fro/fro humerus. This latter phenotype is largely unaffected in the fro/fro;Col1a1-Smpd3 long bones. (C) Skeletal preparations indicate a full rescue of the fro/fro bone deformities (arrows) in the newborn fro/fro;Col1a1-Smpd3 mice. (D) Radiographical analysis shows that the limb abnormalities are absent in 1-mo-old fro/fro;Col1a1-Smpd3 mice. (E) Von Kossa and van Gieson staining of vertebral bone sections from 1-mo-old fro/fro and fro/fro;Col1a1-Smpd3 littermates demonstrate a complete rescue of the mineralization defects in the latter genotype. (F) Table showing genotyping data. Skeletal abnormalities were not seen in any of the fro/fro;Col1a1-Smpd3 mice analyzed.
Finally, we compared the expression of Enpp1, Ank, Mgp, and Col1a1 by qRT-PCR in the parietal bones of newborn fro/fro and fro/fro;Col1a1-Smpd3 mice. We observed significant up-regulation of Enpp1 and Ank, but not Mgp, expression. Col1a1 gene expression was mildly up-regulated in fro/fro;Col1a1-Smpd3 bones in comparison with fro/fro bones (Fig. S2).
Normal bone mineralization in fro/fro;Col1a1-Smpd3 mice
We next examined the skeletal phenotype in fro/fro;Col1a1-Smpd3 mice. Micro-CT analysis of the humerus from 15.5-d-old fro/fro embryos showed poorly mineralized cortical bones, which were fully mineralized in the fro/fro;Col1a1-Smpd3 embryos at the same developmental stage. Interestingly, a reduced presence of mineral in the marrow compartment of both fro/fro and fro/fro;Col1a1-Smpd3 long bones was noted, indicating that the chondrocyte phenotype was largely unaffected in the latter genotype (Fig. 6 A). The aforementioned observation was further confirmed by Alcian blue staining of the humeri sections from WT, fro/fro, and fro/fro;Col1a1-Smpd3 mice. We observed that, as was the case in the long bones from the fro/fro mice, the marrow compartment in the long bones of fro/fro;Col1a1-Smpd3 mice was full of densely packed chondrocytes within a cartilage matrix (Fig. 6 B). We next examined the skeleton of newborn fro/fro;Col1a1-Smpd3 mice. Osteoblast-specific expression of Smpd3 completely corrected the fro/fro skeletal abnormalities (Fig. 6 C). X-ray analysis showed that there was no recurrence of the skeletal abnormalities in the adult fro/fro;Col1a1-Smpd3 mice (Fig. 6 D). Also, we observed an absence of abnormally high osteoid volume in the bones of this latter model (Fig. 6 E).
We analyzed a total of 144 mice from the aforementioned breeding experiments, of which 17 were fro/fro. All of these mice had limb deformities, and 11 of them died perinatally. On the other hand, out of a total of 28 fro/fro;Col1a1-Smpd3 mice generated through this breeding, only three died perinatally, whereas none of them showed any kind of skeletal abnormalities (Fig. 6 F). We tested the significance of these data using the standard Pearson’s χ2 test and found that for the rescue of both skeletal phenotype and perinatal death, the p-values were far below the commonly accepted 5% threshold for significance.
Discussion
Analysis of novel, genetically modified mouse models with skeletal and dental mineralization defects may provide critical information on as yet unidentified regulators of ECM mineralization and, thereby, improve our understanding of this important physiological process. Recently, a loss-of-function mutation in the Smpd3 gene has been identified in a mouse model (fro/fro), which shows severe bone and tooth mineralization defects (Guenet et al., 1981; Aubin et al., 2005). The fro/fro skeletal abnormalities are similar to the skeletal pathology seen in patients with certain forms of osteogenesis imperfecta that do not involve any mutation in collagen genes (Glorieux et al., 2002). As is the case with these patients, the most common parameters affecting ECM mineralization, e.g., serum calcium, Pi, and alkaline phosphatase levels, are not decreased in fro/fro mice. Furthermore, when analyzed by histology, the unmineralized bone matrix appears to be secreted normally in these mice. Collectively, these observations suggest that Smpd3 might affect ECM mineralization through a novel mechanism.
In an earlier study, the skeletal phenotype of Smpd3−/− mice has been described as a form of chondrodysplasia (Stoffel et al., 2005). In agreement with this finding, we observed a significantly impaired apoptosis of hypertrophic chondrocytes, possibly caused by reduced ceramide levels during early skeletal development in fro/fro embryos. Additionally, we also observed poor mineralization of the matrix secreted by osteoblasts that severely affects the strength of the cortical bones in these embryos. This novel finding explains the long bone deformities in fro/fro mice.
Our data suggest that hypomineralization of bone ECM in fro/fro mice is not caused by the elevated levels of mineralization inhibitors MGP or PPi (Fig. 3, I and J). We observed a mild down-regulation of Enpp1 in fro/fro bones, whereas both Ank and Enpp1 expressions were significantly up-regulated in the bones of fro/fro;Col1a1-Smpd3 mice. Interestingly, up-regulation of these two genes in the latter model did not prevent the rescue of the bone mineralization defects. These data suggest that there might be a compensatory interplay between the positive (nSMase2) and negative (ANK and ENPP1) regulators of bone ECM mineralization.
Our current study establishes an osteoblast-specific role of Smpd3 in bone mineralization and several lines of evidence suggest that the Smpd3-encoded enzyme nSMase2 acts as a local modulator of ECM mineralization in bone. First, Smpd3 is highly expressed in bone, and its expression progressively increases as osteoblasts mature. Second, loss of Smpd3 expression in both siRNA-treated MC3T3-E1 preosteoblasts and in fro/fro primary osteoblasts causes impaired mineral deposition in cultures. Finally, osteoblast-specific expression of Smpd3 in fro/fro bones completely rescues the skeletal abnormalities. Collectively, all these findings provide unambiguous demonstration of a direct osteoblast/mineralization effect for the locally synthesized nSMase2 in osteoblasts. Furthermore, a normal skeletal appearance in fro/fro;Col1a1-Smpd3 mice suggests that the loss of nSMase2 activity in osteoblasts is the major cause of the fro/fro phenotype.
At this point, we cannot fully rule out an indirect systemic effect of nSMase2 enzymatic activity from other tissues on the developing skeleton. However, in view of our findings that osteoblast-specific expression of Smpd3 in fro/fro mice corrects the bone but not the cartilage phenotype, we do not consider this as a likely possibility. This latter finding also suggests a tissue-specific role for nSMase2 in the developing skeleton with apparent independent roles in bone and cartilage.
Both fro/fro and the gene-targeted Smpd3−/− mice share similar gross skeletal abnormalities, i.e., short-limbed dwarfism, deformation of long bones, abnormally formed rib cages, and abnormalities in growth plate cartilage. However, Stoffel et al. (2005, 2007), in their gene-targeted model, did not observe bone and tooth mineralization abnormalities, which are seen in all fro/fro mice. This apparent discrepancy can be explained by differences in the analytical methods used to characterize the two mouse lines. Stoffel et al. (2005, 2007) analyzed the mineralization status of the Smpd3−/− mice solely by bone mineral density analysis, which determines the total bone mineral content and is not suitable for detecting an increase in unmineralized bone matrix. In contrast, we analyzed the fro/fro bones using a histomorphometric technique on undecalcified samples, commonly used to detect skeletal mineralization defects.
Although Stoffel et al. (2005, 2007) suggested that the phenotypic variations in fro/fro and Smpd3−/− mice can be attributable to the presence of any additional genetic alterations in the former strain, such mutations would have been fully segregated during the propagation of fro/fro mice in multiple laboratories over the last two decades. This inference, together with the observation that the fully penetrant fro/fro phenotype, including the severe hypomineralization defect, is always associated with the Smpd3 deletion mutation reported by Aubin et al. (2005), clearly identifies the loss of nSMase2 function as the sole cause of the fro/fro phenotype. Indeed, our current data showing a complete rescue of the skeletal phenotypes in fro/fro;Col1a1-Smpd3 mice expressing Smpd3 in osteoblasts rules out the possibility of the presence of any additional mutation in fro/fro mice that may cause the observed mineralization defects.
Our results demonstrate an intrinsic loss of nSMase activity attributable to the fro mutation. The translocation of nSMase2 from the Golgi compartment to the plasma membrane and its recycling back to the Golgi is a highly dynamic and regulated process. Notably, preventing nSMase2 recycling has been shown to increase nSMase activity and ceramide levels, suggesting adverse physiological consequences for alterations in localization of this enzyme (Milhas et al., 2010). Considering the critical nature of this process, we examined whether the reduced nSMase activities in fro/fro tissues are attributable to an impaired nSMase2 localization or are caused by the loss of its catalytic activity. Our cell culture data confirm that the mutant nSMase2 localizes identically to WT nSMase2 and indicate that the reduced tissue nSMase activity in fro/fro mice is likely to be caused by the disruption of the enzyme’s catalytic site.
nSMase2 cleaves sphingomyelin to generate the lipid second messenger ceramide (Merrill et al., 1997; Wu et al., 2010). Thus, a loss of functional nSMase2 could have dual effects, i.e., both a decrease in ceramide levels and an increase in sphingomyelin levels in tissues in which Smpd3 is normally expressed. Indeed, a recent study suggested a crucial role for sphingomyelin and its degradation in bone and dentin mineralization (Goldberg et al., 2008). However, as sphingomyelin, being an integral component of all cell membranes, is present in all tissues in relatively high amounts and because only a small fraction of it is cleaved by the nSMase2 enzymatic activity, a loss-of-function mutation in Smpd3 as such may not have any significant effects on the total tissue sphingomyelin levels. Indeed, we did not observe any difference in total sphingomyelin levels between +/fro and fro/fro bones. This observation suggests that increased sphingomyelin levels in bone attributable to the loss of nSMase2 activity may not account for the ECM mineralization defects in fro/fro mice. Although we could not detect any significant alteration in tissue sphingomyelin levels, we found a remarkable decrease of various ceramide species, particularly those with long chains, in fro/fro bones in comparison to the control +/fro bones. Currently, it is not clear how ceramide might affect bone ECM mineralization. Ceramide acting as a second messenger can affect several signaling pathways and may alter as yet unknown downstream regulators critical for bone ECM mineralization.
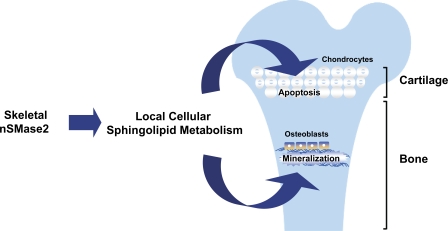
In conclusion, the fro/fro mice lacking a functional nSMase2 provide a unique opportunity to investigate a novel mechanism involved in vertebrate hard tissue mineralization. The data we present here suggest that a local nSMase2 function is required for a normal bone mineralization and for the normal apoptosis of hypertrophic chondrocytes in the cartilage during early skeletal development (Fig. 7). Collectively, these data demonstrate, for the first time, the tissue-specific roles for this enzyme in the developing skeleton. Further analyses of the mouse models reported here may reveal the molecular mechanisms underlying the pathophysiology of certain forms of osteomalacia and osteogenesis imperfecta in humans.
Figure 7.
A model depicting the local activities of nSMase2 in skeletal tissues. The cell-autonomous activity of nSMase2 in bone promotes ECM mineralization. In the cartilage, nSMase2 enzymatic activity is necessary for the normal apoptosis of hypertrophic chondrocytes.
Materials and methods
DNA constructs
The DNA construct for osteoblast-specific expression of the Smpd3 transgene was generated using a 2.3-kb Col1a1 promoter fragment (Rossert et al., 1995). A full-length Smpd3 cDNA (American Type Culture Collection) preceded by a rabbit β-globin intron was inserted in between the Col1a1 promoter fragment and a SV40 polyadenylation signal. The transgene sequence was released from the plasmid backbone by SacII restriction digestion and was used for pronuclear injection. A PCR-based technique was used to introduce the fro mutation into the WT cDNA. Both WT and mutant (fro) Smpd3 cDNAs were cloned in pIRES-hrGFP-1α (Agilent Technologies).
Mice
Generation of fro/fro mice was described previously (Aubin et al., 2005). Transgenic founders were generated by pronuclear injections at the McIntyre Cancer Center Transgenic Core Facility at McGill University following standard techniques. All mice were maintained in a pathogen-free standard animal facility, and the experimental procedures were performed following an animal use protocol approved by the Animal Care Committee of McGill University. Genotypes were determined by PCR on genomic DNAs isolated from the tail biopsies. The following primers were used for the genotyping of the fro mutation: 5′-GGGACGACGTCTGCCTCAGG-3′, 5′-TTAGAGGTCCCAACCACAGG-3′, and 5′-CCCAGGTGCTGGGCAGAAGG-3′. With these three primers, it is possible to amplify specific WT (145 bp) and mutant (189 bp) DNA fragments. The Col1a1-Smpd3 transgene integration was detected using the following primer pair specific for the SV40 polyadenylation signal: 5′-CAGCTCTCCATCAAGATGGT-3′ and 5′-CCGGTTTGGACTCAGAGTAT-3′.
Gene expression analysis
Gene expression analyses were performed using a qRT-PCR system (model 7500; Applied Biosystems). Total RNA was extracted from different tissues with TRIZOL reagent (Invitrogen) and subjected to DNase I (Invitrogen) treatment. The first-strand cDNA synthesis and qRT-PCR were performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems) and SYBR green quantitative PCR master mix (Maxima; Fermentas), respectively. The following primer pairs were used: 5′-AGAAACCCGGTCCTCGTACT-3′ and 5′-CCTGACCAGTGCCATTCTTT-3′ for Smpd3 expression and 5′-AAGCAGGAGGGCAATAAGGT-3′ and 5′-CAAGCAGGGTTAAGCTCACA-3′ for Bglap1 expression. For in situ hybridization analyses, embryos were fixed in 4% PFA, embedded in paraffin, and sectioned at 5-µm thickness. A full-length Smpd3 cDNA was used to generate 35S-labeled sense and antisense riboprobes.
Skeletal preparation and histological analysis
Skeletal tissues from newborn and adult mice were fixed overnight in 95% ethanol, stained in 0.015% Alcian blue dye (Sigma-Aldrich) in a 1:4 solution of glacial acetic acid and absolute ethanol for 24 h, and treated with 2% potassium hydroxide until the soft tissues were dissolved. The mineralized tissues were stained by 0.005% Alizarin red (Sigma-Aldrich) solution in 1% potassium hydroxide and clarified in 1% potassium hydroxide/20% glycerol for ≥2 d. For plastic sectioning, vertebrae were fixed overnight in 4% PFA/PBS, embedded in methyl methacrylate, and sectioned (7-µm thickness), and von Kossa and van Gieson staining was applied. Unmineralized bone sections were analyzed using Osteomeasure software (Osteometrics, Inc.). Mouse embryos were fixed in 4% PFA/PBS, pH 7.4, overnight and embedded in paraffin. 5-µm-thick sections were submitted to von Kossa, Alcian blue, and van Gieson staining. Images were taken at room temperature using a light microscope (DM200; Leica) with a 20× (numerical aperture of 0.40) or 40× (numerical aperture of 0.65) objective. All histological images were captured using a camera (DP72; Olympus), acquired with DP2-BSW software (XV3.0; Olympus), and processed using Photoshop (Adobe). The TUNEL assay was performed on E15.5 embryos to evaluate in vivo chondrocyte apoptosis as per the manufacturer’s instructions (Deadend Fluorometric TUNEL System kit; Promega).
Immunofluorescence and confocal microscopy
MCF-7 cells (15 × 104/dish) were seeded in 35-mm confocal dishes (MatTek), and after 24 h, cells were transiently transfected with 1 µg Smpd3 or mSmpd3 cloned in the pIRES-hrGFP-1α expression vector. After 24 h, cells were fixed with 3.7% PFA for 10 min, permeabilized with 100% methanol for 5 min at −20°C, and blocked with 2% human serum in PBS for 30 min at room temperature. Cells were probed with or without anti-FLAG (1:1,000; Sigma-Aldrich) antibody in 2% serum for 2 h at room temperature, washed with 3× PBS, and probed with fluorescent secondary antibody (1:200 anti–mouse Alexa Fluor 555; 30–45 min at room temperature). After washing with 3× PBS, nuclei were visualized with DRAQ5 staining (1:500 in PBS). Images were captured with a confocal microscope (LSM 510 Meta; Carl Zeiss).
Immunoblotting
Protein samples were separated on 4–20% gradient Tris-HCl gels (Bio-Rad Laboratories) at a constant current of 40 mA before transfer to nitrocellulose membrane in Tris/glycine buffer (100 V for 30 min at 4°C). Membranes were blocked (5% milk in 0.1% Tween in PBS for 30 min) and incubated overnight at 4°C with anti-FLAG (Sigma-Aldrich), anti-nSMase2 (Santa Cruz Biotechnology, Inc.), or antiactin (Sigma-Aldrich) primary antibodies at a 1:1,000, 1:500, or 1:20,000 dilution, respectively. Membranes were washed (3× in 0.1% Tween in PBS), probed with HRP-conjugated mouse or rabbit secondary antibody (1:5,000 in 5% milk in 0.1% Tween in PBS) for 30–45 min at room temperature, and washed (3× in 0.1% Tween in TBS). Proteins were visualized by enhanced chemiluminescence (Thermo Fisher Scientific).
Transient transfection with siRNAs, cell culture, and in vitro mineralization
MC3T3-E1 cells were transfected with 50 ng/µl of Smpd3 (SI01426999; QIAGEN) or control (1027284; QIAGEN) annealed double-stranded siRNAs and cultured in α-MEM (Invitrogen) supplemented with 10% FBS (PAA Laboratories) and 100 U/ml penicillin-streptomycin at 37°C under 5% CO2 in a humidified incubator. Primary osteoblast isolation from calvaria, in vitro differentiation and culture, and Alizarin red staining for mineral deposition were performed as described previously (Li et al., 2011).
Radiography and micro-CT analysis
Radiography and micro-CT analyses of the skeletal samples were performed at the Centre for Bone and Periodontal Research Core Facility at McGill University using an x-ray imaging system (XPERT; Kubtec) and micro-CT system (SkyScan), respectively. For micro-CT analyses, the x-ray source was operated at 45 kV and at 222 µA (maximum power). Images were captured using a 12-bit, cooled charge-coupled device camera (1,024 by 1,024 pixels) coupled by a fiber optics taper to the scintillator. Samples were scanned at a magnification resulting in a pixel size of 4.79 µm. Using a rotation step of 0.9° and an exposition time of 2,240 ms for each step, images were generated, giving a scanning time of 30 min. The cross sections along the specimen long axis were reconstructed using NRecon software (SkyScan), with a distance between each cross section of 9.58 µm. Each cross section was reduced in half-size to facilitate the analysis, giving of a voxel of 9.58 × 9.58 × 9.58 µm3. CT-Analyser and 3D Creator software (both from SkyScan) were used to analyze and to perform 3D rendering, respectively.
Serum biochemistry
Serum calcium and Pi levels were measured using commercially available kits (Diagnostic Chemicals Limited). Serum ALPL levels were measured as described previously (Li et al., 2011), whereas tissue PPi levels were measured using a fluorogenic sensor following the manufacturer’s instructions (Advancing Assay Technologies Bioquest, Inc.).
Sphingomyelinase assays and lipid measurements
Limbs and skullcaps were snap frozen in liquid nitrogen and crushed before further homogenization in 20 mM Tris buffer containing protease inhibitors using an autohomogenizer. Brain tissue was homogenized directly in the same buffer. Aliquots of homogenate were removed for the estimation of protein concentration by the Bradford assay. In vitro analysis of nSMase activity was performed using a mixed micelle assay as described previously (Marchesini et al., 2003). In brief, duplicate aliquots (20–30 µg protein) of homogenate were diluted to 100 µl in neutral buffer containing 25 mM Tris, pH 7.4, 5 mM EDTA, 0.2% Triton X-100, and protease inhibitors. The reaction was started by adding 100 µl assay buffer containing 200 µM sphingomyelin, 100 µM phosphatidylserine, and 100,000 cpm 14C-labeled methyl-sphingomyelin reconstituted in 25 mM Tris, pH 7.4, 10 mM MgCl2, 5 mM DTT, and 0.2% Triton X-100. After incubation for 30 min at 37°C, reactions were stopped by the addition of 1.5 ml chloroform/methanol (2:1). 400 µl of water was added, and samples were vortexed and spun at 3,000 rpm for 5 min at room temperature. Next, 800 µl of the upper phase was added to 4 ml scintillation fluid, vortexed, and counted. 10 µl assay buffer, representing 2 nmol sphingomyelin, was also counted to allow conversion of results from counts per minute to picomoles of ceramide per milligram of protein per hour. For lipid analysis by mass spectrometry, after homogenization, lysate containing 200 µg–1 mg protein was analyzed for sphingomyelin, ceramide, and dihydroceramide levels by tandem liquid chromatography/mass spectrometry as previously described (Bielawski et al., 2010). Lipid levels were normalized to cellular protein.
Data analysis
All results are shown as means ± the standard deviation. Statistical analyses were performed by Student’s t test, with P < 0.05 considered significant as indicated by a single asterisk (**, P < 0.01). Standard Pearson’s χ2 test was used to test the significance of the rescue of both skeletal phenotype and perinatal death.
Online supplemental material
Fig. S1 shows qRT-PCR analysis of Runx2 and Atf4 expression in newborn fro/fro bones. Fig. S2 shows qRT-PCR analysis of Enpp1, Ank, Mgp, and Col1a1 expression in the bones from newborn fro/fro and fro/fro;Col1a1-Smpd3 mice. Fig. S3 shows qRT-PCR analysis of Runx2, Col1a1, Atf4, and Bglap1 expression in the control and Smpd3 siRNA-treated MC3T3-E1 cells and Runx2, Atf4, Bglap1, and Smpd3 expression in WT and +/fro mice. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201102051/DC1.
Acknowledgments
We thank Drs. Geoffrey Hendy and Houman Homayoun for critical reading of the manuscript.
This work was supported by operating grant 216548 from the Canadian Institutes of Health Research and a seed grant from the Osteogenesis Imperfecta Foundation to M. Murshed. Z. Khavandgar receives a stipend from the McGill University Health Centre Research Institute, and M. Murshed receives salary support from the Fonds de la Recherche en Santé du Québec grant 16302. Additional support for this work was provided by National Institutes of Health grant GM43825 to Y.A. Hannun. M. Murshed and M.D. McKee are members of the Centre for Bone and Periodontal Research, and additional support is gratefully acknowledged from the Fonds de la Recherche en Santé du Québec Réseau de Recherche en Santé Buccodentaire et Osseuse.
Footnotes
Abbreviations used in this paper:
- MGP
- matrix gla protein
- micro-CT
- microcomputed tomography
- nSMase
- neutral sphingomyelinase
- qRT-PCR
- quantitative real-time PCR
- WT
- wild type
References
- Aubin I., Adams C.P., Opsahl S., Septier D., Bishop C.E., Auge N., Salvayre R., Negre-Salvayre A., Goldberg M., Guénet J.L., Poirier C. 2005. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat. Genet. 37:803–805 10.1038/ng1603 [DOI] [PubMed] [Google Scholar]
- Bielawski J., Pierce J.S., Snider J., Rembiesa B., Szulc Z.M., Bielawska A. 2010. Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv. Exp. Med. Biol. 688:46–59 10.1007/978-1-4419-6741-1_3 [DOI] [PubMed] [Google Scholar]
- Bose R., Verheij M., Haimovitz-Friedman A., Scotto K., Fuks Z., Kolesnick R. 1995. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 82:405–414 10.1016/0092-8674(95)90429-8 [DOI] [PubMed] [Google Scholar]
- Fleisch H., Bisaz S. 1962. Mechanism of calcification: inhibitory role of pyrophosphate. Nature. 195:911 10.1038/195911a0 [DOI] [PubMed] [Google Scholar]
- Glorieux F.H., Ward L.M., Rauch F., Lalic L., Roughley P.J., Travers R. 2002. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J. Bone Miner. Res. 17:30–38 10.1359/jbmr.2002.17.1.30 [DOI] [PubMed] [Google Scholar]
- Goldberg M., Opsahl S., Aubin I., Septier D., Chaussain-Miller C., Boskey A., Guenet J.L. 2008. Sphingomyelin degradation is a key factor in dentin and bone mineralization: lessons from the fro/fro mouse. The chemistry and histochemistry of dentin lipids. J. Dent. Res. 87:9–13 10.1177/154405910808700103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenet J.L., Stanescu R., Maroteaux P., Stanescu V. 1981. Fragilitas ossium: a new autosomal recessive mutation in the mouse. J. Hered. 72:440–441 [DOI] [PubMed] [Google Scholar]
- Hessle L., Johnson K.A., Anderson H.C., Narisawa S., Sali A., Goding J.W., Terkeltaub R., Millan J.L. 2002. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA. 99:9445–9449 10.1073/pnas.142063399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.M., Johnson M.D., Kingsley D.M. 2000. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 289:265–270 10.1126/science.289.5477.265 [DOI] [PubMed] [Google Scholar]
- Kolak M., Westerbacka J., Velagapudi V.R., Wågsäter D., Yetukuri L., Makkonen J., Rissanen A., Häkkinen A.M., Lindell M., Bergholm R., et al. 2007. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 56:1960–1968 10.2337/db07-0111 [DOI] [PubMed] [Google Scholar]
- Li J., Khavandgar Z., Lin S.H., Murshed M. 2011. Lithium chloride attenuates BMP-2 signaling and inhibits osteogenic differentiation through a novel WNT/GSK3- independent mechanism. Bone. 48:321–331 10.1016/j.bone.2010.09.033 [DOI] [PubMed] [Google Scholar]
- Luo G., Ducy P., McKee M.D., Pinero G.J., Loyer E., Behringer R.R., Karsenty G. 1997. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 386:78–81 10.1038/386078a0 [DOI] [PubMed] [Google Scholar]
- Marchesini N., Luberto C., Hannun Y.A. 2003. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J. Biol. Chem. 278:13775–13783 10.1074/jbc.M212262200 [DOI] [PubMed] [Google Scholar]
- Merrill A.H., Jr, Schmelz E.M., Dillehay D.L., Spiegel S., Shayman J.A., Schroeder J.J., Riley R.T., Voss K.A., Wang E. 1997. Sphingolipids—the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 142:208–225 10.1006/taap.1996.8029 [DOI] [PubMed] [Google Scholar]
- Milhas D., Clarke C.J., Idkowiak-Baldys J., Canals D., Hannun Y.A. 2010. Anterograde and retrograde transport of neutral sphingomyelinase-2 between the Golgi and the plasma membrane. Biochim. Biophys. Acta. 1801:1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed M., Schinke T., McKee M.D., Karsenty G. 2004. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell Biol. 165:625–630 10.1083/jcb.200402046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed M., Harmey D., Millán J.L., McKee M.D., Karsenty G. 2005. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 19:1093–1104 10.1101/gad.1276205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt T., Fujiwara I., Thomas R., Xiao Z.S., Quarles L.D., Drezner M.K. 1999. Coordinated maturational regulation of PHEX and renal phosphate transport inhibitory activity: evidence for the pathophysiological role of PHEX in X-linked hypophosphatemia. J. Bone Miner. Res. 14:2027–2035 10.1359/jbmr.1999.14.12.2027 [DOI] [PubMed] [Google Scholar]
- Obeid L.M., Linardic C.M., Karolak L.A., Hannun Y.A. 1993. Programmed cell death induced by ceramide. Science. 259:1769–1771 10.1126/science.8456305 [DOI] [PubMed] [Google Scholar]
- Okawa A., Nakamura I., Goto S., Moriya H., Nakamura Y., Ikegawa S. 1998. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 19:271–273 10.1038/956 [DOI] [PubMed] [Google Scholar]
- Richard A., Bourgoin S., Naccache P.H., L’Heureux G.P., Krump E., McColl S.R., Pelletier G. 1996. C2-ceramide primes specifically for the superoxide anion production induced by N-formylmethionylleucyl phenylalanine (fMLP) in human neutrophils. Biochim. Biophys. Acta. 1299:259–266 [DOI] [PubMed] [Google Scholar]
- Rossert J., Eberspaecher H., de Crombrugghe B. 1995. Separate cis-acting DNA elements of the mouse pro-α1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J. Cell Biol. 129:1421–1432 10.1083/jcb.129.5.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkute K., Asmis R.H., Nikolova-Karakashian M.N. 2007. Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. J. Lipid Res. 48:2443–2452 10.1194/jlr.M700227-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M., Tabarean I.V., Behrens M.M., Bartfai T. 2006. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc. Natl. Acad. Sci. USA. 103:2904–2908 10.1073/pnas.0510960103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Jenke B., Blöck B., Zumbansen M., Koebke J. 2005. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc. Natl. Acad. Sci. USA. 102:4554–4559 10.1073/pnas.0406380102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Jenke B., Holz B., Binczek E., Günter R.H., Knifka J., Koebke J., Niehoff A. 2007. Neutral sphingomyelinase (SMPD3) deficiency causes a novel form of chondrodysplasia and dwarfism that is rescued by Col2A1-driven smpd3 transgene expression. Am. J. Pathol. 171:153–161 10.2353/ajpath.2007.061285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H.F. 1974. Role of 1,25-dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc. Natl. Acad. Sci. USA. 71:1040–1044 10.1073/pnas.71.4.1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier E., Nègre-Salvayre A., Bocquet B., Itohara S., Hannun Y.A., Salvayre R., Augé N. 2007. Role for furin in tumor necrosis factor alpha-induced activation of the matrix metalloproteinase/sphingolipid mitogenic pathway. Mol. Cell. Biol. 27:2997–3007 10.1128/MCB.01485-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R.A. 2001. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Cell Physiol. 281:C1–C11 [DOI] [PubMed] [Google Scholar]
- Waymire K.G., Mahuren J.D., Jaje J.M., Guilarte T.R., Coburn S.P., MacGregor G.R. 1995. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat. Genet. 11:45–51 10.1038/ng0995-45 [DOI] [PubMed] [Google Scholar]
- Whyte M.P. 1994. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr. Rev. 15:439–461 [DOI] [PubMed] [Google Scholar]
- Wu B.X., Clarke C.J., Hannun Y.A. 2010. Mammalian neutral sphingomyelinases: regulation and roles in cell signaling responses. Neuromolecular Med. 12:320–330 10.1007/s12017-010-8120-z [DOI] [PMC free article] [PubMed] [Google Scholar]