Abstract
Despite widespread use of antiretroviral therapies to control replication of the human immunodeficiency virus (HIV), dysfunctions of cognition that are collectively termed HIV-associated neurocognitive disorders (HAND) still occur in approximately 50% of those infected by the virus. Currently there is not a biomarker that can identify HIV-infected people who are at risk for the development of HAND. Previous studies have identified particular sphingolipid species that are dysregulated in HAND, but the neurocognitive correlates of these biochemical findings are not currently understood. To address this question, we compared cerebrospinal fluid (CSF) levels of sphingomyelin, ceramide, and sterol species with performance on standard neurological tests designed to assess the function of multiple cognitive and motor domains in HIV-infected subjects. We found that sphingomyelin:ceramide ratios for acyl chain lengths of C16:0, C18:0, C22:0, and C24:0 were associated with worse performance on several indices of memory. The most striking finding was for the acyl chain of C18:0 that consistently associated with performance on multiple tests of memory. These findings suggest that the sphingomyelin:ceramide ratio for C18:0 may be a reasonable surrogate marker for memory dysfunction in HIV-infected subjects.
Keywords: ceramide, CSF, HAND, HIV, mass spectrometry, memory, neuron, RAVLT, sphingolipids, sphingomyelin
Introduction
Despite continued advances in highly active antiretroviral therapy (HAART) used to combat infection by the human immunodeficiency virus (HIV), a considerable number of people infected with HIV still develop neurocognitive impairment (for reviews, see Boisse et al, 2008; Nath et al, 2008). Collectively, these disorders are known as HIV-associated neurocognitive disorders (HAND) (Antinori et al, 2007). Although the severity of cognitive impairment in the post-HAART era is generally milder than was observed pre-HAART, neurocognitive impairment eventually occurs in approximately 30% to 50% of people infected with HIV (Cardenas et al, 2009; Tozzi et al, 2007) and there is evidence that the prevalence of HAND may not be decreasing, in part due to an extension of average lifespan as the result of HAART (Valcour et al, 2008). In HIV-infected people on stable HAART, there remains evidence of ongoing brain volume loss and white matter injury that is not associated with HIV viral load, suggesting that there is ongoing cerebral injury despite effective control of viral replication (Cardenas et al, 2009; Gongvatana et al, 2009; McMurtray et al, 2008). These data highlight the importance of discovering biomarkers that can potentially be used to identify individuals at greater risk for neurocognitive impairment, and also to assess the effectiveness of neuroprotective therapy at early stages of dysfunction when neural damage may be reversible.
A potential role for dysregulated sphingolipid metabolism in the pathogenesis of HAND was first identified through the analysis of brain tissues from HIV-infected patients (Haughey et al, 2004). Patients with mild cognitive impairments showed accumulations in multiple forms of sphingomyelin that were prominent in frontal and temporal lobes. In subjects with more severe cognitive impairments, there were also accumulations in several forms of ceramide and lipid peroxidation products, in addition to sphingomyelin. These data suggest that declines of neurocognitive function in HIV-infected subjects may involve a progressive deregulation in sphingolipid metabolism with accumulations of potentially toxic ceramides, sterols, and lipid peroxidation products. This concept is supported by evidence that increased levels of multiple sphingomyelin species in cerebrospinal fluid (CSF) are associated with stable cognitive dysfunction, and increased levels of ceramide species and 4-hydroxynonenal are associated with actively progressing dementia (Bandaru et al, 2007). Recent pathological observations in white matter from subjects with HAND found expansions in the lysosomal apparatus that are consistent with our biochemical findings of perturbed sphingolipid balance, since the molecular response to an overabundance of sphingolipids is to sequester them into lysosomes with a resultant impairment in lysosmal function (Gelman et al, 2005). Recent imaging studies have also demonstrated increased lipid + Lac:Cr ratio in HIV-positive subjects with dementia compared with seronegative controls (Roc et al, 2007). In each of these studies, a global dementia score or a Memorial Sloan-Kettering (MSK) score were used to categorize subjects based on severity of cognitive impairment. Because these indices are composite scores that combine multiple cognitive domains and motor functions, it is not currently known if alterations in brain sphingolipid metabolism are associated with deficits in particular cognitive and/or motor functions.
Sphingolipids are especially enriched in the central nervous system (CNS) where in addition to structural roles, these lipids function as regulators of protein scaffolding, and second messengers that shape cellular signaling events. Ceramides are the simplest sphingolipid, synthesized in the endoplasmic reticulum by a series of enzymatic steps that can produce multiple species of ceramide. These forms of ceramide differ by the acyl chain length, which can vary from 16 to 26 carbons, depending on the ceramide synthase involved in their production (Pewzner-Jung et al, 2006). Ceramides can be deacylated to sphingosine, converted to more complex sphingolipids including glycosphingolipids with the addition of one or more sugar residues or sphingomyelins with the addition of a phosphorylcholine or phosphoethanolamine to the 1-hydroxy group of ceramide. Each of these reactions is bidirectional, and there is evidence that the relative balance of ceramide to these other ceramide-derived lipids can play important roles in regulating cellular events (Haughey 2010).
In the current study, we examined the relationships between CSF levels of sphingomyelin, ceramide, and the sphingomyelin:ceramide ratio and cognition. We found that alterations in the sphingomyelin:ceramide ratio specifically associated with defects in memory as measured by the Rey Auditory Verbal Learning Test (RAVLT).
Results
Subject demographics, plasma or CSF viral load, and immune markers do not associate with performance on cognitive or motor tasks
Of the 48 participants in the NEAD cohort who met the criteria for the current study, 31 participants had both CSF and neuropsychological testing available. Compared to participants for whom CSF was unavailable, those from whom CSF was available had lower Instrumental Activities of Daily Living (IADL) scores (22.2 versus 23.5; p = 0.041) but did not differ on any other demographic or health-related characteristics (Table 1). The average age of participants was 41.3 years (SD = 5.4) and a mean education of 12.1 years (SD = 1.7). A majority of subjects were male (67.7%), African American (83.9%), currently prescribed antiretroviral therapy (80.7%), and had used illicit drugs in the past (100%), although only about half reported active use of illicit drugs (48.4%). At the time of lumbar puncture the average duration of self-reported HIV infection was 7.5 years (SD = 4.5), the average CSF viral load was 2.7 log10 copies/ml (SD = 1.1) and the average plasma viral load was 3.8 log10 copies/ml (SD = 1.5). Duration of HIV, plasma and CSF viral load, and CD4 or CD8 counts were not associated with performance on any of the cognitive or motor tests (data not shown) and were therefore not included as covariates in any statistical models.
Table 1.
Demographic and health-related characteristics of those with and without CSF in the NEAD study
| Characteristics | With CSF and cognitive testing
|
No CSF and/or cognitive testing
|
|||
|---|---|---|---|---|---|
| Mean (SD)/n (%) | n | Mean (SD)/n (%) | n | p value | |
| Age at baseline, years | 41.3 (5.4) | 31 | 40.5 (7.2) | 17 | 0.646 |
| Male | 21 (67.7%) | 31 | 13 (76.5%) | 17 | 0.386 |
| Race | |||||
| Caucasian | 4 (12.9%) | 31 | 3 (17.7%) | 17 | 0.801 |
| African American | 26 (83.9%) | 14 (82.4%) | |||
| Hispanic | 1 (3.2%) | 0 | |||
| Education, years | 12.1 (1.7) | 31 | 12.0 (1.6) | 17 | 0.802 |
| Any R2 Therapy | 25 (80.7%) | 31 | 8 (53.3%) | 17 | 0.082 |
| MSK | |||||
| 0 | 7 (22.6%) | 31 | 8 (53.3) | 15 | 0.224 |
| 0.5 | 5 (16.13%) | 2 (13.3%) | |||
| 1 | 12 (38.7%) | 2 (13.3%) | |||
| 2 | 6 (19.4%) | 3 (20.0%) | |||
| 3 | 1 (3.2%) | 0 | |||
| Beck Depression Inventory | 12.5 (8.9) | 31 | 13.4 (11.1) | 0.767 | |
| IADL score | 22.2 (2.3) | 31 | 23.5 (1.1) | 15 | 0.041 |
| UPDRS total score | 8.4 (8.1) | 30 | 4.5 (5.6) | 15 | 0.107 |
| Ever used drugs | 31 (100%) | 31 | 15 (100%) | 15 | 1.000 |
| Currently use drugs | 15 (48.4%) | 31 | 8 (53.3%) | 15 | 1.000 |
| Currently use alcohol | 10 (32.3%) | 31 | 4 (26.7%) | 15 | 1.000 |
| CD4 cell count | 154.1 (136.1) | 29 | 190.9 (181.0) | 16 | 0.445 |
| CD8 cell count | 755.0 (605.8) | 25 | 751.2 (417.3) | 13 | 0.984 |
| Using Antiretroviral therapy | 25 (80.7%) | 31 | 8 (53.3%) | 15 | 0.082 |
| Plasma HIV RNA (log10 copies/mL) | 3.8 (1.5) | 29 | 4.5 (1.0) | 13 | 0.143 |
| CSF HIV RNA (log10 copies/mL) | 2.7 (1.1) | 28 | |||
| Duration of HIV, years | 7.5 (4.5) | 31 | 7.3 (4.8) | 16 | 0.861 |
| Symptomatic DSP | 18 (58.1%) | 31 | 4 (28.6%) | 14 | 0.108 |
| Asymptomatic DSP | 10 (32.3%) | 31 | 9 (64.3%) | 14 | 0.057 |
Note. MSK = Memorial Sloan-Kettering (MSK); IADL = Instrumental Activities of Daily Living; UPDRS = Unified Parkinson’s Disease Rating Scale; DSP = Distal Sensory Polyneuropathy.
Increased sphingomyelin:ceramide ratios are specifically associated with worse performance in the Rey Auditory Verbal Learning Test
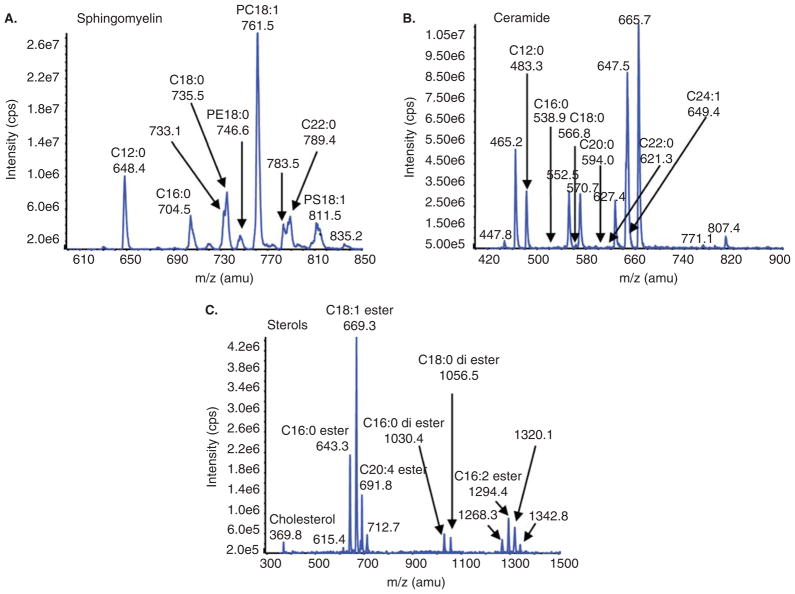
To investigate the relationship between CSF levels of sphingomyelins, ceramides, sterols, and performance on cognitive and motor tasks, we isolated a crude lipid extract from CSF samples and quantitatively analyzed multiple lipid species by high-performance liquid chromatography/triple-stage quadrupole tandem mass spectrometry (HPLC/ESI/MS/MS). Sphingolipid and ceramide species were identified by acyl chain length that varies from 16 to 28 carbons (Figure 1). Because sphingomyelin and ceramide are directly associated by metabolic pathways (ceramide is a precursor to sphingomyelin and sphingomyelin can be hydrolyzed to create ceramide), we used a ratio of sphingomyelin:ceramide as a single variable to explore the association between CSF levels of these sphingolipids and performance on memory and motor tasks.
Figure 1.
Example spectra for sphingomylein, ceramide ans sterol species. A crude lipid extract from CSF was first separated by HPLC and analyzed by multiple reaction monitoring (MRM) using ESI/MS/MS. Combined spectra and individual analytes for (A) sphingomyelins, (B) ceramides, and (C) sterols as identified by acyl chain length (C16:0 to C24:1) are shown.
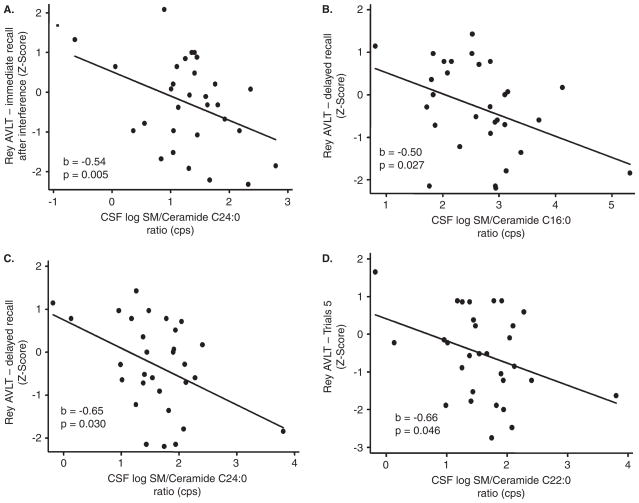
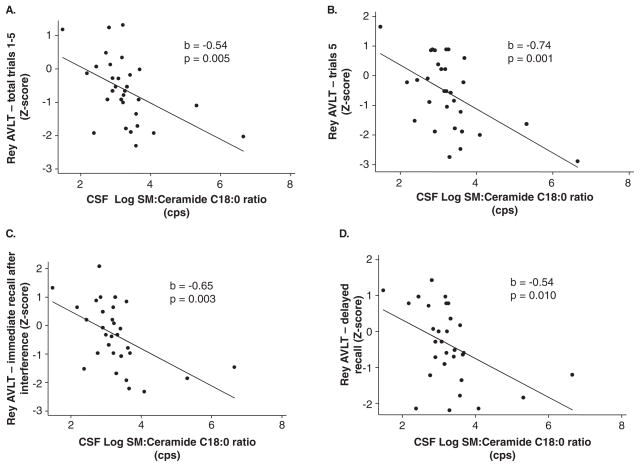
We first examined cross-sectional association between CSF levels of sphingomyelin, ceramide, sphingomyelin:ceramide ratios, and tests scores for each individual task that assessed cognitive and motor functions. There were no associations between individually analyzed sphingomyelin or ceramide species and performance on individual cognitive or motor tasks (Table 2 and data not shown). Greater sphingomyelin:ceramide ratios for acyl chain lengths of C16:0, C18:0, C22:0, and C24:0 were significantly associated with worse performance on several indices of verbal memory as measured by the RAVLT, including the total, trial 5, and immediate and delayed recall scores (Figure 2, Table 2). In particular, higher sphingomyelin:ceramide ratios for C24:0 were specifically associated with deficits in RAVLT immediate recall (p = 0.038), whereas higher C16:0 and C22:0 ratios were significantly associated with deficits in delayed recall (p = 0.027 and p = 0.030, respectively) (Table 2). The most robust association was with the sphingomyelin:ceramide ratio for C18:0, which was significantly increased in conjunction with worse performance in the total score (p = 0.005), trail 5 score (p = 0.001), immediate recall (p = 0.003), and delayed recall (p = 0.01) (Table 2, pFigure 3). None of the sphingomyelin:ceramide ratios examined were associated with verbal recognition on the RAVLT (Table 2) or with performance on cognitive tests assessing visual memory, verbal fluency, executive function, or motor control (Table 3). However, there were trends toward significance the sphingomylein:ceramide ratio C18:0 and recall on the Rey Complex Figure Test ( = 0.086). Controlling for gender, race, antiretroviral therapy, CD4 and CD8 cell counts, current drug or alcohol use, depressive symptoms, and plasma or CSF HIV RNA did not alter the associations.
Table 2.
Associations between CSF lipids and performance on the Rey Auditory Verbal Learning Test
| Log lipid | RAVLT
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total score: Trials 1–5
|
Trial 5 score
|
Immediate recall
|
Delayed recall
|
Recognition
|
||||||
| b | p value | b | p value | b | p value | b | p value | b | p value | |
| Total SM | −0.25 | 0.266 | −0.38 | 0.161 | −0.34 | 0.196 | −0.42 | 0.084 | −0.15 | 0.704 |
| Total Cholesterol | 0.09 | 0.864 | 0.00 | 0.996 | −0.46 | 0.444 | −0.45 | 0.426 | 0.48 | 0.579 |
| SM:Cholesterol | −0.61 | 0.052 | −0.86 | 0.018 | −0.55 | 0.133 | −0.71 | 0.037 | −0.58 | 0.284 |
| Ceramide C16:0 | −0.24 | 0.663 | −0.45 | 0.499 | 0.32 | 0.610 | 0.00 | 1.000 | −1.12 | 0.209 |
| Ceramide C18:0 | 0.46 | 0.189 | 0.37 | 0.394 | 0.72 | 0.069 | 0.40 | 0.290 | 0.07 | 0.902 |
| Ceramide C22:0 | 0.24 | 0.624 | 0.39 | 0.521 | 0.20 | 0.730 | 0.05 | 0.916 | −0.10 | 0.901 |
| Ceramide C24:0 | 0.01 | 0.967 | −0.14 | 0.631 | 0.17 | 0.522 | −0.13 | 0.622 | −0.42 | 0.267 |
| SM:Ceramide C16:0 | −0.31 | 0.135 | −0.45 | 0.071 | −0.46 | 0.058 | −0.50 | 0.027 | −0.16 | 0.661 |
| SM:Ceramide C18:0 | −0.54 | 0.005 | −0.74 | 0.001 | −0.65 | 0.003 | −0.54 | 0.010 | −0.34 | 0.310 |
| SM:Ceramide C22:0 | −0.44 | 0.115 | −0.66 | 0.046 | −0.54 | 0.092 | −0.65 | 0.030 | −0.19 | 0.687 |
| SM:Ceramide C24:0 | −0.33 | 0.204 | −0.31 | 0.322 | −0.61 | 0.038 | −0.35 | 0.218 | 0.34 | 0.448 |
Note. RAVLT = Rey Auditory Verbal Learning Test. Data in bold indicate significance at p < 0.05 or better.
Figure 2.
CSF levels of sphingomylein:ceramide ratios for acyl chain lengths C16:0, C22:0, and C24:0 were associated with worse performance on immediate and delayed recall in the Rey Auditory Verbal Learning Tests. Correlations for CSF levels of sphingomylein: ceramide ratios for acyl chain length (A) C24:0 and immediate recall, (B) C16:0 and (C) C22:0 with delayed recall, and (D) C22:0 with trial 5 scores are shown. Correlation coefficient (b) and exact significance levels (p) are indicated in insets. n = 31 subjects. Data are linear regressions with age- and education-adjusted z-scores for each cognitive test.
Figure 3.
CSF levels of sphingomylein:ceramide ratios for acyl chain lengths of C18:0 are associated with performance on immediate recall, delayed recall, and trial 5 of the Rey Auditory Verbal Learning Test. Correlations for CSF levels of the sphingomyelin:ceramide ratio for C18:0 and (A) total, (B) trial 5, (C) immediate recall, and (D) delayed recall on the Rey Auditory Verbal Learning Test. Correlations for CSF levels of the sphingomylein:ceramide ratio for C18:0 and (D) copy and (E) recall on the Rey Complex Figure Test. The correlation coefficient (b) and exact significance levels (p) are indicated in insets. For D, excluding the data point in the box had little impact on the association (b = −0.32 compared with p = 0.353). n = 31 subjects. Linear regressions with age- and education-adjusted z-scores for each cognitive test.
Table 3.
Cross-sectional association between CSF lipids and other cognitive test z-scores
| Log lipid | Rey-Osterrieth Complex Figure Test—Copy
|
Rey-Osterrieth Complex Figure Test—Recall
|
Odd Man Out
|
Verbal Fluency
|
||||
|---|---|---|---|---|---|---|---|---|
| b | p value | b | p value | b | p value | b | p value | |
| Total SM | −0.34 | 0.593 | 0.09 | 0.756 | −0.03 | 0.941 | −0.23 | 0.375 |
| Total Cholesterol | 0.10 | 0.943 | 0.52 | 0.403 | 0.48 | 0.549 | −0.31 | 0.591 |
| SM/Cholesterol | −0.80 | 0.357 | −0.12 | 0.745 | −0.19 | 0.722 | −0.35 | 0.335 |
| Ceramide C16:0 | −0.96 | 0.500 | −0.28 | 0.666 | −0.83 | 0.265 | −0.21 | 0.728 |
| Ceramide C18:0 | 0.09 | 0.928 | 0.73 | 0.077 | 0.23 | 0.644 | 0.44 | 0.251 |
| Ceramide C22:0 | −1.56 | 0.231 | 0.36 | 0.550 | −1.07 | 0.110 | 0.15 | 0.791 |
| Ceramide C24:0 | −0.94 | 0.115 | 0.07 | 0.788 | −0.14 | 0.655 | −0.08 | 0.769 |
| SM/Ceramide C16:0 | −0.39 | 0.505 | −0.02 | 0.939 | 0.19 | 0.620 | −0.25 | 0.299 |
| SM/Ceramide C18:0 | −0.48 | 0.381 | −0.41 | 0.086 | 0.09 | 0.774 | −0.31 | 0.159 |
| SM/Ceramide C22:0 | 0.00 | 1.000 | 0.01 | 0.978 | 0.57 | 0.263 | −0.31 | 0.339 |
| SM/Ceramide C24:0 | 0.74 | 0.297 | −0.03 | 0.917 | 0.16 | 0.696 | −0.14 | 0.652 |
|
| ||||||||
| Digit Symbol
|
Grooved Pegboard Dominant Hand
|
Mean Choice Reaction Time
|
Mean Sequential Reaction Time
|
|||||
| Log lipid | b | p value | b | p value | b | p value | b | p value |
|
| ||||||||
| Total SM | −0.03 | 0.915 | −0.47 | 0.478 | 0.09 | 0.778 | 0.03 | 0.939 |
| Total Cholesterol | 0.08 | 0.902 | 0.78 | 0.595 | 0.21 | 0.766 | −0.49 | 0.550 |
| SM/Cholesterol | −0.14 | 0.724 | −1.16 | 0.208 | 0.00 | 0.997 | 0.12 | 0.810 |
| Ceramide C16:0 | −1.06 | 0.098 | −1.83 | 0.225 | −0.63 | 0.307 | −0.51 | 0.477 |
| Ceramide C18:0 | 0.17 | 0.685 | 0.30 | 0.763 | 0.24 | 0.533 | 0.31 | 0.521 |
| Ceramide C22:0 | −0.62 | 0.301 | −1.57 | 0.260 | −0.44 | 0.477 | −0.10 | 0.888 |
| Ceramide C24:0 | −0.18 | 0.509 | −1.14 | 0.068 | −0.19 | 0.474 | 0.08 | 0.802 |
| SM/Ceramide C16:0 | 0.08 | 0.776 | −0.28 | 0.656 | 0.11 | 0.723 | −0.06 | 0.870 |
| SM/Ceramide C18:0 | −0.23 | 0.346 | −0.11 | 0.851 | −0.21 | 0.402 | −0.41 | 0.173 |
| SM/Ceramide C22:0 | 0.19 | 0.596 | −0.09 | 0.912 | 0.38 | 0.384 | 0.12 | 0.802 |
| SM/Ceramide C24:0 | 0.22 | 0.499 | 0.73 | 0.334 | 0.43 | 0.193 | −0.10 | 0.804 |
Increased sphingomyelin:cholesterol ratios are associated with worse performance on the Rey Auditory Verbal Learning Test trial 5 and delayed recall
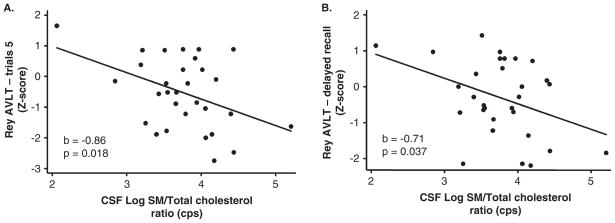
Cholesterol is tightly associated with sphingomyelin and both are critical components of membrane microdomains known as lipid rafts. Perturbations in the relative content of either sphingomyelin or cholesterol can affect neural function by deregulating signaling and the cellular response to stress (Clement et al, 2009; Quinn and Wolf, 2009). Because cholesterol associates with sphingomyelin regardless of the acyl chain length, we combined all forms of sphingomyelin (C16:0 to C24:0) into a single variable for comparison with total cholesterol. Higher sphingomyelin:cholesterol ratios were significantly associated with worse performance on the RAVLT trail 5 score (p = 0.018) and delayed recall (p = 0.037) (Figure 4, Table 2). Sphingomyelin:cholesterol ratios were not associated with performance on tasks that assess visual memory, verbal fluency, executive function, or motor function (data not shown). Controlling for gender, race, antiretroviral therapy, CD4 and CD8 cell counts, current drug or alcohol use, depressive symptoms, and plasma or CSF HIV RNA did not alter any of the associations.
Figure 4.
CSF levels of sphingomyelin:cholesterol ratios are associated with worse performance on trial 5 and delayed recall in the Rey Auditory Verbal Learning Test. Correlations for CSF levels of sphingomyelin (total):cholesterol (total) ratios are shown. The correlation coefficient (b) and exact significance level (p) are indicated in insets. n = 31 subjects. Linear regression with age- and education-adjusted z-scores.
Discussion
It has been demonstrated that the sphingomyelin-ceramide balance in brain becomes progressively disrupted with HAND in accordance with the severity of neurocognitive dysfunction (Haughey et al, 2004). These studies found that multiple species of sphingomyelin were increased in the middle frontal and temporal gyrus of HIV-infected patients with mild cognitive dysfunction, and multiple forms of sphingomylein, ceramide, and the lipid peroxidation product 4-hydroxynoneal were increased in those with moderate to severe cognitive dysfunction (Haughey et al, 2004). Measures of CSF sphingolipids (as surrogate markers for brain sphingolipid metabolism) in patients living with HIV demonstrated that sphingomyelin accumulated with mild and stable cognitive dysfunction, and increased ceramide levels were associated with ongoing cognitive decline (Bandaru et al, 2007). Additionally, there were greater accumulations of sterols sphingomyleins and ceramides in brains of subjects with an apolipoprotein E4 (ApoE4) genotype, suggesting that a genetic component may contribute to perturbed sphingolipid metabolism in HAND (Cutler et al, 2004a). Because each of these studies used a combinational score (Memorial Sloan Kettering Scale) that combines data from tasks that assess multiple cognitive domains and motor functions, it was not known if alterations in sphingomyelin and ceramide content associated with deficits in particular cognitive and/or motor functions. In this study we addressed this question and found that changes in the sphingomyelin:ceramide ratio for acyl chain lengths of C16, C18, C22, and C24 were associated with worse performance on one or more indices of memory, including the total, trial 5, and immediate and delayed recall scores, on the RAVLT. The strongest of these associations was for the sphingomylein: ceramide ratio of C18:0 that was associated with performance on each of the RAVLT memory tasks. These findings suggest that the sphingomyelin:ceramide ratio for C18 may be a sensitive marker for neurocognitive impairment in HIV-infected subjects, and that the levels of C18 may be a reasonable surrogate marker for memory function. However, we cannot rule out possible associations for sphingolipids with other cognitive functions that were not specifically tested in this group of subjects.
To our knowledge, this is the first report to use the ratio of sphingomyelin to ceramide as a diagnostic/prognostic marker. We reasoned that because sphingomyelin and ceramide are directly related metabolic pathways (ceramide is a precursor to sphingomyelin and sphingomyelin can be hydrolyzed to create ceramide), the ratio of sphingomyelin to ceramide would be a useful metric to measure the status of sphingomyelin metabolism. Because these reactions most often modify the head group and not the acyl chain length, we only compared identical tail compositions. Using this metric we found that the sphingomyelin:ceramide ratio for C16:0, C18:0, C22:0, and C24:0 were associated with worse performance on several indices of memory, with C18:0 showing the most consistent effects. A ration of sphingomyelin:ceramide for the particular chain length of C18:0 may be a useful marker for HAND, since other neurodegenerative disease with a known disruption in ceramide metabolism have reported that very long chain ceramide accumulate in brain and CSF. Perturbed sphingolipid balance has been identified in a number of neurodegenerative conditions that include multiple sclerosis, Alzheimer’s disease (AD), amyotrophic lateral sclerosis, and HAND (Cutler et al, 2002, 2004a, 2004b; Bandaru et al, 2007, 2009; Haughey et al, 2004; Wheeler et al, 2008). In each of these neurodegenerative conditions there appears to be disease-specific alterations in particular species and forms of sphigolipids. For example, C24:0 ceramide and sphingomylein are inversely and progressively disrupted in AD with increasing disease severity (Cutler et al, 2004b; He et al, 2008). In contrast, subjects with HAND who showed evidence of mild and stable cognitive impairment had increased C18:0 to C24:0 sphingomylein with no apparent changes in ceramide. Those with HAND and frank dementia showed increased C18:0 to C24:0 sphingomylein and ceramide (Bandaru et al, 2007; Cutler et al, 2004b; Haughey et al, 2004). Our current findings suggest that a ratio of C18 sphingomylein: ceramide may be a sensitive biomarker for cognitive dysfunction in HAND that specifically relates to memory function. Further studies should be aimed to clarify the potential usefulness of this metric in larger and diverse populations of HIV-infected subjects.
A possible explanation for the specificity of C18:0 to associate with memory impairment in HAND has been provided by findings that suggest differential functions that depend on the biophysical properties of sphingolipids with particular carbon chain lengths. For instance, the length and saturation of acyl chains can influence the width of bilayers, fluidity of membranes, interactions with other lipids, and lipid-protein interactions (Aittoniemi et al, 2007; Niemela et al, 2006). Additionally, there is evidence of considerable tissue, cellular, and subcellular specificities for particular molecular species of sphingolipids, suggesting that the pattern of disruption in sphingolipid metabolism may identify specific organ and organelle dysfunctions (Kaiser et al, 2009; Meer and Hoetzl, 2009). Consistent with the regulated subcellular distribution of sphingolipids, there is an increasing amount of data that suggest that sphingolipids with particular carbon chain lengths can differentially regulate signal transduction, cell motility, neurite outgrowth, synpatogenesis, and cellular fusion events important for protein trafficking and synaptic transmitter release (Gallegos et al, 2008; Galvan et al, 2005; Nygren et al, 2005; Wheeler et al, 2009). The findings from these mechanistic studies together with our biochemical and clinical observations suggest that disrupted sphingomyelin:ceramide balance in HAND may reflect perturbed neural function that becomes manifest as deficits in verbal memory. Although we cannot make any definite conclusions why verbal memory was better correlated to surrogate markers of sphingoipid metabolism compared with visual memory, a review of 56 studies that used neuropsychological tests to investigate areas of function affected by central nervous system dysfunction in HIV-infected subjects found evidence for some dysfunction in verbal memory (27% of studies), executive function (43%), motor performance (20%), and information processing (44%) among subjects who were otherwise asymptomatic. Subjects with more advanced HIV infection showed consistent evidence of abnormal functioning in the areas of verbal (48% of studies) and visual (43%) memory, executive functioning (71%), complex attention (62%), motor performance (37%), and information processing (69%) (Dunbar and Brew, 1997). Thus, in HIV-infected subjects there may be impairments of verbal memory early in disease progression that occur independent of visual memory.
The specific association of sphingomylein:ceramide ratios for C18 and impairment on memory in the RAVLT also provides clues to the specific enzymes responsible for perturbations of sphingomyelin:ceramide balance in HAND. There are a multitude of enzymes involved in sphingomyelin and ceramide metabolism, including the following: Sphingomyelinases that mediate the hydrolysis of sphingomyelin to ceramide; five different sphingomyelinase that differ in pH optimum, metal dependence, and subcellular localization are known (Marchesini and Hannun, 2004). Ceramidases that cleave fatty acids from ceramide to produce sphingosine; seven different ceramidase have been identified (Mao and Obeid, 2008). Sphingomyelin synthases (SMS) that catalyze the conversion of ceramide and phosphatidylcholine to sphingomyelin and diacylglycerol; two SMS have been identified, SMS1 that is localized to the Golgi, SMS2 that resides primarily at the plasma membrane (Huitema et al, 2004; Takeuchi et al, 1995). Dihydroceramide desaturases (ceramide synthase; CerS) that are involved in the de novo production of ceramide; six CerS have been identified (Pewzner-Jung et al, 2006). Of these enzymes, only CerS are known to have preferences for particular acyl chain lengths, with each of CerS family members utilizing a relatively restricted subset of fatty acyl–coenzyme As (CoAs) (see Mizutani et al, 2006, for a review). Of particular interest to the present work are CerS1 and CerS3 that preferentially use C18 as a substrate. Our findings suggest that a defect in CerS1 and/or CerS3 may be prominently involved in the perturbation of the sphingomylein:ceramide C18:0 ratio in subjects with HAND. The identification of specific enzymes involved with disturbances of sphingomylein metabolism in HAND may identify new therapeutic targets for this neurodegenerative disease.
Conclusions
In patients infected with HIV there are perturbations in brain sphingolipid metabolism that are detectable in CSF. In this study we found that CSF levels of sphingomyleins and ceramides with the restricted carbon chain lengths of C18:0 were associated with verbal memory as determined by performance on the RAVLT memory tasks. These findings suggest that the sphingomyelin:ceramide ratio for C18:0 may be a sensitive marker for neurocognitive impairment in HIV-infected subjects, and that the levels of C18:0 may be a reasonable surrogate marker for memory function in these patients.
Materials and methods
Subjects
The North East AIDS Dementia (NEAD) cohort was initiated in March 1998 at four sites (Johns Hopkins University, Columbia University, University of Rochester, and Northwestern University). The present study includes 31 subjects from the NEAD cohort for whom both CSF and cognitive testing results were obtained and available from the Johns Hopkins HIV NeuroAIDS repository. Subjects provided written informed consent for their participation in NEAD, and studies were approved by the Johns Hopkins University internal review board governing human subjects research. Patient demographics are listed in Table 1. To be included in the North Eastern AIDS Dementia (NEAD), cohort subjects had to be HIV-positive and have either a CD4+ cell count below 200/μl or a CD4+ count below 300/μl and evidence of cognitive impairment on a neuropsychological test battery. Exclusion criteria for subjects in the NEAD cohort included current or past opportunistic CNS infection at study entry (with the exception of treated neurosyphilis), a history of or current severe affective disorder or history of a chronic neurologic disorder such as multiple sclerosis (MS), head injury with loss of consciousness for longer than 1 hour, stroke, or uncontrolled epilepsy. Current alcohol or drug use were not grounds for exclusion. Neither drug nor alcohol use were associated with cognitive performance.
Assessments and sample collection
Clinical assessments were performed every 6 months for up to 48 months. At each visit research subjects underwent a medical history, a standardized neurological examination, a neuropsychological battery, functional and psychiatric assessments, and a blood draw for laboratory studies including viral load and immune activation markers. CSF samples were obtained every 12 months. After collection, CSF was immediately centrifuged and cell-free CSF was aliquoted and immediately frozen at −80°C. For this study we used baseline CSF samples collected from a single time point.
Cognitive testing
The neuropsychological test battery was administered by trained neuropsychometricians and consisted of eight tests covering seven domains (Albert et al, 2003; Marder et al, 2003). Tests included a computerized simple– and choice–reaction time test (CALCAP) (Miller et al, 1991), Digit Symbol test (Wechsler, 1981), Grooved Pegboard (Dominant Hand) for psychomotor performance (Klove, 1963), Odd Man Out test for assessment of executive function (Flowers and Robertson, 1985), Controlled Verbal Fluency Task (FAS) test for verbal fluency (Benton, 1951), Rey-Osterrieth Complex Figure Test (Rey, 1941) for visual memory, and verbal memory was assessed with the Rey Auditory Verbal Learning Test (Rey, 1941). Age- and education-adjusted z-scores were used to quantify performance for each of the neuropsychological tests. Two sets of published normative data were used: AIDS Link to Intravenous Experience (ALIVE) study cohort (Concha et al, 1995) for respondents with 12 years of education or less, and normative data from the Multicenter AIDS Cohort Study (MACS) (Selnes et al, 1991) for subjects with educational equivalent to a high school diploma or greater.
Lipid extraction from cerebral spinal fluid
A crude lipid extraction from CSF was conducted using a modified Bligh and Dyer procedure as previously described (Haughey et al, 2004). To control for slight differences in extraction efficiencies and day-to-day variations in the efficiency of the mass spectrometer, purified standards of sphingomyelin C12:0, ceramide C12:0, and deuterated cholesterol (10 nM each; Avanti Polar Lipids, Alabaster, AL) were added directly to CSF prior to extraction. Extractions were conduction by the addition of three volumes 100% methanol containing 53 mM ammonium formate to 300 μl CSF. Four volumes of chloroform were added, the mixture was vortexed and centrifuged at 1000 × g for 10 min. The chloroform layer was removed into a glass storage vial and dried under a stream of nitrogen. Dried extracts were sealed and stored at −80°C. For analysis, dried extracts were resuspended in 100% methanol.
Mass spectrometry
Mass spectrometry analyses were performed using a Sciex API 3000 triple-stage quadrupole tandem mass spectrometer (ESI/MS/MS) from Sciex (Thornhill, Ontario, Canada), using methods similar to those described in previous studies (Bandaru et al, 2007; Haughey et al, 2004). Samples were injected using a Harvard Apparatus pump at the rate of 15 ml/min into an electrospray ionization (i.e., Turbo Ion Spray module) Sciex API 3000 triple-stage quadrupole tandem mass spectrometer (ES/MS/MS) operated in the positive mode. Each species of sphingomyelin, ceramide, and sterols were identified by a Q1 mass scan, then by precursor ion scanning or neutral loss scanning of a purified standard. Samples were injected into the ESI/MS/MS for 3 min, where the mass counts accumulated and the sum of the total counts under each peak were used to quantitate each species. Cholesterol and cholesterol ester standards C16:0, C18:0, C18:1, and cholesteryl-arachidonate (C20:0) were purchased from Sigma (St. Louis, MO). Sphingomyelins and ceramides with carbon chain lengths ranging from C16:0 to C24:1 were purchased from Avanti Polar Lipids (Alabaster, AL).
Data analysis
Sphingomyelin analytes were highly correlated after adjusting for Bonferroni correction (p < 0.0001). We therefore summed all sphingomyelin analytes to create a single (total) sphingomyelin variable. Ceramides were not highly correlated, so we examined each analyte separately. When examining the sphingomyelin:ceramide ratio, individual sphingomyelin analytes were used and only species with identical acyl chain lengths and saturation were compared. For example, sphingomyelin:ceramide 16:0 was the ratio of sphingomyelin 16:0 to ceramide 16:0. Counts per second (Cps) were log-transformed prior to analyses and data that were 3 standard deviations beyond the mean were excluded. Using this criteria, one subject was excluded from each of the sphingomyelin and sphingomyelin:ceramide ratio analyses.
t-Tests and Fisher’s exact test were used to examine differences between subjects who did and did not have both CSF and neuropsychological testing available. Analyses of variance (ANOVAs) were used to examine differences in sphingomyelin and ceramide levels by MSK scores. Linear regressions were used to examine the relationship between sphingomyelin and ceramide levels to age- and education-adjusted z-scores for each cognitive test. Gender, race, antiretroviral therapy, CD4 and CD8 cell counts, current drug or alcohol use, depressive symptoms (as measured by the Beck Depression Inventory), plasma and CSF HIV RNAs were examined as potential confounders. As none of these variables affected the association between the CSF sphingomyelin, ceramide, or sphingomylein:ceramide ratios and cognitive tests, the final statististical models did not include any of these covariates. The a priori p value was set at p < 0.05. Analyses were conducted using Stata Version 9.2 (StataCorp, College Station, TX).
Acknowledgments
The authors acknowledge Ms. Fang Yang for technical assistance, the staff associated with the NEAD study, and the other sites at Northwestern University, Columbia University, and the University of Rochester. The sterling efforts of the research subjects who provided time and samples are appreciated.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aittoniemi J, Niemela PS, Hyvonen MT, Karttunen M, Vattulainen I. Insight into the putative specific interactions between cholesterol, sphingomyelin, and palmitoyl-oleoyl phosphatidylcholine. Biophys J. 2007;92:1125–1137. doi: 10.1529/biophysj.106.088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert SM, Flater SR, Clouse R, Todak G, Stern Y, Marder K. Medication management skill in HIV: I. Evidence for adaptation of medication management strategies in people with cognitive impairment. II. Evidence for a pervasive lay model of medication efficacy. AIDS Behav. 2003;7:329–338. doi: 10.1023/a:1025404105378. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, Haughey NJ. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Troncoso J, Wheeler D, Pletnikova O, Wang J, Conant K, Haughey NJ. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiol Aging. 2009;30:591–599. doi: 10.1016/j.neurobiolaging.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL. The Visual Retention Test. New York: Psychological Corporation; 1951. [Google Scholar]
- Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26:799–819. x. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Cardenas V, Meyerhoff D, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant R, Chao L, Truran D, Weiner M. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J NeuroVirol. 2009;15:1–10. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AB, Gamerdinger M, Tamboli IY, Lutjohann D, Walter J, Greeve I, Gimpl G, Behl C. Adaptation of neuronal cells to chronic oxidative stress is associated with altered cholesterol and sphingolipid homeostasis and lysosomal function. J Neurochem. 2009;111:669–682. doi: 10.1111/j.1471-4159.2009.06360.x. [DOI] [PubMed] [Google Scholar]
- Concha M, Selnes OA, McArthur J, Nance-Sproson T, Updike ML, Royal W, Solomon L, Vlahov D. Normative data for a brief neuropsychological test battery in a cohort of injecting drug users. Int J Addict. 1995;30:832–841. doi: 10.3109/10826089509067009. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Haughey NJ, Tammara A, McArthur JC, Nath A, Reid R, Vargas DL, Pardo CA, Mattson MP. Dysregulation of sphingolipid and sterol metabolism by ApoE4 in HIV dementia. Neurology. 2004a;63:626–630. doi: 10.1212/01.wnl.0000134662.19883.06. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004b;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- Dunbar N, Brew B. Neuropsychological dysfunction in HIV infection: a review. J NeuroAIDS. 1997;1:73–102. doi: 10.1300/j128v01n03_05. [DOI] [PubMed] [Google Scholar]
- Flowers KA, Robertson C. The effects of Parkinson’s disease on the ability to maintain a mental set. J Neurol Neurosurg Neuropsychiatry. 1985;48:517–529. doi: 10.1136/jnnp.48.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos CE, Pediconi MF, Barrantes FJ. Ceramides modulate cell-surface acetylcholine receptor levels. Biochim Biophys Acta. 2008;1778:917–930. doi: 10.1016/j.bbamem.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Galvan C, Camoletto PG, Dotti CG, Aguzzi A, Ledesma MD. Proper axonal distribution of PrP(C) depends on cholesterol-sphingomyelin-enriched membrane domains and is developmentally regulated in hippocampal neurons. Mol Cell Neurosci. 2005;30:304–315. doi: 10.1016/j.mcn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Holzer CE, 3rd, Fabian RH, Schuenke KW, Keherly MJ, Richey FJ, Lahart CJ. Potential role for white matter lysosome expansion in HIV-associated dementia. J Acquir Immune Defic Syndr. 2005;39:422–425. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, Jacobus J, Woods SP, Jernigan TL, Ellis RJ, Frank LR, Grant I. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J NeuroVirol. 2009;15:187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ. Sphingolipids in neurodegeneration. [Accessed: Aug 25 2010];Neuromol Med. 2010 doi: 10.1007/s12017-010-8135-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging. 2008;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser HJ, Lingwood D, Levental I, Sampaio JL, Kalvodova L, Rajendran L, Simons K. Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci U S A. 2009;106:16645–16650. doi: 10.1073/pnas.0908987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. Med Clin North Am. 1963;47:1647–1658. [PubMed] [Google Scholar]
- Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008;1781:424–434. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- Marder K, Albert SM, McDermott MP, McArthur JC, Schifitto G, Selnes OA, Sacktor N, Stern Y, Palumbo D, Kieburtz K, Cohen B, Orme C, Epstein LG. Inter-rater reliability of a clinical staging of HIV-associated cognitive impairment. Neurology. 2003;60:1467–1473. doi: 10.1212/01.wnl.0000064172.46685.82. [DOI] [PubMed] [Google Scholar]
- McMurtray A, Nakamoto B, Shikuma C, Valcour V. Cortical atrophy and white matter hyperintensities in HIV: the Hawaii Aging with HIV Cohort Study. J Stroke Cerebrovasc Dis. 2008;17:212–217. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer GV, Hoetzl S. Sphingolipid topology and the dynamic organization and function of membrane proteins. FEBS Lett. 2009;584:1800–1805. doi: 10.1016/j.febslet.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Miller EN, Satz P, Visscher B. Computerized and conventional neuropsychological assessment of HIV-1-infected homosexual men. Neurology. 1991;41:1608–1616. doi: 10.1212/wnl.41.10.1608. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Kihara A, Igarashi Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem J. 2006;398:531–538. doi: 10.1042/BJ20060379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- Niemela PS, Hyvonen MT, Vattulainen I. Influence of chain length and unsaturation on sphingomyelin bilayers. Biophys J. 2006;90:851–863. doi: 10.1529/biophysj.105.067371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren H, Borner K, Hagenhoff B, Malmberg P, Mansson JE. Localization of cholesterol, phosphocholine and galactosylceramide in rat cerebellar cortex with imaging TOF-SIMS equipped with a bismuth cluster ion source. Biochim Biophys Acta. 2005;1737:102–110. doi: 10.1016/j.bbalip.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- Quinn PJ, Wolf C. Thermotropic and structural evaluation of the interaction of natural sphingomyelins with cholesterol. Biochim Biophys Acta. 2009;1788:1877–1889. doi: 10.1016/j.bbamem.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychological dans les cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Roc AC, Ances BM, Chawla S, Korczykowski M, Wolf RL, Kolson DL, Detre JA, Poptani H. Detection of human immunodeficiency virus induced inflammation and oxidative stress in lenticular nuclei with magnetic resonance spectroscopy despite antiretroviral therapy. Arch Neurol. 2007;64:1249–1257. doi: 10.1001/archneur.64.9.noc60125. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Jacobson L, Machado AM, Becker JT, Wesch J, Miller EN, Visscher B, McArthur JC. Normative data for a brief neuropsychological screening battery. Multicenter AIDS Cohort Study. Percept Mot Skills. 1991;73:539–550. doi: 10.2466/pms.1991.73.2.539. [DOI] [PubMed] [Google Scholar]
- Takeuchi J, Okada M, Toh-e A, Kikuchi Y. The SMS1 gene encoding a serine-rich transmembrane protein suppresses the temperature sensitivity of the htr1 disruptant in Saccharomyces cerevisiae. Biochim Biophys Acta. 1995;1260:94–96. doi: 10.1016/0167-4781(94)00188-9. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long--term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J NeuroVirol. 2008;14:362–367. doi: 10.1080/13550280802216494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131:3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]