Abstract
Shear stress plays a critical role in the regulation of vascular biology and diseases, such as atherosclerosis, via modulation of signal transduction and redox balance. Atherosclerosis preferentially occurs in a site-specific manner linked to disturbed flow. In this Forum on Vascular Shear Stress, emerging role of redox-dependent molecular mechanisms by which shear stress regulates pro- and antiatherogenic responses in endothelial cells both in vitro and in vivo are reviewed in depth by experts. This Forum also provides comprehensive reviews regarding experimental apparatus and in vivo, ex vivo, and in vitro systems used for shear stress studies. Antioxid. Redox Signal. 15, 1367–1368.
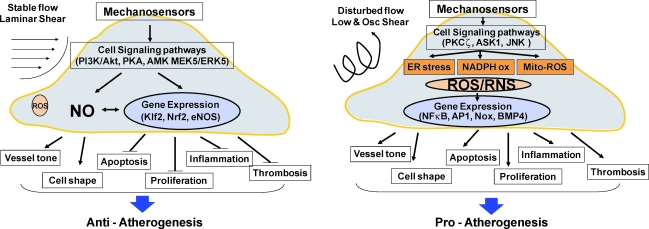
Vascular shear stress is generated by blood flowing over endothelial layer and plays a critical role in vascular biology and diseases such as atherosclerosis. Atherosclerosis preferentially occurs in branched or curved arterial regions associated with unstable blood flow, whereas straight vessels that are exposed to stable flow are spared from the disease (4, 13, 15). Further, recent studies using Apolipoprotein E−/− mice showed that acute exposure to disturbed flow, characterized with low and oscillating shear stress, rapidly induces robust atherosclerosis in mouse carotid artery, providing direct evidence that disturbed flow can indeed induce atherosclerosis in hyperlipidemic condition (6). The mechanisms by which shear stress either induces or inhibits atherosclerosis have been studied by numerous groups, leading to better molecular insights. Shear stress is recognized by endothelial cells via various mechanosensors including the gap junctional protein platelet/endothelial cell adhesion molecule 1, cell surface glycocalyx, primary cilia, ion channels, caveolae and G-proteins, cytoskeletal proteins, and integrins (2, 10). Upon mechanosensing, shear stress regulates endothelial function and structure by inducing cascades of cell signaling events and changing gene expression patterns. Regulation of redox balance through production and removal of reactive oxygen species (ROS) and nitric oxide is a critical mechanism by which shear stress acts as a potent pro- or antiatherogenic stimulus. In this Forum on Vascular Shear Stress, a series of review articles and research articles have been put together to summarize current understanding in mechanisms by which shear stress regulates vascular biology and disease and to highlight the role of redox balance in this evolving field (Fig. 1). Ando and Yamamoto (3) and Berk and colleagues (8) provide general reviews regarding the role of shear stress and an additional vascular stress, stretching, in endothelial biology and atherosclerosis. The antiatherogenic role of a mechanosensitive gene Klf2 is reviewed by Jain's group (7), and Noguchi's group (12) discusses the role of Nrf2 in regulation of antioxidant level and antiatherogenesis by stable shear stress. In contrast, Davies' group (5) highlights the emerging role of ER stress and redox environment in inflammation and atherosclerosis in flow-disturbed arterial regions in vivo. Hsiai and colleagues (11) report the role of JNK and mitochondrial ROS in regulation of endothelial inflammation in response to oscillatory shear stress. Jo and colleagues (9) summarize various in vitro, ex vivo, and in vivo experimental models used to study the role of shear stress in vascular biology and atherosclerosis. Taylor's group (14) reports a novel model of disturbed flow and ROS using coarcted mouse abdominal aorta. Last, Garcia-Cardeña's group (1) discusses the emerging role of shear stress in stem cell differentiation. With this Forum, we attempted to provide comprehensive reviews on the current insights into the role of vascular shear stress in vascular biology and diseases as well as the experimental tools and cell, tissue, and animal models used for shear experiments.
FIG. 1.
Opposing roles of stable flow and disturbed flow in endothelial biology and atherosclerosis. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Abbreviation Used
- ROS
reactive oxygen species
Acknowledgments
N.N.'s work was supported by Special Coordination Fund for Science and Technology and the Academic Frontier Research Project on “New Frontier of Biomedical Engineering Research” of the Ministry of Education, Culture, Sports, Science, and Technology. H.J.'s work was supported by funding from NIH grants HL87012 and HL75209 and a World Class University Project from the Ministry of Science, Technology, and Education of South Korea.
References
- 1.Adamo L. García-Cardeña G. Directed stem cell differentiation by fluid mechanical forces. Antioxid Redox Signal. 2011;15:1463–1473. doi: 10.1089/ars.2011.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali MH. Schumacker PT. Endothelial responses to mechanical stress: where is the mechanosensor? Crit Care Med. 2002;30(5 Suppl):S198–S206. doi: 10.1097/00003246-200205001-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ando J. Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal. 2011;15:1389–1403. doi: 10.1089/ars.2010.3361. [DOI] [PubMed] [Google Scholar]
- 4.Asakura T. Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 5.Davies PF. Civelek M. Endoplasmic reticulum stress, redox and a proinflammatory environment in athero-susceptible endothelium in vivo at sites of complex hemodynamic shear stress. Antioxid Redox Signal. 2011;15:1427–1432. doi: 10.1089/ars.2010.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nam D. Ni CW. Rezvan A. Suo J. Budzyn K. Llanos A. Harrison D. Giddens D. Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nayak L. Lin Z. Jain MK. “Go with the flow”: how Krüppel-like factor 2 regulates the vasoprotective effects of shear stress. Antioxid Redox Signal. 2011;15:1449–1461. doi: 10.1089/ars.2010.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nigro P. Abe Ji. Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid Redox Signal. 2011;15:1405–1414. doi: 10.1089/ars.2010.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezvan A. Ni CW. Alberts-Grill N. Jo H. Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: role of oxidative stress. Antioxid Redox Signal. 2011;15:1433–1448. doi: 10.1089/ars.2010.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubanyi GM. Freay AD. Kauser K. Johns A. Harder DR. Mechanoreception by the endothelium: mediators and mechanisms of pressure- and flow-induced vascular responses. Blood Vessels. 1990;27:246–257. doi: 10.1159/000158816. [DOI] [PubMed] [Google Scholar]
- 11.Takabe W. Jen N. Ai L. Hamilton R. Wang S. Holmes K. Dharbandi F. Khalsa B. Bressler S. Barr ML. Li R. Hsiai TK. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal. 2011;15:1379–1388. doi: 10.1089/ars.2010.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takabe W. Warabi E. Noguchi N. Anti-atherogenic effect of laminar shear stress via Nrf2 activation. Antioxid Redox Signal. 2011;15:1415–1426. doi: 10.1089/ars.2010.3433. [DOI] [PubMed] [Google Scholar]
- 13.VanderLaan PA. Reardon CA. Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 14.Willett NJ. Kundu K. Knight SF. Dikalov S. Murthy N. Taylor WR. Redox signaling in an in vivo murine model of low magnitude oscillatory wall shear stress. Antioxid Redox Signal. 2011;15:1369–1378. doi: 10.1089/ars.2010.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarins CK. Giddens DP. Bharadvaj BK. Sottiurai VS. Mabon RF. Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]