Abstract
Endothelial phenotype heterogeneity plays an important role in the susceptibility of arteries to atherosclerosis. Regions of blood flow disturbance correlate with the development of disease. Here, we briefly outline the association of endoplasmic reticulum stress with endothelium in regions of athero-susceptibility in vivo. It is an important example of susceptible cell phenotype that is likely linked to proinflammatory and oxidative stress pathways. The endothelium in such regions is chronically exposed to complex hemodynamic shear stresses that may be considered as a risk factor for atherosclerosis via these mechanisms. Antioxid. Redox Signal. 15, 1427–1432.
Introduction
Atherosclerosis is not a diffuse disease; it has been noted for centuries that lesion development is associated with arterial curvatures, asymmetries, and branches where the nonuniform arterial geometry generates patterns of blood flow that are considerably more complex than elsewhere. It is well established that endothelial cells are highly sensitive to flow/shear stress; therefore, a biomechanical contribution to localized susceptibility is likely. Athero-susceptible endothelium in vivo expresses a different repertoire of cell phenotypes than that in nearby protected locations [reviewed in (5)]. Identification of important differences in gene and protein expression and the mechanisms responsible requires both global profiling and classic cell and molecular approaches. Recently, the chronic activation of a common signature of cellular endoplasmic reticulum (ER) stress in endothelium has emerged as a potential underlying contributor to athero-susceptibility (1).
ER Stress and the Unfolded Protein Response
ER stress is an adaptive protective mechanism that arises because of excessive protein biosynthesis or interference with normal protein folding mechanisms in the ER lumen in response to multiple kinds of cellular stress (10). Excessive newly synthesized and/or misfolded polypeptides in the ER lumen exceed its protein folding capacity. It results in the activation of the unfolded protein response (UPR), an ubiquitous adaptive cell response that assists cell survival in an adverse environment by activating a set of intracellular signaling pathways. The UPR signals a coordinated transcriptional upregulation of ER chaperones and folding enzymes to promote the correct assembly of unfolded polypeptides and prevent incompletely folded proteins from aggregating.
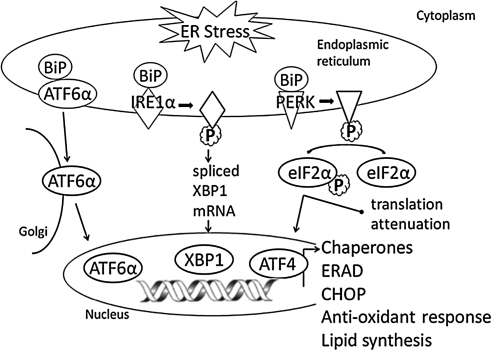
In the unstressed state, the ER chaperone binding protein (BiP; also known as heat-shock protein A5 [HSPA5] and glucose-related protein 78 [GRP78]) binds to each of three ER stress transducers. These are ER transmembrane proteins each having an ER luminal domain for the sensing of unfolded proteins and a cytosolic domain for signaling. While bound, BiP maintains the inactive state of the transducers; however, the UPR is activated when an imbalance occurs in the luminal flux of newly synthesized unfolded or misfolded peptides as a result of cell stress. To bind unfolded/misfolded polypeptides in the ER lumen, BiP dissociates from the chaperones causing their phosphorylation or translocation. Activation of activating transcription factor 6α (ATF6α), inositol requiring kinase 1α (IRE1α), and protein kinase-like ER kinase (PERK) and the downstream consequences of their activation constitute the UPR (Fig. 1).
FIG. 1.
Endoplasmic reticulum (ER) stress and unfolded protein response (UPR). UPR is activated to restore the ER homeostasis in response to the accumulation of unfolded proteins. The UPR canonical pathway is signaled through the three ER transmembrane sensors: protein kinase-like ER kinase (PERK), inositol requiring kinase 1α (IRE1α), and activating transcription factor 6α (ATF6α).
In the first branch of UPR, BiP dissociation results in the dimerization and autophosphorylation of IRE1α, which gains endoribonuclease activity. It excises a 26 base-pair fragment from X-box binding protein 1 (XBP1) mRNA and forms the spliced XBP1 (sXBP1), which is translated into the active transcription factor XBP1 that translocates to the nucleus, where it binds the UPR element. This leads to the transcription of protein folding chaperones as well as ER-associated degradation genes that include ubiquitination and the proteasome. In the second branch of UPR, BiP dissociation exposes a sequence within the 90 kDa protein ATF6α that triggers its localization to the Golgi, where the protein is cleaved. The active 50 kDa form of ATF6α translocates to the nucleus, where it binds to the ATF/cAMP response element and ER stress element to induce the transcription of protein folding chaperones and XBP1. In the third branch of UPR, similar to IRE1α, BiP dissociation results in the dimerization and autophosphorylation of PERK. Active PERK phosphorylates the serine 51 residue of eukaryotic translation initiation factor 2α (eIF2α), causing translation attenuation of most proteins with the exception of ATF4. ATF4 protein binds to the UPR element that leads to the transcription of several UPR genes, including C/ERB homologous protein (CHOP). Additional control mechanisms of UPR activation are also thought to exist (18).
The products of these activated UPR transducers converge as transcriptional regulators in the nucleus to reduce protein synthesis, to upregulate ER chaperones and UPR transducer synthesis, and to ubiquitinate unfolded proteins for degradation through the proteosome, processes that relieve ER stress accumulation of unfolded proteins and restore homeostasis. Failure to restore ER protein equilibrium to a normal range leads to apoptosis through transcriptional induction of CHOP, inflammation through activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), and generation of reactive oxygen species (ROS) through excessive protein oxidation in the ER.
The links between site-specific endothelial ER stress in vivo, hemodynamics, and athero-susceptibility have converged in three recent studies.
Unbiased global genomics
In a multisite study in 45 normal adult swine, endothelium in susceptible regions of the aortic arch (AA), proximal brachiocephalic artery, aorto-renal branch region, and abdominal aorta were analyzed relative to protected sites of the common carotid artery, descending thoracic aorta, and the distal renal artery (1). All athero-susceptible regions are associated with complex disturbed blood flow. From this multisite study the most abundant common feature of the endothelium of all athero-susceptible regions was the upregulation of genes associated with ER processing of proteins, ER stress, and the UPR. Differential gene expression analysis identified 133 genes, 73% of which are involved in ER protein processing and folding and which form a highly connected and coordinated network of genes upregulated in the susceptible regions. Three independent and unbiased pathway mining approaches—Gene Ontology using the program database for annotation, visualization, and integrated discovery (DAVID), gene set enrichment analysis (GSEA), and ingenuity pathway analysis—identified ER stress and the UPR to be over-represented functional categories in athero-susceptible endothelium, including genes that function in protein folding, synthesis, and post-translational protein modification.
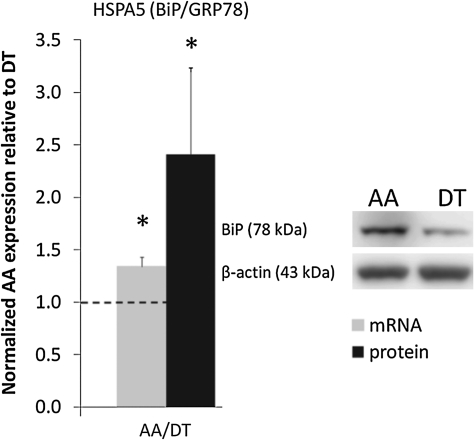
To validate the global genomics analyses, endothelial cell proteins were isolated from AA and descending thoracic aorta and also from the athero-susceptible aorto-renal branch and the protected distal renal artery. At each athero-susceptible disturbed flow site, BiP transcript and/or protein expression was significantly upregulated (Fig. 2). Western blot demonstrated significantly elevated levels of cleaved ATF6α, phospho-IRE1α, and its target, sXBP1. However, the third transducer pathway PERK was not activated. Additional evidence for chronic ER stress and UPR activation in endothelium at an aorto-renal site of athero-susceptibility (referenced to protected renal endothelium) revealed higher expressions of BiP, ATF4, and XBP1. Overall, this study, which approached without preconceived expectations of differential expression of genes and proteins associated with ER stress/UPR, strongly suggests that stresses associated with flow disturbance in vivo elicit partial activation of the UPR, an ER response common to other forms of stress, and that chronic stress is a signature for athero-susceptible endothelial phenotype in vivo.
FIG. 2.
Binding protein (BiP) expression in athero-susceptible arterial endothelium. Heat-shock protein A5 (HSPA5) (BiP/glucose-related protein 78 [GRP78]) gene and protein (78 kDa) expression in aortic arch (AA) normalized to descending thoracic aorta (DT) for each paired sample based on their animal origin. Gene (n = 6 paired samples) and protein (n = 12 paired samples) expression was normalized to GAPDH and β-actin, respectively. Dashed line at 1.0 indicates equal expression of AA and DT. Values > or < 1.0 indicate higher or lower expression in AA, respectively. Data represent mean ± SEM. *p ≤ 0.05 one-sample, one-sided, paired Wilcoxon test. Adapted from Civelek et al. (1).
Flow characteristics in vitro induce BiP activation
Using an in vitro model to simulate human arterial shear stress waveforms, athero-susceptible or atheroprotective flow was applied to human endothelial cells (7). BiP (GRP78) was found to be significantly upregulated in a sustained manner under athero-susceptible, but not atheroprotective flow up to 24 h. This response was dependent on both sustained activation of p38, as well as integrin α2β1. Increased BiP expression correlated with the activation of the ER stress-sensing element promoter by athero-susceptible flow as a marker of the UPR. Shear stress regulation of BiP was through increased protein stability when compared to other flow regulated proteins, such as connexin-43 and vascular cell adhesion molecule (VCAM)-1. Increased endothelial expression of BiP was also observed in athero-susceptible versus atheroprotective regions of C57BL6-strain mice. The study supports a role for the hemodynamic environment in preferentially inducing BiP and the UPR in athero-susceptible regions before lesion development.
sXBP1 chaperone pathway of UPR
sXBP1 encodes the XBP1 transcription factor that translocates to the nucleus to activate ER chaperones and selective proapoptotic target genes as one of the three transduction arms of the UPR (23). Following the observation of endothelial expression of the XBP1 pathway of UPR in branching regions of apolipoprotein E knock out (apoE−/−) mice arteries and in atherosclerotic lesions that developed there, this study reported that athero-susceptible flow waveforms induced XBP1 splicing in cultured endothelial cells. Overexpression of (activated) sXBP1 induced apoptosis in cultured human endothelial cells. To extend the findings to an in vivo assay for atherogenesis, adenoviral-mediated overexpression of sXBP1 was induced in an apoE−/− murine aortic isograft model. In these animals, enhanced intimal hyperplasia and atherosclerosis developed in normally protected regions of the aorta, suggesting that when the XBP1 UPR pathway is greatly overstimulated, the adaptive protective function of UPR reverts to a pathological imbalance. While overexpression was not entirely limited to the endothelium in the isograft model, the data are supportive for a prominent role for endothelial sXBP1.
These three different but complementary approaches to endothelial ER stress provide compelling evidence for the association of hemodynamics with site-specific chronic adaptive UPR in endothelial cells in vivo. The biomechanical mechanisms, including shear stress characteristics associated with athero-susceptibility, are accessible through the application of arterial flow profiles to cultured endothelial cells (20, 23).
ER Stress and Oxidative Mechanisms
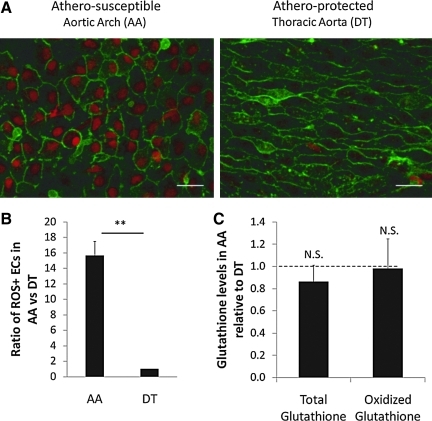
An association between ER stress and oxidative stress exists through the accumulation of ROS during increased protein folding through the formation of intramolecular and intermolecular disulfide bonds (21); molecular oxygen is the terminal electron recipient during disulfide bond formation (22). ROS scavenger molecules such as reduced glutathione that neutralize ROS are depleted in the UPR, further adding oxidative stress (23). However, UPR includes an antioxidant defense mechanism via PERK signaling. The antioxidant transcription factor NF-E2-related factor 2 (Nrf2) is a substrate for active PERK and its nuclear translocation occurs in a PERK-dependent manner under ER stress conditions (4). Nrf2−/− cells are sensitive to ER stress inducing reagents, and overexpression of Nrf2 enhances survival during ER stress (3). Moreover, antioxidants reduce ER stress in vivo and in vitro (16). Although ER stress leads to ROS generation, it is probable that ROS in turn induce ER stress (15). For example, disulfide bonds between the two conserved cysteine residues in the luminal domain of ATF6 are reduced, which lead to the translocation of ATF6 to the Golgi body, suggesting a role of cellular redox status in regulating the UPR activation (17). Further, hydrogen peroxide, the end product of multiple protective responses, is a potent ER stress inducer, and oxidized phospholipids induce ATF4 protein levels, XBP1 splicing, and ATF6 cleavage in cultured human arterial cells (8). In endothelium, BiP (GRP78; HSPA5) sensitivity to peroxynitrite and its colocalization with 3-nitrotyrosine in atherosclerotic lesions of apo E−/− mice has been demonstrated (8). En face confocal imaging of swine artery endothelium ex vivo to measure ROS accumulation as nuclear accumulation of dihydroethidium demonstrated that many more cells (16-fold) in the athero-susceptible AA accumulate ROS than cells in a nearby (protected) descending thoracic aorta (Fig. 3A, B). The ability of endothelial cells to cope with accumulated ROS is crucial for homeostasis of the arterial wall. Although ER stress is present in the athero-susceptible endothelium, upregulation of protective antioxidant genes was also noted in the same cells (19). Oxidative stress is buffered by intracellular glutathione, with the reduced form in a 30:1 excess over the oxidized form in the cytosol (19). A decrease in glutathione levels correlates with increased oxidative stress in cells. Consistent with the presence of antioxidative mechanisms in athero-susceptible endothelium, neither reduced nor oxidized glutathione levels were different between AA and descending thoracic aorta (Fig. 3C). However, ROS accumulation may activate inflammation in these regions.
FIG. 3.
Reactive oxygen species (ROS) detection in aorta. (A) Freshly harvested arteries were incubated with dihydroethidium (red) and isolectin B4 (green). They were imaged en face using confocal microscopy under identical conditions (n = 8 paired samples). (B) Percentages of ROS-positive endothelial cell nuclei were used to calculate a ratio for each pair of samples. (C) Fresh cell lysates were analyzed for glutathione and oxidized glutathione using an enzymatic assay utilizing glutathione reductase and 5,5′-dithiobis(2-nitrobenzoic acid) from Cayman Chemicals. Ratios of glutathione in AA were normalized to DT (n = 11 paired samples). Dashed line at 1.0 indicates equal amounts at AA and DT. Data represent mean ± SEM. Scale bar = 25 μm. N.S., not significant. **p < 0.01, one-sample, one-sided, paired Wilcoxon test. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
ER Stress and Inflammation Through NFκB Activation
In addition to ROS, events in the ER are linked to inflammation through multiple mechanisms (24). They include ER calcium regulation, activation of the NFκB pathway, mitogen-activated protein kinases, and acute immunological reactions (11). In the context of endothelial athero-susceptibility, components of the NFκB pathway have been implicated in athero-susceptibility for more than a decade, initially through the activation of NFκB and transcription factor activator protein 1 (AP1) by flow in vitro (13, 14), subsequently by direct measurement of nuclear translocation of NFκB in mouse AA (9), and the strong association between NFκB pathway genes and athero-susceptible endothelium in swine aorta (19). NFκB is normally held inactive in the cytosol as a complex with inhibitor of nuclear factor kappa-B (IκB), a family of inhibitors of NFκB. Upon phosphorylation, IκB is degraded, releasing NFκB for translocation to the nucleus, where it regulates proinflammatory genes. An increased ER processing load and oxidative stress have been proposed as a link between ER stress and NFκB activation. The UPR transduction pathway through PERK-eIF2α inhibits translation and has been proposed to favor activation of NFκB by causing an increase in the NFκB:IκB ratio because of the longer half-life of NFκB compared to IκBα (6, 12). However, a more direct mechanism linking UPR to NFκB may occur through IRE1α which as noted above is one of the two chronically activated transducer pathways in swine athero-susceptible endothelium (12).
When IRE1α is activated by autophosphorylation in the UPR, a conformational shift allows it to bind tumor necrosis factor-alpha receptor-associated factor 2 (TRAF2) (12). The IRE1α-TRAF2 complex binds IκB kinase (IKK), which phosphorylates IκBα, leading to its degradation, releasing NFκB for translocation to the nucleus. The IRE1α-TRAF2 complex also binds the kinase c-Jun N-terminal kinase (JNK), which in turn phosphorylates transcription factor AP1. In the nucleus, both NFκB and AP1 induce transcription of inflammatory genes.
In summary, the endothelial phenotypes in undiseased but patho-susceptible regions of arteries share some fundamental characteristics that distinguish them from patho-protected sites. Spatially sensitive differential expression of intracellular pathways may represent a chronic adapted state; in other words, the dynamic states of many individual steps in interconnected pathways are regionally different and the cells are in a different equilibrium state overall but still within a tolerable range of normal function. The presence of disturbed blood flow may sensitize or prime these regions through biomechanical forces and transport imbalances (e.g., the retention of locally generated ROS). Whatever the primary mechanism(s), the endothelium in these regions in arteries is experiencing a local environment that increases the protein biosynthetic load and promotes ER stress and the UPR. These are linked to classic pathways of ROS, inflammation, cell proliferation, and apoptosis, which are not fully activated unless additional risk factors are introduced. A recent review of Redox regulation of ER function can be found in reference (2).
Abbreviations Used
- AA
aortic arch
- AP1
transcription factor activator protein-1
- apoE−/−
apolipoprotein E knock out
- ATF4
activating transcription factor 4
- ATF6α
activating transcription factor 6α
- BiP
binding protein
- cAMP
cyclic adenosine monophosphate
- CHOP
C/ERB homologous protein
- DAVID
database for annotation, visualization and integrated discovery (NIH)
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- GRP78
glucose-related protein 78
- GSEA
gene set enrichment analysis
- HSPA5
heat-shock protein A5
- IκB
inhibitor of nuclear factor kappa-B
- IKK
inhibitor of nuclear factor kappa-B kinase
- IRE1α
inositol requiring kinase 1α
- JNK
c-Jun N-terminal kinase
- kDa
kilo daltons
- mRNA
messenger ribonucleic acid
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2
nuclear factor-E2 related factor 2
- PERK
protein kinase-like ER kinase
- Redox
oxidation-reduction (reactants)
- ROS
reactive oxygen species
- (s)XBP1
(spliced) X-box binding protein
- TRAF2
tumor necrosis factor-alpha receptor-associated factor 2
- UPR
unfolded protein response
- VCAM-1
vascular cell adhesion molecule-1
Acknowledgments
The authors gratefully acknowledge the bioinformatics contributions of Drs. Elisabetta Manduchi, Gregory Grant, and Chris Stoeckert of the University of Pennsylvania Computational Biology and Informatics Laboratory in support of the authors' research. The authors thank the National Institutes of Health for grants HL36049 MERIT, HL64388, and HL62250 (P.F.D.), and the American Heart Association for Fellowship support (AHA0315286U; M.C.).
References
- 1.Civelek M. Manduchi E. Riley RJ. Stoeckert CJ., Jr. Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–461. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csala M. Margittai E. Banhegyi G. Redox control of endoplasmic reticulum function. Antioxid Redox Signal. 2010;13:77–108. doi: 10.1089/ars.2009.2529. [DOI] [PubMed] [Google Scholar]
- 3.Cullinan SB. Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Cullinan SB. Zhang D. Hannink M. Arvisais E. Kaufman RJ. Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng J. Lu PD. Zhang Y. Scheuner D. Kaufman RJ. Sonenberg N. Harding HP. Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feaver RE. Hastings NE. Pryor A. Blackman BR. GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1534–1541. doi: 10.1161/ATVBAHA.108.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gargalovic PS. Gharavi NM. Clark MJ. Pagnon J. Yang WP. He A. Truong A. Baruch-Oren T. Berliner JA. Kirchgessner TG. Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 9.Hajra L. Evans AI. Chen M. Hyduk SJ. Collins T. Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotamisligil GS. Endoplasmic reticulum stress and atherosclerosis. Nat Med. 2010;16:396–399. doi: 10.1038/nm0410-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu P. Han Z. Couvillon AD. Kaufman RJ. Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khachigian LM. Resnick N. Gimbrone MA., Jr. Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan Q. Mercurius KO. Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra JD. Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra JD. Miao H. Zhang K. Wolfson A. Pennathur S. Pipe SW. Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadanaka S. Okada T. Yoshida H. Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol. 2007;27:1027–1043. doi: 10.1128/MCB.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oikawa D. Kimata Y. Kohno K. Self-association and BiP dissociation are not sufficient for activation of the ER stress sensor Ire1. J Cell Sci. 2007;120:1681–1688. doi: 10.1242/jcs.002808. [DOI] [PubMed] [Google Scholar]
- 19.Passerini AG. Polacek DC. Shi C. Francesco NM. Manduchi E. Grant GR. Pritchard WF. Powell S. Chang GY. Stoeckert CJ., Jr. Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee WJ. Ni C-W. Zheng Z. Chang K. Jo H. Bao G. HuR regulates the expression of stress-sensitive genes and mediates inflammatory response in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107:6858–6863. doi: 10.1073/pnas.1000444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter C. Gogvadze V. Laffranchi R. Schlapbach R. Schweizer M. Suter M. Walter P. Yaffee M. Oxidants in mitochondria: from physiology to diseases. Biochim Biophys Acta. 1995;1271:67–74. doi: 10.1016/0925-4439(95)00012-s. [DOI] [PubMed] [Google Scholar]
- 22.Tu BP. Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng L. Zampetaki A. Margariti A. Pepe AE. Alam S. Martin D. Xiao Q. Wang W. Jin ZG. Cockerill G. Mori K. Li YS. Hu Y. Chien S. Xu Q. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc Natl Acad Sci U S A. 2009;106:8326–8331. doi: 10.1073/pnas.0903197106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K. Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]