Abstract
Fluid shear stress is intimately linked with vascular oxidative stress and atherosclerosis. We posited that atherogenic oscillatory shear stress (OSS) induced mitochondrial superoxide (mtO2•−) production via NADPH oxidase and c-Jun NH2-terminal kinase (JNK-1 and JNK-2) signaling. In bovine aortic endothelial cells, OSS (±3 dyn/cm2) induced JNK activation, which peaked at 1 h, accompanied by an increase in fluorescein isothiocyanate-conjugated JNK fluorescent and MitoSOX Red (specific for mtO2•− production) intensities. Pretreatment with apocynin (NADPH oxidase inhibitor) or N-acetyl cysteine (antioxidant) significantly attenuated OSS-induced JNK activation. Apocynin further reduced OSS-mediated dihydroethidium and MitoSOX Red intensities specific for cytosolic O2•− and mtO2•− production, respectively. As a corollary, transfecting bovine aortic endothelial cells with JNK siRNA (siJNK) and pretreating with SP600125 (JNK inhibitor) significantly attenuated OSS-mediated mtO2•− production. Immunohistochemistry on explants of human coronary arteries further revealed prominent phosphorylated JNK staining in OSS-exposed regions. These findings indicate that OSS induces mtO2•− production via NADPH oxidase and JNK activation relevant for vascular oxidative stress. Antioxid. Redox Signal. 15, 1379–1388.
Introduction
Atherosclerosis is a systemic disease; however, its manifestations tend to be focal and eccentric (11, 18, 38). The spatial (∂τ/∂x) and temporal (∂τ/∂t) components of shear stress largely determine the focal characteristics of vascular oxidative stress, leading to proinflammatory states (1, 18, 20, 21). In the arterial regions exposed to atheroprotective hemodynamics, pulsatile flow develops, whereas at the lateral wall of arterial bifurcations, disturbed flow, including atherogenic oscillatory shear stress (OSS), prevails (20, 21). The latter flow characteristics, defined as bidirectional net zero forward flow, are implicated in the production of reactive oxygen species (ROS) via NADPH oxidase systems (20, 24), and an elevated level of ROS production contributes to endothelial dysfunction relevant for the initiation of atherosclerosis (16).
Numerous sources of cellular superoxide (O2•−) production contribute to vascular oxidative stress, including NADPH oxidase systems, mitochondria, xanthine oxidase, eNOS uncoupling, p450 isoenzymes, and peroxisomes (11, 16, 32). NADPH oxidase is considered the major source of ROS generation in vascular endothelial cells (ECs) (16). A low level of ROS is implicated in normal cellular functions, such as cell survival (13, 42), differentiation (22, 44), post-translational protein modifications (18), and host defense (26), whereas a high level of ROS production engenders oxidative stress and tissue damage (4, 21).
Both biomechanical and biochemical stimuli mediate mitochondrial ROS generation. Atheroprotective pulsatile shear stress induces endothelial mitochondrial membrane potential (ΔΨm) and a reduction in mitochondrial superoxide (mtO2•−) production via an increase in manganese superoxide dismutase (Mn-SOD) activities (28). In contrast, oxidized low-density lipoprotein (oxLDL) induces mtO2•− production, leading to apoptosis via JNK-mediated Mn-SOD ubiquitination and protein degradation (41). However, the mechanisms whereby atherogenic OSS regulates mtO2•− remain unknown.
Mounting evidence supports the notion that shear stress activates c-Jun NH2-terminal kinase (JNK-1 and JNK-2) in cultured vascular ECs (9, 29, 40). JNK is one of the signaling molecules in the mitogen-activated protein kinase super family, and is implicated in stress responses to inflammatory cytokines, growth factors (7) and ROS (40). Li et al. reported that laminar shear stress (LSS) at 12 dyn/cm2 induced a transient and rapid activation of JNK-1 and JNK-2 via extracellular signal-regulated kinases (ERK-1 and ERK-2) (29). Moreover, Li et al. showed that LSS inhibited tumor necrosis factor-mediated JNK activation via MEK5-BMK1 in vascular ECs (15, 27). In this context, we proposed that atherogenic OSS induced mtO2•− production via NADPH oxidase and JNK activation.
In this study, we demonstrated in bovine aortic ECs (BAECs) that OSS (±3 dyn/cm2) induced JNK activation peaked at 1 h, accompanied by an increase in mtO2•− production. Pretreatment with apocynin (an inhibitor to NADPH oxidase assembly) or N-acetyl cysteine (NAC) (an antioxidant) resulted in a significant attenuation of OSS-induced JNK activation. Apocynin further reduced OSS-mediated cytosolic O2•− production. As a corollary, transfecting BAECs with JNK siRNA (siJNK), pretreating with SP600125 (JNK inhibitor), or with apocynin significantly reduced OSS-mediated mtO2•− production. Immunohistochemistry on explants of human coronary arteries also revealed prominent phosphorylated JNK staining in the arterial regions prone to atherogenic hemodynamics. Hence, our findings support the notion that OSS induced mtO2•− production via NADPH oxidase and JNK activation is relevant for vascular oxidative stress.
Materials and Methods
EC culture and inhibitor study
Confluent BAECs between passages 4 and 7 were seeded on Collagen Type I (BD Biosciences)–coated glass slides (5 cm2) at 1.5 × 105 cells per slide and grown to confluent monolayers in high glucose (4.5 g/l) Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone) and 100 U/ml L-glutamine–penicillin–streptomycin (Sigma) for 48 h in 5% CO2 at 37°C. Before shear stress exposure, the cells were starved in Dulbecco's modified Eagle's medium with 0.5% FBS overnight to reduce phosphorylative background. For inhibitor studies, the cells were pretreated with either JNK inhibitor SP600125 (10 μM) for 30 min, NADPH oxidase inhibitor apocynin (1 mM) for 2 h, or antioxidant (5 mM) before shear stress exposure.
Dynamic flow system to simulate OSS in the arterial bifurcation
A dynamic flow channel was used to implement OSS by simulating hemodynamics in human carotid arterial bifurcations. The flow system was designed to generate well-defined flow profiles across the width of the parallel flow chamber at various temporal gradients (∂τ/∂t), frequencies, and amplitudes (17). BAECs were exposed to two conditions: (i) control at static conditions, and (ii) OSS at τave = 0.02 and ∂τ/∂t at ± 3 dyn/cm2/s1. At the lateral wall of arterial bifurcations, flow separation and migrating stagnation points create low and oscillating shear stress (OSS: bidirectional net zero forward flow), which is commonly considered as an inducer of vascular oxidative stress (6, 25).
Detection of intracellular superoxide
The intracellular superoxide (O2•−) production was measured using dihydroethidium (DHE). BAEC were exposed to OSS for 1 h and cells were washed with the culture medium without phenol red. Cells were then incubated with DHE (5 μM) for 20 min followed by five times of washing. Images were acquired from three chosen fields using an inverted epifluorescence microscope and using a ProgRes C3 digital microscope camera.
siRNA transfection
The siRNA target sequence for Bovine JNK-1 was 5°-CATGGAGCTCATGGATGCAAATCTT-3° and Bovine JNK-2 was 5°-CATGAAAGAATGTCCTACCTTCTTT-3°. siRNA (each 30 nM) was transfected to BAEC with Lipofectamine RNAiMAX (Invitrogen) as described previously (28). Cells were used for confirmation of gene knockdown or function assay 48 h after transfection. Negative control siRNA (Qiagen) was used as the scramble siRNA. There was no observable damage due to the transfection procedure.
Western blots analysis
After OSS exposure, BAECs were assessed for JNK phosphorylation. The cells were rinsed with phosphate-buffered saline (PBS) and lysed using RIPA buffer supplemented with protease and phosphatase inhibitors. Protein concentration was measured using the Bio-Rad DC assay and 50 μg of protein was loaded for Western blot. Activated JNK (p-JNK) was measured using an anti-phosphor-JNK antibody (Upstate Cell Signaling Solutions). Parallel blots were performed with anti-total JNK (Upstate Cell Signaling Solutions) and/or anti-β-actin (Millipore Corp.) to standardize protein abundance. Densitometry was performed using NIH Scion Image Software (Scion Corp.).
Flow cytometry analysis to quantify mtO2•− production
MitoSOX Red superoxide indicator (Invitrogen) is a fluorogenic dye that is selective for mtO2•− in live cells (37). It localizes into cellular mitochondria and is readily oxidized by superoxide, but not other sources of ROS or nitrogen species. The oxidation of the probe is prevented by superoxide dismutase and exhibits a bright red fluorescence upon binding to nucleic acids (excitation/emission maxima = 510/580 nm). After OSS exposure, BAECs were incubated with MitoSOX Red (3 μM) for 10 min at 37°C. The cells were collected by trypsinization and washed in PBS supplemented with 2% FBS. Cells were fixed in 2% paraformaldehyde and suspended in PBS. Measurements were performed in duplicates using the BD LSR II flow cytometer (BD Biosciences) at the USC Center for Stem Cell and Regenerative Medicine FACS Core. MitoSOX Red was excited at 488 nm, and the data were collected by a 575/26 nm (FL2) channel. The data were presented by histograms in terms of the mean intensity of MitoSOX fluorescence normalized to those of the static controls.
Immunohistochemistry analyses of phosphorylated JNK in explants of human coronary arteries
Three explants of human coronary arteries were isolated from the transplant patients with ischemic cardiomyopathy. The protocol was approved by the USC Institutional Review Board for identifier-stripped specimens. Cross sections of the left and right coronary arteries with and without atherosclerotic lesions were stained for phosphor-JNK, cytochrome c, and succynyl dehydrogenase. The latter two antibodies were specific mitochondrial inner membrane proteins. Immunostaining was performed in frozen sections using anti-p-JNK antibody (Santa Cruz Biotech.), anti-cytochrome c (Abcam), biotinylated secondary antibodies, and horseradish peroxidase-conjugated streptavidin (Sigma-Aldrich Corp.). Diaminobenzidine (DAB) was used as a chromogen and the sections were counterstained with hematoxylin for observation of intima, media, and adventitia. Counterstaining with α-smooth muscle actin antibody (ab5694 at 1:100 dilution; Abcam) allowed for distinguishing smooth muscle cells (SMCs) in the media and intima. EC were stained with monoclonal antibodies (Dakocytomation) for EC-specific von Willebrand factor at a dilution of 1:25. Negative controls were performed by omitting the primary antibody. Positive controls were established by using the brain and kidney tissues.
Statistical analysis
Data are expressed as mean ± standard deviation and compared among separate experiments. Comparisons of multiple values were made by one-way analysis of variance, and statistical significance for pairwise comparison was determined using the Tukey test. p-Values of <0.05 are considered statistically significant.
Results
OSS transiently induced JNK activation
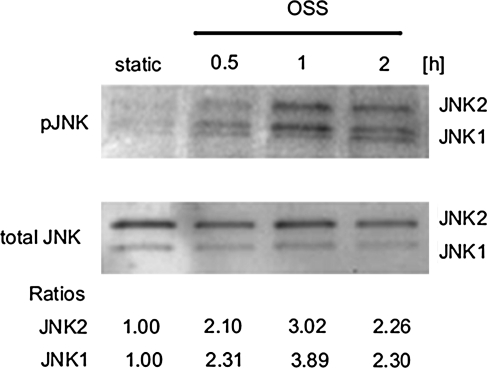
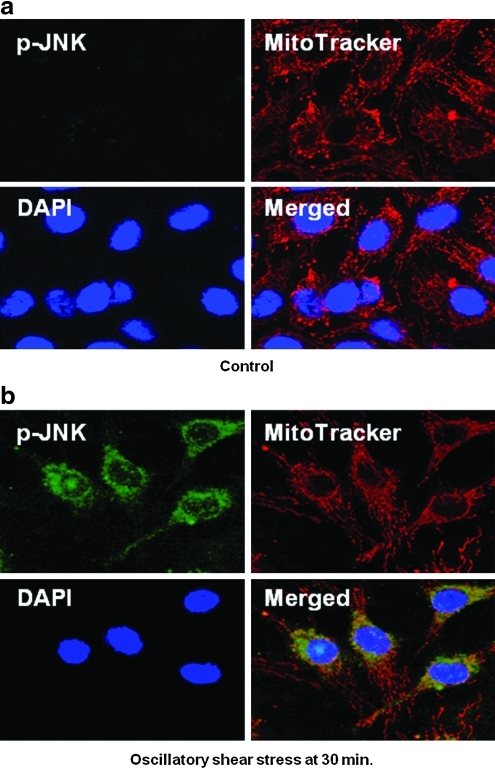
The characteristics of shear stress regulate JNK activation in BAECs. OSS induced both JNK-1 and -2 activations (Fig. 1). The intensities of individual JNK isoforms were normalized to total JNK, and they peaked by 3.06-fold and 3.89-fold, respectively, as compared to the static condition at 1 h. Confocal fluorescence microscopy also supported OSS-induced JNK activation (Fig. 2). Activated JNK was stained with fluorescein isothiocyanate-conjugated anti-phosphorylated JNK (green), the mitochondria with MitoTracker Red (red), and the nuclei with DAPI (blue). Under static conditions, BAECs did not exhibit visible JNK green fluorescence. In response to OSS, BAECs displayed a significant increase in JNK intensity after 30 min. The merged images showed orange/yellow signals, implicating a potential role of activated JNK in the mitochondrial redox status.
FIG. 1.
Oscillatory shear stress (OSS) induced transient c-Jun NH2-terminal kinase (JNK) activation. Bovine aortic endothelial cells (BAEC) monolayers were exposed to static condition or OSS for 30 min, 1 h, or 2 h. Phosphorylated JNK was then analyzed by Western blot analysis, quantified by densitometry, and expressed as fold ratios relative to total JNK and static conditions. OSS induced a peaked JNK activation at 1 h (both JNK isoforms) (p < 0.01 vs. static condition). The experiments were performed in triplicates.
FIG. 2.
JNK activation in response to OSS. p-JNK was stained with fluorescein isothiocyanate-anti-p-JNK (green). Active cellular mitochondria were localized using MitoTracker Red (red). Nuclei were stained with DAPI (blue). (a) Under static conditions, JNK green fluorescence was hardly visible. (b) In response to OSS, a significant JNK green fluorescence developed after 30 min, accompanied by yellowish/orange signals as a result of merged spectra between fluorescein isothiocyanate and MitoTracker Red. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
NADPH oxidase-mediated cytosolic O2•− production induced JNK activation
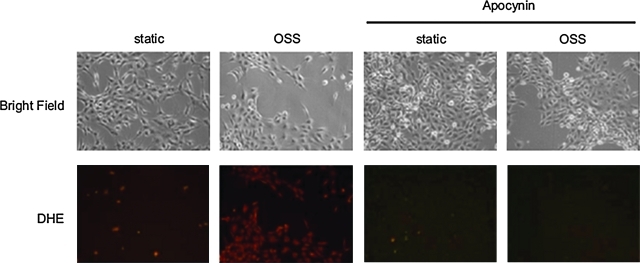
OSS induced NADPH oxidase, which, in turn, generated cytosolic O2•− production (20, 21). In the presence of NADPH oxidase inhibitor, apocynin (1 mM), OSS-induced JNK-1 and -2 activations were significantly attenuated from 3.35- to 2.30-fold and 4.57- to 3.27-fold, respectively, as compared to the static condition (Fig. 3a). As a corollary, pretreatment with antioxidant, N-acetylcysteine (NAC; 5 mM), further reduced OSS-mediated JNK-1 and -2 activations from 2.95- to 2.11-fold and 3.02- to 2.0-fold, respectively, as compared to the static condition (Fig. 3b). Apocynin also reduced OSS-mediated cytosolic O2•− production as illustrated by a reduction in DHE staining (Fig. 4). These findings suggest that NADPH oxidase-mediated cytosolic O2•− production was implicated in OSS-induced JNK phosphorylation.
FIG. 3.
Apocynin or NAC attenuated OSS-induced JNK phosphorylation. (a) BAECs were pretreated with apocynin (1 mM) for 2 h before OSS exposure. Apocynin significantly reduced OSS-induced JNK phosphorylation expressed as fold change relative to total JNK in comparison with the untreated condition (*p < 0.01 vs. static conditions. #p < 0.01 vs. OSS, n = 3). (b) Pretreatment with 5 mM of N-acetyl cysteine (NAC) also significantly reduced OSS-induced JNK phosphorylation (*p < 0.01 vs. static conditions. #p < 0.01 vs. OSS, n = 3). All studies were performed in duplicates.
FIG. 4.
Apocynin attenuated oscillatory shear-induced dihydroethedium (DHE) intensities. OSS exposure increased DHE staining compared to the static conditions. This increase in DHE intensity was attenuated in response to Apocynin treatment (1 mM). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
OSS induced mtO2•− production via NADPH oxidase and JNK activation
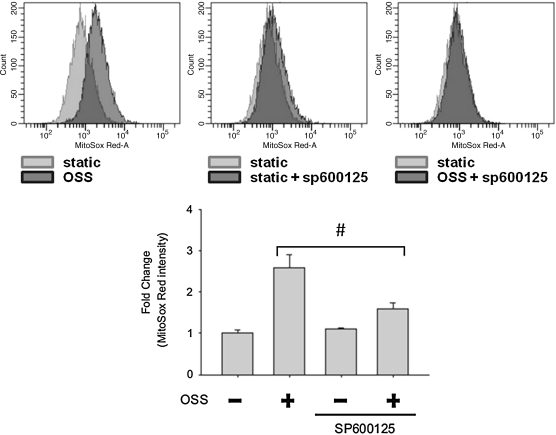
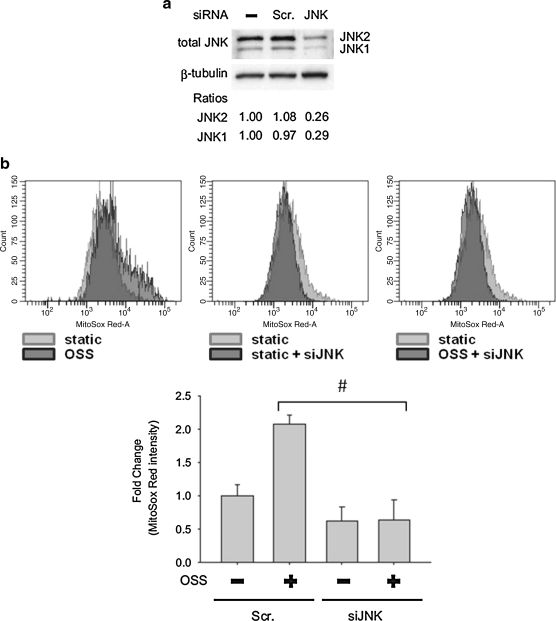
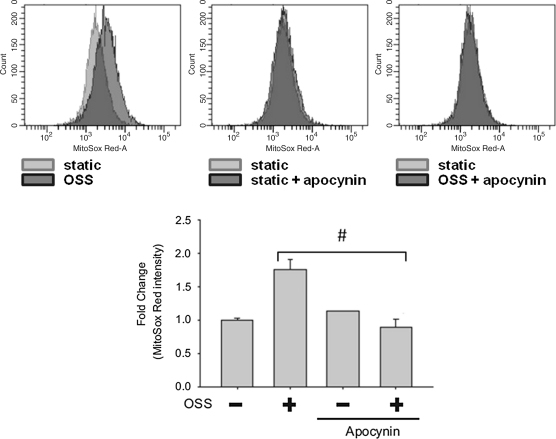
To assess whether NADPH oxidase and JNK activation were implicated in the mitochondrial redox status, we tested the effects of Apocynin, JNK inhibitor (SP600125), and JNK knock-down (with JNK siRNA, siJNK) on mtO2•− production. Flow cytometry was employed to quantify MitoSOX Red intensities specific for mtO2•−. OSS induced a 2.57-fold increase in MitoSOX Red intensity as compared to the static condition (Fig. 5). This induction was inhibited by SP600125 (10 μM) by 62%. Next, we further demonstrated the effect of JNK on mtO2•− production with siJNK. The effect of siRNA was confirmed by Western blot analysis (Fig. 6a). JNK-1 protein level was decreased by 71% and JNK-2 by 74% following siJNK transfection. JNK knockdown with siJNK completely inhibited OSS-induced MitoSOX Red intensity as compared to the static condition (Fig. 6b). Pretreatment with Apocynin (1 mM) further reduced OSS-mediated MitoSOX Red intensity from 1.80- to 0.89-fold as compared to the static conditions (Fig. 7). Taken together, these findings demonstrated the notion that OSS mediated mtO2•− production via NADPH oxidase and JNK activation.
FIG. 5.
Inhibition of JNK attenuated MitoSOX Red intensities. BAEC monolayers were pretreated with JNK inhibitor, SP600125, and mitochondrial superoxide (mtO2•−)-specific dye, MitoSOX Red, before flow exposure. Measurements were performed using BD LSR II flow cytometer. (Top) The data were presented by histograms in terms of the mean intensity of MitoSOX fluorescence normalized to those of the static conditions. (Bottom) OSS-induced MitoSOX intensity was significantly attenuated in response to SP600125 (p < 0.01 vs. static condition. #p < 0.01 vs. OSS, n = 3).
FIG. 6.
Knockdown of JNK reduced OSS-mediated MitoSOX Red intensities. (a) BAEC were transfected with siJNK or scramble (scr) siRNA for 48 h. Cell lysate was used to verify the efficiency of siJNK on the protein level of JNK. The blots were representative of two independent experiments with similar results. (b, top) The data were presented by histograms in terms of the mean intensity of MitoSOX fluorescence normalized to those of the static controls. (b, bottom) While scrambled JNK did not affect OSS-mediated MitoSOX intensity (p < 0.01 vs. static with scr siRNA, n = 3), transfecting BAECs with siJNK significantly reduced OSS-mediated MitoSOX intensity (#p < 0.01 vs. OSS with scr siRNA, n = 3).
FIG. 7.
Inhibition of NADPH oxidase attenuated OSS-induced MitoSOX Red intensities. Measurements were performed using BD LSR II flow cytometer. (Top) The data were presented by histograms in terms of the mean intensity of MitoSOX Red fluorescence normalized to those of the static conditions. (Bottom) While OSS induced an increase in MitoSOX Red fluorescence by 1.75 ± 0.2 (p < 0.01 vs. static conditions, n = 3), pretreatment with apocynin significantly attenuated OSS-induced MitoSOX Red intensity (#p < 0.01 vs. OSS, n = 3).
Activated JNK was present in the OSS-exposed regions of human coronary arteries
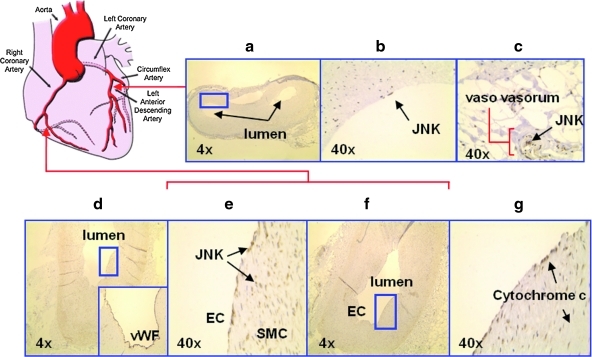
Explants of human coronary arteries isolated from heart transplant patients with ischemic cardiomyopathy were analyzed for JNK activation (Fig. 8). Cytochrome c staining identified mitochondria, and von Willebrand factor staining identified ECs (18). JNK activation was present in the left main bifurcation (Fig. 8a, b). Also prominent was activated JNK staining in the vaso vasorum (brown) (Fig. 8c). Cross sections from the greater curvature of the right coronary artery further revealed prominent phosphorylated JNK staining, accompanied by cytochrome c staining in the ECs (Fig. 8d–g). These immunohistochemistry findings supported the aforementioned in vitro findings of OSS-induced JNK activation in explants of human coronary arteries.
FIG. 8.
Immunohistochemistry of explants of human coronary arteries. The blue boxes indicate areas of interest that are subsequently magnified in successive panels. (a–c) In the OSS-exposed regions such as the left main coronary bifurcation, endothelial cells were stained positive for activated JNK. (c) Vaso vasorum from the same cross section also revealed prominent activated JNK staining (brown). (d–g) Cross section from the greater curvature of the right coronary artery revealed prominent activated JNK and cytochrome c staining in the endothelial cells. Positive von Willebrand factor (vWF) staining indicated presence of endothelial cells, while cytochrome c revealed presence of mitochondria. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Discussion
In this study, we demonstrated that OSS induced mtO2•− production via NADPH oxidase and JNK activation. We showed that (i) siJNK and SP600125 (inhibitor of JNK) reduced OSS-induced mtO2•− generation (mtO2•−), (ii) antioxidant, NAC, attenuated OSS-induced JNK activation, and (iii) Apocynin (inhibitor of NADPH oxidase assembly) reduced OSS-induced cytosolic O2•− production, JNK activation, and mtO2•− generation. Ex vivo analyses of human coronary arteries further supported the notion that JNK activation was present in the OSS-exposed ECs. Collectively, the novelty of our observations is that OSS-activated JNK plays an important role in modulating mtO2•− generation relevant for vascular oxidative stress.
JNK, a stress-activated protein kinase, plays a role in EC activation and macrophage recruitment. JNK activation was also linked with atherosclerosis. Marked decrease of macrophage and foam cell infiltration was observed in the arterial wall of ApoE−/−JNK-2−/− double knockout mice as compared to ApoE−/− mice (36), whereas oxLDL activated JNK in macrophages in ApoE−/− mice (36). Osto et al. further showed that JNK-2−/− knockout mice displayed an elevated level of Mn-SOD (33), supporting the notion that oxLDL-activated JNK mediated Mn-SOD ubiquitination/protein degradation with relevance to atherogenesis (41).
Mitochondrial function is intimately linked with endothelial metabolic homeostasis (35). Oxidative phosphorylation through complexes I to II, III, and IV drives the proton translocation across the inner membrane to mitochondrial intermembrane space. While mitochondrial electron transport chain drives the synthesis of ATP, ∼1.5%–2% of electrons leak out to form superoxide anion (O2•−) (30). Activated JNK colocalizes with mitochondria (2), inhibiting electron transport through mitochondrial complexes II, III, and IV, and promoting EC apoptosis (19) in response to ischemia-reperfusion injury (7). Mitochondrial dysfunction as a result of increased mtO2•− production has been well implicated in Diabetes Mellitus and neurodegernative diseases (34). Knocking out JNK-1 and -2 protected mice (jnk1–/–jnk2–/–) from apoptosis (3). Hence, activated JNK plays an important role in modulating mitochondrial redox status.
NADPH oxidase is an important source of vascular endothelial superoxide production, and has been implicated in the development of atherosclerosis. OSS induces cytosolic superoxide production through the upregulation of NADPH oxidase expression, which, in turn, induces monocyte chemoattractant protein 1 (20, 21). In this study, inhibition of NADPH oxidase and its superoxide production reduced OSS-induced JNK activation, suggesting the role of NADPH oxidase in JNK activation.
The use of Apocynin to inhibit NADPH oxidase remains controversial. Apocynin is commonly used to inhibit p47phox translocation and assembly of NADPH oxidase complexes (39). In the phagocytic cells, the inhibitor activity of apocynin requires oxidation by myeloperoxidase (MPO) and H2O2 to form an apocynin radical, which, in turn, oxidizes thiols in NADPH oxidase (43). In HEK293 cells that overexpressed Nox1, Nox2, or Nox4, apocynin was reported to act as an antioxidant, and it does not inhibit NADPH oxidase in the absence of MPO (14). In vascular ECs, specific NADPH oxidase inhibitor gp91ds-tat attenuated superoxide production in response to shear stress (10, 20, 21), and oxidation of apocynin was mediated by other peroxidases (rather than MPO) (23). In in vivo systems, apocynin has been used to reduce NAPDH oxidase-mediated superoxide production in ApoE–/– mice (12, 45). Taken together, the use of apocynin is cell type dependent and a viable inhibitor to link OSS-mediated superoxide production with JNK activation.
Previous work by Berk and colleagues (15) and Jo and colleagues (9) suggest that the time-dependent JNK phosphorylation was activated by different signal pathways. Berk's group reported that H2O2 induced JNK activation in ECs (15). Our current study also supports the notion that OSS induced JNK activation via NADPH-mediated cytosolic O2•− production. Go et al. and Jo and colleagues previously demonstrated that LSS-mediated ·NO production was required for JNK activation after 60 min. By transfecting BAEC with Akt mutant, Go et al. and Jo and colleagues showed that LSS at 10 dyn/cm2 stimulated JNK by activating the cascade of PI3K-Akt- eNOS and ·NO production. However, the precise mechanisms whereby pulsatile versus OSS modulates JNK activation remain to be defined.
JNK activation is prominent in the atherosclerotic lesions (31). Our ex vivo findings revealed prominent activated JNK staining in OSS-exposed regions of human coronary arteries. In the left main bifurcation, activated JNK staining was prominent in ECs, SMCs, and macrophages/foam cells. JNK staining was also prominent in the vaso vasorum in the adventitia (Fig. 8c). Unlike our in vitro cultured BAEC model, human coronary arteries were constantly exposed to the circulating cytokines and growth factors as well as the paracrine effects of SMCs, all of which may contribute to JNK activation (5, 8).
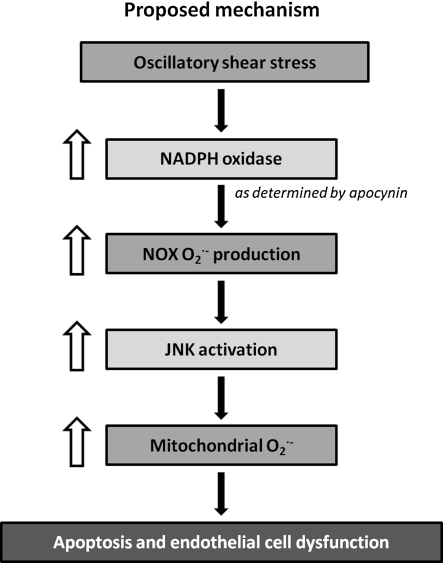
In summary, our data support the hypothesis that OSS increased cytosolic superoxide production via NADPH oxidase. Cytosolic superoxide subsequently activated JNK, which in turn induced the production of mtO2•− (Fig. 9). Hence, atherogenic OSS induced mtO2•− production via NADPH oxidase-JNK signaling pathway is relevant for initiation of atherosclerosis.
FIG. 9.
Proposed mechanism of OSS-mediated mtO2•− production. OSS increased cytosolic superoxide production via NADPH oxidase. Cytosolic superoxide subsequently activated JNK, which in turn induced the production of mtO2•−.
Abbreviations Used
- ΔΨm
mitochondrial membrane potential
- BAECs
bovine aortic endothelial cells
- DHE
dihydroethidium
- ECs
endothelial cells
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- JNK
c-Jun NH2-terminal kinase
- LSS
laminar shear stress
- Mn-SOD
manganese superoxide dismutase
- MPO
myeloperoxidase
- mtO2•−
mitochondrial superoxide
- NAC
N-acetyl cysteine
- O2•−
superoxide
- OSS
oscillatory shear stress
- oxLDL
oxidized low-density lipoprotein
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- SMCs
smooth muscle cells
- vWF
von Willebrand factor
Acknowledgments
These studies were supported by National Institutes of Health HL-068689 and HL-083015 (to T.K.H.).
Author Disclosure Statement
All authors have no disclosures.
References
- 1.Ai L. Rouhanizadeh M. Wu J. Takabe W. Yu H. Alavi M. Li R. Chu Y. Miller J. Heistad D. Hsiai T. Pulsatile and oscillatory shear stress influences spatial variations in vascular Mn-SOD expression and nitrotyrosine formation. Am J Physiol Cell Physiol. 2008;294:C1576–C1585. doi: 10.1152/ajpcell.00518.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki H. Kang PM. Hampe J. Yoshimura K. Noma T. Matsuzaki M. Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- 3.Arita Y. Harkness SH. Kazzaz JA. Koo HC. Joseph A. Melendez JA. Davis JM. Chander A. Li Y. Mitochondrial localization of catalase provides optimal protection from H2O2-induced cell death in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L978–L986. doi: 10.1152/ajplung.00296.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bochkov V. Kadl A. Huber J. Gruber F. Binder B. Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 5.Chiu JJ. Chen LJ. Lee CI. Lee PL. Lee DY. Tsai MC. Lin CW. Usami S. Chien S. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood. 2007;110:519–528. doi: 10.1182/blood-2006-08-040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Keulenaer GW. Chappell DC. Ishizaka N. Nerem RM. Alexander RW. Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 7.Dougherty CJ. Kubasiak LA. Frazier DP. Li H. Xiong WC. Bishopric NH. Webster KA. Mitochondrial signals initiate the activation of c-Jun N-terminal kinase (JNK) by hypoxia-reoxygenation. FASEB J. 2004;18:1060–1070. doi: 10.1096/fj.04-1505com. [DOI] [PubMed] [Google Scholar]
- 8.Gimbrone MA., Jr. Topper JN. Biology of the vessel wall: endothelium. In: Chein KR, editor. Molecular Basis of Cardiovascular Disease. Philadelphia: W.B. Saunders Company; 1998. pp. 331–348. [Google Scholar]
- 9.Go YM. Park H. Maland MC. Darley-Usmar VM. Stoyanov B. Wetzker R. Jo H. Phosphatidylinositol 3-kinase gamma mediates shear stress-dependent activation of JNK in endothelial cells. Am J Physiol. 1998;275:H1898–H1904. doi: 10.1152/ajpheart.1998.275.5.H1898. [DOI] [PubMed] [Google Scholar]
- 10.Godbole A. Lu X. Guo X. Kassab G. NADPH oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol. 2009;296:H152–H158. doi: 10.1152/ajpheart.01251.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison D. Griendling K. Landmesser U. Hornig B. Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7–11. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 12.Hattori Y. Hattori S. Wang X. Satoh H. Nakanishi N. Kasai K. Oral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:865–870. doi: 10.1161/01.ATV.0000258946.55438.0e. [DOI] [PubMed] [Google Scholar]
- 13.Heistad DD. Unstable coronary-artery plaques. N Engl J Med. 2003;349:2285–2287. doi: 10.1056/NEJMp038161. [DOI] [PubMed] [Google Scholar]
- 14.Heumuller S. Wind S. Barbosa-Sicard E. Schmidt H. Busse R. Schroder K. Brandes R. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 15.Hojo Y. Saito Y. Tanimoto T. Hoefen RJ. Baines CP. Yamamoto K. Haendeler J. Asmis R. Berk BC. Fluid shear stress attenuates hydrogen peroxide-induced c-Jun NH2-terminal kinase activation via a glutathione reductase-mediated mechanism. Circ Res. 2002;91:712–718. doi: 10.1161/01.res.0000037981.97541.25. [DOI] [PubMed] [Google Scholar]
- 16.Hsiai T. Berliner JA. Oxidative stress as a regulator of murine atherosclerosis. Curr Drug Targets. 2007;8:1222–1229. doi: 10.2174/138945007783220678. [DOI] [PubMed] [Google Scholar]
- 17.Hsiai TK. Cho SK. Reddy S. Hama S. Navab M. Demer LL. Honda HM. Ho CM. Pulsatile flow regulates monocyte adhesion to oxidized lipid-induced endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1770–1776. doi: 10.1161/hq1001.097104. [DOI] [PubMed] [Google Scholar]
- 18.Hsiai TK. Hwang J. Barr ML. Correa A. Hamilton R. Alavi M. Rouhanizadeh M. Cadenas E. Hazen SL. Hemodynamics influences vascular peroxynitrite formation: implication for low-density lipoprotein apo-B-100 nitration. Free Radic Biol Med. 2007;42:519–529. doi: 10.1016/j.freeradbiomed.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu YL. Li S. Shyy JY. Chien S. Sustained JNK activation induces endothelial apoptosis: studies with colchicine and shear stress. Am J Physiol. 1999;277:H1593–H1599. doi: 10.1152/ajpheart.1999.277.4.H1593. [DOI] [PubMed] [Google Scholar]
- 20.Hwang J. Ing MH. Salazar A. Lassegue B. Griendling K. Navab M. Sevanian A. Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang J. Saha A. Boo YC. Sorescu GP. McNally JS. Holland SM. Dikalov S. Giddens DP. Griendling KK. Harrison DG. Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 22.Jang Y. Sharkis S. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson D. Schillinger K. Kwait D. Hughes C. McNamara E. Ishmael F. O'Donnell R. Chang M. Hogg M. Dordick J. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium. 2002;9:191–203. doi: 10.1080/10623320213638. [DOI] [PubMed] [Google Scholar]
- 24.Ku DN. Blood flow in arteries. Annu Rev Fluid Mech. 1997;29:399–434. [Google Scholar]
- 25.Ku DN. Giddens DP. Zarins CK. Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 26.Lambeth J. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 27.Li L. Tatake RJ. Natarajan K. Taba Y. Garin G. Tai C. Leung E. Surapisitchat J. Yoshizumi M. Yan C. Abe J. Berk BC. Fluid shear stress inhibits TNF-mediated JNK activation via MEK5-BMK1 in endothelial cells. Biochem Biophys Res Commun. 2008;370:159–163. doi: 10.1016/j.bbrc.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li R. Beebe T. Cui J. Rouhanizadeh M. Ai L. Wang P. Gundersen M. Takabe W. Hsiai T. Pulsatile shear stress increased mitochondrial membrane potential: implication of Mn-SOD. Biochem Biophys Res Commun. 2009;388:406–412. doi: 10.1016/j.bbrc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YS. Shyy JY. Li S. Lee J. Su B. Karin M. Chien S. The Ras-JNK pathway is involved in shear-induced gene expression. Mol Cell Biol. 1996;16:5947–5954. doi: 10.1128/mcb.16.11.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madamanchi NR. Vendrov A. Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 31.Metzler B. Hu Y. Dietrich H. Xu Q. Increased expression and activation of stress-activated protein kinases/c-Jun NH(2)-terminal protein kinases in atherosclerotic lesions coincide with p53. Am J Pathol. 2000;156:1875–1886. doi: 10.1016/S0002-9440(10)65061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordberg J. Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 33.Osto E. Kouroedov A. Mocharla P. Akhmedov A. Besler C. Rohrer L. von Eckardstein A. Iliceto S. Volpe M. Luscher T. Inhibition of protein kinase C {beta} prevents foam cell formation by reducing scavenger receptor A expression in human macrophages. Circulation. 2008;118:2174–2182. doi: 10.1161/CIRCULATIONAHA.108.789537. [DOI] [PubMed] [Google Scholar]
- 34.Pieczenik S. Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Quintero M. Colombo SL. Godfrey A. Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci R. Sumara G. Sumara I. Rozenberg I. Kurrer M. Akhmedov A. Hersberger M. Eriksson U. Eberli FR. Becher B. Boren J. Chen M. Cybulsky MI. Moore KJ. Freeman MW. Wagner EF. Matter CM. Luscher TF. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 37.Robinson KM. Janes MS. Pehar M. Monette JS. Ross MF. Hagen TM. Murphy MP. Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorescu GP. Song H. Tressel SL. Hwang J. Dikalov S. Smith DA. Boyd NL. Platt MO. Lassegue B. Griendling KK. Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 39.Stefanska J. Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surapisitchat J. Hoefen RJ. Pi X. Yoshizumi M. Yan C. Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci U S A. 2001;98:6476–6481. doi: 10.1073/pnas.101134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takabe W. Li R. Ai L. Yu F. Berliner J. Hsiai T. Oxidized low-density lipoprotein-activated c-Jun NH2-terminal kinase regulates manganese superoxide dismutase ubiquitination. Implication for mitochondrial redox status and apoptosis. Arterioscler Thromb Vasc Biol. 2010;30:436–441. doi: 10.1161/ATVBAHA.109.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thannickal VJ. Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 43.Touyz R. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension. 2008;51:172–174. doi: 10.1161/HYPERTENSIONAHA.107.103200. [DOI] [PubMed] [Google Scholar]
- 44.Wei Z. Costa K. Al-Mehdi A. Dodia C. Muzykantov V. Fisher A. Simulated ischemia in flow-adapted endothelial cells leads to generation of reactive oxygen species and cell signaling. Circ Res. 1999;85:682–689. doi: 10.1161/01.res.85.8.682. [DOI] [PubMed] [Google Scholar]
- 45.Xu X. Gao X. Potter B. Cao J. Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]