Abstract
Claudins are a family of nearly two dozen transmembrane proteins that are a key part of the tight junction barrier that regulates solute movement across polarized epithelia. Claudin family members interact with each other, as well as with other transmembrane tight junction proteins (such as occludin) and cytosolic scaffolding proteins (such as zonula occludens-1 (ZO-1)). Although the interplay between all of these different classes of proteins is critical for tight junction formation and function, claudin family proteins are directly responsible for forming the equivalent of paracellular ion selective channels (or pores) with specific permeability and thus are essential for barrier function. In this review, we summarize current progress in identifying structural elements of claudins that regulate their transport, assembly, and function. The effects of oxidant stress on claudins are also examined, with particular emphasis on lung epithelial barrier function and oxidant stress induced by chronic alcohol abuse. Antioxid. Redox Signal. 15, 1179–1193.
Introduction
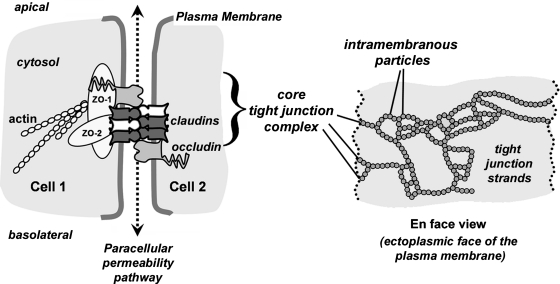
Tight junctions ring the lateral membrane of polarized epithelial cells and establish the border of the apical plasma membrane. Traditionally known as the zonula occludens (ZO), this subcomponent of the junctional complex was initially recognized functionally. By electron microscopy, tight junctions appear as sites of cell–cell contact where the plasma membranes are close enough to appear fused (101). Subsequent freeze-fracture electron microscopy demonstrated that these sites of near fusion were formed by strands of intramembranous particles corresponding to tight junctions (Fig. 1) (22).
FIG. 1.
Core protein constituents of tight junctions. A diagram depicting the cell–cell contact site where tight junctions regulate paracellular permeability (dashed line and arrows). The core tight junction unit consists of transmembrane proteins (claudins, occludin) and scaffold proteins (ZO-1, ZO-2) which link claudins to the actin cytoskeleton. Head-to-head binding between claudins on adjacent cells provides the structural basis for the paracellular permeability barrier. Cartoon of an en face view which represents the appearance of tight junction strands as intramembraneous particles (circles) within the plasma membrane as visualized by freeze fracture electron microscopy. Each of the intramembraneous particles represents a complex containing several proteins, including claudins.
Tight junction protein complex
Tight junctions are composed of several different classes of proteins which interact in a coordinated manner to form epithelial barriers. Of these, the major functional transmembrane proteins that confer specific paracellular barrier properties are tetraspan proteins known as claudins (3, 5, 73). The indispensable role for claudin-family transmembrane proteins in forming tight junctions was demonstrated by showing that strands of intramembranous particles appear when claudin-null fibroblasts are transfected to express different claudins (40).
For claudins to form functional tight junction strands requires additional proteins. Most notably are the cytoplasmic scaffold proteins ZO-1 and ZO-2 which bind directly to claudins and link them to the actin cytoskeletal network (Fig. 1) [for review, see Gonzalez–Mariscal et al. (42a)] (121). Another tetraspan transmembrane protein, occludin, interacts directly with claudins and helps to regulate tight junction formation [reviewed by Blasig et al. (15a)]. While claudins, occludin, ZO-1, and ZO-2 can be thought of as a core tight junction protein complex, specialized tight junctions contain other proteins. For instance, tricellulin, which is structurally similar to occludin, promotes tight junction assembly by interacting with occludin and remains associated localized to sites where three cells are in direct contact (74, 132). Tight junctions are also regulated by other plasma membrane proteins which are not part of the core tight junction complex, such as single pass transmembrane immunoglobulin superfamily proteins such as junction adhesion molecules (77) [reviewed by Bazzoni (11a)]. Despite the complex network of proteins which are needed to properly regulate the paracellular barrier, claudins are clearly essential tight junction proteins.
Claudin family proteins and paracellular permeability
To date, 23 human claudins have been identified, including two splice variants (Fig. 2). Epithelial and endothelial cells express anywhere from two to ten claudin isoforms in a tissue-specific manner; expression of multiple claudins by the same cell provides the ability to form tight junction strands of unique composition. Thus, claudin composition determines whether tight junctions will be relatively permeable, as is the case for kidney epithelium (47), or relatively impermeable, as is the case for microvascular endothelium of the blood–brain barrier (120).
FIG. 2.
Homology between human claudins. A homology dendrogram was calculated by comparing the full length amino acid sequences of human claudins (hCldn) using ClustalW (19). Cldn-10a, cldn-10b, cldn-18, and cldn-18A2.1 are mRNA splice variants derived from the same gene product. The length of each branch line and point of divergence is proportional to the amount of inferred time of evolutionary divergence between different claudin genes (106). The dendrogram is restricted to human claudins known to be structural tight junction components; some claudin-related proteins in the Epithelial Membrane Protein family (68) were not included in this analysis.
Claudins fall into two functional subcategories: 1) claudins which specifically increase paracellular permeability through the formation of paracellular channels (pore forming claudins), and 2) claudins which more generally reduce paracellular permeability (sealing claudins) (73). Of the pore forming claudins, one of the best characterized is claudin-2 (cldn-2), which was initially discovered to cause Madin Darby Canine Kidney (MDCK) cells to assume a “leaky” phenotype (38) and was subsequently found to form pores specific for monovalent cations (1) and water (104). Other pore forming claudins include cldn-7 and cldn-15 and −16. Cldn-10 is a particularly interesting pore forming claudin in that the two mRNA splice variants, cldn-10a and cldn-10b, form pores with preferential permeability for ions of opposite charge (125).
Claudins that have been associated with a more general barrier tightening function, include cldn-1, −3, −5, −11, and −19. However, the ability of these claudins to enhance barrier function is not universal. For instance, in the vascular endothelium, cldn-5 is a critical component to maintain the blood brain barrier [reviewed by Carrano et al., (17a) and Lehner et al. (77a)] (88). While cldn-5 has also been associated with a tightening of the paracellular barrier in other cell types (2, 131), this is not universal since increased cldn-5 expression by lung airway and alveolar epithelia correlates with decreased barrier function (25, 32, 127). The most likely explanation for this disparity is that cldn-5, and claudins in general, are sensitive to the tight junction microenvironment and their function depends upon interactions with other claudins present. Thus, the context of claudin expression needs to be considered in addition to the absolute level of expression when considering their effects of paracellular permeability.
Claudin Structure
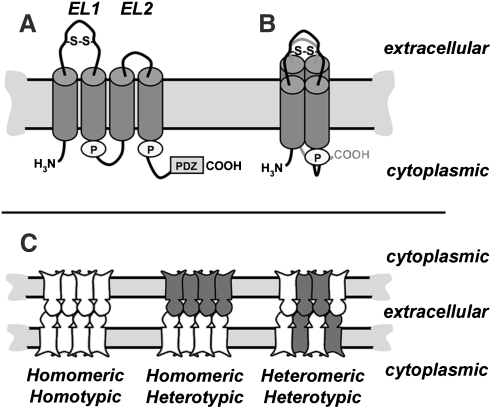
Claudins are proteins that are composed of four transmembrane domains, two extracellular loops, a short cytosolic N-terminus, and longer cytosolic C-terminus. Although detailed high resolution structures for claudins are not yet available, the four transmembrane domains are predicted to form individual alpha helices which span the membrane bilayer (Fig. 3). Although typically drawn as an extended “snake” in the membrane, the alpha helices of claudins are much more likely to be tightly packed, based on structures of other tetra-span membrane proteins, such as Cluster of Differentiation protein 81 (CD81), and Connexin26 (Cx26) (81, 107) and modeling of the claudin second extracellular loop domain (EL2) (99).
FIG. 3.
Claudin secondary structure and classes of claudin-claudin interactions. (A) Line diagram showing key features of a typical claudin in the plane of the membrane, including the two extracellular loop (EL) domains, where EL1 contains a putative disulfide bond (S–S). Cylinders represent predicted transmembrane alpha helical domains. Also illustrated are two palmitoylation motifs (“P”) and the PDZ binding motif at the extreme C-terminus of the protein. (B) Proposed conformation of an individual claudin in the plane of the membrane, showing the four transmembrane alpha helical domains as a tightly packed complex. (C) Classes of claudin–claudin interactions within a tight junction strand. Depicted are tight junctions at sites where two cells are in contact composed of a single claudin (homomeric/homotypic) or multiple claudins (homomeric/heterotypic; heteromeric/heterotypic). Claudins can interact via head-to-head binding in the extracellular environment between adjacent cells (heterotypic interactions) and within the plane of the plasma membrane in the same cell (heteromeric interactions).
Claudins are oriented in the membrane so that they have both N- and C-terminal cytosolic domains. The N-terminus is short, ∼7 amino acids, and has no known function. By contrast, the C-terminus varies in both length (21 to 63 amino acids) and sequence between the various claudin isoforms. Despite sequence divergence amongst family members, nearly all claudin family members contain a Post-synaptic density 95, Discs large, ZO-1 (PDZ) binding motif in the C-terminal tail (122). The PDZ binding motif interacts most notably with the scaffold proteins ZO-1 and ZO-2, which tethers claudins into tight junction strands by linking them to the actin cytoskeleton (121, 123). However, other scaffold proteins such as multi-PDZ domain protein (MUPP)-1 (50), and Pals1 Associated Tight Junction protein (PATJ) (103) have been found to associate with claudins and regulate their function.
The first extracellular loop is ∼ 50 amino acids and is responsible for the selective permeability of tight junctions (23, 131). Additionally, the first extracellular loop also contains a conserved motif [Gly-Leu-Trp-x-x-Cys-(8–10aa)-Cys] which also is found in the closely-related peripheral myelin protein 22 (PMP22), membrane protein 40 (MP40), and the epithelial membrane proteins (EMPs) (68). It is hypothesized that the cysteines in this motif form a disulfide bond which stabilizes the secondary structure of the first extracellular loop. While the presence of a disulfide bond in the first extracellular loop has not been conclusively demonstrated, the paracellular barrier function of cldn-2 is resistant to thiol-reactive agents, such as methanethiosulfonate (8). In addition, mutating either or both of the cysteines in the first extracellular loop to serine disrupts the ability of cldn-5 to form high resistance tight junctions (131).
A disulfide bond in the extracellular loop of claudins is expected to be necessary for intercellular binding to occur, analogous to the requirement for disulfide bonds in connexin extracellular loop domains (11). Thus, the first extracellular loop represents a site which could be sensitive to changes in redox potential (61). The second extracellular loop is smaller (∼16 amino acids) and has been modeled to form a helix-turn-helix structure (99). The selectivity of claudin paracellular permeability is predominantly dictated by the first extracellular loop domain, however, both extracellular loop domains are critical for the regulation of claudin–claudin interactions. Claudin motifs which regulate the ion selectivity of paracellular diffusion and formation of tight junction pores have been discussed in recent reviews (3, 5, 73); here we will focus on interactions between claudins and pathways for claudin assembly into tight junction strands.
Interactions Between Claudins
Homotypic and heterotypic interactions
Claudin extracellular loop domains bridge the space between adjacent cells at tight junctions. While it is known that claudins on abutting cells interact with each other, the specific characteristics of these interactions are an active area of study.
Head-to-head interactions between claudins on opposing cells are known as either homotypic or heterotypic, depending on whether they are between the same type of claudin or between different claudin gene products. By and large, most claudins seem to interact homotypically, based largely on co-localization at cell–cell contact sites when expressed by fibroblasts or other claudin-null cells. However, this has not been exhaustively examined.
Heterotypic interactions are much less frequent (Table 1). To date, the only heterotypic claudin interactions that have been characterized have involved cldn-3 (25, 33, 41). By contrast, several pairwise combinations of claudins have been found to be heterotypically incompatible, even though they are heteromerically compatible when expressed by the same cell (e.g., cldn-3 and cldn-4 or cldn-16 and cldn-19). In addition to the claudins listed in Table 1, there is also evidence from transfected MDCK cells that cldn-2 and cldn-8 are heterotypically incompatible, although this is difficult to interpret since cells transfected with cldn-8 show decreased expression of endogenous cldn-2 (6).
Table 1.
Heterotypic Claudin Interactions
| Claudin | 1 | 2 | 3 | 4 | 5 | 16 | 19 |
|---|---|---|---|---|---|---|---|
| 1 | + | − | + | − | − | nd | nd |
| 2 | − | + | + | nd | nd | nd | nd |
| 3 | + | + | + | − | + | nd | nd |
| 4 | − | nd | − | + | − | nd | nd |
| 5 | − | nd | + | − | + | nd | nd |
| 16 | nd | nd | nd | nd | nd | + | − |
| 19 | nd | nd | nd | nd | nd | − | + |
One of the few claudins shown to be heterotypically incompatible with cldn-3 is cldn-4, despite the fact that these two claudins have nearly identical extracellular loop domains (33). Heterotypic incompatibility was found to require a single amino acid residue, Asn44 which, when converted to Thr, created a mutant form of cldn-3 which was able to interact with cldn-4 (33). Notably, this point mutation did not ablate the ability of cldn-3(N44T) to interact with cldn-1 or cldn-5, underscoring that several elements of the extracellular loop domains help mediate heterotypic interactions. Consistent with these findings, a conserved motif in the second extracellular loop of cldn-5 contains several bulky hydrophobic amino acids which have been shown to stabilize antiparallel homotypic interactions (99) that could also play a role in stabilizing heterotypic interactions as well.
The functional ramifications of heterotypic claudin compatibility are just beginning to be explored, however, it is most likely to enable epithelial cells to fine tune their paracellular permeability, comparable to heteromeric gap junction channels (24). As an example of this, MDCK cells transfected to overexpress cldn-3 showed a decrease in cldn-2 based cation pore activity (84), which could be due to heterotypic interaction between these two compatible claudins (41). Interestingly, MDCK cells overexpressing cldn-3 had comparable paracellular water pore activity as untransfected MDCK cells (104), indicating that pores formed by heterotypic cldn-2/cldn-3 had unique properties unattainable from a homomeric cldn-2 channels. By contrast, claudins with limited or no heterotypic compatibility should be able to form pores which are less influenced by other claudins present in tight junction strands. This could be important to enable an acute change in paracellular permeability, such as that recently demonstrated for cldn-4, which is upregulated in response to acute lung injury (70, 134). A complete heterotypic compatibility profile will help determine whether this is the case.
Heteromeric interactions
In addition to head-to-head interactions, claudins also interact side by side in the plane of the membrane, referred to as homomeric and heteromeric interactions. Homomeric interactions have been most extensively studied using cldn-5 (15, 99). Using fluorescence resonance energy transfer (FRET), which offers the ability to assess claudin homomers in the native context of the cell membrane, C-terminal Cyan Fluorescent Protein (CFP) or Yellow Fluorescent Protein (YFP) tagged cldn-5 proteins were shown to interact at a distance of <6 angstroms at the plasma membrane (15, 99). Importantly, the C-terminal tag blocked the ability of these claudins to interact with ZO-1 or ZO-2, ruling out a contribution of the tight junction scaffold in mediating homotypic interactions.
By contrast with heterotypic interactions, heteromeric interactions are more frequent. For example, cldn-3 and cldn-4 heteromerically interact, as do cldn-16 and cldn-19, despite the fact that these pairs are heterotypically incompatible. In fact, heteromeric interactions are likely to be required for these pairs of claudins to form functional tight junction strands by enabling a homotypic/heteromeric complex to form.
Cldn-16 (110) and cldn-19 (67) have both been implicated in familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC). When co-expressed in LLC-PK1 cells, cldn-16 and cldn-19 acted synergistically to produce sodium-selective pores in the tight junction barrier rather than magnesium-selective pores (53). Thus, FHHNC is associated with a decrease with sodium permeability rather than a direct change in magnesium, which suggests that when either cldn-16 or cldn-19 are disrupted in the kidney, the defect in magnesium resorption is indirect and due to a decrease in transepithelial potential by decreased sodium permeability. Interestingly, incorporation of cldn-16 to tight junctions depends on cldn-19 and vice versa, suggesting that these claudins act as co-chaperones (53). Other claudins expressed by the kidney, such as cldn-10a and cldn-18, retain the ability to form tight junctions, regardless of whether cldn-16 or −19 are present, consistent with a model where subsets of claudins are coordinately regulated.
How the specificity of claudin hetero-oligomerization is regulated by the kidney remains an open question. In particular, a yeast two-hybrid assay was used to demonstrate that cldn-16 is unable to homo-oligomerize and seems to be restricted to hetero-oligomerization with cldn-19, whereas cldn-19 homo-oligomerizes and also hetero-oligomerizes with cldn-10a and cldn-18 (53). Assessing claudin compatibility using yeast two-hybrid assays should be interpreted with caution; for instance, cldn-16 homo-oligomerizes when expressed by L cell fibroblasts (54). Regardless of mechanism, one implication of this model is that tight junction strands are not composed of simple homogeneous mixtures of claudins and instead have fine structure which could have functional ramifications for epithelial barriers.
Claudin-Occludin Interactions
Ever since occludin-deficient mice were found to have near normal barrier function (105), it has become clear that occludin is more prominently involved in regulating tight junctions as opposed to being a strict structural element [for review, see Blasig et al. (15a)]. Differential roles for claudins and occludin in maintaining tight junctions is further underscored by fluorescence recovery after photobleaching (FRAP) and fluorescence loss In photobleaching (FLIP) experiments performed with Green Fluorescent Protein (GFP) fusion proteins, where cldn-1 is a relatively stable component of tight junctions, with a mobile fraction of ∼ 25% of the total signal (109). Interestingly, cldn-1 stability was unaffected by deleting the C terminal PDZ binding domain, suggesting that a direct interaction with ZO-1 or ZO-2 is not needed to stabilize cldn-1 once assembled into tight junction strands; instead, claudins lacking the PDZ binding motif are more likely to be stabilized by heterotypically binding to claudins on adjacent cells with the capacity to interact with ZO-1. By contrast, occludin is significantly more dynamic and has a mobile fraction approaching ∼ 80%. The rate of cldn-1 recovery is about twice as rapid as that of occludin, suggesting that cldn-1 is more freely diffusing through the plasma membrane than occludin.
Despite these differences in mobility, an extracellular loop mimetic peptide corresponding to the EL1 domain of cldn-1 stably binds to cldn-1, cldn-3, and occludin, which correlates with the ability of the peptide to disrupt tight junctions (91). While not definitive, this does suggest that the EL domains of claudins and occludin are close enough to interact which could help strengthen the tight junction barrier. Other evidence in support of direct claudin–occludin interactions comes from studies of the mechanism of action for Clostridium perfringens enterotoxin (CPE), which binds to several claudin EL2 domains with high affinity. There are three major biochemically isolatable complexes that represent the sequence of interaction of CPE with cell tight junction proteins, a small complex containing CPE and claudins which subsequently oligomerizes and then incorporates occludin (111). Since CPE lacks a high affinity binding site for occludin, recruitment of occludin into the CPE complex is most likely to reflect a claudin–occludin interaction, rather than a direct interaction with CPE.
Post-Translational Modification of Claudins
Palmitoylation
Palmitoylation is likely a universal feature of claudin assembly, since signature di-cysteine palmitoylation motifs are conserved throughout the claudin protein family (124) (Fig. 3). Palmitoylated cldn-14 more efficiently partitioned into detergent-resistant membranes as compared to a mutant, nonpalmitoylated cldn-14 construct (124), which suggests that membrane microdomains, or lipid “rafts”, play a role in tight junction assembly (34, 97). Although claudin partitioning into microdomains is established, roles for microdomains in regulating the trafficking and oligomerization of claudins are not well understood at present.
Tetraspanins, transmembrane proteins that enhance membrane assembly and trafficking to the plasma membrane, are also palmitoylated which enhances tetraspanin partitioning into microdomains and oligomerization (52, 78). Intriguingly, tetraspanin palmitoylation occurs in the Golgi apparatus and is linked to the formation of higher order membrane protein complexes (136). Although tetraspanins and claudins both span the bilayer four times and have similar protein orientation in the bilayer, they are structurally distinct and not homologous (107). Despite these differences in structure, claudins were found to weakly interact with the tetraspanin CD9, indicating that these two classes of proteins partition into comparable membrane microdomains (72). However, siRNA depletion of CD9 has little effect on epithelial barrier function, thus the functional role for a putative CD9-claudin interaction remains obscure.
Palmitoylation of the tetraspanin CD81 is inhibited by oxidant stress induced by glutathione depletion or treatment with hydrogen peroxide (21). This, in turn, promotes CD81 binding to a 14-3-3 scaffold protein and alters downstream signaling (21). Whether claudin palmitoylation is regulated by oxidative stress is an open question. However, this possibility is underscored by the demonstration that treatment of MDCK cells with hydrogen peroxide disrupts claudin localization to tight junctions and decreases barrier function (42).
Phosphorylation
Members of the claudin family are also directly phosphorylated on the C-terminal cytosolic domain which has dramatic and divergent effects on claudin activity and integration into tight junctions. For instance, phosphorylation of cldn-5 (57) or cldn-16 (56) by protein kinase A stimulates a redistribution of intracellular pools of these claudins to incorporate into tight junction strands, as assessed by immunofluorescence microscopy. These increases in claudin assembly into tight junctions are also associated with increased barrier function. However, this effect is not universal, since lysophosphatidic acid stimulates rho kinase-mediated phosphorylation of cldn-5 which causes decreased incorporation into tight junctions and decreased barrier function (135). Further, protein kinase A has been shown to decrease assembly of cldn-3 into tight junctions resulting in decreased barrier function (26).
Protein kinase C shows a comparable specificity in regulating the phosphorylation and integration of cldn-1 and cldn-4, but not other claudins, into intestinal epithelial tight junctions (10). Consistent with a role for cldn-1 phosphorylation in promoting assembly into tight junctions and increasing barrier function, protein phosphatase 2A has been shown to dephosphorylate cldn-1 and decrease barrier function (96).
Clearly, the potential for claudin phosphorylation to affect their function is just beginning to be addressed, and tools such as antibodies that recognize claudin-specific phosphorylation events will prove to be valuable reagents (135). Although phosphorylation has been correlated with changes in claudin assembly and function, the mechanisms which underlie this are not well defined. In particular, little is known about whether claudin phosphorylation affects interactions with scaffold proteins or other tight junction components.
Claudin Trafficking and Oligomerization
Based on freeze-fracture electron microscopy analysis, claudins are organized into tight junctions as a network of “beaded” strands, where the individual beads are roughly the diameter of a gap junction channel (117). This led to the suggestion that claudins are organized into a basic hexameric unit, similar to connexins in gap junction hemichannels (69). However, there is little biochemical evidence in support of claudins organizing into stable hexamers. For instance, native gel electrophoresis shows that claudins form ladders of stable oligomers in increasing in molecular mass up to a hexamer (25, 85). These results should be interpreted with caution, since this method of analysis does not distinguish claudin aggregates or heterotypic complexes from bona fide hexamers that would be properly assembled in a membrane bilayer.
There are several examples where claudin mutations disrupt delivery to the plasma membrane and instead accumulate in intracellular compartments, mainly the endoplasmic reticulum (ER) and Golgi apparatus, presumably due to misfolding (64, 99). Inefficient trafficking is not restricted to mutant claudins. For instance, cldn-16 and cldn-19 act as apparent co-chaperones, since trafficking to the plasma membrane is more efficient in cells expressing both of these claudins as opposed to cells expressing only one or the other (53). All of these observations have direct relevance to how claudin misprocessing can disrupt tissue barrier function, since the ability of one class of mutant claudin to perturb other normal claudins may play a significant role in the pathologic weakening of tight junctions in human disease.
Little is known about how claudins interact prior to assembly into tight junction strands. If claudins parallel classical multimeric transmembrane proteins, then proper folding and oligomerization would be a prerequisite to further transport along the secretory pathway (35, 112). However, it is not known whether claudins interact as early as the endoplasmic reticulum.
Connexin family gap junction proteins may provide one clue to whether claudins oligomerize early in the secretory pathway. Connexins represent an interesting exception to the classical pathway, since it is well established that Cx43 is transported from the endoplasmic reticulum as a monomer to the Golgi apparatus where it subsequently oligomerizes into biochemically stable hexameric hemichannels (69, 75). The recent discovery that a chaperone protein, ERp29, stabilizes monomeric Cx43 underscores the existence of a connexin specific quality control pathway which promotes oligomerization in the trans Golgi network (27). The extent of claudin oligomerization which occurs prior to delivery to the plasma membrane remains an open question.
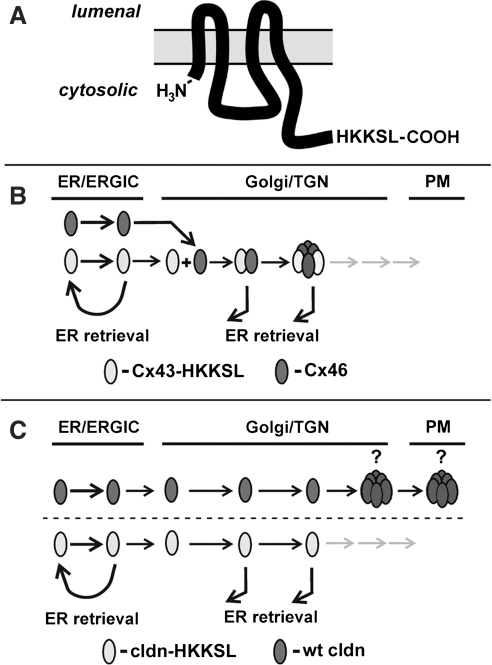
As a tool to study connexin trafficking, we previously developed a series of connexins tagged with an ER retention/retrieval motif, His-Lys-Lys-Ser-Leu (HKKSL) (83) (Fig. 4). Cx43-HKKSL shows steady state localization to the endoplasmic reticulum as monomers stabilized by ERp29 (27–29, 82, 83). However, Cx43-HKKSL also has the capacity to act as a dominant negative and prevent the transport of untagged connexins to the plasma membrane, provided that they are compatible to hetero-oligomerize and can be retrieved from the Golgi apparatus (Fig. 4B). Specifically, Cx43-HKKSL was previously found to prevent the transport of Cx46 to the plasma membrane due to a direct interaction (Figs. 5A–5C) (30, 31). Thus, HKKSL-tagged connexins provide a method to define heteromeric compatibility amongst connexins in the context of a native membrane microenvironment.
FIG. 4.
Use of a dilysine motif to produce ER-retained transmembrane proteins. (A) Placement of an HKKSL motif on the carboxyl-terminus of a tetraspan transmembrane protein, such as a claudin or connexin, acts as an ER-retention/retrieval signal (83). (B) For proteins that are heteromerically compatible to interact early in the secretory pathway, such as Cx43 and Cx46, the HKKSL-tagged protein acts as a dominant negative to prevent transport of wild-type proteins to the plasma membrane (PM). (C) By contrast, if an HKKSL-tagged claudin does not have the ability to inhibit the transport of a wild-type claudin, this indicates that the two claudins do not interact in the ER, Golgi intermediate compartment (ERGIC), or Golgi apparatus. (C) depicts the potential for claudin oligomerization occurring in either the trans Golgi network (TGN) or at the plasma membrane (PM). Immunofluorescence images corresponding to the models depicted in (B) and (C) are shown in Figure 5.
FIG. 5.
ER-retained claudins lack the ability to interact with untagged claudins. (A–L) Henrietta Lacks (HeLa) cells transfected to express either Cx43-HKKSL and Cx46 (A–C), cldn-4 and cldn-3 (D–F), cldn-4 and cldn-3-HKKSL (G–I), or cldn-4-HKKSL and cldn-3 (J–L) were fixed, permeabilized, and immunostained using mouse anti-Cx43 and rabbit anti-Cx46 (A–C) or mouse anti-cldn-4 and rabbit anti-cldn-3 (D–L) and Cy3-goat anti-mouse IgG and Cy2-goat anti-rabbit IgG. Arrowheads show areas where intracellular Cx43-HKKSL and Cx46 co-localize at the ER (A–C) and where cldn-3 and cldn-4 co-localize at the plasma membrane (D–F). There was little co-localization between the HKKSL tagged and untagged claudins (G–L). (M–O) MDCK cells were transiently transfected with myc-tagged cldn-4-HKKSL, fixed, permeabilized, and then immunostained using rabbit anti-cldn-3 and mouse anti-myc and the fluorescent secondary antibodies described above. Again, there was little co-localization between endogenously expressed cldn-3 and myc-cldn-4-HKKSL. Bar, 10 μm.
With the original intention of performing a parallel analysis to identify subclasses of heteromerically compatible claudins, we produced a series of HKKSL-tagged claudins. To our surprise, we found that, regardless of context, none of the cldn-HKKSL constructs we developed had the capacity to act as dominant negatives against native claudins. As shown in Figure 5, despite the fact that cldn-3 and cldn-4 are heteromerically compatible claudins (33), neither cldn-3-HKKSL nor cldn-4-HKKSL had a dominant negative effect on cldn-4 or cldn-3, respectively. Note that cldn-4-HKKSL also had no effect on endogenous cldn-3 expressed by MDCK cells (Fig. 5). In fact, expression of cldn-3-HKKSL or cldn-5-HKKSL lacked the ability to inhibit transport of any endogenous claudins expressed by MDCK cells (not shown).
The inability of cldn-HKKSL constructs to act as dominant negatives is in striking contrast to Cx43-HKKSL, which inhibits Cx46 trafficking to the plasma membrane, even though Cx43 and Cx46 oligomerize in the trans Golgi network (71). One potential interpretation of this result is that claudins oligomerize in an extremely late secretory subcompartment, or even at the plasma membrane. For this to occur would require a quality control pathway with the capacity to prevent premature claudin–claudin interactions, analogous to the connexin quality control pathway (69). Alternatively, claudins may not readily form stable oligomers in isolation and instead require interactions with the tight junction scaffold and/or heterotypic interactions with other claudins to maintain a higher order structure. Finally, these observations are subject to the caveat that the HKKSL tag could interfere with normal claudin–claudin interactions, even though this was not the case for connexins. One potential approach to distinguish between these possibilities would be to determine whether claudin overexpression drives premature oligomerization or tight junction fibril formation in an intracellular compartment, since this would imply a saturation of a putative quality control pathway, provided that overexpression does not cause the claudins to aggregate. Alternatively, a detailed analysis of claudin–claudin interactions in a native membrane context using techniques such as FRET could help establish whether claudins are close enough to assemble in the endoplasmic reticulum and/or Golgi apparatus.
Claudin Mutations in the Pathology of Human Disease
The importance of claudins in mammalian physiology is reflected by human diseases due to claudin mutations, as well as the phenotype of claudin-deficient mice (Table 2). Although claudins are frequently expressed in several different tissues, the phenotype resulting from claudin mutations or deficiencies tends to reflect a major effect on a single or limited set of organ systems. For instance, mutations in cldn-1 have been associated with neonatal ichthyosis, a rare skin disorder combined with sclerosing cholangitis, a liver disorder caused by blockage of bile ducts (49). Additionally, cldn-1 null mice have an embryonic lethal phenotype due to increased skin permeability which leads to neonatal dehydration (39). Cldn-14 mutations have been shown to cause nonsyndromic deafness in both humans and mice (129). It is currently hypothesized that cldn-14 is a vital component in the cation restrictive barrier responsible for maintaining proper cation levels in endolymph in the cochlea of the ear (13).
Table 2.
Human Diseases and Transgenic Mouse Phenotype Attributable to Altered Claudin Expression
| Gene | Human disease | Mouse phenotype | Reference |
|---|---|---|---|
| Cldn-1 | Ichthyosis and sclerosing cholangitis | Skin barrier dysfunction, neonatal lethal | (39, 49) |
| Cldn-2 | Renal proximal tubule defect | (94) | |
| Cldn-5 | Blood–brain barrier defect | (95) | |
| Cldn-6 (overexpression) | Skin barrier defect | (119) | |
| Cldn-7 | Dehydration and severe renal NaCl wasting | (116) | |
| Cldn-11 | Myelination defect in CNS | (43) | |
| Cldn-11 | Cochlear potential defects | (66) | |
| Cldn-14 | Nonsyndromic deafness | Endolymph potential defects | (13) |
| Cldn-16 Cldn-19 |
Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC) | Defective renal Mg+ reabsorption | (53, 64, 130) |
| Cldn-19 | Defect in Schwann cell barriers | (86) |
Unless otherwise stated, the human diseases and mouse phenotype refer to claudin mutations or claudin-deficient mouse models.
Failure to absorb magnesium in the thick ascending loop of Henle was shown to be the underlying cause of FHHNC (14, 102). Mutations in cldn-16 lead to recessive renal hypomagnesaemia (67) and cldn-19 mutations show a similar deficiency in regulating magnesium reabsorption (130). Interestingly, cldn-16 interacts with cldn-19 to increase paracellular selectivity to sodium, rather than having a direct effect on paracellular magnesium permeability (54). This leads to a model for indirect control of ion permeability where a gradient of cldn-16/cldn-19 expression in the thick ascending limb of the kidney establishes a NaCl concentration gradient which creates a lumen positive transepithelial diffusion potential that drives paracellular magnesium reabsorption, as opposed to a direct change in magnesium permeability (7).
To date, the only mutant claudins to be associated with human disease are cldn-1, −14, −16, and −19. Based on the phenotypes for claudin-deficient mice that have been discovered (Table 2), it seems likely that mutations in other claudins will also be found to have human pathology, although another strong possibility is that claudin mutations will result in developmental lethality rather than disease.
Effects of Oxidant Stress on Claudins
Alcoholic lung syndrome and glutathione depletion
Oxidant stress is associated with several different classes of barrier failure where claudin misregulation plays a critical role, including inflammatory bowel disease [reviewed by John et al. (59a)] and disruption of the blood-brain barrier [reviewed by Carrano et al. (17a) and Lehner et al. (77a)]. However, the lung is uniquely susceptible to oxidant stress in that it is directly exposed to environmental oxygen.
Of particular relevance to public health, clinical studies have linked excess dietary ethanol to oxidant stress in the lung (89) which contributes to the severity of acute respiratory distress syndrome (ARDS) (62, 90). Since lungs are particularly sensitive to oxidant stress, high levels of the antioxidant glutathione are required in the airspaces as a protective mechanism (59). As a significant factor in alcoholic lung syndrome, ethanol metabolism to acetaldehyde depletes the reduced glutathione pool and is a direct source of oxidant stress in response to chronic alcohol abuse (17, 90). Conversely, a diet enriched in the glutathione precursor procystine prevents the alcoholic lung phenotype from developing in an animal model for chronic alcohol ingestion (16, 44).
The intense oxidant load on alcoholic lungs provides causes the alveolar epithelium to be prone to injury and apoptosis (16). Since inflammation, the concomitant infiltration of neutrophils and activation of alveolar macrophages all further contribute to oxidant stress in response to acute lung injury (20), the alcoholic lung is highly susceptible to a second hit leading to more severe ARDS than a healthy lung which is not already under an oxidant burden.
A primary risk factor in the severity of ARDS is a defect in alveolar barrier function, since this promotes alveolar flooding (128). Consistent with this, prolonged ethanol ingestion creates a baseline condition where an otherwise healthy alcoholic has defective lung barrier function due to a dysregulation of lung claudin expression, based on an analysis of claudin expression by rats fed alcohol for 6 weeks (36, 46). The effect of alcohol was most striking for type I alveolar epithelial cells (Fig. 6), which comprise the majority of the lung air–liquid barrier. Specifically, chronic alcohol ingestion decreased expression of several claudins, including cldn-1, cldn-7, and cldn-18 (36). The effect of alcohol on claudin expression is not restricted to lung epithelium, since dietary ethanol has also been shown to increase gut permeability through a decrease in cldn-1, primarily in the ileum, which was also accompanied by decreases in occludin and ZO-1 (138). Cldn-1 expression by intestinal epithelia showed a linear correlation with expression of the transcription factor hepatocyte nuclear factor 4α; whether this is the case in pulmonary epithelia remains to be determined.
FIG. 6.
Changes in claudin expression in response to chronic alcohol ingestion. (A) Organization of alveolar epithelium, showing the organization of type I and type II alveolar epithelial cells in the terminal airspaces of the normal lung. The image presents an accurate representation of the relative size, distribution, and pattern of localization for alveolar epithelial cells in a healthy, adult lung. (B, C) Relative expression of cldn-1, −3, −4, −5, −7, and −18 by alveolar epithelial type II cells freshly isolated from rats (B) or cultured for 6 days to generate model type I cells (C). Rats were fed for 6 weeks using a calorically matched Liber-DeCarli liquid diet with 36% of the calories from maltodextrin (control) or ethanol (alcohol), harvested, and then analyzed by immunoblot, normalized to the level of GAPDH expression. Each claudin in the alcohol-fed experimental group was calculated as the mean+SE of six independent preparations, expressed as the percent change vs. control fed rats; p values are shown above pairs of bars. Chronic exposure to dietary alcohol had a significant effect on cldn-5 expression by type II cells; type I cells also showed decreases in several claudins, including cldn-1, cldn-7, and cldn-18. Data in (B, C) were modified from Fernandez, et al. (36).
In parallel to the alcohol-induced decreases in claudins expressed by alveolar epithelial cells, alcohol also increased the protein expression of cldn-5 (36). Surprisingly, we and others have found increased cldn-5 to be associated with increasing the permeability of lung epithelial barriers (25, 33, 127). The mechanism for increased barrier permeability associated with increased cldn-5 expression by alveolar epithelia is not known at present and may reflect a tissue specific role for cldn-5, since cldn-5 is crucial for endothelial barrier function (95) and overexpression of cldn-5 by other epithelia has also been found to improve the function tight junction barriers (2). Nonetheless, these results underscore the concept that the effect of alcohol on alveolar barrier function is due to a remodeling of tight junction claudin composition as opposed to a generalized decrease in tight junction assembly.
Several studies from other systems suggest that incorporation of claudins into tight junction strands is sensitive to cell glutathione content, as well as the relative pool sizes for sulfur-containing amino acids. For instance, renal epithelial cells cultured in medium containing reduced levels of cysteine show altered pattern of claudin expression, and a modest increase in barrier function (114). Feeding mice a mixture of probiotic bacteria (Lactobacillus and Bifidobacterium strains), which produce significant levels of glutathione in the gut, significantly increased systemic plasma glutathione, and enabled mice to resist the deleterious effects of acute pancreatitis on intestinal barrier function. Probiotic bacteria helped intestinal epithelium maintain normal levels of cldn-1 and cldn-2 incorporated into tight junctions, which is expected to help maintain the intestinal barrier and reduce the potential for sepsis (80). Ingested probiotic bacteria (Lactobacillus plantarum) were also found to prevent the downregulation of cldn-1 and cldn-4 by hepatocytes in an animal model of obstructive jaundice (137). In this model, Lactobacillus prevented plasma glutathione oxidation, further underscoring the ability of probiotic bacteria to have beneficial systemic effects. Nonetheless, it remains an open question how probiotic signals from bacteria can be transmitted from the gut lumen to the circulatory system.
Angiotensin II
In addition to its metabolic effects on the antioxidant glutathione pool, ethanol also induces cell-signaling pathways that contribute to oxidant stress that are linked to alterations in tight junction permeability. In particular, ethanol stimulates angiotensin II activity (12) which, in turn, upregulates nicotinamide adenine dinucleotide phosphate-oxidase (Nox) (108). Angiotensin II has also been shown to reduce blood-brain barrier function through a protein kinase C-mediated pathway, although there was no obvious effect on cldn-5 expression or localization (37). Thus, by analogy angiotensin II may play a direct role in compromising the barrier function of the pulmonary circulation as well.
Transforming Growth Factor β
As a response to oxidant stress, alveolar epithelial cells increase expression and secretion of transforming growth factor β (TGF-β) (12, 62). TGF-β has previously been shown to exacerbate the acute phase of lung injury (92, 100) and influence alveolar epithelial function by promoting epithelial-to-mesenchyme transition (EMT) (63, 65, 133). Alveolar epithelial cells undergoing EMT will directly affect the lung barrier by simply downregulating expression of tight junction proteins, including claudins.
TGF-β also increases oxidant stress by decreasing γ-glutamylcysteine synthetase expression (9, 58), thus reducing the antioxidant glutathione reserves of the lung. TGF-β also increases oxidant stress by increasing Nox expression (55, 115) and H2O2 production (126). Increased oxidant stress has the added potential to exacerbate alveolar injury by creating a positive-feedback loop, particularly if TGF-β expression and activation are driven by a second insult, such as sepsis or direct trauma (45). In addition to reactive oxygen species, reactive nitrogen species, including peroxynitrite, are generated during acute lung injury (48, 113) which could potentially interfere with tyrosine phosphorylation and function of claudins.
Other redox sensitive pathways affecting claudins
Although it remains to be determined whether claudins are direct targets of oxidants, there are several lines of evidence which show that oxidant stress can impair barrier function. For instance, several oxidized lipids have the capacity to disrupt tight junctions (18), most notably 1-palmitoyl-2-(5-oxo-valeroyl)-sn-glycero-3-phosphocholine, which is relevant to cardiovascular disease (60). Oxidants such as hydrogen peroxide and chloramine have also been found to disrupt tight junctions when tested on cultured cells. This is most frequently manifested as a disruption of claudin localization to tight junctions (93, 98). A similar effect has also been observed in alveolar epithelial cells isolated from alcohol fed rats (36).
In one study, the effect of oxidant stress on claudin localization was attributed to p38 mitogen activated protein (MAP) kinase which correlated with an increase in the ability to extract cldn-4 using Triton X-100, indicating a change in association with the tight junction scaffold (98). Moreover, ZO-1 expression was found to decrease in response to chloramine which also disrupts tight junction morphology (93), consistent with the central role for ZO-1 in stabilizing claudins to tight junctions (121, 123) [for review, see Gonzalez–Mariscal et al. (42a)]. However, the effect of oxidant stress is not necessarily due to a global effect of tight junction proteins. For instance, gastric epithelial cells challenged with hydrogen peroxide show a specific decrease in barrier function and cldn-3 expression, while expression of other claudins such as cldn-4 and cldn-7 are unchanged (51).
Oxidant stress has also been found to be induced by human immunodeficiency virus proteins, such as glycoprotein 120 (HIV gp120), which can have an adverse effect on tissue barrier function (4, 76, 79). In particular, HIV gp120-induced oxidant stress increases expression of matrix metalloproteinases which degrade cldn-5 and thus compromise the blood-brain barrier (79). Antioxidant enzymes had a protective effect (79), suggesting a potential therapeutic approach to the prevention of HIV-associated dementia.
Conclusions
In the past decade, there has been considerable progress from identifying claudins as key components that regulate tight junction permeability to understanding how claudins function at a detailed molecular level. Several areas require further investigation. For example, the compatibility profile for claudin–claudin interactions remains incomplete. Current understanding of claudin assembly into tight junctions and identifying roles for other transmembrane tight junction proteins also requires additional study. Of particular importance is to determine how the complexity of claudin expression and organization into tight junctions can have significant effects on paracellular permeability. There also is an emerging literature correlating changes in tumor cell claudin expression to their metastatic potential (87, 118). Thus, in addition to their central role in regulating tight junctions, it seems likely that future studies will elucidate other aspects of cell behavior regulated by claudins.
Alterations in redox state clearly have the capacity to alter paracellular barrier function. There are both direct and indirect potential pathways where claudins can be affected by redox state, ranging from intracellular signaling via reactive oxygen species to redox sensitive motifs present in claudins such as extracellular loop domains stabilized by disulfide bonds. Identifying control points where redox alters claudin expression and function is anticipated to enable antioxidant strategies to be developed which might prove as suitable approaches to improve barrier function.
Abbreviations Used
- ARDS
acute respiratory distress syndrome
- CD81
cluster of differentiation protein 81
- CFP
cyan fluorescent protein
- Cldn
claudin
- CPE
Clostridium perfringens enterotoxin
- Cx
connexin
- EL1
first extracellular loop domain
- EL2
second extracellular loop domain
- EMP
epithelial membrane protein
- EMT
epithelial-to-mesenchyme transition
- ER
endoplasmic reticulum
- ERGIC
ER, Golgi intermediate compartment
- FHHNC
familial hypomagnesemia with hypercalciuria and nephrocalcinosis
- FLIP
fluorescence loss in photobleaching
- FRAP
fluorescence recovery after photobleaching
- FRET
fluorescence resonance energy transfer
- GFP
green fluorescent protein
- HeLa
Henrietta Lacks
- HIV gp120
human immunodeficiency virus glycoprotein 120
- HKKSL
His-Lys-Lys-Ser-Leu ER retention/retrieval motif
- MAP
mitogen activated protein
- MDCK
Madin Darby canine kidney
- MP40
membrane protein 40
- MUPP
multi-PDZ domain protein
- Nox
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase
- PATJ
Pals1 associated tight junction protein
- PDZ
post-synaptic density 95, discs large, ZO-1
- PM
plasma membrane
- PMP22
peripheral myelin protein 22
- TGF-β
transforming growth factor β
- TGN
trans Golgi network
- YFP
Yellow Fluorescent Protein
- ZO
zonula occludens
Acknowledgments
This work was supported by National Institutes of Health Grants HL-083120, AA-013757 (to MK) and AA-013528 (to CEO and LAM).
References
- 1.Amasheh S. Meiri N. Gitter AH. Schoneberg T. Mankertz J. Schulzke JD. Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 2.Amasheh S. Schmidt T. Mahn M. Florian P. Mankertz J. Tavalali S. Gitter AH. Schulzke JD. Fromm M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM. Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andras IE. Pu H. Deli MA. Nath A. Hennig B. Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–265. doi: 10.1002/jnr.10762. [DOI] [PubMed] [Google Scholar]
- 5.Angelow S. Ahlstrom R. Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelow S. Schneeberger EE. Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol. 2007;215:147–159. doi: 10.1007/s00232-007-9014-3. [DOI] [PubMed] [Google Scholar]
- 7.Angelow S. Yu AS. Claudins and paracellular transport: An update. Curr Opin Nephrol Hypertens. 2007;16:459–464. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- 8.Angelow S. Yu AS. Cysteine mutagenesis to study the structure of claudin-2 paracellular pores. Ann NY Acad Sci. 2009;1165:143–147. doi: 10.1111/j.1749-6632.2009.04038.x. [DOI] [PubMed] [Google Scholar]
- 9.Arsalane K. Dubois CM. Muanza T. Begin R. Boudreau F. Asselin C. Cantin AM. Transforming growth factor-β1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: Transcriptional effect on the GSH rate-limiting enzyme γ-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17:599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- 10.Banan A. Zhang LJ. Shaikh M. Fields JZ. Choudhary S. Forsyth CB. Farhadi A. Keshavarzian A. Theta Isoform of protein kinase C alters barrier function in intestinal epithelium through modulation of distinct claudin isotypes: A novel mechanism for regulation of permeability. J Pharmacol Exp Ther. 2005;313:962–982. doi: 10.1124/jpet.104.083428. [DOI] [PubMed] [Google Scholar]
- 11.Bao X. Reuss L. Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 11a.Bazzoni G. Pathobiology of junctional adhesion molecules. Antioxid Redox Signal. 2011;15:1221–1234. doi: 10.1089/ars.2010.3867. [DOI] [PubMed] [Google Scholar]
- 12.Bechara RI. Pelaez A. Palacio A. Joshi PC. Hart CM. Brown LA. Raynor R. Guidot DM. Angiotensin II mediates glutathione depletion, transforming growth factor-β1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:L363–370. doi: 10.1152/ajplung.00141.2005. [DOI] [PubMed] [Google Scholar]
- 13.Ben–Yosef T. Belyantseva IA. Saunders TL. Hughes ED. Kawamoto K. Van Itallie CM. Beyer LA. Halsey K. Gardner DJ. Wilcox ER. Rasmussen J. Anderson JM. Dolan DF. Forge A. Raphael Y. Camper SA. Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard A. Jeunemaitre X. Coudol P. Dechaux M. Froissart M. May A. Demontis R. Fournier A. Paillard M. Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 2001;59:2206–2215. doi: 10.1046/j.1523-1755.2001.00736.x. [DOI] [PubMed] [Google Scholar]
- 15.Blasig IE. Winkler L. Lassowski B. Mueller SL. Zuleger N. Krause E. Krause G. Gast K. Kolbe M. Piontek J. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci. 2006;63:505–514. doi: 10.1007/s00018-005-5472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Blasig IE. Bellmann C. Cording J. del Vecchio G. Zwanziger D. Huber O. Haseloff RF. Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal. 2011;15:1195–1219. doi: 10.1089/ars.2010.3542. [DOI] [PubMed] [Google Scholar]
- 16.Brown LA. Harris FL. Guidot DM. Chronic ethanol ingestion potentiates TNF-α-mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L377–386. doi: 10.1152/ajplung.2001.281.2.L377. [DOI] [PubMed] [Google Scholar]
- 17.Brown LA. Harris FL. Ping XD. Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: A role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 17a.Carrano A. Hoozemans JJM. van der Vies SM. Rozemuller AJM. van Horssen J. de Vries HE. Amyloid beta induces oxidative stress-mediated blood–brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal. 2011;15:1167–1178. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- 18.Chen–Quay SC. Eiting KT. Li AW. Lamharzi N. Quay SC. Identification of tight junction modulating lipids. J Pharm Sci. 2009;98:606–619. doi: 10.1002/jps.21462. [DOI] [PubMed] [Google Scholar]
- 19.Chenna R. Sugawara H. Koike T. Lopez R. Gibson TJ. Higgins DG. Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christofidou–Solomidou M. Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5:47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Clark KL. Oelke A. Johnson ME. Eilert KD. Simpson PC. Todd SC. CD81 associates with 14-3-3 in a redox-regulated palmitoylation-dependent manner. J Biol Chem. 2004;279:19401–19406. doi: 10.1074/jbc.M312626200. [DOI] [PubMed] [Google Scholar]
- 22.Claude P. Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colegio OR. Van Itallie CM. McCrea HJ. Rahner C. Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 24.Cottrell GT. Burt JM. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta. 2005;1711:126–141. doi: 10.1016/j.bbamem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Coyne CB. Gambling TM. Boucher RC. Carson JL. Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 26.D'Souza T. Agarwal R. Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–26240. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 27.Das S. Smith TD. Das Sarma J. Ritzenthaler JD. Maza J. Kaplan BE. Cunningham LA. Suaud L. Hubbard MJ. Rubenstein RC. Koval M. ERp29 restricts connexin43 oligomerization in the endoplasmic reticulum. Mol Biol Cell. 2009;20:2593–2604. doi: 10.1091/mbc.E08-07-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das Sarma J. Das S. Koval M. Regulation of connexin43 oligomerization is saturable. Cell Commun Adhes. 2005;12:237–247. doi: 10.1080/15419060500511875. [DOI] [PubMed] [Google Scholar]
- 29.Das Sarma J. Kaplan BE. Willemsen D. Koval M. Identification of rab20 as a potential regulator of connexin43 trafficking. Cell Commun Adhes. 2008;15:65–74. doi: 10.1080/15419060802014305. [DOI] [PubMed] [Google Scholar]
- 30.Das Sarma J. Meyer RA. Wang F. Abraham V. Lo CW. Koval M. Multimeric connexin interactions prior to the trans-Golgi network. J Cell Sci. 2001;114:4013–4024. doi: 10.1242/jcs.114.22.4013. [DOI] [PubMed] [Google Scholar]
- 31.Das Sarma J. Wang F. Koval M. Targeted gap junction protein constructs reveal connexin-specific differences in oligomerization. J Biol Chem. 2002;277:20911–20918. doi: 10.1074/jbc.M111498200. [DOI] [PubMed] [Google Scholar]
- 32.Daugherty BL. Mateescu M. Patel AS. Wade K. Kimura S. Gonzales LW. Guttentag S. Ballard PL. Koval M. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1266–1273. doi: 10.1152/ajplung.00423.2003. [DOI] [PubMed] [Google Scholar]
- 33.Daugherty BL. Ward C. Smith T. Ritzenthaler JD. Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem. 2007;282:30005–30013. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 34.Edidin M. The state of lipid rafts: From model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 35.Ellgaard L. Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez AL. Koval M. Fan X. Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol. 2007;41:371–379. doi: 10.1016/j.alcohol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleegal-DeMotta MA. Doghu S. Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:640–647. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- 38.Furuse M. Furuse K. Sasaki H. Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuse M. Hata M. Furuse K. Yoshida Y. Haratake A. Sugitani Y. Noda T. Kubo A. Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuse M. Sasaki H. Fujimoto K. Tsukita S. A single gene product, claudin-1 or −2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuse M. Sasaki H. Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez JE. DiGeronimo RJ. Arthur DE. King JM. Remodeling of the tight junction during recovery from exposure to hydrogen peroxide in kidney epithelial cells. Free Radic Biol Med. 2009;47:1561–1569. doi: 10.1016/j.freeradbiomed.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a.González-Mariscal L. Quirós M. Díaz-Coránguez M. ZO proteins and redox-dependent processes. Antioxid Redox Signal. 2011;15:1235–1253. doi: 10.1089/ars.2011.3913. [DOI] [PubMed] [Google Scholar]
- 43.Gow A. Southwood CM. Li JS. Pariali M. Riordan GP. Brodie SE. Danias J. Bronstein JM. Kachar B. Lazzarini RA. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 44.Guidot DM. Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res. 2000;24:1070–1076. [PubMed] [Google Scholar]
- 45.Guidot DM. Folkesson HG. Jain L. Sznajder JI. Pittet JF. Matthay MA. Integrating acute lung injury and regulation of alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2006;291:L301–306. doi: 10.1152/ajplung.00153.2006. [DOI] [PubMed] [Google Scholar]
- 46.Guidot DM. Modelska K. Lois M. Jain L. Moss IM. Pittet JF. Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol. 2000;279:L127–135. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- 47.Gunzel D. Yu AS. Function and regulation of claudins in the thick ascending limb of Henle. Pflugers Arch. 2009;458:77–88. doi: 10.1007/s00424-008-0589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haddad IY. Pataki G. Hu P. Galliani C. Beckman JS. Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hadj–Rabia S. Baala L. Vabres P. Hamel–Teillac D. Jacquemin E. Fabre M. Lyonnet S. De Prost Y. Munnich A. Hadchouel M. Smahi A. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterology. 2004;127:1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Hamazaki Y. Itoh M. Sasaki H. Furuse M. Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–461. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto K. Oshima T. Tomita T. Kim Y. Matsumoto T. Joh T. Miwa H. Oxidative stress induces gastric epithelial permeability through claudin-3. Biochem Biophys Res Commun. 2008;376:154–157. doi: 10.1016/j.bbrc.2008.08.140. [DOI] [PubMed] [Google Scholar]
- 52.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 53.Hou J. Renigunta A. Gomes AS. Hou M. Paul DL. Waldegger S. Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou J. Renigunta A. Konrad M. Gomes AS. Schneeberger EE. Paul DL. Waldegger S. Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu YC. Wang LF. Chien YW. Nitric oxide in the pathogenesis of diffuse pulmonary fibrosis. Free Radic Biol Med. 2007;42:599–607. doi: 10.1016/j.freeradbiomed.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 56.Ikari A. Ito M. Okude C. Sawada H. Harada H. Degawa M. Sakai H. Takahashi T. Sugatani J. Miwa M. Claudin-16 is directly phosphorylated by protein kinase A independently of a vasodilator-stimulated phosphoprotein-mediated pathway. J Cell Physiol. 2008;214:221–229. doi: 10.1002/jcp.21178. [DOI] [PubMed] [Google Scholar]
- 57.Ishizaki T. Chiba H. Kojima T. Fujibe M. Soma T. Miyajima H. Nagasawa K. Wada I. Sawada N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood–brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res. 2003;290:275–288. doi: 10.1016/s0014-4827(03)00354-9. [DOI] [PubMed] [Google Scholar]
- 58.Jardine H. MacNee W. Donaldson K. Rahman I. Molecular mechanism of transforming growth factor (TGF)-β1-induced glutathione depletion in alveolar epithelial cells. Involvement of AP-1/ARE and Fra-1. J Biol Chem. 2002;277:21158–21166. doi: 10.1074/jbc.M112145200. [DOI] [PubMed] [Google Scholar]
- 59.Jean JC. Liu Y. Brown LA. Marc RE. Klings E. Joyce–Brady M. γ-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L766–776. doi: 10.1152/ajplung.00250.2000. [DOI] [PubMed] [Google Scholar]
- 59a.John LJ. Fromm M. Schulzke JD. Epithelial barriers in intestinal inflammation. Antioxid Redox Signal. 2011;15:1255–1270. doi: 10.1089/ars.2011.3892. [DOI] [PubMed] [Google Scholar]
- 60.Johnstone SR. Ross J. Rizzo MJ. Straub AC. Lampe PD. Leitinger N. Isakson BE. Oxidized phospholipid species promote in vivo differential cx43 phosphorylation and vascular smooth muscle cell proliferation. Am J Pathol. 2009;175:916–924. doi: 10.2353/ajpath.2009.090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 62.Joshi PC. Guidot DM. The alcoholic lung: Epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol. 2007;292:L813–823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- 63.Kasai H. Allen JT. Mason RM. Kamimura T. Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kausalya PJ. Amasheh S. Gunzel D. Wurps H. Muller D. Fromm M. Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim KK. Kugler MC. Wolters PJ. Robillard L. Galvez MG. Brumwell AN. Sheppard D. Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitajiri S. Miyamoto T. Mineharu A. Sonoda N. Furuse K. Hata M. Sasaki H. Mori Y. Kubota T. Ito J. Furuse M. Tsukita S. Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J Cell Sci. 2004;117:5087–5096. doi: 10.1242/jcs.01393. [DOI] [PubMed] [Google Scholar]
- 67.Konrad M. Schaller A. Seelow D. Pandey AV. Waldegger S. Lesslauer A. Vitzthum H. Suzuki Y. Luk JM. Becker C. Schlingmann KP. Schmid M. Rodriguez–Soriano J. Ariceta G. Cano F. Enriquez R. Juppner H. Bakkaloglu SA. Hediger MA. Gallati S. Neuhauss SC. Nurnberg P. Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koval M. Claudins: Key pieces in the tight junction puzzle. Cell Commun Adhes. 2006;13:127–138. doi: 10.1080/15419060600726209. [DOI] [PubMed] [Google Scholar]
- 69.Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006;16:159–166. doi: 10.1016/j.tcb.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koval M. Tight junctions, but not too tight: Fine control of lung permeability by claudins. Am J Physiol Lung Cell Mol Physiol. 2009;297:L217–218. doi: 10.1152/ajplung.00196.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koval M. Harley JE. Hick E. Steinberg TH. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol. 1997;137:847–857. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kovalenko OV. Yang XH. Hemler ME. A novel cysteine cross-linking method reveals a direct association between claudin-1 and tetraspanin CD9. Mol Cell Proteomics. 2007;6:1855–1867. doi: 10.1074/mcp.M700183-MCP200. [DOI] [PubMed] [Google Scholar]
- 73.Krause G. Winkler L. Mueller SL. Haseloff RF. Piontek J. Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 74.Krug SM. Amasheh S. Richter JF. Milatz S. Gunzel D. Westphal JK. Huber O. Schulzke JD. Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lassiter C. Fan X. Joshi PC. Jacob BA. Sutliff RL. Jones DP. Koval M. Guidot DM. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res Ther. 2009;6:1. doi: 10.1186/1742-6405-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laukoetter MG. Nava P. Lee WY. Severson EA. Capaldo CT. Babbin BA. Williams IR. Koval M. Peatman E. Campbell JA. Dermody TS. Nusrat A. Parkos CA. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77a.Lehner C. Gehwolf R. Tempfer H. Krizbai I. Hennig B. Bauer HC. Bauer H. Oxidative stress and blood–brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid Redox Signal. 2011;15:1305–1323. doi: 10.1089/ars.2011.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levy S. Shoham T. Protein–protein interactions in the tetraspanin web. Physiology (Bethesda) 2005;20:218–224. doi: 10.1152/physiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- 79.Louboutin JP. Agrawal L. Reyes BA. Van Bockstaele EJ. Strayer DS. HIV-1 gp120-induced injury to the blood–brain barrier: Role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol. 2010;69:801–816. doi: 10.1097/NEN.0b013e3181e8c96f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lutgendorff F. Nijmeijer RM. Sandstrom PA. Trulsson LM. Magnusson KE. Timmerman HM. van Minnen LP. Rijkers GT. Gooszen HG. Akkermans LM. Soderholm JD. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLoS One. 2009;4:e4512. doi: 10.1371/journal.pone.0004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maeda S. Nakagawa S. Suga M. Yamashita E. Oshima A. Fujiyoshi Y. Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 82.Maza J. Das Sarma J. Koval M. Defining a minimal motif required to prevent connexin oligomerization in the endoplasmic reticulum. J Biol Chem. 2005;280:21115–21121. doi: 10.1074/jbc.M412612200. [DOI] [PubMed] [Google Scholar]
- 83.Maza J. Mateescu M. Sarma JD. Koval M. Differential oligomerization of endoplasmic reticulum-retained connexin43/connexin32 chimeras. Cell Commun Adhes. 2003;10:319–322. doi: 10.1080/cac.10.4-6.319.322. [DOI] [PubMed] [Google Scholar]
- 84.Milatz S. Krug SM. Rosenthal R. Gunzel D. Muller D. Schulzke JD. Amasheh S. Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010;1798:2048–2057. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 85.Mitic LL. Unger VM. Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci. 2003;12:218–227. doi: 10.1110/ps.0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyamori H. Takino T. Kobayashi Y. Tokai H. Itoh Y. Seiki M. Sato H. Tight junctions in Schwann cells of peripheral myelinated axons: A lesson from claudin-19-deficient mice. J Cell Biol. 2005;169:527–538. doi: 10.1083/jcb.200501154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morin PJ. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 88.Morita K. Sasaki H. Furuse M. Tsukita S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moss M. Guidot DM. Wong–Lambertina M. Ten Hoor T. Perez RL. Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 90.Moss M. Parsons PE. Steinberg KP. Hudson LD. Guidot DM. Burnham EL. Eaton S. Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 91.Mrsny RJ. Brown GT. Gerner–Smidt K. Buret AG. Meddings JB. Quan C. Koval M. Nusrat A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am J Pathol. 2008;172:905–915. doi: 10.2353/ajpath.2008.070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munger JS. Huang X. Kawakatsu H. Griffiths MJ. Dalton SL. Wu J. Pittet JF. Kaminski N. Garat C. Matthay MA. Rifkin DB. Sheppard D. The integrin αv β6 binds and activates latent TGF β1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 93.Musch MW. Walsh–Reitz MM. Chang EB. Roles of ZO-1, occludin, and actin in oxidant-induced barrier disruption. Am J Physiol Gastrointest Liver Physiol. 2006;290:G222–231. doi: 10.1152/ajpgi.00301.2005. [DOI] [PubMed] [Google Scholar]
- 94.Muto S. Hata M. Taniguchi J. Tsuruoka S. Moriwaki K. Saitou M. Furuse K. Sasaki H. Fujimura A. Imai M. Kusano E. Tsukita S. Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA. 2010;107:8011–8016. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nitta T. Hata M. Gotoh S. Seo Y. Sasaki H. Hashimoto N. Furuse M. Tsukita S. Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nunbhakdi–Craig V. Machleidt T. Ogris E. Bellotto D. White CL., 3rd Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nusrat A. Parkos CA. Verkade P. Foley CS. Liang TW. Innis–Whitehouse W. Eastburn KK. Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 98.Oshima T. Sasaki M. Kataoka H. Miwa H. Takeuchi T. Joh T. Wip1 protects hydrogen peroxide-induced colonic epithelial barrier dysfunction. Cell Mol Life Sci. 2007;64:3139–3147. doi: 10.1007/s00018-007-7268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piontek J. Winkler L. Wolburg H. Muller SL. Zuleger N. Piehl C. Wiesner B. Krause G. Blasig IE. Formation of tight junction: Determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 100.Pittet JF. Griffiths MJ. Geiser T. Kaminski N. Dalton SL. Huang X. Brown LA. Gotwals PJ. Koteliansky VE. Matthay MA. Sheppard D. TGF-β is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reese TS. Karnovsky MJ. Fine structural localization of a blood–brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez–Soriano J. Vallo A. Garcia–Fuentes M. Hypomagnesaemia of hereditary renal origin. Pediatr Nephrol. 1987;1:465–472. doi: 10.1007/BF00849255. [DOI] [PubMed] [Google Scholar]
- 103.Roh MH. Liu CJ. Laurinec S. Margolis B. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J Biol Chem. 2002;277:27501–27509. doi: 10.1074/jbc.M201177200. [DOI] [PubMed] [Google Scholar]
- 104.Rosenthal R. Milatz S. Krug SM. Oelrich B. Schulzke JD. Amasheh S. Gunzel D. Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 105.Saitou M. Furuse M. Sasaki H. Schulzke JD. Fromm M. Takano H. Noda T. Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saitou N. Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 107.Seigneuret M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: Conserved and variable structural domains in the tetraspanin superfamily. Biophys J. 2006;90:212–227. doi: 10.1529/biophysj.105.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seshiah PN. Weber DS. Rocic P. Valppu L. Taniyama Y. Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: Upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 109.Shen L. Weber CR. Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simon DB. Lu Y. Choate KA. Velazquez H. Al–Sabban E. Praga M. Casari G. Bettinelli A. Colussi G. Rodriguez–Soriano J. McCredie D. Milford D. Sanjad S. Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 111.Singh U. Van Itallie CM. Mitic LL. Anderson JM. McClane BA. CaCo-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species, one of which contains the tight junction protein occludin. J Biol Chem. 2000;275:18407–18417. doi: 10.1074/jbc.M001530200. [DOI] [PubMed] [Google Scholar]
- 112.Sitia R. Molteni SN. Stress, protein (mis)folding, and signaling: The redox connection. Sci STKE. 2004;2004:pe27. doi: 10.1126/stke.2392004pe27. [DOI] [PubMed] [Google Scholar]
- 113.Sittipunt C. Steinberg KP. Ruzinski JT. Myles C. Zhu S. Goodman RB. Hudson LD. Matalon S. Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–510. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- 114.Skrovanek S. Valenzano MC. Mullin JM. Restriction of sulfur-containing amino acids alters claudin composition and improves tight junction barrier function. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1046–1055. doi: 10.1152/ajpregu.00072.2007. [DOI] [PubMed] [Google Scholar]
- 115.Sturrock A. Cahill B. Norman K. Huecksteadt TP. Hill K. Sanders K. Karwande SV. Stringham JC. Bull DA. Gleich M. Kennedy TP. Hoidal JR. Transforming growth factor-β1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 116.Tatum R. Zhang Y. Salleng K. Lu Z. Lin JJ. Lu Q. Jeansonne BG. Ding L. Chen YH. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Renal Physiol. 2010;298:F24–34. doi: 10.1152/ajprenal.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsukita S. Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14:531–536. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 118.Tsukita S. Yamazaki Y. Katsuno T. Tamura A. Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–6938. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]
- 119.Turksen K. Troy TC. Permeability barrier dysfunction in transgenic mice overexpressing claudin 6. Development. 2002;129:1775–1784. doi: 10.1242/dev.129.7.1775. [DOI] [PubMed] [Google Scholar]
- 120.Ueno M. Mechanisms of the penetration of blood-borne substances into the brain. Curr Neuropharmacol. 2009;7:142–149. doi: 10.2174/157015909788848901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Umeda K. Ikenouchi J. Katahira–Tayama S. Furuse K. Sasaki H. Nakayama M. Matsui T. Tsukita S. Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 122.Van Itallie CM. Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 123.Van Itallie CM. Fanning AS. Bridges A. Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van Itallie CM. Gambling TM. Carson JL. Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci. 2005;118:1427–1436. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 125.Van Itallie CM. Rogan S. Yu A. Vidal LS. Holmes J. Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol. 2006;291:F1288–1299. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 126.Waghray M. Cui Z. Horowitz JC. Subramanian IM. Martinez FJ. Toews GB. Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 127.Wang F. Daugherty B. Keise LL. Wei Z. Foley JP. Savani RC. Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29:62–70. doi: 10.1165/rcmb.2002-0180OC. [DOI] [PubMed] [Google Scholar]
- 128.Ware LB. Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 129.Wattenhofer M. Reymond A. Falciola V. Charollais A. Caille D. Borel C. Lyle R. Estivill X. Petersen MB. Meda P. Antonarakis SE. Different mechanisms preclude mutant CLDN14 proteins from forming tight junctions in vitro. Hum Mutat. 2005;25:543–549. doi: 10.1002/humu.20172. [DOI] [PubMed] [Google Scholar]
- 130.Weber S. Schneider L. Peters M. Misselwitz J. Ronnefarth G. Boswald M. Bonzel KE. Seeman T. Sulakova T. Kuwertz–Broking E. Gregoric A. Palcoux JB. Tasic V. Manz F. Scharer K. Seyberth HW. Konrad M. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 131.Wen H. Watry DD. Marcondes MC. Fox HS. Selective decrease in paracellular conductance of tight junctions: Role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Westphal JK. Dorfel MJ. Krug SM. Cording JD. Piontek J. Blasig IE. Tauber R. Fromm M. Huber O. Tricellulin forms homomeric and heteromeric tight junctional complexes. Cell Mol Life Sci. 2010;67:2057–2068. doi: 10.1007/s00018-010-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Willis BC. Borok Z. TGF-β-induced EMT: Mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 134.Wray C. Mao Y. Pan J. Chandrasena A. Piasta F. Frank JA. Claudin 4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamamoto M. Ramirez SH. Sato S. Kiyota T. Cerny RL. Kaibuchi K. Persidsky Y. Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang X. Kovalenko OV. Tang W. Claas C. Stipp CS. Hemler ME. Palmitoylation supports assembly and function of integrin–tetraspanin complexes. J Cell Biol. 2004;167:1231–1240. doi: 10.1083/jcb.200404100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang M. Wang XQ. Zhou YK. Ma YL. Shen TY. Chen HQ. Chu ZX. Qin HL. Effects of oral Lactobacillus plantarum on hepatocyte tight junction structure and function in rats with obstructive jaundice. Mol Biol Rep. 2010;37:2989–2999. doi: 10.1007/s11033-009-9866-y. [DOI] [PubMed] [Google Scholar]
- 138.Zhong W. Zhao Y. McClain CJ. Kang YJ. Zhou Z. Inactivation of hepatocyte nuclear factor-4α mediates alcohol-induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2010;299:G643–651. doi: 10.1152/ajpgi.00515.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]