Abstract
Laminar shear stress is known to confer potent anti-inflammatory, antithrombotic, and antiadhesive effects by differentially regulating endothelial gene expression. The identification of Krüppel-like factor 2 as a flow-responsive molecule has greatly advanced our understanding of molecular mechanisms governing vascular homeostasis. This review summarizes the current understanding of Krüppel-like factor 2 action in endothelial gene expression and function. Antioxid. Redox Signal. 15, 1449–1461.
Introduction
Arteriosclerosis and its attendant consequences are the leading cause of morbidity and mortality worldwide (28). Pathological studies reveal that the earliest atherosclerotic lesions often occur at branch points and curvatures in the vasculature (37, 106). Blood flow at bends and curvatures are disturbed with characteristic low and oscillatory shear stress. On the other hand, straight parts of arteries are subjected to laminar blood flow that is unidirectional and high shear stress (24). The endothelial cells (ECs) lining the vasculature are transducers of various physiological stimuli and hence are actively involved in many physiological processes such as blood coagulation and vascular permeability (37). Studies reveal that different type of shear stress dictates a distinct endothelial phenotype. Laminar shear stress, through the induction of factors such as endothelial nitric oxide synthase (eNOS) and thrombomodulin (TM), confers an atheroprotective, anticoagulant, and anti-inflammatory phenotype. In contrast, disturbed blood flow can render the endothelium dysfunctional by increasing expression of proinflammatory proteins such as vascular cell adhesion molecule 1 (VCAM-1) and reducing anticoagulant factors such as eNOS (87).
Accumulating evidence implicate the Krüppel-like factors (KLFs) as key regulators of endothelial biology. In particular, KLF2 and more recently KLF4 have been identified as laminar flow inducible factors that play important roles in the regulation of endothelial function. In this review, we discuss published studies to date that support a critical role of KLFs in endothelial biology.
The KLF Family of Transcription Factors
The KLFs are a subclass of the zinc-finger family of transcriptional regulators implicated in the regulation of cellular growth and differentiation and tissue development. The term “Krüppel” is a German word meaning “cripple.” This is based on the observation that Drosophila embryos homozygous for Krüppel exhibit altered thoracic and anterior abdominal segments (45, 69, 72, 75). To date, a total of 21 members have been identified, including four specificity protein (Sp) factors (Sp1–4) and 17 KLFs (KLF1–17). KLFs contain 3 Cys2/His2 zinc fingers at the C terminus of the protein (10, 11, 88). The interfinger space contains a 7-aa sequence, TGEKP(Y/F)X, which is highly conserved (21). Each zinc finger contains three critical residues, enabling the KLFs to bind to a consensus DNA sequence (GC-box and CACCC sequence) (13, 90) on target genes. Members of the KLF family differ from one another in their non-DNA-binding domains via which they exert transcriptional activation or repression (5). In addition, Sp/KLF members can affect one another's function by physically competing for the same DNA-binding site or regulating each other's expression (13, 22, 39, 108).
Krüppel-Like Factor 2
KLF2 was cloned by Lingrel and colleagues in 1995 using the zinc-finger domain of KLF1 as hybridization probe (3). Since it is highly expressed in the lungs, KLF2 was initially termed lung KLF. Mouse KLF2 is a 354-aa protein (355-aa in humans) with a molecular weight of 38 kDa. Human KLF2 is >85% homologous to the mouse counterpart, sharing >90% amino acid similarity. In addition, the proximal promoter and untranslated 3′ regions between the two species are also highly conserved. Further, a 75 bp element within the proximal KLF2 promoter is essentially identical between human and mouse, and appears critical for the transcriptional regulation of KLF2 expression (94). Structure and function analyses demonstrate that KLF2 contains an N-terminal transcriptional activation domain between amino acids 1 and 110 and an inhibitory domain between amino acids 110 and 267. This inhibitory domain can bind to a WW domain-containing E3 ubiquitin protein ligase 1 (WWP1), leading to ubiquitination and proteasomal degradation of KLF2 (20, 109).
KLF2 expression is developmentally regulated. Expression is initially noted at embryonic day 7 in mouse, followed by a decrease at day 11, and reactivation at day 15 (3). Gene targeting studies identify an essential role for KLF2 for organismal development. KLF2 knockout mice exhibit abnormal blood vessel formation due to insufficient smooth muscle cells recruitment, resulting in embryonic hemorrhage and death between days 12.5 and 14.5 (53, 95). These mice also display abnormal lung morphogenesis, supporting an essential role for this factor in lung development (96).
In adult, KLF2 expression is noted in the endothelium as well as several additional cell types, particularly hematopoietic cells. While the observations in the endothelium will be discussed in great detail below, for the purpose of completeness, we summarize a few key observations in non-EC lineages here. First, studies from several laboratories have shown that KLF2 plays a critical role in T-cell maturation, thymocyte, and T-cell trafficking (16, 54, 78, 102). Second, KLF2 is expressed in the monocyte/macrophage lineage, where it prevents proinflammatory monocyte activation and macrophage foam cell formation via inhibition of nuclear factor kappa B (NFκB) and activator protein 1 (AP-1) (6, 23). Finally, KLF2 has been implicated in adipogenesis (7, 103), stem cell renewal (47), and erythropoiesis (8).
Role of KLF2 in the Endothelium
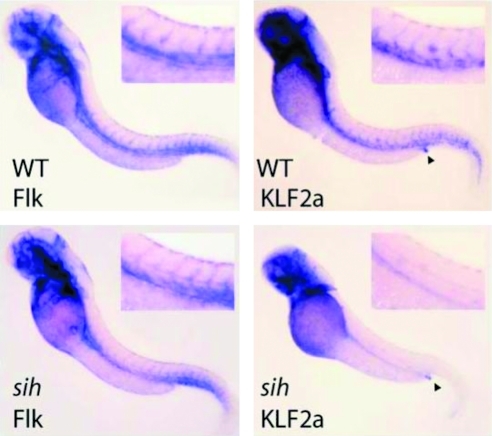
Within the vessel wall, studies in zebrafish (Fig. 1), mouse, and humans (Fig. 2B) indicate that KLF2 expression is limited to the endothelium (26, 93). However, KLF2 expression in the endothelium is not uniform. As elegantly demonstrated by Dekker and colleagues, KLF2 expression was robust in linear segments of the human aorta but notably lower at branch points (and near the bifurcation in the iliac and carotid arteries) (Fig. 2B, D, E) and in the mouse carotid artery exposed to disturbed blood flow by partial ligation (26). Parmar et al. used fetal liver kinase 1 (Flk-1) or vascular endothelial growth factor receptor 2 (VEGFR2) as an EC marker in the experiment involving zebrafish (Fig. 1). This study was performed to critically assess the dependence of endothelial KLF2 expression on blood flow. Zebrafish with silent heart (sih) mutation have a noncontractile heart. While Flk-1 expression in sih is indistinguishable from that in wild type zebrafish, the vascular expression of KLF2a (a zebrafish homolog of KLF2) is lost. As an internal control, labeling of the anal sphincter by the KLF2a probe was noted to be similar in wild type and sih embryos (69). These observations suggest that hemodynamics affect KLF2 expression (70). Indeed, KLF2 expression is induced in response to laminar flow; further, knockdown studies reveal that induction of ∼15% of all flow-related genes is KLF2-dependent (93). Outlined below are further details that emphasize the critical role of KLF2 in vascular biology and how it regulates endothelial homeostasis.
FIG. 1.
Flow-dependent regulation of KLF2. Whole-mount in situ hybridization of WT or sih mutant zebrafish embryos at 48 h, probed for Flk (VEGFR2) or KLF2a. Insets show close-ups of the trunk vasculature. Anal sphincter staining is indicated by arrowheads. Reprinted with permission from Parmar et al. (70). Flk, fetal liver kinase; KLF2, Krüppel-like factor 2; sih, silent heart; VEGFR2, vascular endothelial growth factor receptor 2; WT, wild type. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
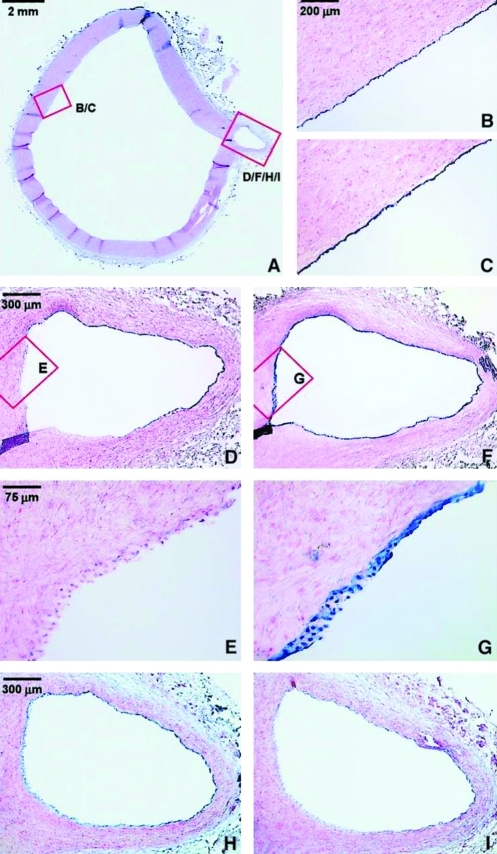
FIG. 2.
Endothelial-specific expression of LKLF and claudin-5 in the normal human aorta. (A) Overview of a section of the thoracic aorta of a 13-year-old female stained with nuclear fast red. Nonradioactive mRNA in situ hybridizations was performed on consecutive sections, using antisense riboprobes of LKLF (B, D, E), claudin-5 (H), cytochrome P450 1B1 (I), and the endothelial-specific marker von Willebrand factor (C, F, G). The detection of the mRNA–probe hybrid results in a blue color associated with the nuclei. Panels (E) and (G) show no significant expression of the LKLF mRNA (E), whereas von Willebrand factor (G) is consistently and specifically expressed in the endothelium of the entire specimen. Stacks of nuclei are visible because of the thickness of the sections (16 μm) and the conical shape of the branching artery. Claudin-5 was specifically expressed in the endothelium (H), but detection of the cytochrome P450 1B1 mRNA was too close to background hybridization levels (I). Reprinted with permission from Dekker et al. (26). LKLF, lung Krüppel-like factor. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Anti-inflammatory and atheroprotective actions
Disturbed flow at arterial bifurcations and partially ligated mouse carotid artery leads to endothelial dysfunction and proinflammatory gene expression (25, 68). Alternatively, laminar shear stress induces expression of protective genes such as eNOS and TM (Fig. 3) (34). Hence, the endothelial phenotype is dependent on interplay of pro- and anti-inflammatory gene expression. Studies in our laboratory in 2004 demonstrated that KLF2 regulates endothelial inflammation by inhibiting expression of VCAM-1 and E-selectin (Fig. 3) in response to various proinflammatory cytokines, which correlated with decreased T cell attachment and rolling on endothelial monolayers. Subsequent studies indicate that KLF2 can inhibit the NFκB pathway at multiple levels. In the case of stimuli such as interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNFα), inhibition occurs via recruitment of transcriptional coactivators (e.g., cyclic AMP response element-binding protein, CBP/p300). However, in the case of thrombin, a second mechanism is also operative (Fig. 3). We found that KLF2 potently inhibits thrombin-mediated induction of multiple cytokines/chemokines (e.g., monocyte chemotactic protein 1, IL-6, and IL-8) by inhibiting expression of its principal receptor protease-activated receptor 1 (23, 58, 80).
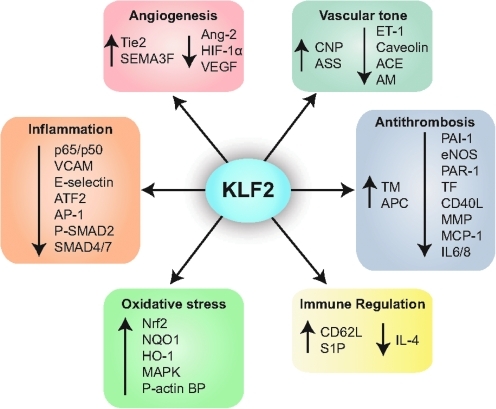
FIG. 3.
Schematic diagram of the functions of KLF2. CNP, C-natriuretic peptide; ET-1, endothelin-1; ASS, arginosuccinate synthase; AM, adrenomedullin; ACE, angiotensin converting enzyme; TM, thrombomodulin; APC, activated protein C; PAI-1, plasminogen activator inhibitor-1; eNOS, endothelial nitric oxide synthase; PAR-1, protease-activated receptor 1; TF, tissue factor; CD40L, CD40 ligand; MMP, matrix metalloproteinase; MCP-1, monocyte chemotactic protein 1; IL-6/8, interleukin 6/8; CD62L, CD62 ligand; S1P, sphingosine-1 phosphate; IL-4, interleukin-4; Nrf2, nuclear factor erythroid 2-like; NQO1, NAD(P)H:quinine oxidoreductase-1; HO-1, heme oxygenease-1; MAPK, mitogen-activated protein kinase; P-actin BP, phosphorylated actin-binding protein; VCAM, vascular cell adhesion molecule; ATF2, activating transcription factor 2; AP-1, activator protein 1; SMAD, Sma and Mad related protein; Ang, angiopoetin; SEMA3F, semaphorin 3F; HIF-1α, hypoxia-inducible factor 1 alpha. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Another mechanism by which KLF2 mediates its anti-inflammatory effects is via inhibition of nuclear activity of activating transcription factor 2 (ATF2) (Fig. 3) (34). While increased amounts of phosphorylated ATF2 are noted in the endothelium overlying atherosclerotic plaques, a KLF2-dependent reduction in ATF2 nuclear-binding activity was noted when human umbilical vein EC (HUVEC) were subjected to shear stress. While loss-of-function studies using KLF2 siRNA decreased the inhibition of ATF2 by shear stress, ATF2 knockdown suppressed proinflammatory gene expression under no-flow conditions, suggesting that shear stress and KLF2 protect the endothelium from basal proinflammatory and atherogenic stimuli. ATF2, together with c-Jun, forms a heterodimer called AP-1, which induces proinflammatory and procoagulant gene expression. By preventing nuclear localization of phosphorylated ATF2, KLF2 prevents downstream expression of inflammatory and prothrombotic pathways (34).
It is also known that AP-1 functions as a critical mediator for transforming growth factor beta (TGF-β)-dependent proinflammatory gene expression. Examination of gene expression profiles by Boon et al. in KLF2 overexpressed HUVECs revealed suppression of many established TGF-β-inducible genes. Further gain- and loss-of-function studies as well as promoter activity assays demonstrated that KLF2 inhibits TGF-β signaling by suppressing AP-1, decreasing phosphorylation and nuclear localization of Sma and Mad-related protein (SMAD)-2, subsequent inhibition of TGF-β-dependent transcription of SMAD-4 and TGF-β-independent activation of SMAD-7 (Fig. 3) (14).
Laminar fluid shear stress is associated with EC elongation and atheroprotective endothelial phenotype. On the other hand, low oscillatory fluid shear stress is associated with cobblestone-shaped ECs with randomly oriented cytoskeletons and proinflammatory, proatherosclerotic endothelial phenotype. Vartanian et al. used micropatterned lanes to demonstrate that EC elongation in the absence of fluid shear stress (known as micropatterned ECs) was associated with downregulated VCAM-1 while having no effect on E-selectin and intercellular adhesion molecule 1, and significantly upregulated KLF2. This suggests that cellular elongation and cytoskeletal alignment of micropatterned ECs act synergistically with fluid shear stress to confer atheroprotective, anti-inflammatory properties to the endothelium (91). A recent study by Horrevoets and colleagues linked the specific effects of shear-induced KLF2 on endothelial morphology to the suppression of Jun NH2-terminal kinase and mitogen-activated protein kinase signaling leading to inhibition of phosphorylation of actin cytoskeleton-associated proteins (15). Using antibody arrays and mRNA microarray analysis, they demonstrated that KLF2-mediated inhibition of Jun NH2-terminal kinase and its downstream targets ATF2/c-Jun is dependent on the cytoskeleton.
The collective insights from the aforementioned studies indicate that KLF2 has anti-inflammatory properties. However, these results are based almost exclusively on in vitro studies. In this regard, the studies by Atkins and colleagues assessing the effect of genetic deficiency of KLF2 on atherosclerosis are of central importance to the field. It was found that hemizygous deficiency of KLF2 promotes diet-induced atherosclerosis in apolipoprotein E-deficient mice (Fig. 4) (6). Remarkably, no significant changes were found in the levels of eNOS, TM, and VCAM-1 in KLF2 heterozygous mice. However, an important role for KLF2 in macrophage activation was identified and likely contributed to the observed phenotype. These observations highlight not only the importance of partial KLF2 deficiency but also the need for additional studies analyzing the effect of endothelial-specific deletion of KLF2 on identified target genes.
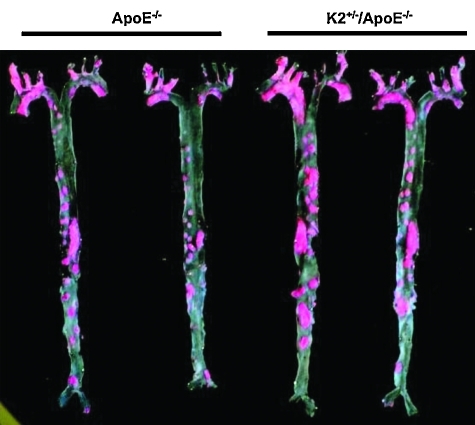
FIG. 4.
KLF2 heterozygous mice develop more atherosclerosis. Male littermate KLF2+/+/ApoE–/– and KLF2+/–/ApoE–/– mice at 6 weeks of age were fed a high-fat, high-cholesterol diet for 20 weeks. Aortas were harvested and Sudan IV–stained for lipid. Two representative pairs of fixed and stained aortas en face. K2+/– indicates KLF2+/–. Reprinted with permission from Atkins et al. (6). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Antithrombotic functions
The endothelium maintains antithrombotic luminal surface by expression of factors that inhibit platelet aggregation and blood coagulation. Further, platelets and leukocytes interact with the endothelium through a complex and dynamic molecular and cellular network, which when dysregulated leads to pathological inflammation and thrombosis. Studies conducted in our lab reveal that KLF2 modulates expression of several genes critical in maintaining an antithrombotic endothelial surface (52). KLF2 overexpression strongly increases eNOS and TM, a cell surface factor essential in generating activated protein C via interactions with thrombin, leading to potent inhibition of coagulation. Additionally, KLF2 inhibits cytokine-mediated tissue factor (TF) expression and plasminogen activator inhibitor 1 production, both of which are potent procoagulants. As expected, KLF2 overexpression increased blood clotting times and blood flow rates under both basal and inflammatory conditions, whereas siRNA knockdown of KLF2 reduced time for clot formation (Fig. 3).
Additional evidence implicating KLF2 in regulating endothelial thrombosis is derived from studies using thrombin as stimuli. Thrombin is a critical player in the coagulation cascade and induces multiple prothrombotic factors such as TF, CD40 ligand, plasminogen activator inhibitor 1, chemokines and cytokines such as monocyte chemotactic protein 1, IL-6, IL-8, and matrix degrading enzymes such as matrix metalloproteinases 1, 2, and 9. As mentioned above, Lin and colleagues have demonstrated that KLF2 inhibits thrombin-mediated endothelial action (58). Cumulatively, the above studies support an antithrombotic role for KLF2 (Fig. 3). However, a role for KLF2 in regulating thrombosis in vivo has not been reported.
Regulation of vascular tone
Endothelial KLF2 transcriptionally regulates several endothelial genes implicated in maintaining vascular tone (Fig. 3) (18). SenBanerjee et al. were first to demonstrate strong induction of eNOS in response to sustained KLF2 expression. Deletion and mutational analysis of the eNOS promoter revealed a KLF2-binding region (−644 to −652) that was critical for mediating induction of the eNOS promoter. Further studies identified this KLF2-mediated activation to be dependent on CBP/p300 recruitment as a cofactor to the eNOS promoter (58). Caveolin-1 is an endogenous regulator of eNOS, and whereas KLF2 inhibits expression of caveolin-1, it also induces arginosuccinate synthase, a limiting enzyme in eNOS substrate bioavailability (25, 27, 38, 73). Further, sustained expression of KLF2 inhibits expression of endothelin-1, adrenomedullin, and angiotensin converting enzyme, all of which increase vascular contractile tone (14). Despite these observations and contrary to expectations, endothelial-specific knockout of KLF2 is embryonically lethal and demonstrates high output cardiac failure. This vascular defect can be rescued by increasing vascular tone with phenylephrine, indicating that the cardiac failure is a result of profound loss of peripheral vascular resistance. The molecular basis for this observation is still to be elucidated as no differences were noted in target gene expression known to affect vascular tone such as eNOS, adrenomedullin, and endothelin-1 in both control and knockout mouse embryos (56). While additional studies are clearly required, these findings stress the importance of KLF2 in hemodynamic regulation, cardiovascular development, and function.
Angiogenesis
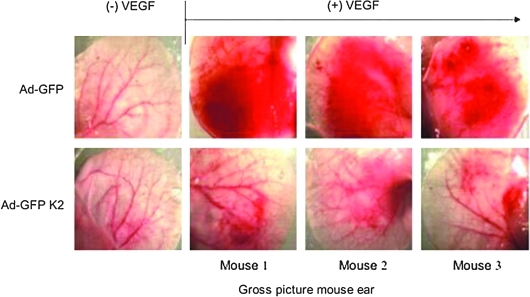
Angiogenesis is essential for normal growth and repair of organs with an imbalance contributing to various malignant, inflammatory, ischemic, infectious, and immune disorders (17, 35, 46). EC migration in response to certain stimuli (e.g., VEGF) is an important feature of both normal physiological (e.g., menstrual cycle) and pathological states (e.g., inflammation and cancer) (35, 46). Sustained expression of KLF2 potently inhibited VEGF-A-mediated tissue edema and angiogenesis in a nude mouse ear model (Fig. 5). It inhibits endothelial activation and proliferation as noted by reduced VEGF-mediated calcium influx and induction of proinflammatory factors such as VCAM-1, TF, and cyclooxygenase (9). Further studies identified KLF2 as a regulator of the key VEGF receptor VEGFR2 (also known an Flk1 or KDR). Mechanistically, KLF2 binds to VEGFR2 promoter by competing with Sp1 and decreased its expression. A second mechanism by which KLF2 may reduce angiogenesis is through effects on semaphorins. For example, semaphorins have been shown to inhibit EC migration, and semaphorin 3F (SEMA3F) has been found to be a potent metastasis inhibitor that targets both tumor and stromal cells (12). Dekker and colleagues have demonstrated that KLF2 inhibits EC migration by virtue of its ability to induce expression of SEMA3F. Thus, by reducing EC proliferation and migration, KLF2 can inhibit angiogenesis (Fig. 3). However, more recently, work by Meadows et al. reveals that KLF2 associates with the E-twenty six (ETS) family protein ets-related gene (ERG) to synergistically activate VEGFR2 in the embryo and disruption of KLF2 results in abnormal vascular development (65). Thus, the effects of KLF2 on angiogenesis are clearly complex and differential effects may be observed based on developmental stage or cellular context.
FIG. 5.
KLF2 inhibits VEGF-A-mediated angiogenesis. Photographs of nude mouse ears before [(–)VEGF] and after [(+)VEGF] treatment with VEGF-A in the presence (Ad-K2) and absence (Ad-GFP) of adenoviral KLF2. Reprinted with permission from Bhattacharya et al. (9). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
The angiopoietins (Ang) Ang-1 and Ang-2 are ligands of the receptor tyrosine kinase Tie-2. Ang-1 regulates vascular quiescence and angiogenesis via Tie-2. Recent studies demonstrate that Ang1/Tie2 signal induces the activity of myocyte enhancer factor 2 (MEF-2) via a PI3/AKT pathway to increase KLF2 expression (Fig. 6) (77). Ang-2 acts as an autocrine regulator of EC inflammatory responses and antagonizing the actions of Ang-1 and Tie-2. By destabilizing the quiescent endothelium, Ang-2 facilitates endothelial activation by inflammatory (TNF and IL-1) and angiogenic (VEGF) cytokines. Ang-2 is stored in the Weibel-Palade bodies in the endothelium and can be released immediately following stimulation, highlighting its role in controlling the transition of the resting quiescent endothelium toward an activated and responsive endothelium (31, 32). KLF2 not only inhibits cytokine-mediated Ang-2 expression in ECs, but has also been shown to induce Tie-2 expression (52).
FIG. 6.
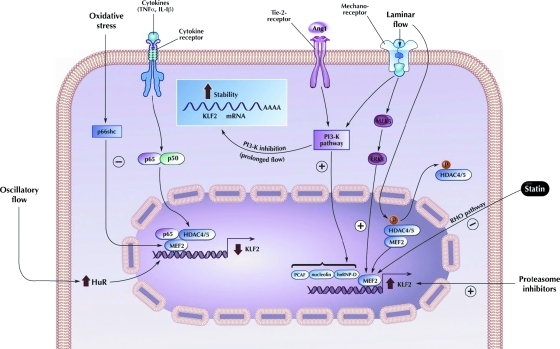
Schematic diagram showing upstream regulation of KLF2 in endothelial cells. PI3K, phosphoinositide-3 kinase; MEF2, myocyte enhancer factor 2; PCAF, P 300/CBP-associated factor; HDAC4/5, histone deacetylase 4/5; Rho pathway, Ras homolog pathway; hnRNP-D, heterogeneous nuclear ribonucleoprotein D; ERK, extracellular signal-regulated kinase; TNFα, tumor necrosis factor alpha; IL-1β, interleukin 1 beta. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Hypoxia-inducible factor 1 (HIF-1) is a central regulator of hypoxic response in many cell types and induces the expression of proangiogenic factors in the endothelium, thus promoting angiogenesis. Studies in our lab identified KLF2 as an inhibitor of HIF-1α expression and function (Fig. 3). Overexpression of KLF2 inhibited HIF-1α and its target genes (IL-8, Ang-2, and VEGF) expression in the endothelium. KLF2 also inhibited hypoxia-induced endothelial tube formation. As expected, ECs from KLF2+/− mice demonstrated increased tube formation under hypoxic conditions. Further, KLF2+/− mice brains revealed increased microvessel density. Mechanistically, KLF2 promotes HIF-1α degradation in a von Hippel-Lindau protein independent, p53-independent but proteasome-dependent manner by disrupting the interaction between HIF-1α and its chaperone heat shock protein 90 (Hsp90) (49). Thus, KLF2 inhibits hypoxia-mediated angiogenesis by regulating HIF-1α expression.
Vascular development and remodeling
Vascular structures readily remodel in response to hemodynamic stimuli associated with changes in blood flow (55). Current evidence suggests that hemodynamic forces are necessary to induce vessel remodeling in the mammalian yolk sac (60). In 1997, Leiden and colleagues showed that KLF2−/− was embryonically lethal. Although angiogenesis and vasculogenesis were grossly normal, these mice embryos died between E12.5 and E14.5 secondary to severe intraembryonic and intra-amniotic hemorrhage. Umbilical veins and arteries had abnormally thin tunica media and aneurysmal dilatation leading to ruptured vessels. Smooth muscle cells in the aorta also failed to form a compact tunica media, suggesting that KLF2 played a critical role in assembly of vascular tunica media and vessel wall stabilization during embryogenesis (53). More recently, Wu et al. noted that in KLF2−/− mice, there was failure of mural cells to migrate around ECs in the dorsal aorta. These investigators also showed that mouse embryonic fibroblasts from KLF2-null mice exhibit both growth and migratory defects in response to platelet-derived growth factor B. Taken together, these results highlight the importance of an interaction between platelet-derived growth factor and KLF2 in mural cell biology and vascular maturation (101).
Oxidative stress and vascular injury
Long-term laminar shear stress exposure confers anti-inflammatory and anticoagulant properties to ECs (37). Several studies have been performed to identify the differences in gene transcription of ECs exposed to laminar shear stress versus no flow control conditions. While Dekker et al. (8) identified KLF2 to be specifically induced by laminar shear stress in ECs, another study identified nuclear factor erythroid 2-like (Nrf2) to be the transcription factor responsible for antioxidant gene expression (19). Laminar flow also induces expression of genes that protect against antioxidant stress, including NAD(P)H:quinine oxidoreductase-1 (NQO1), and heme oxygenase-1 (HO-1). Studies by Chen et al. revealed that these genes contain an antioxidant response element in their promoters and are downstream targets of Nrf2. Expression of Nrf2 and its target genes NQO1 and HO-1 are upregulated by KLF2 (Fig. 3). KLF2 overexpression resulted in nuclear localization of Nrf2, leading to more efficient activation of Nrf2 and expression of Nrf2-dependent genes, leading to antioxidant activity. Further, studies by Warabi et al. show that while laminar shear stress is associated with induction of Nrf2 regulating genes, marked increase in Nrf2 protein levels occur primarily by stabilizing Nrf2 protein via lipid peroxidation as mRNA levels of Nrf2 remain unchanged (96, 97). KLF2 and Nrf2 act in synergy to control ∼70% of the genes induced by laminar shear stress (19, 33, 97, 98).
Although the complement cascade is an essential defense against bacterial infection, its catalytic activity has the potential to inflict injury on bystander host tissues, including vascular endothelium. Studies indicate that ECs respond robustly to complement activation by expression of cell adhesion molecules, cytokines, and chemokines that are similar to those induced by TNFα and lipopolysaccharide, inducing proinflammatory and procoagulant activities on the endothelium (50, 76, 86). Since CD59 blocks the terminal pathway of complement activation, Mason and colleagues investigated the effects of laminar shear stress on endothelial expression of complement inhibitory proteins such as CD59 and decay accelerating factor. Steady laminar shear stress-induced CD59 mRNA and was accompanied by reduced C9 deposition and complement-mediated lyses of flow-conditioned ECs. While CD59 induction was independent of PI3-K, extracellular signal-regulated kinase (ERK)1/2, and nitric oxide, knockdown studies revealed dependence upon an ERK5/KLF2 signaling pathway. Additionally, CD59 expression on vascular endothelium was significantly higher in atheroprotected regions of the aorta exposed to unidirectional laminar shear stress, as opposed to atheroprone areas exposed to disturbed flow. This suggests that CD59 is upregulated via ERK5/KLF2 conferring endothelial resistance to complement-mediated injury and atheroprotection in areas exposed to laminar shear stress (50).
Regulation of KLF2 Expression in ECs
Flow-mediated regulation of KLF2
The mechanistic basis for flow-mediated induction of KLF2 has been the subject of intense investigation. Promoter deletion studies have revealed that the proximal region of KLF2 promoter can be induced by flow. A breakthrough observation first reported by Kumar and colleagues was that this region contains a functional consensus-binding site for a family of transcription factors termed myocyte enhancer factor 2 (MEF2) proteins. MEF2 factors are members of the MADS box (MCM1 Agamous–Deficiens–Serum response factor) family of transcription factors that bind to A/T rich sequences. Although best known for their role in muscle development, emerging literature implicate MEF2A and MEF2C as critical regulators of endothelial biology. For example, Wang et al. identified mutations in MEF2A in an inherited disorder with features of coronary artery disease. Further, MEF2C has been implicated as a regulator of endothelial integrity and permeability. The mechanisms underlying the favorable effects of MEF2 factors in ECs are not understood, but the link to KLF2 provides a potential explanation. ERK5, a kinase that has been well characterized to be activated by laminar flow, can activate MEF2 pathway. Therefore, it is possible that the following axis may be operative: flow → ERK5 → MEF2 → KLF2 (Fig. 6). Indeed, this pathway has been substantiated by the following studies. Loss-of-function studies by Sohn and colleagues showed that ERK5 is essential for embryonic KLF2 expression (82a). Consistent with this paradigm, Parmar et al. showed that overexpression of a dominant negative MEF2 (or mutant MEK5—an upstream activator of ERK5) prevented flow-mediated induction of KLF2 expression in ECs, confirming the necessity of MEK5 activation in flow-mediated KLF2 expression.
Building on the discovery of essential role of MEF2 in regulating flow-mediated KLF2 expression, Wang et al. demonstrated a novel role for histone deacetylase 5 (HDAC5) in flow-mediated KLF2 expression through regulating MEF2A activity (56, 93). HDAC5, a class IIa histone deacetylase, negatively regulates MEF2 expression in cardiomyocytes and skeletal muscles differentiation and cardiac growth (63, 64). In their studies, Wang et al. showed that shear stress-induced HDAC5 phosphorylation and nuclear export in ECs via a calcium/calmodulin-dependent pathway, leading to dissociation of HDAC5 and MEF2 and enhancing MEF2 activity. MEF2 subsequently increased expression of KLF2 (Fig. 6) and eNOS. More importantly, overexpression of a mutant form of HDAC5 (defective in phosphorylation by replacing Ser259/Ser498 with Ala259/Ala498) abrogated flow-mediated MEF2 activity and subsequent KLF2 and eNOS expression. Taken together, these observations highlight a novel role of HDAC5 in mediating flow-induced KLF2 and eNOS expression.
Several other recent studies shed more light on our understanding on the regulation of KLF2 by shear stress. Young et al. demonstrated that AMP-activated protein kinase activation by pulsatile laminar flow is able to activate ERK5/MEF2 pathway, leading to the increase of KLF2 expression. While pulsatile flow induces sustained expression of KLF2 in cultured ECs, oscillatory flow causes prolongs suppression after initial transient induction. Studies by Wang et al. reveals that this KLF2 downregulation is mediated by the inhibitory effect of the Src signaling pathway (92).
While the majority of studies show that laminar flow increase KLF2 mRNA transcription, several studies also support a role for shear stress-induced mRNA stabilization. For example, van Thienen et al. demonstrated that shear stress could also sustain KLF2 expression through mRNA stabilization via phosphoinositide-3 kinase-dependent pathway. In addition, Bao and colleagues identified a role for the shear-sensitive regulator gene HuR in KLF2 regulation (Fig. 6). HuR is ubiquitously expressed and belongs to the embryonic lethal abnormal visual family of RNA-binding proteins. HuR binds to AU-rich elements of the 3′ untranslated region of certain RNAs to increase their stability and translation. Analysis of HUVECs revealed that as compared to static conditions, oscillatory flow increased HuR mRNA levels by ∼1.7-fold and laminar shear flow by ∼1.3-fold. This was accompanied by a similar degree of change in the proatherosclerotic gene bone morphogenetic protein 4 (BMP-4). Loss-of-function studies of HuR revealed an increase in KLF2 and eNOS and a 60% decrease in BMP-4. An inhibition of inflammatory response was also noted as evidenced by decreased intercellular adhesion molecule 1 and VCAM-1, NFκB phosphorylation, and monocyte adhesion. Further, tissue staining in mouse aorta revealed increased HuR expression in the lesser curvature of the aortic arch that is known to be exposed to disturbed flow. In addition, HuR knockdown increased KLF2 mRNA by 2.5-fold. Mechanistically, HuR does not directly bind to KLF2, eNOS, or BMP-4 as revealed by immunoprecipitation analysis of HuR protein and mRNA complexes. Surprisingly, HuR knockdown does increase the stability of KLF2 mRNA. The underlying mechanism on how HuR regulates KLF2 is not entirely clear and awaits further studies (74).
In addition to the MEF2 factors and HuR pathways mentioned above, several other factors have been identified to contribute to KLF2 expression. Using promoter analyses, Lingrel and colleagues in 2004 discovered that shear stress induced specific nuclear binding to a proximal 62bp element in the KLF2 promoter (44). Huddleson et al. used DNA affinity chromatography and mass spectrometry to identify p300/CBP-associated factor, heterogeneous nuclear ribonucleoprotein D, and nucleolin bind to the KLF2 and enhance transcriptional activity via phosphoinositide-3 kinase signaling (Fig. 6) (42, 43). However, the importance of these factors in regulating endogenous KLF2 expression under basal conditions or in the context of flow remains to be defined.
Cytokine-mediated inhibition of KLF2
Proinflammatory cytokines such as TNFα and IL-1β have been shown to repress KLF2 expression in ECs. This is an important observation, as reduction in KLF2 expression may lead to unopposed NFκB activity and endothelial proinflammatory activation. Using chemical and genetic inhibitors, Kumar and colleagues determined that the TNFα-mediated reduction of KLF2 was dependent on both the NFκB and HDAC pathways (Fig. 6). Next using a combination of promoter deletion and mutation analyses, chromatin immunoprecipitation assays, and siRNA-mediated knockdown studies, evidence was provided that p65 (a component of NFκB) and HDAC4 cooperate to inhibit the ability of MEF2 factors to induce KLF2 expression (52). These studies coupled with the observation that KLF2 can inhibit NFκB raise the possibility that the balance of these two transcriptional pathways may regulate the state of endothelial activation (52).
Reduction of KLF2 by oxidative stress
H2O2 and advanced glycation end products are well known as mediators of diabetes vasculopathy. Studies by Woo et al. demonstrate that SUMOylation of ERK5 by H2O2 and advanced glycation end products inhibits KLF2 expression by decreasing MEF2 activity. While ERK5 transcriptional activity was suppressed by expression of Ubc9 (SUMO E2 conjugase) or protein inhibitor of activated STAT-1, kinase activity was unaffected. Further, point mutation and knockdown studies confirmed that SUMOylation exclusively affected ERK5 transcriptional repression without change in its kinase activity (100). Recently, an adaptor protein p66shc, a member of the Shc protein family of molecular adaptors that promotes cellular oxidative stress and apoptosis, has been demonstrated to reduce KLF2 expression through suppression of MEF2A expression (51).
Pharmacological Implications of KLF2
Statins (3-hydroxy-3methylglutaryl coenzyme A or HMG-CoA inhibitors)
Given the favorable properties that KLF2 confers to the endothelium, one would in principle like to identify mechanisms to exploit this as a therapeutically meaningful manner (Fig. 6). In this regard, studies from our group and others have identified a novel link between KLF2 and a commonly prescribed class of medications termed statins. Statins are antihyperlipidemic agents that possess anti-inflammatory and antithrombotic effects. The effects of statins overlap with that of KLF2 (71, 81, 89). Consistent with this observation, we and others have reported that multiple statins can induce KLF2 expression. This induction was abrogated by geranylgeranyl pyrophosphate but not farnesyl pyrophosphate, thereby implicating the Ras homolog pathway in KLF2 induction (Fig. 6). Consistent with this observation, we found that overexpression of a constitutively active form of Rho strongly reduced KLF2 expression. Further, we demonstrated that the induction of KLF2 by statins occurs through the MEF2 site in proximal promoter region. Finally, siRNA-mediated knockdown studies demonstrated that statin-mediated induction of eNOS and TM mRNA and protein accumulation within ECs is KLF2 dependent. Taken together, these data identify KLF2 as an important nuclear effector of statin effects in ECs.
Recent studies indicate that endothelial responsiveness to statins depends on biomechanical stimuli shear stress. As demonstrated by Ali et al., when ECs were subjected to laminar shear stress, their sensitivity to statins was increased (2). KLF2 was implicated to play a role in this process. HO-1 is an antiatherogenic factor upregulated by statins and laminar shear stress. EC subjected to laminar shear stress demonstrated enhanced HO-1-mediated antioxidant effects at lower concentrations of statins (2, 57). Importantly, KLF2 deficiency in ECs led to blunted induction of HO-1 by the combination of shear stress and statins.
Proteasome inhibitors
Proteasome inhibitors are used in treatment of multiple myeloma and other hematological malignancies and appear to decrease the risk of thromboembolic events (66). Proteasome inhibitors induce expression of TM and protein C activating capacity of the endothelium. Although NFκB is a principal target of proteasome inhibitors, this effect was noted to be independent of NFκB signaling and due to direct increase in KLF2 (Fig. 6) and KLF4 expression (41).
Krüppel-Like Factor 4
While most of the attention to date has focused on KLF2, there is now increasing momentum in field that additional members of this gene family may also regulate EC biology. KLF4 was initially termed gut-enriched KLF or epithelial zinc-finger protein owing to its high expression in the gut and skin epithelium (36, 82). It is a single-copy gene on chromosome 9p31, and contains a repression domain in between amino acids 181 and 388, and an activation domain between amino acids 91 and 117. KLF4 is highly expressed in the differentiating layers of the epidermis, and KLF4−/− causes neonatal death due to loss of skin barrier function (79). KLF4 also functions to maintain normal gastric epithelial homeostasis. Deletion of KLF4 in the gastrointestinal epithelium leads to altered differentiation and increased proliferation. Additionally, expression of KLF4 is nearly absent in gastric cancer (48). KLF4 is also found to be highly expressed in the corneal epithelium, where it plays a critical role in development and maintenance of the cornea (83, 84). Numerous articles describe the critical role of KLF4 in stem cell biology (4, 61, 67, 85, 99). KLF4 functions as an upstream regulator of Nanog, maintains pluripotency, and prevents embryonic stem cell differentiation (107). KLF4 also plays a critical role in the endothelium (40), monocyte/macrophage (29, 30), and vascular smooth muscle biology (1, 59, 104, 105).
KLF4 was first described in ECs by Yet et al. in 1998 (104). Subsequently, KLF4 has been shown to be induced by shear stress (62). Studies from our lab implicate that KLF4 serves as an important regulator of key factors involved in endothelial function. For example, KLF4 increases atheroprotective genes such as eNOS and TM, while reducing adhesion molecules such as VCAM-1 and procoagulant factors (e.g., TF). As a functional consequence, KLF4 decreases inflammatory cell adhesion to the endothelial surface and prolongs clotting time (40). Although similarities to the actions and effects of KLF2 exist, KLF4 differs in its expression in response to inflammatory stimuli. In response to inflammatory stimuli such as TNFα, KLF4 is highly induced. In their article on atherogenesis in KLF2+/− ApoE null mice, Atkins et al. noted an increased expression of KLF4 (6). This raises the possibility that, in the context of inflammation, KLF4 may serve as a back-up to modulate endothelial inflammation when KLF2 levels are strongly attenuated.
Future Directions
The discovery of KLFs as mediators of flow-dependent responses in ECs was an important advance in the field of vascular biology. To date much attention has focused on KLF2, and momentum is building for KLF4 as well. Although collectively the observations are intriguing, much of our understanding has been based on in vitro observations. Importantly, in vivo studies are just starting to emerge and have been surprisingly mixed in their confirmation of the in vitro data. For example, while KLF2 clearly regulates a number of endothelial genes in vitro, in vivo confirmation has not been forthcoming. There are a number of explanations that may account for the discrepancy between in vitro and in vivo observations ranging from technical considerations to the potential compensatory role of KLF4. This lack of clarity is not uncommon for a nascent field and it is anticipated that, with additional research, a better understanding of their role in vascular biology will emerge. Some key issues that need to be addressed for KLFs in vascular biology are as follows.
First, while hemizygous deficiency of KLF2 promotes atherosclerosis, the relative contribution of the endothelium and various hematopoietic lineages that contribute to this phenotype (e.g., macrophages and T-lymphocytes) remains unknown. Conditional deletion approaches are clearly needed to address this issue. In addition, the establishment of such in vivo models will also facilitate efforts to understand the role of endothelial (or immune cell) KLF2 in a host of vascular processes such as angiogenesis and thrombosis. Essentially, identical considerations apply to KLF4, which is also expressed in the endothelium and various hematopoietic lineages. It is hoped that through such efforts, an understanding of the relative contributions of each factor to specific physiologic and pathologic processes will be gleaned. Additionally, given the concerns about redundancy, it is quite possible that cell-type-specific compound mutants of KLF2 and KLF4 will ultimately be required to fully understand the role of this family in vascular biology. Second, both KLF2 and KLF4 have been shown to differentially affect the expression of factors that confer anti-inflammatory, antithrombotic, and antiproliferative effects in ECs. However, detailed mechanistic insights remain poorly understood. For example, what are the factors that KLF2, KLF4, or both cooperate with to regulate endothelial targets? Are these interacting factors the same or distinct? Third, KLF2 and KLF4 are regulated by a number of pharmacologic agents (e.g., statins, AMP-activated protein kinase activators, and proteasome inhibitors). The importance of KLFs in mediating the effects of these agents in the context of cellular or organismal biology remains unclear. Finally, there are scattered reports that alterations in KLF levels correlate with disease. For example, a ∼30% reduction in KLF2 levels are seen in circulating monocytes from patients with atherosclerosis. Additional studies are clearly needed to understand if KLF2 and/or KLF4 levels or activity are dysregulated in human disease.
Abbreviations Used
- ACE
angiotension converting enzyme
- AM
adrenomedullin
- Ang
angiopoietin
- AP-1
activator protein-1
- APC
activated protein C
- ASS
arginosuccinate synthase
- ATF2
activating transcription factor 2
- BMP
bone morphogenetic protein
- CBP/p300
cyclic AMP response element-binding protein
- CD40L
CD40 ligand
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- ERG
ets-related gene
- ERK
extracellular signal-regulated kinase
- ET-1
endothelin-1
- ETS
E-twenty six
- Flk-1
fetal liver kinase 1
- HDAC
histone deacetylase
- HIF-1α
hypoxia-inducible factor 1 alpha
- hnRNP-D
heterogeneous nuclear ribonucleoprotein D
- HO-1
heme oxygenase 1
- Hsp
heat shock protein
- HUVECs
human umbilical vein endothelial cells
- IL
interleukin
- KLF
Krüppel-like factor
- LKLF
lung Krüppel-like factor
- MADS
MCM1 Agamous–Deficiens–Serum response factor
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein 1
- MEF2
myocyte enhancer factor 2
- MMP
matrix metalloproteinase
- NFκB
nuclear factor kappa B
- NQO1
NAD(P)H:quinine oxidoreductase-1
- Nrf2
nuclear factor erythroid 2-like
- PAI-1
plasminogen activator inhibitor-1
- PAR-1
protease-activated receptor 1
- PI3K
phosphoinositide-3 kinase
- Rho
Ras homolog
- SEMA3F
semaphorin 3F
- sih
silent heart
- SMAD
Sma and Mad related protein
- Sp
specificity protein
- TF
tissue factor
- TGF
transforming growth factor
- TM
thrombomodulin
- TNF
tumor necrosis factor
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- WT
wild type
- WWP1
WW domain-containing E3 ubiquitin ligase 1
Acknowledgments
This work was supported by NIH grants HL 72952, HL 75427, HL 76754, HL 086548, HL 084154, and P01 HL 48743 (to M.K. Jain), HL 087595 (to Z. Lin).
References
- 1.Adam PJ. Regan CP. Hautmann MB. Owens GK. Positive- and negative-acting Krüppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22alpha in vivo. J Biol Chem. 2000;275:37798–37806. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- 2.Ali F. Zakkar M. Karu K. Lidington EA. Hamdulay SS. Boyle JJ. Zloh M. Bauer A. Haskard DO. Evans PC. Mason JC. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem. 2009;284:18882–18892. doi: 10.1074/jbc.M109.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson KP. Kern CB. Crable SC. Lingrel JB. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Krüppel-like factor: identification of a new multigene family. Mol Cell Biol. 1995;15:5957–5965. doi: 10.1128/mcb.15.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoi T. Yae K. Nakagawa M. Ichisaka T. Okita K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 5.Atkins GB. Jain MK. Role of Krüppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 6.Atkins GB. Wang Y. Mahabeleshwar GH. Shi H. Gao H. Kawanami D. Natesan V. Lin Z. Simon DI. Jain MK. Hemizygous deficiency of Krüppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee SS. Feinberg MW. Watanabe M. Gray S. Haspel RL. Denkinger DJ. Kawahara R. Hauner H. Jain MK. The Krüppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J Biol Chem. 2003;278:2581–2584. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- 8.Basu P. Morris PE. Haar JL. Wani MA. Lingrel JB. Gaensler KM. Lloyd JA. KLF2 is essential for primitive erythropoiesis and regulates the human and murine embryonic beta-like globin genes in vivo. Blood. 2005;106:2566–2571. doi: 10.1182/blood-2005-02-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya R. Senbanerjee S. Lin Z. Mir S. Hamik A. Wang P. Mukherjee P. Mukhopadhyay D. Jain MK. Inhibition of vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis by the Krüppel-like factor KLF2. J Biol Chem. 2005;280:28848–28851. doi: 10.1074/jbc.C500200200. [DOI] [PubMed] [Google Scholar]
- 10.Bieker JJ. Isolation, genomic structure, and expression of human erythroid Krüppel-like factor (EKLF) DNA Cell Biol. 1996;15:347–352. doi: 10.1089/dna.1996.15.347. [DOI] [PubMed] [Google Scholar]
- 11.Bieker JJ. Krüppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 12.Bielenberg DR. Hida Y. Shimizu A. Kaipainen A. Kreuter M. Kim CC. Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black AR. Black JD. Azizkhan-Clifford J. Sp1 and Krüppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 14.Boon RA. Fledderus JO. Volger OL. van Wanrooij EJ. Pardali E. Weesie F. Kuiper J. Pannekoek H. ten Dijke P. Horrevoets AJ. KLF2 suppresses TGF-beta signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler Thromb Vasc Biol. 2007;27:532–539. doi: 10.1161/01.ATV.0000256466.65450.ce. [DOI] [PubMed] [Google Scholar]
- 15.Boon RA. Leyen TA. Fontijn RD. Fledderus JO. Baggen JM. Volger OL. van Nieuw Amerongen GP. Horrevoets AJ. KLF2-induced actin shear fibers control both alignment to flow and JNK signaling in vascular endothelium. Blood. 2010;115:2533–2542. doi: 10.1182/blood-2009-06-228726. [DOI] [PubMed] [Google Scholar]
- 16.Carlson CM. Endrizzi BT. Wu J. Ding X. Weinreich MA. Walsh ER. Wani MA. Lingrel JB. Hogquist KA. Jameson SC. Krüppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan SD. Nilsson H. Ahluwalia A. Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XL. Varner SE. Rao AS. Grey JY. Thomas S. Cook CK. Wasserman MA. Medford RM. Jaiswal AK. Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 20.Conkright MD. Wani MA. Lingrel JB. Lung Krüppel-like factor contains an autoinhibitory domain that regulates its transcriptional activation by binding WWP1, an E3 ubiquitin ligase. J Biol Chem. 2001;276:29299–29306. doi: 10.1074/jbc.M103670200. [DOI] [PubMed] [Google Scholar]
- 21.Dang DT. Pevsner J. Yang VW. The biology of the mammalian Krüppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang DT. Zhao W. Mahatan CS. Geiman DE. Yang VW. Opposing effects of Krüppel-like factor 4 (gut-enriched Krüppel-like factor) and Krüppel-like factor 5 (intestinal-enriched Krüppel-like factor) on the promoter of the Krüppel-like factor 4 gene. Nucleic Acids Res. 2002;30:2736–2741. doi: 10.1093/nar/gkf400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das H. Kumar A. Lin Z. Patino WD. Hwang PM. Feinberg MW. Majumder PK. Jain MK. Krüppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A. 2006;103:6653–6658. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davids N. Finite element methods of studying mechanical factors in blood flow. Neurol Res. 1981;3:83–105. doi: 10.1080/01616412.1981.11739593. [DOI] [PubMed] [Google Scholar]
- 25.Dekker RJ. Boon RA. Rondaij MG. Kragt A. Volger OL. Elderkamp YW. Meijers JC. Voorberg J. Pannekoek H. Horrevoets AJ. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 26.Dekker RJ. van Soest S. Fontijn RD. Salamanca S. de Groot PG. VanBavel E. Pannekoek H. Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 27.Dekker RJ. van Thienen JV. Rohlena J. de Jager SC. Elderkamp YW. Seppen J. de Vries CJ. Biessen EA. van Berkel TJ. Pannekoek H. Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farb A. Tang AL. Burke AP. Sessums L. Liang Y. Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92:1701–1790. doi: 10.1161/01.cir.92.7.1701. [DOI] [PubMed] [Google Scholar]
- 29.Feinberg MW. Cao Z. Wara AK. Lebedeva MA. Senbanerjee S. Jain MK. Krüppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- 30.Feinberg MW. Wara AK. Cao Z. Lebedeva MA. Rosenbauer F. Iwasaki H. Hirai H. Katz JP. Haspel RL. Gray S. Akashi K. Segre J. Kaestner KH. Tenen DG. Jain MK. The Krüppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiedler U. Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Fiedler U. Reiss Y. Scharpfenecker M. Grunow V. Koidl S. Thurston G. Gale NW. Witzenrath M. Rosseau S. Suttorp N. Sobke A. Herrmann M. Preissner KT. Vajkoczy P. Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 33.Fledderus JO. Boon RA. Volger OL. Hurttila H. Yla-Herttuala S. Pannekoek H. Levonen AL. Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811. [DOI] [PubMed] [Google Scholar]
- 34.Fledderus JO. van Thienen JV. Boon RA. Dekker RJ. Rohlena J. Volger OL. Bijnens AP. Daemen MJ. Kuiper J. van Berkel TJ. Pannekoek H. Horrevoets AJ. Prolonged shear stress and KLF2 suppress constitutive proinflammatory transcription through inhibition of ATF2. Blood. 2007;109:4249–4257. doi: 10.1182/blood-2006-07-036020. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 36.Garrett-Sinha LA. Eberspaecher H. Seldin MF. de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- 37.Gimbrone MA., Jr. Endothelial dysfunction, hemodynamic forces, and atherosclerosis. Thromb Haemost. 1999;82:722–726. [PubMed] [Google Scholar]
- 38.Goodwin BL. Solomonson LP. Eichler DC. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem. 2004;279:18353–18360. doi: 10.1074/jbc.M308160200. [DOI] [PubMed] [Google Scholar]
- 39.Haldar SM. Ibrahim OA. Jain MK. Krüppel-like Factors (KLFs) in muscle biology. J Mol Cell Cardiol. 2007;43:1–10. doi: 10.1016/j.yjmcc.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamik A. Lin Z. Kumar A. Balcells M. Sinha S. Katz J. Feinberg MW. Gerzsten RE. Edelman ER. Jain MK. Krüppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 41.Hiroi T. Deming CB. Zhao H. Hansen BS. Arkenbout EK. Myers TJ. McDevitt MA. Rade JJ. Proteasome inhibitors enhance endothelial thrombomodulin expression via induction of Kruppel-like transcription factors. Arterioscler Thromb Vasc Biol. 2009;29:1587–1593. doi: 10.1161/ATVBAHA.109.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huddleson JP. Ahmad N. Lingrel JB. Up-regulation of the KLF2 transcription factor by fluid shear stress requires nucleolin. J Biol Chem. 2006;281:15121–15128. doi: 10.1074/jbc.M513406200. [DOI] [PubMed] [Google Scholar]
- 43.Huddleson JP. Ahmad N. Srinivasan S. Lingrel JB. Induction of KLF2 by fluid shear stress requires a novel promoter element activated by a phosphatidylinositol 3-kinase-dependent chromatin-remodeling pathway. J Biol Chem. 2005;280:23371–23379. doi: 10.1074/jbc.M413839200. [DOI] [PubMed] [Google Scholar]
- 44.Huddleson JP. Srinivasan S. Ahmad N. Lingrel JB. Fluid shear stress induces endothelial KLF2 gene expression through a defined promoter region. Biol Chem. 2004;385:723–729. doi: 10.1515/BC.2004.088. [DOI] [PubMed] [Google Scholar]
- 45.Jackle H. Rosenberg UB. Preiss A. Seifert E. Knipple DC. Kienlin A. Lehmann R. Molecular analysis of Krüppel, a segmentation gene of Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1985;50:465–473. doi: 10.1101/sqb.1985.050.01.058. [DOI] [PubMed] [Google Scholar]
- 46.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 47.Jiang J. Chan YS. Loh YH. Cai J. Tong GQ. Lim CA. Robson P. Zhong S. Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 48.Katz JP. Perreault N. Goldstein BG. Actman L. McNally SR. Silberg DG. Furth EE. Kaestner KH. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–945. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Kawanami D. Mahabeleshwar GH. Lin Z. Atkins GB. Hamik A. Haldar SM. Maemura K. Lamanna JC. Jain MK. Krüppel-like factor 2 inhibits hypoxia-inducible factor 1alpha expression and function in the endothelium. J Biol Chem. 2009;284:20522–20530. doi: 10.1074/jbc.M109.025346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinderlerer AR. Ali F. Johns M. Lidington EA. Leung V. Boyle JJ. Hamdulay SS. Evans PC. Haskard DO. Mason JC. KLF2-dependent, shear stress-induced expression of CD59: a novel cytoprotective mechanism against complement-mediated injury in the vasculature. J Biol Chem. 2008;283:14636–14644. doi: 10.1074/jbc.M800362200. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A. Hoffman TA. Dericco J. Naqvi A. Jain MK. Irani K. Transcriptional repression of Krüppel like factor-2 by the adaptor protein p66shc. FASEB J. 2009;23:4344–4352. doi: 10.1096/fj.09-138743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar A. Lin Z. SenBanerjee S. Jain MK. Tumor necrosis factor alpha-mediated reduction of KLF2 is due to inhibition of MEF2 by NF-kappaB and histone deacetylases. Mol Cell Biol. 2005;25:5893–5903. doi: 10.1128/MCB.25.14.5893-5903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo CT. Veselits ML. Barton KP. Lu MM. Clendenin C. Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuo CT. Veselits ML. Leiden JM. LKLF: a transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 55.Langille BL. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol. 1993;21(Suppl 1):S11–S17. doi: 10.1097/00005344-199321001-00003. [DOI] [PubMed] [Google Scholar]
- 56.Lee JS. Yu Q. Shin JT. Sebzda E. Bertozzi C. Chen M. Mericko P. Stadtfeld M. Zhou D. Cheng L. Graf T. MacRae CA. Lepore JJ. Lo CW. Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Lee TS. Chang CC. Zhu Y. Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 58.Lin Z. Hamik A. Jain R. Kumar A. Jain MK. Krüppel-like factor 2 inhibits protease activated receptor-1 expression and thrombin-mediated endothelial activation. Arterioscler Thromb Vasc Biol. 2006;26:1185–1189. doi: 10.1161/01.ATV.0000215638.53414.99. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y. Sinha S. McDonald OG. Shang Y. Hoofnagle MH. Owens GK. Krüppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 60.Lucitti JL. Jones EA. Huang C. Chen J. Fraser SE. Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maherali N. Sridharan R. Xie W. Utikal J. Eminli S. Arnold K. Stadtfeld M. Yachechko R. Tchieu J. Jaenisch R. Plath K. Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 62.McCormick SM. Eskin SG. McIntire LV. Teng CL. Lu CM. Russell CG. Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2001;98:8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKinsey TA. Zhang CL. Lu J. Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKinsey TA. Zhang CL. Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meadows SM. Salanga MC. Krieg PA. Krüppel-like factor 2 cooperates with the ETS family protein ERG to activate Flk1 expression during vascular development. Development. 2009;136:1115–1125. doi: 10.1242/dev.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musallam KM. Dahdaleh FS. Shamseddine AI. Taher AT. Incidence and prophylaxis of venous thromboembolic events in multiple myeloma patients receiving immunomodulatory therapy. Thromb Res. 2009;123:679–686. doi: 10.1016/j.thromres.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 67.Nakatake Y. Fukui N. Iwamatsu Y. Masui S. Takahashi K. Yagi R. Yagi K. Miyazaki J. Matoba R. Ko MS. Niwa H. Klf4 cooperates with Oct3/4 and Sox2 to activate the Lefty1 core promoter in embryonic stem cells. Mol Cell Biol. 2006;26:7772–7782. doi: 10.1128/MCB.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nam D. Ni CW. Rezvan A. Suo J. Budzyn K. Llanos A. Harrison D. Giddens D. Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nusslein-Volhard C. Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 70.Parmar KM. Larman HB. Dai G. Zhang Y. Wang ET. Moorthy SN. Kratz JR. Lin Z. Jain MK. Gimbrone MA., Jr. García-Cardeña G. Integration of flow-dependent endothelial phenotypes by Krüppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parmar KM. Nambudiri V. Dai G. Larman HB. Gimbrone MA., Jr. Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 72.Preiss A. Rosenberg UB. Kienlin A. Seifert E. Jackle H. Molecular genetics of Krüppel, a gene required for segmentation of the Drosophila embryo. Nature. 1985;313:27–32. doi: 10.1038/313027a0. [DOI] [PubMed] [Google Scholar]
- 73.Razani B. Engelman JA. Wang XB. Schubert W. Zhang XL. Marks CB. Macaluso F. Russell RG. Li M. Pestell RG. Di Vizio D. Hou H., Jr. Kneitz B. Lagaud G. Christ GJ. Edelmann W. Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 74.Rhee WJ. Ni CW. Zheng Z. Chang K. Jo H. Bao G. HuR regulates the expression of stress-sensitive genes and mediates inflammatory response in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107:6858–6863. doi: 10.1073/pnas.1000444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenberg UB. Preiss A. Seifert E. Jackle H. Knipple DC. Production of phenocopies by Krüppel antisense RNA injection into Drosophila embryos. Nature. 1985;313:703–706. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- 76.Saadi S. Holzknecht RA. Patte CP. Stern DM. Platt JL. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–1814. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sako K. Fukuhara S. Minami T. Hamakubo T. Song H. Kodama T. Fukamizu A. Gutkind JS. Koh GY. Mochizuki N. Angiopoietin-1 induces Krüppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem. 2009;284:5592–5601. doi: 10.1074/jbc.M806928200. [DOI] [PubMed] [Google Scholar]
- 78.Sebzda E. Zou Z. Lee JS. Wang T. Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 79.Segre JA. Bauer C. Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 80.SenBanerjee S. Lin Z. Atkins GB. Greif DM. Rao RM. Kumar A. Feinberg MW. Chen Z. Simon DI. Luscinskas FW. Michel TM. Gimbrone MA., Jr. Garcia-Cardena G. Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sen-Banerjee S. Mir S. Lin Z. Hamik A. Atkins GB. Das H. Banerjee P. Kumar A. Jain MK. Krüppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 82.Shields JM. Christy RJ. Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82a.Sohn SJ. Li D. Lee LK. Winoto A. Transcriptional regulation of tissue-specific genes by the ERKS mitogen-activated protein kinase. Mol Cell Biol. 2005;25:8553–8566. doi: 10.1128/MCB.25.19.8553-8566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swamynathan SK. Davis J. Piatigorsky J. Identification of candidate Klf4 target genes reveals the molecular basis of the diverse regulatory roles of Klf4 in the mouse cornea. Invest Ophthalmol Vis Sci. 2008;49:3360–3370. doi: 10.1167/iovs.08-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swamynathan SK. Katz JP. Kaestner KH. Ashery-Padan R. Crawford MA. Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–194. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 86.Tedesco F. Pausa M. Nardon E. Introna M. Mantovani A. Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–1627. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Topper JN. Cai J. Falb D. Gimbrone MA., Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turner J. Crossley M. Basic Krüppel-like factor functions within a network of interacting haematopoietic transcription factors. Int J Biochem Cell Biol. 1999;31:1169–1174. doi: 10.1016/s1357-2725(99)00067-9. [DOI] [PubMed] [Google Scholar]
- 89.van Thienen JV. Fledderus JO. Dekker RJ. Rohlena J. van Ijzendoorn GA. Kootstra NA. Pannekoek H. Horrevoets AJ. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc Res. 2006;72:231–240. doi: 10.1016/j.cardiores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 90.van Vliet J. Crofts LA. Quinlan KG. Czolij R. Perkins AC. Crossley M. Human KLF17 is a new member of the Sp/KLF family of transcription factors. Genomics. 2006;87:474–482. doi: 10.1016/j.ygeno.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Vartanian KB. Berny MA. McCarty OJ. Hanson SR. Hinds MT. Cytoskeletal structure regulates endothelial cell immunogenicity independent of fluid shear stress. Am J Physiol Cell Physiol. 2010;298:C333–C341. doi: 10.1152/ajpcell.00340.2009. [DOI] [PubMed] [Google Scholar]
- 92.Wang N. Miao H. Li YS. Zhang P. Haga JH. Hu Y. Young A. Yuan S. Nguyen P. Wu CC. Chien S. Shear stress regulation of Krüppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006;341:1244–1251. doi: 10.1016/j.bbrc.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 93.Wang W. Ha CH. Jhun BS. Wong C. Jain MK. Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010;115:2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wani MA. Conkright MD. Jeffries S. Hughes MJ. Lingrel JB. cDNA isolation, genomic structure, regulation, and chromosomal localization of human lung Krüppel-like factor. Genomics. 1999;60:78–86. doi: 10.1006/geno.1999.5888. [DOI] [PubMed] [Google Scholar]
- 95.Wani MA. Means RT., Jr. Lingrel JB. Loss of LKLF function results in embryonic lethality in mice. Transgenic Res. 1998;7:229–238. doi: 10.1023/a:1008809809843. [DOI] [PubMed] [Google Scholar]
- 96.Wani MA. Wert SE. Lingrel JB. Lung Krüppel-like factor, a zinc finger transcription factor, is essential for normal lung development. J Biol Chem. 1999;274:21180–21185. doi: 10.1074/jbc.274.30.21180. [DOI] [PubMed] [Google Scholar]
- 97.Warabi E. Takabe W. Minami T. Inoue K. Itoh K. Yamamoto M. Ishii T. Kodama T. Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 98.Warabi E. Wada Y. Kajiwara H. Kobayashi M. Koshiba N. Hisada T. Shibat M. Ando J. Tsuchiya M. Kodama T. Noguchi N. Effect on endothelial cell gene expression of shear stress, oxygen concentration, and low-density lipoprotein as studied by a novel flow cell culture system. Free Radic Biol Med. 2004;37:682–694. doi: 10.1016/j.freeradbiomed.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 99.Wernig M. Meissner A. Foreman R. Brambrink T. Ku M. Hochedlinger K. Bernstein BE. Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 100.Woo CH. Shishido T. McClain C. Lim JH. Li JD. Yang J. Yan C. Abe J. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008;102:538–545. doi: 10.1161/CIRCRESAHA.107.156877. [DOI] [PubMed] [Google Scholar]
- 101.Wu J. Bohanan CS. Neumann JC. Lingrel JB. KLF2 transcription factor modulates blood vessel maturation through smooth muscle cell migration. J Biol Chem. 2008;283:3942–3950. doi: 10.1074/jbc.M707882200. [DOI] [PubMed] [Google Scholar]
- 102.Wu J. Lingrel JB. Krüppel-like factor 2, a novel immediate-early transcriptional factor, regulates IL-2 expression in T lymphocyte activation. J Immunol. 2005;175:3060–3066. doi: 10.4049/jimmunol.175.5.3060. [DOI] [PubMed] [Google Scholar]
- 103.Wu J. Srinivasan SV. Neumann JC. Lingrel JB. The KLF2 transcription factor does not affect the formation of preadipocytes but inhibits their differentiation into adipocytes. Biochemistry. 2005;44:11098–11105. doi: 10.1021/bi050166i. [DOI] [PubMed] [Google Scholar]
- 104.Yet SF. McA'Nulty MM. Folta SC. Yen HW. Yoshizumi M. Hsieh CM. Layne MD. Chin MT. Wang H. Perrella MA. Jain MK. Lee ME. Human EZF, a Krüppel-like zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J Biol Chem. 1998;273:1026–1031. doi: 10.1074/jbc.273.2.1026. [DOI] [PubMed] [Google Scholar]
- 105.Yoshida T. Gan Q. Owens GK. Krüppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Physiol Cell Physiol. 2008;295:C1175–C1182. doi: 10.1152/ajpcell.00288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoshida T. Kaestner KH. Owens GK. Conditional deletion of Krüppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zarins CK. Giddens DP. Bharadvaj BK. Sottiurai VS. Mabon RF. Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 108.Zhang P. Andrianakos R. Yang Y. Liu C. Lu W. Krüppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating Nanog gene expression. J Biol Chem. 2010;285:9180–9189. doi: 10.1074/jbc.M109.077958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang X. Srinivasan SV. Lingrel JB. WWP1-dependent ubiquitination and degradation of the lung Krüppel-like factor, KLF2. Biochem Biophys Res Commun. 2004;316:139–148. doi: 10.1016/j.bbrc.2004.02.033. [DOI] [PubMed] [Google Scholar]