Abstract
Embelin is an active ingredient of traditional herbal medicine that exhibits anti-tumor effects in human prostate cancer cells. However, therapeutic effect of embelin in combination with conventional radiation therapy is not yet determined. In this study, we evaluate the sensitizing potential of embelin on ionizing radiation (IR) in a human prostate cancer model. In vitro, embelin combined with radiation potently suppressed prostate cancer PC-3 cell proliferation that was associated with S and G2/M arrest in cell cycle. Moreover, the combination treatment promoted caspase-independent apoptosis, as evidenced by the increased apoptotic cell death without caspase-3 activation, but not autophagy. Clonogenic survival assay showed that S-phase arrest was required for embelin-mediated radiosensitization. In vivo, embelin significantly improved tumor response to X-ray radiation in the PC-3 xenograft model. Combination therapy produced enhanced tumor growth delay and prolonged time to progression, with minimal systemic toxicity. Immunohistochemistry studies showed that embelin plus IR significantly inhibited cell proliferation, induced apoptosis, and decreased microvessel density in tumors as compared with either treatment alone, suggesting an enhanced combinatory inhibition on tumor suppression and angiogenesis. Our results demonstrate that embelin significantly facilitates tumor suppression by radiation therapy both in vitro and in vivo in the prostate cancer model. This finding warrants embelin as a novel adjuvant therapeutic candidate for the treatment of hormone-refractory prostate cancer that is resistant to radiation therapy.
Keywords: IAP inhibitor, Embelin, prostate cancer, ionizing radiation therapy
Introduction
Prostate cancer is the most common malignancy and the second leading cause of cancer-related death in men in the United States. Current therapeutics for advanced human prostate cancer is limited by the propensity of the disease to progress from an androgen-dependent to an androgen-independent phenotype. Anti-cancer therapeutic approaches such as ionizing radiation can activate cellular apoptotic machinery in androgen-independent prostate cancer cells [1-3]. However, acquired radiation resistance is developed in hormone-refractory prostate cancer that is associated with apoptosis-resistance [4, 5]. Therefore, apoptosis tends to be involved in the molecular mechanism of ra-diosensitivity in prostatic tumors [6]. Indeed, defects in the apoptosis machinery have been connected to the resistance of cancer cells to current therapeutic interventions including ionizing radiation [7, 8].
Growing evidences have shown that acquired resistance to radiation therapy can be overcome by utilizing small-molecule compounds that target key proteins involved in radiation resistance [9]. The mechanism of drug-mediated radiosensitization can be categorized into several modes [10], in which cell cycle redistribution is demonstrated as one of the crucial mechanisms. As differential response of the cells to radiation depends on the cell cycle phases, an agent that can synchronize tumor cells in a phase of the cell cycle that is sensitive to radiation is supposed to be a potential therapeutic candidate for facilitating radiotherapy efficacy [11, 12]. As such, it is not surprising that apoptosis may be involved as a mechanism for drug-mediated radiosensitization, if the radiosensitivity depends on cell cycle progression [11].
Radiation-produced apoptosis is an active cellular response that is considered to be a cell fate when cells fail to recover from DNA damage repair and cell cycle arrest [13-15]. Although controversial in some circumstances, the relationship between apoptosis and radiosensitivity is widely documented [16, 17]. We have previously reported that (-)-gossypol, a natural Bcl-2 inhibitor, enhances tumor regression by radiotherapy in a prostate cancer model in vivo [6, 18]. Furthermore, in vitro, (-)-gossypol combined with radiation synergistically triggers apoptosis in a schedule-dependent manner [6]. In another study, a synthetic small molecule XIAP inhibitor shows radiosensitizing effect by facilitating radiation-induced apoptosis in the hormone-refractory prostate cancer [19, 20]. These studies provide evidences that in prostate cancer, apoptosis could be a consequential outcome for combining radiation with antagonists of anti-apoptotic proteins.
Embelin is an active ingredient of traditional herbal medicine for cancer and other diseases [21]. We have previously identified embelin as a potent inhibitor of X-linked inhibitor of apoptosis protein (XIAP), making embelin the first reported natural small molecule XIAP-inhibitor [22]. Besides its antibacterial activity, embelin exhibits cytotoxic effect and inhibits cell proliferation in various cancer cell types [22-26], presumably by activating apoptosis machinery [22, 25, 27]. Furthermore, embelin is reported to enhance tumor cell apoptosis induced by tumor necrosis factor alpha (TNFa) [21] or TNF-related apoptosis-inducing ligand (TRAIL) [28, 29], suggesting that embelin can be applied in conjunction with other therapeutic modalities for cancer therapy. However, so far there is no report on combining embelin with radiotherapy for human cancer treatment.
In this study, we aim to evaluate the effect of embelin on prostate cancer radiosensitivity. We investigate the combinatorial efficacy of embelin and ionizing radiation both in vitro and in vivo in a preclinical prostate cancer model. We also explore the underlying mechanism of embelin-mediated radiosensitization.
Materials and methods
Reagents and cell culture
Embelin (98%) was purchased from INDOFINE Chemical Company (Hillsborough, NJ). The powder was reconstituted in dimethyl sulfoxide (DMSO) and stored as aliquots (50 mM) at -70° C. Fluorogenic substrates DEVD-AFC and Staurosporin were purchased from BioVision (Mountain View, CA). Anti-poly(ADP-ribose) poly-merase (PARP) (F-2) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-XIAP antibody was purchased from BD Bio-sciences (San Jose, CA). Anti-β-actin (AC-74) antibody was obtained from Sigma (St. Louis, MO). Protease inhibitor cocktail was from Roche (Indianapolis, IN). Other chemicals were from Sigma unless otherwise indicated. Human prostate cancer cell lines PC-3 and DU-145 were purchased from American Type Culture Collection. Cells were maintained in RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, and incubated in a 5% CO2 humidified incubator at 37°C. All tissue culture reagents were purchased from Invitrogen (Grand Island, NY).
Cell proliferation and cell death assays
The cell proliferation was assessed by cell counting [18, 30, 31]. Briefly, cells were seeded in a 24-well plate at a density of 2×105 cells/ well and treated with embelin, IR or in combination in triplicate. Every 24 h for up to 7 days, attached cells were harvested and counted using a Coulter cell counter (Fullerton, CA). Relative cell number was normalized by dividing cell number at various times to the average number of untreated control on the first day (day 0). For cell death analysis, total cells (including both floating and attached cells) were harvested and stained with trypan blue [32, 33]. Percentage of cell viability was calculated by dividing the number of negative-stained cells to total cells. Data were from two independent samples in triplicate. Data were plotted and analyzed by Graph-Pad Prism 5.0 (San Diego, CA). Autophagy assays were carried out as we described recently [33, 34].
Flow cytometry
Annexin V binding assay was employed to detect apoptosis, using an Annexin V-FITC and propidium iodide (PI) staining kit (Roche) according to the manufacturer's instruction. Cells in the lower right quadrant were scored as “early apoptosis” [Annexin (+)/PI (-)], and in the upper right quadrant were scored as “necrosis/ late apoptosis” [Annexin (+)/PI (+)] [18, 35]. For cell cycle and sub-G1 analysis, the harvested cells were fixed in 70% ethanol at 4°C overnight, then treated with propidium iodide (PI, 50 μg/ ml) and RNase A (1 μg/ml) for 30 min. Samples were analyzed by flow cytometry (FACSCalibur, BD Biosciences). sub-G1 population was calculated from hypodiploid DNA fluorescent in the cell cycle histogram [18, 30, 36]. Data were analyzed using WinMDI 2.8 software (Purdue University Cytometry Laboratory).
Caspase activation assay
Cells were resolved in a lysis buffer (BioVision), and whole cell lysates (40 μg) were incubated with 20 μM of fluorogenic substrate DEVD-AFC in a reaction buffer (BioVision) containing 5 mM DTT at 37°C for 1 h. Proteolytic release of AFC was monitored at λex = 405 nm and λem = 500 nm using a fluorescence microplate reader (BMG, Cary, NC). Fold increase of fluorescence signal was calculated by comparing the normalized signal in each treated sample with that in the untreated control [30, 35].
Western blot analysis
Western blot was carried out as described previously [30, 32, 37]. For xenografted tumor tissue, a homogenizer (Fisher) was used. Total protein extraction was quantified using the Bradford reagent (Bio-Rad, Hercules, CA). Whole cell lysates were resolved by SDS-PAGE (Bio-Rad), electrotransferred to nitrocellulose membranes (Bio-Rad) and probed with respective antibodies.
Clonogenic cell survival assay
Cells were pretreated with various doses of Em for a desired time course and irradiated by X-ray as described [9, 19]. Cells were then seeded in 6-well plates at the desired cell density (200∼10,000 cells/well) based on treatment stringency in the absence of embelin. After 12-14 days incubation, plates were stained with crystal violet (0.1%). Colonies over 50 cells were manually counted. The survival curves were plotted using a standard linear-quadratic model [35, 38]. Triplicated data of each sample were normalized and the enhancement ratio (ER) was calculated as previously described [6, 35].
Animal study
Female athymic NCr-nu/nu mice (5∼6 weeks) were inoculated subcutaneously (s.c.) on both sides of the lower back above the tail with 3×106 cells/0.2 ml of PC-3 cells [9]. When tumors reached around 100 mm3, the mice were randomized into 4 groups with 8∼10 mice per group and treated daily with (a) vehicle control [0.1% carboxymethyl cellulose (CMC)]; (b) X-ray radiation at 2 Gy fraction on days 1 to 5 weekly for 2 weeks; (c) embelin via oral gavage (p.o) at a dose of 60 mg/kg on days 1 to 5 weekly for 3 weeks; and (d) X-ray radiation plus embelin. Embelin was dosed 1 h before irradiation. Tumor size and body weight were measured twice a week using a venier caliper. Tumor volume was calculated using the formula: (length × width2)/2. Tumor doubling time was evaluated by monitoring the first day when the tumor volume was twice baseline volume, and characterized by Kaplan-Meier estimate [9, 18, 30, 35]. All the experiments were done according to the protocols approved by University of Michigan Guidelines for Use and Care of Animals.
Histological analysis
Tumor samples were fixed in 4% paraformaldehyde at 4°C overnight for paraffin embedding. Other than hematoxylin and eosin (H&E) staining, cell proliferation was tested by Ki-67 and proliferating cell nuclear antigen (PCNA) staining, apoptosis was detected by Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) staining using an in situ Apop-Tag kit, and tumor angiogenesis was analyzed by anti-mouse CD31 staining for detection of the intratumoral microvessel density [9, 35].
Statistical analysis
Two-tailed Student's t-test was employed for analyzing both in vitro and in vivo data. Two-way ANOVA was applied to analyze tumor growth. Mantel-Cox (Log-Rank) test was used for survival analysis. All analysis was performed by GraphPad Prism 5.0. A threshold of P < 0.05 was defined as statistically significant.
Results
Embelin combined with IR inhibits cell growth and induces cell death
We have previously reported embelin inhibits prostate cancer cell growth in vitro [22]. Previous reports have shown that small-molecule treatments may help sensitize cancer cells to radiation therapy [9]. To investigate if embelin could work in this manner, we first wanted to monitor if it had any effects on cell proliferation and cell death. We used the human prostate cancer PC-3 cells. Here, we confirm that embelin alone dose-dependently inhibited PC-3 cell growth (Figure 1A) and induced PC-3 cell death (Figure 1B and Table 1). From day 5 post-treatment, 10 μM of embelin exerted significant growth control (>45%), whereas 20 μM of embelin showed drastic cell-killing effect (>60%). These data suggest that embelin is a cytostatic rather than cytotoxic agent since it favors suppressing cell proliferation, with observed cytotoxicity only at higher doses and a longer exposure time. Moreover, embelin plus IR achieved significantly more cell growth inhibition (P<0.001) and cell death (P<0.05) as compared with either treatment alone after day 2 (Figure 1 and Table 1). These data suggest that embelin in combination with ionizing radiation potently reduced cancer cell proliferation as well as enhanced cancer cell death.
Figure 1.

Effect of embelin (Em) combined with IR on PC-3 cell proliferation and cell death. A, Em alone or combined with IR inhibited cell growth. PC-3 cells were treated with 10 μM and 20 μM of Em, 4 Gy irradiation, or in combination. Em remained continuous exposure to the cells throughout the experiments. Attached cells were harvested and counted every day for 7 days. Data are expressed as the ratio of cell number with treatment to the untreated control. Data shown are means ± SD (n=6). B, Em alone or combined with IR promoted cell death. Both attached and floating cells were harvested for trypan blue staining. Cell viability (%) was quantified by dividing the number of unstained cells to total cells. Data shown are means ± SD (n=6). C, cell density was monitored 72 h after treatment. Typical pictures are shown in a phase contrast mode. Original magnification, × 100.
Table 1.
Effect of embelin with IR on PC3 cell growth and cell death
Embelin combined with IR induces cell cycle arrest
As embelin plus IR exhibited potent anti-proliferation effect in vitro, we further tested the cell cycle distribution in the treated cells. While IR induced G2/M arrest, embelin alone triggered S-phase arrest dose-dependently (Figure 2A and Figure 3A). After 72 h, S-phase cell population showed 2.1±0.8 folds at 10 μM and 3.6±1.1 folds at 20 μM, respectively. In addition, embelin plus IR induced both S and G2/M arrest comparing with control (P<0.05) (Figure 2B). This result suggests that embelin alone, as well as combined with IR, induced cell cycle arrest in PC-3 cells.
Figure 2.
Cell cycle analysis of PC-3 after Em and IR treatment. A, cells were treated with Em (20 μM), IR (4 Gy) or in combination for 24 h and 72 h, respectively. Data represent one of three independent experiments. B, cell population (%) in each phase was quantified. Columns, mean of three independent experiments; bars, SD (n=3). *, P< 0.05.
Figure 3.
Dose effect of Em on radiosensitization. A, Em induced S phase arrest in PC-3 dose-dependently. Cells were treated with various doses of Em for 72 h. Cell cycle was analyzed and cell population in each phase is numerically depicted. Gemcitabine (Gem) (Lilly, Indianapolis, Indiana) was used as a positive control, treating cells at a dose of 1 μM for 24 h. Data represent one of two independent experiments. B, clonogenic survival curve of cells pretreated with indicated dose of Em, as described in A. Data are shown as mean ± SD (n=3). C, clonogenic survival curve of cells pretreated with 1 μM and 2 μM of Gem for 24 h. Data are shown as mean ± SD (n=3).
Embelin combined with IR triggers caspase-independent apoptosis
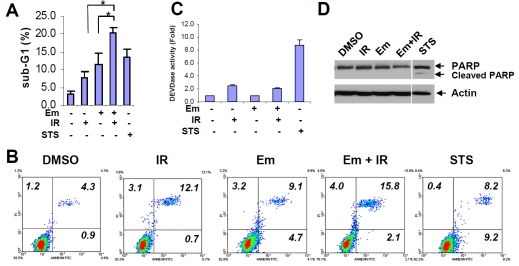
As mentioned above, embelin alone or plus IR exhibited cytotoxic effect (Figure 1B). We then determined whether apoptosis is involved in such cytotoxicity. Embelin plus IR induced significantly more hypodiploid sub-G1 population than either treatment alone (P<0.05), consistent with the known apoptosis-inducer staurosporin (Figure 4A). However, the combination treatment barely increased the Annexin V+ PI-apoptotic cell population (Figure 4B). Instead, Annexin V+ PI+ population was enhanced by the combination treatment when compared with either treatment alone (Figure 4B), suggesting the activation of late apoptosis. Interestingly, neither single nor combined treatment triggered caspase-3 activation (Figure 4C) and PARP cleavage (Figure 4D), which was in contrast with staurosporin that induced caspase-dependent apoptosis. We have also carried out autophagy analysis and found that embelin did not induce significant autophagy in the conditions tested (Figure 5). Furthermore, neither treatment decreases expression of multiple anti-apoptotic proteins including Bcl-2, Bcl-xL, XIAP and cIAP-1, but not Mcl-1, (Figure 6). These data demonstrate that embelin combined with IR augments cell apoptosis that is caspase-independent.
Figure 4.
Combination effect of Em and IR on cell death in vitro. A, PC-3 cells were treated with Em (20 μM), IR (4 Gy) or in combination for 72 h. Cells treated with staurosporin (STS, 1 μM) for 24 h was used as a positive control. Sub-G1 cell population was the hypodiploid DNA contents from the cell cycle diagram by flow cytometry analysis. Columns, mean of two independent experiments; bars, SD (n=2). *, P< 0.05. B, cells were treated with Em (20 μM), IR (4 Gy) or in combination for 48 h, or with STS (1 μM) for 4 h. Cells were stained with Annexin V and PI and analyzed by flow cytometry. Cell population in each indicated quadrant is numerically depicted. Data represent one of two independent experiments. C, cells were treated as described in A, and enzymatic activity of caspase-3 was determined by fluorescence. The activity of the control was defined as “1”. Columns, mean; bars, SD (n=3). D, cells were treated as described in A. Whole cell lysates (50 μg) were analyzed by Western blot and visualized by anti-PARP antibody. Actin is used as a loading control.
Figure 5.
Embelin does not induce autophagy in prostate cancer cells. A, The cells were treated with 5, 10 and 20μM Embelin or DMSO for 16h, then stained with acridine orange. Quantitative data was shown as percent of cells with orange punctuates punctuate (n=5). B, Cells were transfected by LC3-GFP plasmid, treated with 5, 10 and 20μM Embelin or DMSO for 24h, and then analyzed under a fluorescent microscope. Quantitative data was shown as percent of cells with punctuate LC3-GFP (200 cells, n=5).
Figure 6.

Expression of anti-apoptotic proteins after Em and IR treatment in vitro. Samples in Figure 3D were probed with antibodies against Bcl-2 (Santa Cruz), Bcl-xL (BD), Mcl-1 (Santa Cruz), XIAP (BD) and cIAP-1 (Santa Cruz).
Embelin radiosensitizes PC-3 cells via cell cycle arrest at S-phase
We next evaluated radiosensitization potential of embelin in prostate cancer cells. We performed series of clonogenic assays using different schedules to assess the therapeutic effects. The PC-3 cells offered an outstanding opportunity to study this because of the ideal size and morphology of its formed colonies and relative plating efficiency when treated with embelin (Figure 7A and C). Colony formation assay showed that embelin alone minimally inhibited PC-3 colony growth below 10 mM (Figure 7A), thus a concentration of 10 mM was used for later experiments. After pretreatment for 48 h, embelin sensitized radiation-induced clonogenic cell growth inhibition in a dose-dependent mode (Figure 3B), similar to the well-documented radiosensitizer gemcitabine that also induces S arrest (Figure 3C).
Figure 7.
Radiosensitization effect of Em. A, PC-3 cells (200 cell/well) were treated with indicated dose of Em for 72 h and incubated in the fresh medium without Em for another 11 days. Plating efficiency is expressed as a percentage of colonies in treated group by untreated control. Bars, SD (n=3). B, cells were pretreated with 10 μM of Em for 24 h, 48 h, and 72 h, respectively, and irradiated. Cells were then seeded at desired densities according to different doses of IR without Em. The survival curves were plotted using a standard linear-quadratic model. ER, enhancement ratio. Bars, SD (n=3). Data represent one of three independent experiments. C, stained colonies in a typical well by the treatment in B are shown. D, cells were exposed to Em and IR with two different sequences. Em-IR-DMSO: cells were pretreated with 10 μM of Em for 72 h and irradiated. Right after radiation, cells were seeded for clonogenic assay without Em (with DMSO control). Em-IR-Em: cells were pretreated with 10 μM of Em for 1 h and irradiated. Cells were incubated with Em (10 μM) for another 24 h post-irradation and seeded. Clonogenic survival analysis was performed as described in B. Bars, SD (n=3). Data represent one of two independent experiments.
We have shown that embelin alone induces S-phase arrest and exerts enhanced apoptosis when combined with IR. We also evaluated whether S arrest is correlated with radiosensitivity by embelin. With increased pretreatment time, embelin facilitated IR-induced clonogenic cell growth inhibition, as per enhancement ratio (Figure 7B). When cells were pretreated with embelin for 72 h, maximum effect was observed even at a lower IR dose of 2 Gy (Figure 7B and C). The results demonstrate that embelin radiosensitizes PC-3 cells in a dose- and time -dependent manner, and is correlated with embelin-induced S-phase arrest. To explore whether radiation-induced DNA repair is involved in embelin-mediated radiosensitization, we compared two different treatment schedules. Cells pretreated with embelin for 1 h before radiation followed by 24 h post-radiation showed less clonogenic survival compared with 72 h pretreatment before radiation (P<0.01, Figure 7D), suggesting that cell cycle arrest, but not DNA repair, is a major mechanism involved in embelin-mediated radiosensitization. This is further confirmed by the immunostaining of nuclear gH2AX that embelin indeed hardly impaired IR-induced DNA damage repair (Figure 8). Overall, these results indicate that embelin sensitizes PC-3 cells to X-ray radiation is associated with the S-phase arrest.
Figure 8.
Em hardly impaired IR-induced DNA damage repair. Immunostaining of nuclear gH2AX was conducted as described previously [9]. gH2AX foci are shown as green fluorescent dots counterstained with DAPI. Original magnification, ×400. Fifty cells were selected randomly for foci quantification (×400). Data are shown as mean ± SD (n=50).
Embelin potentiates IR-induced tumor regression in PC-3 xenografts
To evaluate the radiosensitization potential of embelin in vivo, we employed the PC-3 xenograft tumor model as we previously described [9]. As shown in Figure 9A, the combination therapy inhibited tumor growth more potently than either treatment alone (P< 0.001, Two-way ANOVA). At the end of treatment (day 18), median tumor size in the combination group was 60% of that in IR alone group (P< 0.001), and 18% of that in control group (P<0.001) (Figure 9B). Notably, the mice body weight loss was less than 15% during the treatment, and rapidly recovered right after the cessation of treatment, suggesting that the accumulated systemic toxicity by the treatment is transient and reversible (Figure 9C). When monitoring tumor growth of each mouse throughout the experiment, the combination treatment significantly increased tumor-doubling time, 5∼6-folds longer than either treatment alone (P<0.001 by Mantel-Cox) (Figure 9D), indicating an improvement of the overall survival. Together, these data demonstrate that embelin plus IR resulted in prostate tumor growth inhibition and prolonged survival with minor systemic toxicity.
Figure 9.
Combinatorial effect of Em and IR on PC-3 xenograft. Nude mice bearing PC-3 tumors were treated with Em, IR, or in combination, as described in Materials and Methods. A, PC-3 tumor growth curve with desired treatments. Data are shown as mean ± SEM (n ≥ 14). ***, P<0.001 vs. IR (two-way ANOVA). B, tumor suppression (T/C) was calculated as the ratio of the median tumor volume in the treated group compared with untreated control when the last dose of Em was applied (day 18). Columns, mean; bars, SEM. Tumor number (n) in each group is shown. ***, P<0.001. C, relative body weight was expressed as the ratio of body weight at various times after treatment compared with the first day of treatment (day 0). Data are mean ± SEM (n=9). Only minus SD bars are shown to simplify the figure. A threshold of “0.85” is set as a solid line to evaluate body weight loss. D, Kaplan-Meier analysis on tumor volume doubling time. The median tumor volume doubling time of each group is depicted numerically. ***, P<0.001 vs. IR (Mantel-Cox test). Tumor numbers (n) in each group are described in B.
Embelin combined with IR reduces cell proliferation and angiogenesis, and induces apoptosis in vivo
To further confirm the anti-tumor efficacy by combination treatment, tumor samples were processed for both histological and biological analyses. Embelin plus IR group showed fewer Ki67- and PCNA-positive cells, more TUNEL-positive cells and fewer CD31-positive microvessels than either treatment alone (Figure 10A). Such difference was significant after quantification between combination and IR alone group (P<0.001 for Ki67 and PCNA; P<0.05 for TUNEL and CD31) (Figure 10B). Consistent with TUNEL staining, the combination group, but not single treatments, induced PARP cleavage in the tumor tissue (Figure 10C). Moreover, embelin plus IR dramatically decreased XIAP expression than either embelin or IR alone (Figure 10C). These data demonstrate that combining embelin with radiation potently suppresses proliferation, triggers apoptosis and reduces angiogenesis in xenograft tumors in vivo.
Figure 10.
Immunohistochemical and molecular analysis of tumor tissues. A, tumor tissues were processed by anti-Ki67, anti-PCNA, TUNEL, anti-mouse CD31 and H&E staining. Original magnification, ×400. B, positive stained cells or microvessels were quantified by counting 8 random fields. Data are presented as a percentage (for Ki67 and PCNA) or an absolute counting (for TUNEL and CD31) per field (Original magnification, ×200). Columns, mean; bars, SEM (n=8). *, P< 0.05; ***, P< 0.001 vs. IR. C, whole cell lysates (50 μg) of tumor tissues were probed with anti-PARP and anti-XIAP antibody. GAPDH was used as a loading control.
Discussion
In this study, we found that embelin enhances therapeutic efficacy of radiation therapy in human prostate cancer PC-3 both in vitro and in vivo. Embelin combined with X-ray radiation achieved increased tumor growth inhibition and apoptosis, which is accompanied by the embelin-induced cell cycle arrest in S-phase. Moreover, the combination treatment increased caspase-independent apoptosis but not autophagy. Clonogenic survival assay showed that S-phase arrest was required and may serve as a major mechanism for embelin-mediated radiosensitization. In vivo, embelin significantly enhanced tumor growth inhibition and time to progression by radiation therapy in PC-3 xenografts with minimal systemic toxicity. Immunohistochemistry studies displayed that embelin plus IR significantly inhibited cell proliferation and microvessel density, and induced apoptosis in tumors as compared with either treatment alone. Together, these data demonstrate the tangible effect of embelin in combination with radiation and provide a potential mechanism of embelin-mediated radiosensitization. To our knowledge, this is the first report on the combinational application of embelin with ionizing radiation for human cancer therapy.
Embelin was identified as the first natural XIAP antagonist by our group and collaborators [22], together with series of related compounds and derivatives [20, 39, 40]. Since XIAP blocks apoptosis by neutralizing caspase activity [41, 42], it is not surprising that embelin exerts pro-apoptotic effect in various cancer cell types [22, 23, 27]. However in our study, a single use of embelin is not sufficient to induce apoptosis, although dramatic growth inhibition is observed. This result suggests that embelin functions as a cytostatic instead of cytotoxic agent that halts cell proliferation without inducing apoptosis or autophagy. We have previously shown that embelin potently induced apoptosis in DU-145 cells, another prostate cancer cell line [22].
Albeit its less apoptotic-inducing effect alone, embelin can potentiate radiation therapy, as evidenced by the results that embelin was able to enhance the tumor regression by ionizing radiation in the PC-3 model. Interestingly, we find that in vitro, the combination treatment leads to enhanced apoptosis that is not correlated with caspase-3 activation, suggesting that apoptosis is caspase-independent; while in vivo in tumor tissues, the combination therapy indeed triggers caspase-dependent apoptosis. Moreover, XIAP expression is reduced in tumors while remains unchanged in cells with either treatment, suggesting that XIAP may likely be interfered only in the presence of tumor micro-environment. This postulation is further confirmed by the fact that intratumoral angiogenesis is reduced along with augmented apoptosis. The discrepancy of the mode of cell death between the in vitro and in vivo setting remains unclear. Nevertheless, the growth inhibition is consistent in cells and in animal model, demonstrating the anti-tumor efficacy generated by embelin plus IR.
Combination of radiation and other agents that achieve radiosensitizing potential has become important interventions for the patients with solid tumors, including the locally advanced, castration resistant prostate cancer [43]. Despite the fact that cells in G2/M phase are the most sensitive to radiation, S-phase arrest is indispensable for drug-mediated radiosensitization in some combination treatments, although the S-phase cells are supposed to be relatively resistant to radiation [11]. For example, it is well documented that gemcitabine-mediated radiosensitization is dependent on S-phase arrest induced by the drug, and such finding implies the importance of both depletion of phosphory-lated deoxynucleotides and redistribution of cells through S-phase [44]. Also, radiosensitization effect of 5-fluorouracil (5-FU) is only achieved when 5-FU is present before radiation, suggesting that S-phase arrest is necessary for its radiosensitivity [45]. These evidence demonstrate that the improvement of radiotherapy combinations with a proper scheduling will help to rationally develop new radiosensitizing strategies.
Consistent with the behaviors of those chemo-therapeutic agents, our study shows that embelin-induced S-phase arrest seems to play a role in its radiosensitizing activity, suggesting that embelin may function as an S-phase radiosensitizer. Also, the dependence of S-phase progression for radiosensitizing potential and acquired apoptosis by embelin precludes a functional role of p53, as suggested in other studies [46]. Our data also reveal that cell cycle modulation but not DNA damage repair seems to be a predominant mechanism involved in embelin-mediated radiosensitization. Moreover, enhanced apoptosis by combination therapy is less likely to be a consequence of radiosensitization as the schedule of apoptosis and clonogenic survival is different. Overall, these two independent outcomes support the therapeutic potential of combining embelin and radiation therapy. In addition, it is worth noting that embelin seems to induce S—phase arrest only in PC-3 cells but not in other cancer cells (data not shown and ref. [23]), indicating that the effect of cell cycle redistribution by embelin is cellular context-dependent. As such, our finding at least provides an example of certain cancer types that may respond to embelin and radiotherapy as long as S-phase arrest can be achieved by embelin in those settings. We are currently delineating the action mode of S-phase arrest to interpret the potential mechanism of embelin-mediated radiosensitization.
In conclusion, our results demonstrate that embelin significantly augments the anti-tumor activity of ionizing radiation both in vitro and in vivo, therefore reveals an encouraging observation that corroborating the natural small-molecule anti-apoptotic agent and conventional radiation therapy will result in enhanced efficacy. The current study supports that embelin represents a promising adjuvant intervention that can improve the outcome of radiotherapy for hormone refractory prostate cancer that are resistant to radiation.
Acknowledgments
Grant support: This study was supported in part by Department of Defense Prostate Cancer Research Program W81XWH-06-1-0010, NIH grants R01 CA121830(S1) and CA134655 (to L. X.), and by NIH through a University of Michigan Cancer Center Support Grant (5 P30 CA46592). J. D. is a University of Michigan Undergraduate Research Opportunity Program (UROP) student.
We wish to thank Susan Harris for help with the manuscript; the University of Michigan Comprehensive Cancer Center (UMCCC) Histology Core for the immunohistology study; the UMCCC Flow Cytometry Core for flow cytometry analysis, and UMCCC Unit of Laboratory Animal Medicine (ULAM) for help with animal experiments.
References
- 1.Kyprianou N. Apoptosis: therapeutic significance in the treatment of androgen-dependent and androgen-independent prostate cancer. World J Urol. 1994;12:299–303. doi: 10.1007/BF00184107. [DOI] [PubMed] [Google Scholar]
- 2.Costello AJ, Bolton DM, Ellis D, Crowe H. Histopathological changes in human prostatic adenoma following neodymium:YAG laser ablation therapy. J Urol. 1994;152:1526–1529. doi: 10.1016/s0022-5347(17)32461-8. [DOI] [PubMed] [Google Scholar]
- 3.Palayoor ST, Bump EA, Teicher BA, Coleman CN. Apoptosis and clonogenic cell death in PC3 human prostate cancer cells after treatment with gamma radiation and suramin. Radiat Res. 1997;148:105–114. [PubMed] [Google Scholar]
- 4.Gjertsen BT, Logothetis CJ, McDonnell TJ. Molecular regulation of cell death and therapeutic strategies for cell death induction in prostate carcinoma. Cancer Metastasis Rev. 1998;17:345–351. doi: 10.1023/a:1006170332301. [DOI] [PubMed] [Google Scholar]
- 5.DiPaola RS, Patel J, Rafi MM. Targeting apoptosis in prostate cancer. Hematol Oncol Clin North Am. 2001;15:509–524. doi: 10.1016/s0889-8588(05)70229-x. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Yang D, Wang S, Tang W, Liu M, Davis M, Chen J, Rae JM, Lawrence T, Lippman ME. (-)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4:197–205. [PubMed] [Google Scholar]
- 7.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 8.Wu CT, Chen WC, Liao SK, Hsu CL, Lee KD, Chen MF. The radiation response of hormone-resistant prostate cancer induced by long-term hormone therapy. Endocr Relat Cancer. 2007;14:633–643. doi: 10.1677/ERC-07-0073. [DOI] [PubMed] [Google Scholar]
- 9.Dai Y, DeSano JT, Meng Y, Ji Q, Ljungman M, Lawrence TS, Xu L. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:1217–1225. doi: 10.1016/j.ijrobp.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oehler C, Dickinson DJ, Broggini-Tenzer A, Hofstetter B, Hollenstein A, Riesterer O, Vuong V, Pruschy M. Current concepts for the combined treatment modality of ionizing radiation with anticancer agents. Curr Pharm Des. 2007;13:519–535. doi: 10.2174/138161207780162935. [DOI] [PubMed] [Google Scholar]
- 11.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Formenti SC, Symmans WF, Volm M, Skinner K, Cohen D, Spicer D, Danenberg PV. Concurrent paclitaxel and radiation therapy for breast cancer. Semin Radiat Oncol. 1999;9:34–42. [PubMed] [Google Scholar]
- 13.Hu Q, Hill RP. Radiosensitivity, apoptosis and repair of DNA double-strand breaks in radiation-sensitive Chinese hamster ovary cell mutants treated at different dose rates. Radiat Res. 1996;146:636–645. [PubMed] [Google Scholar]
- 14.Wang JY. DNA damage and apoptosis. Cell Death Differ. 2001;8:1047–1048. doi: 10.1038/sj.cdd.4400938. [DOI] [PubMed] [Google Scholar]
- 15.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 16.Meyn RE, Stephens LC, Milas L. Programmed cell death and radioresistance. Cancer Metastasis Rev. 1996;15:119–131. doi: 10.1007/BF00049491. [DOI] [PubMed] [Google Scholar]
- 17.Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, Ji M, Pienta K, Lawrence T, Xu L. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther. 2008;7:2192–2202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai Y, Liu ML, Tang WH, Desano J, Burstein E, Davis M, Pienta KJ, Lawrence TS, Xu L. Molecularly Targeted Radiosensitization of Human Prostate Cancer by Modulating Inhibitor of Apoptosis. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-0188. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Liu M, Tomita Y, Pan H, Yoshioka Y, Krajewski K, Roller PP, Wang S. Structure-based design of potent, conformationally constrained Smac mimetics. J Am Chem Soc. 2004;126:16686–16687. doi: 10.1021/ja047438+. [DOI] [PubMed] [Google Scholar]
- 21.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated an-tiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71:209–219. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 22.Nikolovska-Coleska Z, Xu L, Hu Z, Tomita Y, Li P, Roller PP, Wang R, Fang X, Guo R, Zhang M, Lippman ME, Yang D, Wang S. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2004;47:2430–2440. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 23.Xu M, Cui J, Fu H, Proksch P, Lin W, Li M. Embelin derivatives and their anticancer activity through microtubule disassembly. Planta Med. 2005;71:944–948. doi: 10.1055/s-2005-871250. [DOI] [PubMed] [Google Scholar]
- 24.Podolak I, Galanty A, Janeczko Z. Cytotoxic activity of embelin from Lysimachia punctata. Fitoterapia. 2005;76:333–335. doi: 10.1016/j.fitote.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Joy B, Sivadasan R, Abraham TE, John M, Sobhan PK, Seervi M, Santhoshkumar TR. Lysosomal destabilization and cathepsin B contributes for cytochrome c release and caspase activation in embelin-induced apoptosis. Mol Carcinog. 2009 doi: 10.1002/mc.20599. [DOI] [PubMed] [Google Scholar]
- 26.Chitra M, Sukumar E, Suja V, Devi CS. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 1994;40:109–113. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- 27.Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma J, Zou B, Gu Q, Wang J, Pang R, Lan HY, Wong BC. Peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of Embelin on colon carcinogenesis. Cancer Res. 2009;69:4776–4783. doi: 10.1158/0008-5472.CAN-08-4754. [DOI] [PubMed] [Google Scholar]
- 28.Siegelin MD, Gaiser T, Siegelin Y. The XIAP inhibitor Embelin enhances TRAIL-mediated apoptosis in malignant glioma cells by down-regulation of the short isoform of FLIP. Neurochem Int. 2009;55:423–430. doi: 10.1016/j.neuint.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Mori T, Doi R, Kida A, Nagai K, Kami K, Ito D, Toyoda E, Kawaguchi Y, Uemoto S. Effect of the XIAP inhibitor Embelin on TRAIL-induced apoptosis of pancreatic cancer cells. J Surg Res. 2007;142:281–286. doi: 10.1016/j.jss.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 30.Dai Y, Liu M, Tang W, Li Y, Lian J, Lawrence TS, Xu L. A Smacmimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB. BMC Cancer. 2009;9:392. doi: 10.1186/1471-2407-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, Li M, Qu Y, Tang W, Zheng Y, Lian J, Ji M, Xu L. Design and synthesis of novel Gefitinib analogues with improved anti-tumor activity. Bioorg Med Chem. 2010;18:3812–3822. doi: 10.1016/j.bmc.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, Xiang D, Ji M, Lawrence TS, Xu L. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2010 doi: 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian J, Karnak D, Xu L. The Bcl-2-Beclin 1 interaction in (-)-gossypol-induced autophagy versus apoptosis in prostate cancer cells. Autophagy. 2010;6 doi: 10.4161/auto.6.8.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y, Liu M, Tang W, Desano J, Burstein E, Davis M, Pienta K, Lawrence T, Xu L. Molecularly targeted radiosensitization of human prostate cancer by modulating inhibitor of apoptosis. Clin Cancer Res. 2008;14:7701–7710. doi: 10.1158/1078-0432.CCR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu L, Frederik P, Pirollo KF, Tang WH, Rait A, Xiang LM, Huang W, Cruz I, Yin Y, Chang EH. Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum Gene Ther. 2002;13:469–481. doi: 10.1089/10430340252792594. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ, Cruz I, Xiang LM, Pirollo KF, Chang EH. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol Cancer Ther. 2002;1:337–346. [PubMed] [Google Scholar]
- 38.Lawrence TS, Davis MA, Hough A, Rehemtulla A. The role of apoptosis in 2',2'-difluoro-2'-deoxycytidine (gemcitabine)-mediated radiosensitization. Clin Cancer Res. 2001;7:314–319. [PubMed] [Google Scholar]
- 39.Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Tomita Y, Krajewski K, Roller PP, Wang S. Structure-based design, synthesis, and evaluation of conformationally constrained mimetics of the second mitochondria-derived activator of caspase that target the X-linked inhibitor of apoptosis protein/caspase-9 interaction site. J Med Chem. 2004;47:4147–4150. doi: 10.1021/jm0499108. [DOI] [PubMed] [Google Scholar]
- 40.Schimmer AD, Dalili S, Batey RA, Riedl SJ. Targeting XIAP for the treatment of malignancy. Cell Death Differ. 2006;13:179–188. doi: 10.1038/sj.cdd.4401826. [DOI] [PubMed] [Google Scholar]
- 41.Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 42.Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 43.Karnak D, Xu L. Chemosensitization of prostate cancer by modulating Bcl-2 family proteins. Curr Drug Targets. 2010;11:699–707. doi: 10.2174/138945010791170888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence TS, Davis MA, Chang EY, Canman CE, Maybaum J, Radany EH. Lack of dependence of 5-fluorodeoxyuridine-mediated radiosensitization on cytotoxicity. Radiat Res. 1995;143:281–285. [PubMed] [Google Scholar]
- 46.Zellars RC, Naida JD, Davis MA, Lawrence TS. Effect of p53 overexpression on radiation sensitivity of human colon cancer cells. Radiat Oncol Investig. 1997;5:43–49. doi: 10.1002/(SICI)1520-6823(1997)5:2<43::AID-ROI1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]