Abstract
Although the protective mechanisms of delayed ischemic preconditioning have received extensive studies, few have addressed the mechanisms associated with rapid ischemic postconditioning. We investigated whether ischemic tolerance induced by rapid preconditioning is regulated by the Akt survival signaling pathway. Stroke was generated by permanent occlusion of the left distal middle cerebral artery (MCA) plus 30 min or 1 h occlusion of the bilateral common carotid artery (CCA) in male rats. Rapid preconditioning performed 1h before stroke onset reduced infarct size by 69% in rats with 30 min CCA occlusion, but by only 19% with 1 h occlusion. After control ischemia with 30 min CCA occlusion, Western Blot showed that P-Akt was transiently increased while Akt kinase assay showed that Akt activity was decreased. Although preconditioning did not change P-Akt levels at 1h and 5h compared with control ischemia, it attenuated reduction in Akt activity at 5h in the penumbra. However, preconditioning did not change the levels of P-PDK1, P-PTEN, and P-GSK3β in the Akt pathway, all of which were decreased after stroke. At last, the PI3K kinase inhibitor, LY294002, completely reversed the protection from ischemic preconditioning. In conclusion, Akt contributes to the protection of rapid preconditionin against stroke.
Keywords: rapid preconditioning, ischemic tolerance, cerebral ischemia, focal ischemia, neuroprotection, Akt
Introduction
Ischemic preconditioning is considered to be the most robust endogenous form of neuroprotection [5,11,14,15]. Two protective time windows for preconditioning have been identified: rapid preconditioning performed 30 min to 3 hour, and delayed ischemic preconditioning, performed one to several days before the onset of the subsequent severe ischemia [5,9,11,22–24]. The protective effects and mechanisms of delayed preconditioning have received extensive studies; however, relatively few have studied rapid preconditioning [17,18,24,26], and its protective mechanisms.
Whether the Akt/PKB survival signaling pathway contributes to the protective effects of delayed preconditioning against global cerebral ischemia has been controversial (reviewed by [31], and its role in the protective effects of rapid preconditioning against focal ischemia is unknown. Akt activity is deemed to be upregulated by phosphorylation at ser-473 (p-Akt) and thr-308 [6,7,16]. Akt is phosphorylated via several upstream molecules, such as PDK 1 and PTEN. PDK1 directly or indirectly phosphorylates Akt while PTEN dephosphorylates and inactivates Akt. Upon activation, Akt blocks apoptosis by phosphorylating its downstream molecules, such as GSK3β; dephosphorylated or activated GSK3β leads to apoptosis by phosphorylating and degrading β-catenin, a critical transcript factor for cell survival [6,7,16]. Activated GSK3 β causes β-catenin phosphorylation resulting in its degradation and apoptosis [21].
We have previously reported the role of the Akt pathway in the protective effects of moderate hypothermia [32]and ischemic postconditioning [8]. We found that both hypothermia and postconditioning upregulate Akt activity when examined by an Akt kinase assay, and inhibition of Akt activity abolishes their protection. However, Akt activity does not always correlate with Akt phosphorylation. In addition, phosphorylation of other molecules in the Akt pathway does not always change in the same direction with that of Akt [8,32]. Furthermore, changes in total protein of Akt and other proteins in the Akt pathway are largely ignored in most previous studies. How neuroprotectants affect total protein levels in the Akt pathway, regardless phosphorylation, has not been studied. In this study, not only did we examine the effect of rapid preconditioning on phosphorylation of proteins in the Akt pathway and Akt activity, but also analyzed its effect on total protein levels in the Akt pathways.
Materials and Methods
Focal Cerebral Ischemia
Experimental protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care. Focal ischemia was generated as previously described [4,33,34]. Anesthesia was induced with 5% isoflurane maintained with 2–3% isoflurane during surgery in male Sprague-Dawley rats (350–390g). Core temperature was monitored with a rectal probe and maintained at 37°C throughout the experiment. The distal middle cerebral artery (MCA) was occluded permanently above the rhinal fissure while the bilateral common carotid arteries (CCA) were transiently occluded for 30 or 60 min. For Western blot, in vitro kinase assay and drug test, test ischemia was induced by 30 min bilateral CCA occlusion plus permanent MCA occlusion. To monitor blood gas, a catheter was inserted into the left femoral artery. pO2 was controlled between 120–150, pCO2 between 38–50, and pH between 7.3–7.4.
Ischemic preconditioning
Rapid ischemic preconditioning was performed by transiently occluding the left distal MCA for 15 min using a micro-clip and test ischemia was induced 1h later; test ischemia included 30 or 60 min of transient bilateral CCA occlusion combined with permanent MCA occlusion.
General histology and infarct size measurement
For acute infarct size measurement, rats were perfused transcardially with normal saline at two days after ischemia. Brains were sectioned into 5 coronal blocks rostral (level 1) to caudal (level 5) and stained with 1% TTC solution. Infarcts were measured at all 5 levels as previously described [9,25,32]. The percentage of infarct cortex was calculated according to the formula:
(contralateral cortex − (Ipsilateral cortex − infarct cortex)) / contralateral cortex X 100%.
Drug delivery
To study whether Akt inhibition abolishes the protection of rapid preconditioning, 10 µl of the PI3 kinase inhibitor LY294002 (10 mM) or vehicle was infused into the ventricular space ipsilateral to the ischemia (from Bregma: AP= −0.92 mm, ML=1.5mm, DV=3.5mm), as described [32]. The reason we chose intaraventricular administration of this drug, but not intravenously or intraperitoneally, is that the drug is able to directly penetrate into the brain issue, thus avoiding any potential complications of passing blood brain barrier. The drug was dissolved in DMSO a final concentration of 10 mM. This dose has been shown to inhibit Akt kinase activity in our previous study [8]. Four groups were studied. Two groups, control ischemia and preconditioning plus test ischemia, received injection of LY294002. One hour after preconditioning ischemia, the drug was injected before test ischemia, which was generated by permanently occluding the left dMCA combined with 30 minutes of bilateral CCA occlusion. Another two groups, a control ischemia group and a preconditioning group, were treated with the same amount of vehicle, 10ul of DMSO. Infarct size was measured 2 d after stroke as described above.
Western blots
To detect the levels of various proteins in the Akt pathway, rats survived for 1, 5 and 24 h after CCA release were sacrificed by overdose of isoflurane and transcardially perfused with cold PBS. Rat brains were removed and tissue corresponding to the ischemic penumbra and core was dissected for Western blot (Fig 1.). The ischemic penumbra was defined as the tissue saved by preconditioning 2 days post-stroke, and the corresponding region from the control ischemic brain was dissected for comparison. The fresh brain tissue was used for preparation of whole cell protein extraction as previously described [20,32]. Briefly, brain tissues were cut into small pieces and homogenized in a glass homogenizer using 7 volumes of the cold suspension buffer. The homogenate was centrifuged at 10,000g for 20 min, and the supernatant was taken for protein detection. Western blot was performed as previously described [32–34]. Twenty micrograms of protein in each lane was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 4–15% Ready Gel (BIO-RAD Laboratories, Cat# L050505A2) for 1.5 hours. Protein bands were transferred from the gel to polyvinylidinene fluoride (Millipore, Bedford, MA, U.S.A.) membranes for 1 hour.
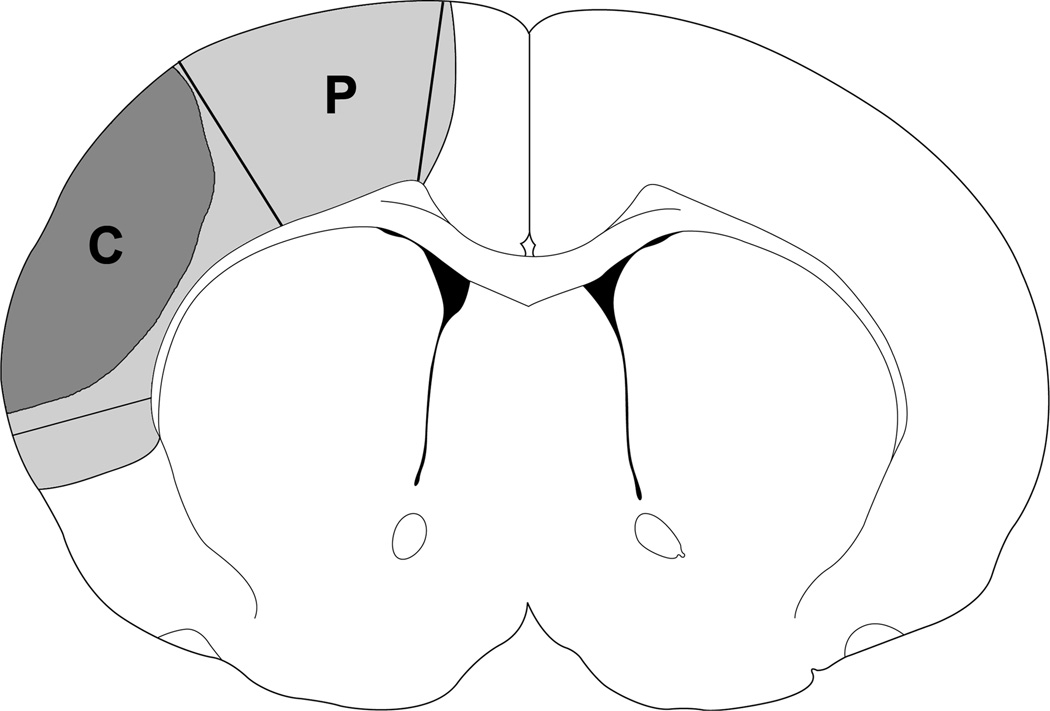
Fig. 1.
A diagram indicates that tissues dissected correspond to the ischemic penumbra and core. Region P plus region C represents ischemic injury in a rat with control ischemia; region P is spared by rapid preconditioning, which is defined as the penumbra, and region C is defined as the ischemic core. These regions were dissected for Western blotting. The corresponding non-ischemic cortex from sham animal without ischemia was dissected for comparison.
For phosphorylated or total protein of Akt, PTEN, PDK1, GSK 3 β, primary antibodies were incubated for 1 hour followed by a horseradish peroxidase-conjugated secondary anti-rabbit antibody (1:1000, Cell signaling) for 1 hour, incubated with ECL plus solution (GE Healthcare, cat. RPN2132) for 5 min. The membranes were scanned using Typhoon trio with the following settings - acquisition mode [8]: Fluorescence; Emission Filter: 520 BP 40 CY2, ECL+, Blue FAM; Laser: ECL+ Excitation; PMT: 600; Pixel size: 100 microns. After scanning without stripping, the same membranes were incubated with β-actin (Sigma-Aldrich, cat. A3854, 1:20000) for 30 min at room temperature, and then incubated with Alexa Fluor®647 donkey anti-mouse IgG(H+L) conjugate secondary antibody (Molecular Probes, cat. A31571, 1:5000) for 1h at room temperature while shielded from light. The membrane was scanned using the same Typhoon trio with the setting of Acquisition mode: Fluorescence; Emission Filter: 670 BP 30 CY5; PMT: 600; Laser: Red (633); Pixel size: 100 microns.
For detecting β-catenin and β-actin, the membranes were incubated in a mixture of primary antibodies consisting of β-catenin (1:1000, mouse mAb-Calbiochem, cat. 219357) and β-actin (1:20000, rabbit, Bethyl, cat. A300-491A) overnight at 4°C with gentle agitation, then incubated with secondary antibodies of Alexa Fluor®647 donkey anti-mouse (1:1500) to detecti β-catenin and Alexa Fluor®488 donkey anti-rabbit (cat. A21206) at 1:5000 to detect β-actin, for 1h at room temperature and shielded from light. Protein bands of β-catenin and β-actin were scanned simultaneously by the Typhoon trio with the setting of Acquisition mode [29,30]: Fluorescence; Emission Filter: 520 BP 40 CY2, ECL+, Blue FAM and 670 BP 30 CY5 for b-catenin; PMT: 600; Laser: blue(488) for b-actin and Red(633) for β-catenin; Pixel size:100 microns. The optical densities of all protein bands were analyzed using the ImageQuant 5.2 software (Typhoon Trio).
In vitro Akt kinase assay
To detect the true Akt activity, the experiment was performed exactly as described [32], following instructions in the Akt kinase assay kit from Cell Signaling Technology (Cat#9840). Rats were sacrificed with an overdose of isoflurane and perfused with iced PBS. The brains were removed and the ischemic penumbra was dissected on ice. Whole cell extraction was prepared. Immobilized Akt primary antibody bead slurry (20ul) was added into 150 µg protein overnight at 4°C. The Akt antibody pellet was washed with cell lysis buffer and suspended in 50 µl kinase buffer supplemented with 1 µl of 10 mM ATP and 1 µg of GSK-3 α/β fusion protein, which was allowed to incubate for 30 min at 30°C. The reaction was terminated with 25 µl 3X SDS sample buffer. The solution was then mixed with Vortex and centrifuged. The supernatant was taken for Western blot detection of phosphorylated GSK3 α/β fusion protein. The protein level of phosphorylated GSK3 α/β fusion protein reflects the Akt activity.
For statistical analyses, two-way ANOVA was used to compare the protective effect of preconditioning on infarct size, on various protein bands from Western blots and on Akt kinase assay. Differences in protein bands from Western blots were analyzed using one-way ANOVA followed by Fisher LSD post-hoc test. All tests are considered statistically significant for p values ≤0.05. Data are presented as means ± SEM.
Results
Rapid preconditioning reduces infarct size according to ischemic severity
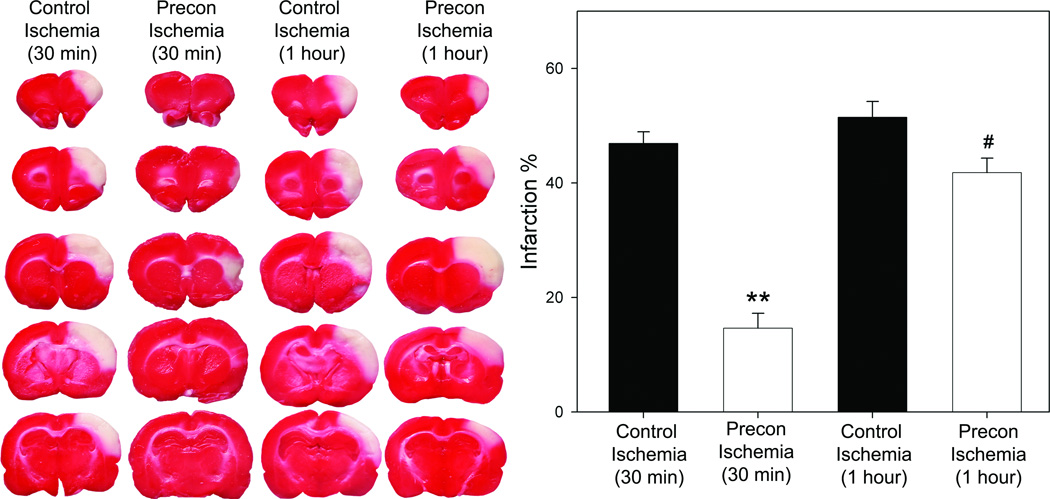
Similar infarction size was detected in ischemia generated by 30 min or 60 min of transient occlusion of the bCCA combined with permanent distal MCA occlusion; however, rapid preconditioning robustly reduced infarction by 69% in the 30 min ischemia, but it only mildly attenuated injury by 19% in the 60 min ischemia (Fig.2).
Fig. 2.
Rapid preconditioning significantly inhibited ischemic damage. Ischemic brains were harvested at 2d after stroke for TTC staining. Representative brain sections stained with TTC are shown in the left column. The bar graphs (the right column) represent the average infarction size normalized to the non-ischemic cortex. Animal numbers in each group from the left to the right on the bar graph are 7, 7, 5 and 6, respectively. *** vs control ischemia (30min), P<0.001; # vs control ischemia (1hour), P<0.05.
Akt activity contributes to ischemic tolerance
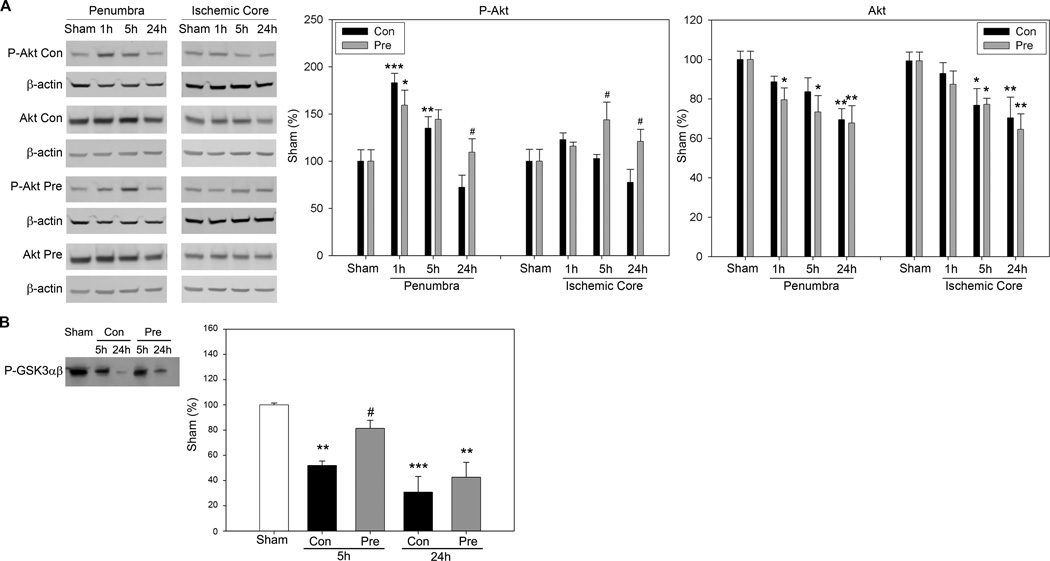
Levels of phosphorylated Akt (P-Akt) and total protein of Akt in the ischemic penumbra and core were measured by Western blot (Fig.3). In the ischemic penumbra, P-Akt was transiently increased at 1h and 5h, but returned to baseline levels at 24h in animals with control ischemia. Preconditioning did not affect P-Akt level at 1 and 5h, but enhanced its level at 24h compared with the control at the same time point. In the ischemic core, P-Akt level did not change significantly after control ischemia; however, preconditioning enhanced P-Akt levels at 5 and 24h compared with control ischemia at the respective time points. Total Akt level decreased in both the ischemic penumbra and core after stroke; preconditioning did not significantly change its levels.
Fig. 3.
The effect of preconditioning on levels of P-Akt and total Akt, and Akt activity. A. Representative protein bands of Western blot for P-Akt and total Akt from the ischemic penumbra and core. β-actin was probed to indicate even loading of protein. The optical density of each protein band was normalized to that of protein bands of sham animals. Average protein levels of P-Akt and Akt are shown in the bar graphs. Con, control ischemia; Pre, preconditioning plus ischemia. The same labels are used for other figures. *, **, *** vs sham, P<0.05, 0.01. 0.001; # vs control at corresponding time point, P<0.05, two way ANOVA, SNK. B. Preconditioning attenuated reduction in Akt activity in the penumbra after stroke. Akt activity was measured using the in vitro Akt kinase assay. Protein levels of phosphorylated GSK3 α/β (P-GSK3 α/β) by Akt represent Akt activity; representative protein bands of P-GSK3 α/β are shown. The bar graph shows average Akt activities. **, *** vs sham, P<0.01, 0.001.
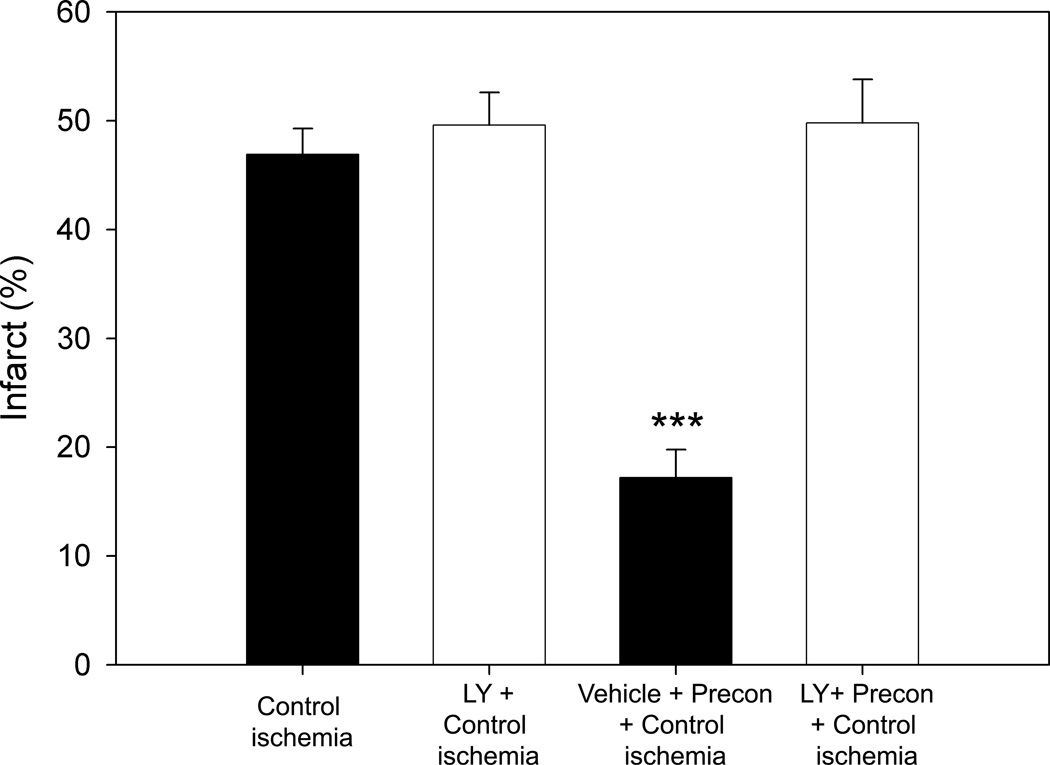
Akt kinase assay showed that the true Akt activity significantly decreased at 5 and 24h after stroke in the control, but it was preserved at 5h by preconditioning (Fig.3B). We further found that the PI3K kinase inhibitor, LY294002, completely reversed the protection from ischemic preconditioning (Fig. 4).
Fig. 4.
The PI3K/Akt inhibitor LY294002 abolished preconditioning’s protection. Representative infarction stained by TTC are presented. The bar graph shows average infarct size (N=7/group). *** vs other groups, P<0.001..
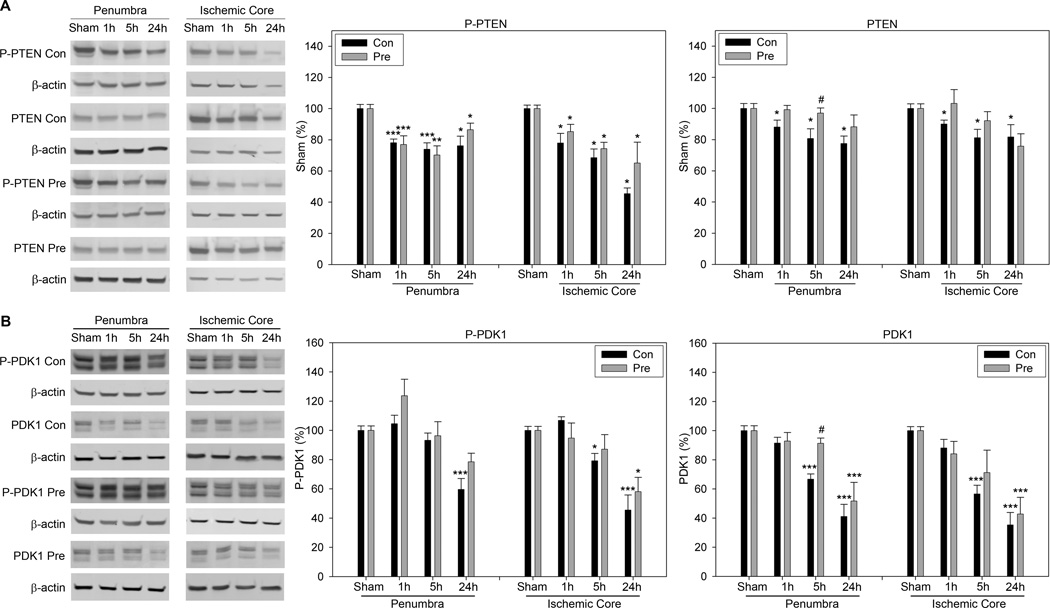
The effects of preconditioning on P-PTEN and P-PDK1 levels after stroke
The results of Western blot show that P-PTEN levels were decreased in both the penumbra and the core from 1 to 24h after stroke, which were not affected by preconditioning (Fig. 5A). Total protein of PTEN was also decreased after stroke, which was significantly prevented by preconditioning at 5h in the penumbra (Fig.5A).
Fig. 5.
A. The effect of preconditioning on P-PTEN and PTEN levels. Representative Western blot for P-PTEN, PTEN and β-actin are shown. The bar graphs stand for statistical results of protein level of P-PTEN and PTEN, respectively. *, **, *** vs sham, P<0.05, 0.01. 0.001; # vs control at corresponding time point, P<0.05. B. The effect of preconditioning on levels of P-PDK1 and total PDK1. The two left columns are representative protein bands of Western blot for P-PDK1, PDK1 and β-actin in the ischemic penumbra and core. The bar graphs in the right two columns represent average densities of P-PDK1 and PDK1, respectively. *, **, *** vs sham, P<0.05, 0.01, 0.001. # vs control at corresponding time point, P<0.05. N=5–6/group.
P-PDK1 was significantly decreased at 24h in both the penumbra and core after control ischemia. Rapid preconditioning had no significant effects on changes in P-PDK1(Fig. 5B). Total protein of PDK1 was decreased from 1 to 24h in both the penumbra and core, which was significantly attenuated by preconditioning at 5h in the penumbra (Fig 5B).
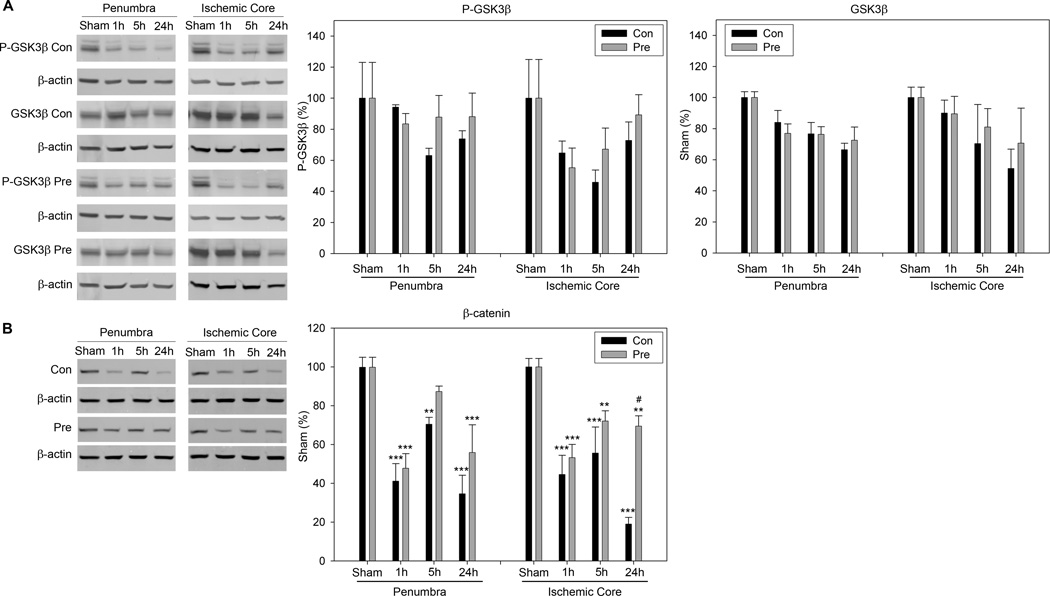
Preconditioning did not change levels of P-GSK 3β, but it allayed ischemia-induced-β-catenin degradation after stroke
Both P-GSK3β and GSK3β levels tended to decrease after stroke in the penumbra and core; preconditioning had no significant effect on both proteins.
Preconditioning showed a trend to inhibit β-catenin degradation caused by stroke. The total protein of β-catenin was significantly decreased after stroke in both the ischemic penumbra and core (Fig. 6). Preconditioning significantly attenuated its reduction at 24h in the ischemic core.
Fig. 6.
A. Protein levels of P-GSK3 β and total GSK3β were not changed by preconditioning compared to control ischemia. The representative protein bands of P-GSK3β, total GSK3β and β-actin in the penumbra and core are shown. The statistical results for P-GSK3β and GSK3β are presented in the bar graphs. Both P-GSK3β and GSK3β levels tended to decrease after stroke in the penumbra and core (no significance was reached probably due to the large variability of the results). Preconditioning had no significant effect on the both proteins. B. The effect of preconditioning on β-catenin degradation after stroke. The two left columns show representative β-catenin protein bands of Western blot from the penumbra and core. Statistical results are shown in the two bar graphs. *, **, *** vs sham, P<0.05, 0.01. 0.001; # vs control at corresponding time point, P<0.05. N=5–6/group.
Discussion
In this study we demonstrated that rapid preconditioning protects against focal cerebral ischemia as a function of ischemic severity in rats. That is, the less severe the ischemia, the stronger the protection. Rapid preconditioning enhanced Akt activity after stroke, and a specific PI3K/Akt inhibitor abolished the protective effect of preconditioning, suggesting that Akt activity contributes to its protection. Nevertheless, Akt phosphorylation (Ser 473) did not correlate with the protective effect of rapid preconditioning, therefore, Akt phosphorylation is not a meaningful marker for neuroprotection.
Our results suggest that Akt phosphorylation (ser 473) may not be used as the “gold standard” marker for Akt activity as it has been used in most previous studies (reviewed by [31]). For example, many studies consistently reported that Akt phosphorylation is transiently increased after cerebral ischemia, leading to the conclusion that Akt activity is increased after stroke [27]. However, together with this current study, we recently demonstrated that P-Akt level does not always represent its true activity reflected by the Akt kinase assay [8,32]. First, we showed that P-Akt is transiently increased after stroke, while Akt activity is actually decreased at the same time points [8,32]. Second, intra-ischemic moderate hypothermia inhibits Akt phosphorylation, while it increases Akt activity compared with normothermia [32]. Third, our current study shows that rapid preconditioning did not increase the level of P-Akt at 5h, but it enhances Akt activity compared with control ischemia. As we have discussed before [31], such a discrepancy may be due to the fact that Akt activity is regulated by two phosphorylation sites (Ser 473 and Thr 308), and P-Akt (Ser473) alone is insufficient to stimulate its activity [1,2,13]. However, the phosphorylation level of P-Akt (Thr 308) is not an ideal marker either for Akt activity after stroke, as we have shown that its levels were not changed by stroke [32]. Furthermore, tyrosine phosphorylation is essential for Akt activation [3]. Taken together, P-Akt levels (ser 473) alone don’t necessarily explain the beneficial effects of Akt activity in mediating neuroprotection.
Whether Akt contributes to the protective effect of delayed preconditioning is controversial in previous reports. In studies concluding that Akt does not contribute to ischemic preconditioning, the authors note that the early P-Akt (Ser473) peak is inhibited by delayed preconditioning [10,19]. In other studies demonstrating that Akt contributes to preconditioning, the authors usually emphasized that P-Akt (Ser473) level is improved by preconditioning at later time points, without interpreting the inhibited early peak of P-Akt (Ser473) after preconditioning [27,28]. Moreover, these studies show that inhibiting Akt activity by the PI3K inhibitor Wortmannin or LY 294002 blocks the protective effect of delayed preconditioning [12,27,28]. Therefore, such controversies are partly derived from the fact that Akt kinase assay was not performed in those studies.
We further showed that total protein levels of Akt, PTEN, PDK1, GSK 3β, and β-catenin were reduced after stroke, and rapid preconditioning had no effect on total proteins of Akt and GSK 3β, but attenuated reductions in PTEN, PDK1 and β-catenin at certain time points after stroke. Previous studies have focused on Akt phosphorylation after stroke, but total Akt protein levels were usually not quantitated. From our current study, total Akt protein doesn’t correlate with the final pathological outcomes. Nevertheless, the total protein levels of PTEN, PDK1 and β-catenin correspond to the reduction in infarction size in rats receiving preconditioning. Although our current study cannot answer whether the preservation of these protein levels is simply the effect of protection or the cause of protection, it is an indication that merely studying their phosphorylation is insufficient.
Conclusion
Rapid preconditioning reduces ischemic damage induced by focal ischemia, and the protection is inversely related to the severity of stroke. In addition, the Akt pathway contributes to the protection of rapid preconditioning.
ACKNOWLEDGEMENTS
The authors wish to thank Ms. Felicia F. Beppu for manuscript assistance and Ms Elizabeth Hoyte for figure preparations. This study was supported by AHA grant SDG 0730113N (HZ) and NIH grant 1R21NS057750-01A2 (HZ), NINDS grants R01 NS27292 (GKS) and P01 NS37520 (GKS).
References
- 1.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 4.Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- 5.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 6.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 7.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Gao G, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, Zhao H. The Akt signaling pathway contributes to postconditioning’s protection against stroke; the protection is associated with the MAPK and PKC pathways. Journal of Neurochemistry. 2008 doi: 10.1111/j.1471-4159.2008.05218.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X, Ren C, Zhao H. Protective effects of ischemic post-conditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008;86:2505–2511. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- 10.Garcia L, Burda J, Hrehorovska M, Burda R, Martin ME, Salinas M. Ischaemic preconditioning in the rat brain: effect on the activity of several initiation factors, Akt and extracellular signal-regulated protein kinase phosphorylation, and GRP78 and GADD34 expression. J Neurochem. 2004;88:136–147. doi: 10.1111/j.1471-4159.2004.02188.x. [DOI] [PubMed] [Google Scholar]
- 11.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 12.Hashiguchi A, Yano S, Morioka M, Hamada J, Ushio Y, Takeuchi Y, Fukunaga K. Up-regulation of endothelial nitric oxide synthase via phosphatidylinositol 3-kinase pathway contributes to ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2004;24:271–279. doi: 10.1097/01.WCB.0000110539.96047.FC. [DOI] [PubMed] [Google Scholar]
- 13.Hill MM, Andjelkovic M, Brazil DP, Ferrari S, Fabbro D, Hemmings BA. Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a staurosporine-insensitive kinase. J Biol Chem. 2001;276:25643–25646. doi: 10.1074/jbc.C100174200. [DOI] [PubMed] [Google Scholar]
- 14.Kato H, Araki T, Kogure K. Preserved neurotransmitter receptor binding following ischemia in preconditioned gerbil brain. Brain Res Bull. 1992;29:395–400. doi: 10.1016/0361-9230(92)90074-8. [DOI] [PubMed] [Google Scholar]
- 15.Kato H, Liu Y, Araki T, Kogure K. MK-801, but not anisomycin, inhibits the induction of tolerance to ischemia in the gerbil hippocampus. Neurosci Lett. 1992;139:118–121. doi: 10.1016/0304-3940(92)90871-4. [DOI] [PubMed] [Google Scholar]
- 16.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meller R, Thompson SJ, Lusardi TA, Ordonez AN, Ashley MD, Jessick V, Wang W, Torrey DJ, Henshall DC, Gafken PR, Saugstad JA, Xiong ZG, Simon RP. Ubiquitin proteasome-mediated synaptic reorganization: a novel mechanism underlying rapid ischemic tolerance. J Neurosci. 2008;28:50–59. doi: 10.1523/JNEUROSCI.3474-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura M, Nakakimura K, Matsumoto M, Sakabe T. Rapid tolerance to focal cerebral ischemia in rats is attenuated by adenosine A1 receptor antagonist. J Cereb Blood Flow Metab. 2002;22:161–170. doi: 10.1097/00004647-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Namura S, Nagata I, Kikuchi H, Andreucci M, Alessandrini A. Serine-threonine protein kinase Akt does not mediate ischemic tolerance after global ischemia in the gerbil. J Cereb Blood Flow Metab. 2000;20:1301–1305. doi: 10.1097/00004647-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Noshita N, Lewen A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1442–1450. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Nusse R. Wnts and Hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development. 2003;130:5297–5305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski RP, Graupner G, Titova E, Zhang J, Chiu J, Dach N, Corleone D, Tang J, Zhang JH. The hyperbaric oxygen preconditioning-induced brain protection is mediated by a reduction of early apoptosis after transient global cerebral ischemia. Neurobiol Dis. 2008;29:1–13. doi: 10.1016/j.nbd.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Pinzon MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr. 2004;36:323–327. doi: 10.1023/B:JOBB.0000041762.47544.ff. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Ren C, Gao X, Steinberg G, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2007.11.056. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Xiong L, Chen S, Liu Y, Zhu X. Rapid tolerance to focal cerebral ischemia in rats is induced by preconditioning with electroacupuncture: window of protection and the role of adenosine. Neurosci Lett. 2005;381:158–162. doi: 10.1016/j.neulet.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Yano S, Morioka M, Fukunaga K, Kawano T, Hara T, Kai Y, Hamada J, Miyamoto E, Ushio Y. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2001;21:351–360. doi: 10.1097/00004647-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Yin XH, Zhang QG, Miao B, Zhang GY. Neuroprotective effects of preconditioning ischaemia on ischaemic brain injury through inhibition of mixed-lineage kinase 3 via NMDA receptor-mediated Akt1 activation. J Neurochem. 2005;93:1021–1029. doi: 10.1111/j.1471-4159.2005.03096.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Gao X, Yan Z, Ren C, Shimohata T, Steinberg GK, Zhao H. Inhibiting caspase-3 activity blocks beta-catenin degradation after focal ischemia in rat. Neuroreport. 2008;19:821–824. doi: 10.1097/WNR.0b013e3282ffda72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Ren C, Gao X, Takahashi T, Sapolsky RM, Steinberg GK, Zhao H. Hypothermia blocks beta-catenin degradation after focal ischemia in rats. Brain Res. 2008;1198:182–187. doi: 10.1016/j.brainres.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H, Yenari MA, Cheng D, Barreto-Chang OL, Sapolsky RM, Steinberg GK. Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:681–692. doi: 10.1097/01.WCB.0000127161.89708.A5. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]