Abstract
The assembly of an HIV-1 particle is a complex, multistep process involving several viral and cellular proteins, RNAs, and lipids. While many macroscopic and fixed cell microscopic techniques have provided important insights into structure of HIV-1 particles and the mechanisms by which they assemble, analysis of individual particles and their assembly in living cells offers the potential of surmounting many of the limitations inherent in other approaches. In this review we discuss how the recent application of live cell microscopic imaging techniques have increased our understanding of the process of HIV-1 particle assembly. In particular, we focus on recent studies that have employed total internal reflection fluorescence microscopy and other single-virion imaging techniques in live cells. These approaches have illuminated the dynamics of Gag protein assembly, viral RNA packaging and ESCRT protein recruitment at the level of individual viral particles. Overall, the particular advantages of individual particle imaging in living cells have yielded findings that would have been difficult or impossible to obtain using macroscopic or fixed cell microscopic techniques.
Introduction
The assembly of an HIV-1 particle is a complex, multistep process involving several viral and cellular proteins, RNAs, and lipids. Each of the components of a virus particle need to move to the site of virion assembly, via diffusion or transport, and then interact with other virion components in specific ways as the particle is assembled1; 2. The application of a variety of techniques from the disciplines of biochemistry, spectroscopy and structural biology as well as various forms of microscopy has resulted in a fairly good understanding of the molecular details of viral and cellular protein recruitment, i.e. how the various components of virions bind to one another. However, some parameters of HIV-1 assembly have been challenging to define, and some have been controversial. These include the location in the cell at which particle assembly is initiated, how and when the various virion components, and required cellular cofactors, move to sites of particle assembly and the overall dynamics of virion genesis.
In this review we discuss how the recent application of live cell microscopic imaging techniques have increased our understanding of the process of HIV-1 particle assembly. In particular, we focus on recent studies that have employed total internal reflection fluorescence microscopy (TIR-FM). These and other imaging approaches, including confocal and epifluorescent microscopy have allowed new insights into the behavior of viral proteins in living cells and the dynamics with which HIV-1 particles are constructed in a physiologically relevant setting, i.e. a living cell. The application of these techniques has allowed, for the first time, the visualization of the assembly of individual virus particles in living cells, from the initiation of particle assembly to virion release, as well as quantitative analysis of the dynamics of particle assembly.
Advantages (and pitfalls) of single particle imaging in the analysis of HIV-1 assembly
Spectroscopic and biochemical methods have long been used to examine both the structure of the components of macromolecular assemblies and their interactions. Indeed, the dynamics of assembly of bacteriophage and viruses have been used as model systems for understanding macromolecular complex assembly3. The strength of spectroscopic and biochemical studies is their ability to obtain large signal-to-noise ratios by pooling many events into a single measurement. However, this strength also leads to a number of limitations in the kinds of information that can be gathered. A major constraint is that dynamic information is usually lost. For example, in the case viruses such as HIV-1, the assembly of particles is generally not synchronized, thus information about the temporal ordering of events is lost by averaging. In contrast, imaging of single virions avoids temporal averaging and the loss of information about the dynamics of individual events.
Further weaknesses of macroscopic measurements include the fact that the signal is derived from all molecules in a sample, whether or not they participate in assembly. Indeed, pulse-chase labeling experiments suggest that only a small fraction of the HIV-1 Gag that is synthesized is actually incorporated into particles4. This problem is potentially exacerbated when host machinery that is involved in viral assembly is analyzed. Moreover, assembly events may be too transient to be reflected in the macroscopic measurements. This would be particularly severe if the assembly time of individual particles is short compared to the overall lifespan of virion components. This is likely to be a problem with imaging HIV-1 assembly, since pulse-chase labeling suggests that several hours can pass between the synthesis of an HIV-1 Gag protein and its appearance in extracellular virions4; 5; 6, while assembly times for individual particles have been estimated to be in the order of minutes (see below).
Conversely, in microscopy, if a set of criteria can be established for determining if a particular signal comes from an assembling virion, analysis of individual particles offers the potential of surmounting many of the problems of macroscopic measurements. Since individual virions do not contains sufficient material for conventional biochemical or spectroscopic analysis, microscopic imaging is employed. Tagging viral and host components with fluorophores can offer the sensitivity to study the formation of these individual virions. By taking this approach, the dynamics of recruitment of components to an individual virion can, in principle, be assayed by measuring the fluorescence associated with each component at a putative site of virion assembly.
Despite the potential advantages, there are a number of important issues to address in the general design and interpretation of imaging experiments: Do the fluorescent tags that are attached to viral or cellular components affect the process to be studied? What kind of imaging modality should be used? How is the level of fluorescence related to the number of molecules present at a particular location in the cell? How can one be confident that the fluorescent signal is actually reporting the event one wishes to study?
Almost all approaches to live-cell imaging of specific molecules involve attaching a tag, usually a fluorophore. For instance, it is common to express proteins as fusions to a fluorescent protein, such as GFP. While such approaches have provided many biological powerful insights7 it is important to recognize that tagged proteins can be non-functional, dominant-negative, or fail to reflect the localization of the native protein, particularly if the fluorescently tagged protein is present at significantly greater than endogenous levels, as is often the case when it is expressed by transient transfection8. Moreover, while the presence of a fluorescent tag on a protein has sometimes been used to quantify the number of molecules of a protein, there are number of associated caveats. For example; untagged endogenous protein is often present in addition to the ectopically expressed fluorescent fusion protein and the ratio of the two at the particular subcellular site being studied is generally not known. Another problem is relating the fluorescence intensity to the number of fluorophores (reviewed in 9). This can be affected by various factors including how effectively the dipole of the fluorophore is excited and how efficiently the photons are captured by the detector, as well as effects of local environment around a fluorophore, leading to differences in fluorescence, even in the absence of differences in the numbers of fluorophores9.
For these reasons, it is important to perform a number of control experiments to demonstrate that molecules (in this case viral and cellular proteins that participate in particle assembly) behave authentically before embarking on a set of imaging experiments. Moreover, quantitative analyses of particle assembly should be interpreted cautiously – while it is clearly possible to track the dynamics of individual components relative to each other, quantifying the numbers of molecules present at a particular location in a cell is challenging.
Biological properties of HIV-1 virion components and imaging HIV-1 assembly
Fluorescent images of putatively assembling HIV-1 particles have been obtained using a number of approaches, including standard fixed cell immunofluorescence microscopy, as well as fixed and live cell imaging combined with fluorescent protein tagging10,11; 12; 13 or biarsenical-tetracysteine tagging of virion components14; 15. In principle, a number of virion components can be labeled with fluorophores, but using the HIV-1 Gag protein has some key advantages. First, it is the most abundant protein in virion particles by a considerable margin – it is estimated that several thousand molecules of Gag are present in each virion16, a number that should be readily detectable using light microscopy if a reasonable fraction of the molecules are labeled with conventional fluorophores. Second, it is the only viral protein that is required to be expressed in cells to cause the formation of virus-like particles1. Indeed, expression of Gag alone in cells causes the formation of particles that are morphologically indistinguishable from bona-fide immature virions. Third, Gag can tolerate the insertion of monomeric fluorescent proteins either at its C-terminus or at the flexible C-terminus of the matrix (MA) domain of Gag, while retaining the ability to assemble into virions11; 13. In both cases, the morphological accuracy of particle assembly is improved by co-expressing unlabelled Gag molecules; if this precaution is taken, fluorescent virus like particles that are morphologically indistinguishable from authentic virions can be assembled and visualized on the surface of cells using fluorescent protein fusions of Gag13; 17. In the case of the MA insertion site, it is even possible to generate virions that are fluorescently labeled and nearly fully infectious. These sites can also tolerate the insertion of a tetracysteine tag for biarsenical fluorophore labeling, and some studies have been carried out using this approach14; 15, although this strategy is generally reckoned to be less effective in obtaining high quality images of particle assembly than fluorescent protein tagging.
In principle, virion components other than Gag (e.g. the Env and Vpr proteins) can also be tagged with fluorophores. However, the fraction of the total protein expressed in virion-producing cells that is associated with particles is significantly less for these virion components than it is for the Gag protein. One other key component of HIV-1 virions that can be labeled is the genomic RNA; this has been achieved by expressing fluorescent protein-RNA binding protein fusions, along with viral RNAs engineered to include multiple copies of the cognate RNA binding site18; 19; 20. This approach has recently been of great utility in imaging the packaging of viral genomes and assessing when and where genomic RNA dimerization occurs in cells.
An important pitfall in imaging the HIV-1 Gag protein is that it can exist in several different states, and different locations in the cell, before, during and after it has assembled into particles21; 22; 23; 24; 25. Light microscopic techniques lack the resolving power to determine whether a fluorescent signal is emitted by unassembled labeled Gag protein, individual virions, or clustered virions. Importantly, if the signal that is examined is truly that of a virion in the act of assembly, then that signal is expected to be rare. A rough calculation, (based on the assumption that 106 infected or transfected cells can produce ~1μg of p24 capsid protein per day) means that individual cells generate ~several thousand particles/day. Current estimates of the time to assemble an HIV-1 particle are of the order of a few minutes (see below). Thus, at any particular time, there may be only few dozen virions in the act of assembly (and at different stages of the process), in a background of hundreds or thousands of particles that have already assembled, as well as a background of virion components that are not yet assembled or, perhaps, never will be assembled. When an assembly event occurs, it is but one source of fluorescent signal in a background that is much larger. Thus, the challenge is how to resolve a genuine assembly event from a fluke anecdotal observation, or from events that precede or follow assembly.
In designing a strategy to maximize the chances of observing assembly events, it is instructive to consider the overall behaviour of the Gag protein. Infected cells, or cells expressing HIV-1 Gag protein alone contain a pool of Gag that appears to be diffusely distributed throughout the cytoplasm25, along with apparent concentrations of the protein at the plasma membrane and/or at intracellular sites21; 22; 24; 26; 27. Experiments that have employed epifluorescence microscopy combined with FRAP and photoactivation indicate that the diffusely distributed pool of Gag is highly mobile, and circulates throughout the cytoplasm within <60 seconds25. It is likely that this mobile pool of Gag is essentially equivalent the cytoplasmic Gag protein that is unprocessed and behaves as monomers or low order multimers when Gag-expressing or HIV-1 infected cells are subjected to biochemical fractionation and membrane flotation analysis28; 29. This mobile, diffusely distributed, cytoplasmic pool of HIV-1 Gag can be reasonably assumed to represent Gag protein that has yet to be incorporated into particles. Conversely, the concentrations of Gag that are visualized at the plasma membrane or coincident with endosomal compartments could potentially represent Gag proteins that are in the act of assembling into virions. Deciding whether accumulations or concentrations of Gag represent populations of the protein before, during or after it has assembled into particles is a critical factor in interpreting an imaging experiment. Indeed, this key decision has been a source of controversy over where in the cell HIV-1 assembly occurs2. However, several studies have revealed that endosomal accumulations arise later than concentrations at the plasma membrane6; 21; 22; 23; 24, and their appearance can inhibited by blocking endocytosis in the absence of effects on extracellular particle yield21; 22. Moreover the accumulation of Gag protein in endosomes is exacerbated by tetherin, a restriction factor that is expressed in cells that are commonly used in imaging experiments, and traps virions at the plasma membrane after they have assembled, and thereafter causes their internalization30. Thus, intracellular concentrations of Gag occur largely through internalization of virions that have already assembled at the cell surface. Alternatively, in macrophages, portions of the plasma membrane are deeply involuted, and therefore assembly at these areas of the plasma membrane can give the illusion that assembly occurs at intracellular compartments31; 32. Overall, it has become quite clear that HIV-1 Gag protein accumulates first at the plasma membrane, and this finding is key for the interpretation of imaging experiments, and selecting optimal techniques for imaging the assembly of individual particles.
Selection of microscopy techniques for imaging HIV-1 assembly
Selecting an optimal imaging modality for the analysis of any given biological process is not always straightforward. Techniques that improve spatial resolution, often do so at the expense of temporal resolution. For example, electron microscopy can resolve finer spatial detail than light microscopy, but at the cost of a complete loss of temporal resolution: the samples are fixed and, thus, not changing. Even the newest super-resolution optical techniques trade spatial for temporal resolution. Confocal microscopy has the advantage of providing sharper images by discarding light that is not derived from the plane of focus. However, there are two fees that must be paid for this advantage. First, since photons are being discarded, the signal is weaker which often necessitates stronger illumination resulting in faster photobleaching. Second, if one is observing a moving object, the narrower the plane of focus then the sooner the object is lost from view. Multiphoton microscopy has the advantage of allowing one to image deeper into tissue. However, it cannot achieve as fine a spatial resolution as other forms of microscopy.
A technique that has proved especially useful in the analysis of HIV-1 assembly is a variation of epi-fluorescence microscopy called total-internal reflection fluorescence microscopy (TIR-FM)9. TIR-FM creates an excitatory field that exponentially decays away from the coverslip with a space constant of ~70 nm. Thus, if one is studying events occurring at the surface of the cell, TIR-FM has the advantage of exciting only those fluorophores that are in close proximity to the coverslip (the ventral surface of the cell). This results in excellent spatial and temporal resolution in imaging experiments and the application of this approach has allowed the visualization of the genesis of individual virions.
Insights into HIV-1 assembly from imaging studies
Dynamics of the assembly Gag to particles
TIR-FM studies have yielded results that are entirely consistent with the notion that HIV-1 particle genesis is initiated and completed at the plasma membrane, both in model cell lines33; 34 and in primary macrophages, a physiological target of HIV-1 infection (Jouvenet, Simon, Bieniasz, unpublished data). Specifically, if particles assemble on the cell surface, then they should appear one at a time, and the time course with which each individual particle appears should reflect the time course of assembly. Two key differences would be seen if virions assembled on internal membranes and were then delivered to the surface. First, a number of particles would be expected appear together simultaneously at the plasma membrane as a vesicle containing pre-assembled virions fuses with it, delivering a bolus of virions to the cell surface. Second, the rate at which virions appeared would reflect the rate of delivery of vesicles to the cell surface (which would appear almost instantaneous relative to the rates at that images are actually acquired during the observation of HIV-1 assembly, which occurs over several minutes).
In its basic form, monitoring the assembly of individual HIV-1 particles in TIR-FM essentially involved measuring the signal associated with a punctum of fluorescently tagged Gag protein at the plasma membrane over time33; 34. These experiments show that following the initial detection of a punctum whose intensity is marginally greater than the surrounding diffuse Gag background, its fluorescence increases steadily until it reaches a plateau (Fig. 1). The ‘assembly time’ is defined as the time elapsed between the initial detection of a punctum and the point at which its intensity reaches a plateau. In the context of Gag-GFP, transiently expressed in HeLa cells, the mean assembly time of individual particles is approximately 8-9 minutes34. However, assembly times are quite variable and range from as little as 4 minutes to as much as 20 minutes, even within the same cell. Importantly, particles generated using HIV-1 Gag-GFP, or using a proviral construct, in which GFP in inserted in the flexible C-terminus of MA assemble with broadly similar kinetics33; 34. The variation in assembly times can be explained in part by variation in Gag expression level, which is known or predicted to influence self interaction between Gag molecules and its propensity to accumulate at the plasma membrane35. Indeed, assembly times are longer for Gag-GFP particles that appear at early times after transfection, but decrease thereafter as Gag concentration in the cell increases, stabilizing at ~5 min34, suggesting that the rate of assembly of individual virions is accelerated as Gag protein accumulates. Another key determinant of assembly time that likely contributes to the observed variability is the number of Gag molecules packaged into virions which is known to vary among individual particles16
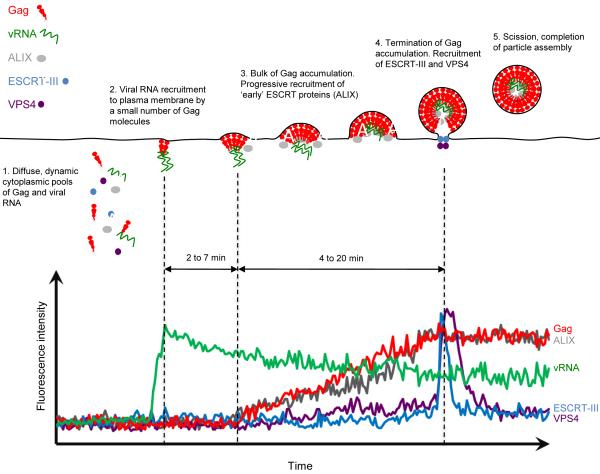
Figure 1.
Sterotypical behavior of viral and cellular components (compiled from several different actual experiments) during sequential steps in the assembly of HIV-1 and EIAV particles, imaged by TIR-FM. The fluorescent intensity over time at a site of virion particle assembly is plotted on the chart and an interpretation of the fluorescence data is represented by the diagram. The various virion components and host factors are color-coded in the same way on the chart and the diagram (Gag, red; viral RNA (vRNA), green; ALIX, grey; ESCRT-III, blue; VPS4, purple).
The aforementioned assembly times are likely to represent underestimates of the true time required to complete assembly. This is because a certain number of Gag molecules must be already assembled in order to be detected as a nascent punctum. The number of Gag molecules required to assemble to cross this detection threshold is unknown, and is dependent on the sensitivity and resolution of the imaging equipment, as well as the ‘background’ level of fluorescent Gag that is yet to be incorporated into virions. Based on the detection limit of our own microscope, we estimate that this first signal of a particle, as defined by a detectable punctum of fluorescent Gag protein, is likely to occur when a few tens of molecules of Gag have assembled. The time required for these molecules to gather at the site of assembly is not defined, but studies in which the viral RNA is also imaged (see below) suggest that it is likely to be in the order of a few minutes.
Analogous assembly times have been measured for other retroviruses, and using other techniques. Specifically, the assembly of individual equine infectious anemia virus (EIAV) Gag-GFP particles, measured using TIR-FM, occurs in a mean of 11.5 minutes36. Additionally, the assembly of individual MLV particles has been imaged using spinning disc confocal microscopy37. Mean assembly times for MLV were measured 15 min or 20 min depending on the specific cell line used, with variation in the range of 8 to 30 minutes37. Individual MLV assembly events have also been characterized using atomic force microscopy, where the progress of assembly was determined by measuring the viral protrusion height the infected cell surface over time38. In these studies the nascent virion bud grew to maximal height, presumably indicating the completion of assembly, in a mean of 20 minutes. Again, these assembly times are likely to represent underestimates, because a detection threshold must be crossed in order to define the commencement of assembly. Nonetheless, it is noteworthy that broadly similar assembly dynamics are evident across a range of retroviruses, and using a variety of imaging techniques.
Use of FRET, FRAP and photoconvertible fluorophores to monitor Gag behavior during viral assembly
A number of refinements of the aforementioned optical techniques have been applied to reveal further insight into the process of Gag assembly, and to provide additional evidence that the appearance of fluorescent puncta represent genuine assembly events. For example, inserting the photoconvertible fluorescent protein mEosFP into the stalk of MA has revealed the origin of Gag proteins that are incorporated into nascent virions33. When excited with 405 nm light, mEosFP irreversibly photoconverts such that its emission changes from green to red. Thus, if cells are subjected to 405nm illumination in a TIR microscope, Gag-mEosFP molecules present at plasma membrane will have red emission thereafter. Analysis of particle assembly following a pulse of 405nm excitation revealed that Gag-mEosFP molecules that are newly recruited to particles emit green light33. This strongly suggests Gag molecules that are recruited into assembling particles do so from the rapidly diffusing cytosolic pool, or from Gag molecules that have only been recently (after the 405nm excitation) been delivered to the plasma membrane.
Analyses of fluorescence resonance energy transfer (FRET) has been used to demonstrate retroviral Gag oligomerization in the cytosol and at the plasma membrane, at the scale of the whole cell24; 39; 40, suggesting that initiating events in particle assembly, specifically Gag multimerization, can occur in the cytoplasm. FRET has also been used to characterize Gag proximity during assembly individual particles34. At the early stages of assembly, when Gag puncta are initially detected, the FRET coefficient between Gag-GFP and Gag-mCherry is similar to the FRET coefficient measured in areas containing only diffuse Gag-GFP and Gag-mCherry. During assembly, the FRET coefficient increases, reaching a maximum at the same time that recruitment terminates. This maximum FRET coefficient in Gag puncta on the plasma membrane is similar FRET coefficients measured within individual cell-free particles, again providing evidence in support of the notion that Gag puncta that appear at the plasma membrane represent bona fide particles34.
FRAP experiments performed on assembling VLPs have demonstrated that the plateau that defines the end of the aforementioned ‘assembly time’ genuinely represent the completion of the assembly of a particle34. Specifically, fluorescence recovery is observed in particles that have increasing fluorescence during the pre-bleach period. Conversely, Gag-GFP particles whose intensity is high and stable during the pre-bleach period do not recover after bleaching. Thus, particles that were recruiting Gag molecules before the bleach continued to do so post-bleach; those with steady fluorescence represent particles in which Gag recruitment was completed and irreversible, the expected signature of virion assembly events.
Overall, such fluorescence-based techniques have strongly suggested that assembly of HIV-1 particles is nucleated at the plasma membrane and proceeds via by a constant recruitment of Gag molecules from a rapidly diffusing cytosolic pool. These Gag molecules become closely, and eventually irreversibly, associated with each other, until some threshold is reached and Gag recruitment terminates. As such, the puncta of fluorescently tagged Gag proteins that appear at the plasma membrane exhibit several properties expected of assembling virion particles.
Behavior of viral genomic RNA during assembly of individual particles
Techniques developed for imaging the localization and movement of cellular mRNAs in living cells41 have been applied in the analysis of HIV-1 genome packaging during particle assembly18; 19; 20. Specifically, an RNA binding protein, from the bacteriophage MS2, is fused to GFP and a nuclear localization signal. The MS2 coat protein ordinarily recognises a stem loop within the phage RNA with high affinity and high specificity. Thus, to visualize viral or cellular RNA, multiple (n=24) copies of this stem loop are inserted into the target RNA, and the RNA expressed in cells that also express the nuclear MS2-GFP fusion protein. Following transcription, the MS2 stem-loop-containing RNAs bind the MS2-GFP fusion protein in the nucleus and the complexes move to the cytoplasm where they can be visualized as fluorescent puncta. For imaging HIV-1 genomes, the MS2 stem loops were inserted into a minimal viral genome, within the gag intron such that only unspliced viral mRNA, which constitutes the viral genome, is labeled. Importantly, these modified viral genomes were found to be incorporated into virions in a packing signal-dependent manner and to be compatible with the generation of infectious virions, suggesting that they should behave similarly to bona fide viral RNA18; 19.
Using such approaches, HIV-1 RNA can be readily visualized in the cytoplasm using a variety of microscopic techniques. Interestingly, there is also some evidence from this technique that HIV-1 RNA, as well as feline immunodeficiency virus (FIV) RNA accumulates at the nuclear envelope, suggesting that transport through the nuclear pore might be rate limiting19. In the cytoplasm, HIV-1 RNA appears highly dynamic. Observation by TIR-FM reveals that individual HIV-1 RNA molecules move rapidly in and out the proximity of the plasma membrane, remaining visible in the TIR field no more than few seconds, with high and variable lateral mobility (0.05 to 0.5 μm/s). However when Gag is coexpressed, a fraction of the RNA molecules dock at the membrane, and remain there for several minutes where they exhibit slower lateral drift (~0.01μm/s)18. Although Gag is not initially detected in association with these membrane anchored RNAs, the fact that this behavior is not observed in the absence of Gag, or when either the Gag myristoylation signal or the viral RNA packaging signal is mutated, strongly suggests that a sub detectable number of Gag molecules are responsible for anchoring viral RNA the plasma membrane18 (Fig. 1). Notably, when Gag assembly is imaged in the presence of labeled viral RNA, ~75% of assembly events are observed to occur coincident with a membrane anchored viral RNA. On average, ~4.5 minutes elapses between the appearance of the viral RNA at the plasma membrane and first detection of a coincident Gag protein signal (Fig 1). The lateral drift of the viral RNA slows further and then ceases as Gag assembly proceeds. Particle assembly appears to be necessary to irreversibly anchor the viral RNA, because a Gag mutant that retains membrane- and RNA- binding activities but does not assemble into particles, causes viral RNAs to dock at the plasma membrane, but these RNAs continue to drift laterally, and then dissociate from the membrane after a mean of ~8 minutes. Overall, these observations indicate that an early intermediate in particle assembly is a membrane bound complex containing viral RNA and a small number of Gag molecules, which nucleates the further recruitment of Gag protein that forms the nascent virion.
Visualizing HIV-1 budding
Imaging HIV-1 particles generated with a Gag fused to pHluorin, a GFP variant whose fluorescence is diminished at acidic pH42, has been used as a tool to determine whether particles have undergone scission from the cell surface34. This assay is based on the assumption that once virions have detached from the cell membrane, they should not be able to exchange any molecules—even protons—with the cell cytoplasm. Because the exceptionally high turnover enzyme, carbonic anhydrase, which catalyses the reaction: CO2 + H2O ↔ H+ + HCO3−, is highly abundant in the cytoplasm, increasing the pCO2 in cell culture medium should acidify the cytosol more rapidly efficiently than the interior of particles that have completed budding and separated from the cytosplasm. Indeed, after a brief pulse of pCO2, two populations of Gag-pHlourin particles on the surface of cells that differ in their sensitivity to pCO2 are detected. The comparatively pCO2–resistant population exhibits the same property as virion particles that are harvested from the cell-free culture supernatant and have, by definition, completed budding34. Importantly, the appearance of these pCO2–resistant, putatively budded, particles is abolished when mutations are introduced into the so called late-domain that recruits ESCRT proteins34. While the pHluorin-based approach does not currently allow precise measurements of the time at which budding occurs, this approach does reveal that the mobility of particles does not increase, nor do they move away from the site of assembly, after budding34 at the ventral surface-coverslip interface. Nonetheless, other investigators have used changes in particle mobility, or their complete disappearance in TIR-FM microscopy experiments as a surrogate for viral budding33. Intuitively, the motility of particles might increase once scission has occurred, since the constraint of a membranous neck is removed. Using this approach, estimates in the order of 10-15 minutes between the completion of Gag accumulation and particle release have been obtained33. However, using this criterion, only a small minority of particles are observed to be released33. It is likely that in most cases the movement of completely budded particles is inhibited by cell surface adhesion molecules as well as by the limited space between the ventral surface of the cell and the coverslip (~40□nm), which is smaller that the typical diameter of a virion (~100□nm). Therefore, increases in particle mobility might be a consequence of their internalization rather than budding. This interpretation is consistent with the observation that simultaneous with a particle beginning to move, it disappears from the evanescent field (which decays over 100 nm from the coverslip) while still being visible under epi-fluorescent illumination, demonstrating that it is moving away from the coverslip33). Thus, the increased particle mobility that can sometimes be observed after assembly has been completed should be interpreted with caution, in our view.
Behavior of ESCRT proteins during assembly of individual particles
Studies of the endosomal sorting complex required for transport (ESCRT) proteins during retrovirus assembly has recently revealed the kinetics of recruitment of the cellular machinery required for budding36; 43. The ESCRT machinery, which is composed of approximately 20 proteins organized into a coherent interaction network, functions in cellular membrane fission events, such as multivesicular body formation and at the terminal stages of cytokinesis44; 45. Thus, studies of viral budding may serve as a model for the function of the ESCRT pathway in cellular processes. The complete ESCRT machinery contains of 3 defined protein complexes, ESCRT-I, ESCRT-II and ESCRT-III, and other associated proteins such as ALIX and the ATPase, VPS444; 45. ESCRT proteins and complexes are soluble and are thought to be recruited at the site of membrane fission in an ordered manner, with certain components, such as the ESCRT-I complex and ALIX acting early in the pathway, and the ESCRT-III complex, which is comprised of a numerous CHMP proteins acting later. In mammalian cells, ALIX bridges ESCRT-I to ESCRT-III proteins via direct interactions 46; 47; 48; 49; 50. Recent in vitro data suggest that ESCRT-III proteins are responsible for the scission of the membrane neck and that VPS4 acts during or after the scission step to recycle the complex51.
Numerous enveloped viruses, including all retroviruses, exploit the ESCRT machinery directly or indirectly using specific sequences, called late domains, that mediate ESCRT protein recruitment. There are various types of late domain among retroviruses, for example HIV-1 encodes two late domains within the p6 domain of Gag; a PTAP motif which binds ESCRT-I52; 53; 54, and an LxxLF motif which binds ALIX with relatively low affinity47; 48. Conversely EIAV encodes a single known late domain motif, YPDL, which binds ALIX with higher affinity46; 47; 48; 55.
Using stable cell lines expressing moderate levels of a subset of GFP-tagged ESCRT proteins36, or transiently expressed VPS443, the dynamics of ESCRT protein recruitment during the assembly of HIV-1 and EIAV have recently been defined with TIR-FM based approaches. Specifically, ALIX progressively accumulates at EIAV assembly sites, with dynamics that are virtually indistinguishable from the accumulation of EIAV Gag (Fig. 1). ALIX is not observed to dissociate from assembly sites and, concordantly, ALIX is found in EIAV and HIV-1 virions47; 48. In stark contrast, the CHMP proteins that have been examined (CHMP1b, CHMP4a and CHMP4c) are only transiently recruited to HIV-1 and EIAV assembly sites at around the time that Gag protein accumulation is completed36, typically remaining detectable at the site of budding for only a ~2 minutes (Fig. 1). The VPS4 protein behaves similarly to the CHMP proteins, presumably because it is recruited by them, and is observed only transiently at the site of assembly around the time that Gag accumulation is completed36; 43. The two completely different behaviors of the ESCRT pathway components suggest different modes of recruitment. In the case of ALIX, the amount recruited appears to be simply proportional to the number of ALIX binding sites that are present at the site of particle assembly. It is even possible that Gag and ALIX are recruited into a nascent virion as a complex that has preformed in the cytoplasm. Conversely, the CHMP and VPS4 proteins behave as if some triggering event that occurs upon termination of Gag accumulation causes their rapid recruitment. This trigger could, perhaps, be the accumulation of a threshold number of CHMP protein binding sites as the ‘early’ ESCRT proteins such as ALIX accumulate to a particular level, or perhaps the attainment of a critical degree membrane curvature. The ‘activation’ of CHMP proteins, whereby a binding site for the next member of a CHMP protein polymer is revealed as each monomer is recruited and activated may also play a role in this characteristic56. ALIX and the CHMP proteins also differ in that ALIX is not efficiently removed through the action of VPS4 while CHMP proteins and VPS4 itself dissociate from assembly sites soon after their deposition36. Since the recruitment of the proteins that are responsible for membrane fission occurs at the time assembly is completed and, because they remain at the site of assembly for ~2 minutes, this leads to the hypothesis that budding occurs quite rapidly after completion of assembly. Notably, catalytically inactive forms of VPS4 that cause budding arrest lead to the accumulation of CHMP proteins at sites of particle budding, demonstrating that VPS4 activity is actually required for the release of virions, and not simply for the recycling of the CHMP proteins and replenishment of soluble pools of ESCRT-III components36.
Concluding remarks and opportunities for further study
Much has been learned about the assembly of HIV-1 and other retroviruses by visualizing the genesis of individual particle in living cells. However, there are several more questions in the biology of HIV-1 assembly that might be usefully tackled using such imaging based approaches. For example, a number of viral and cellular molecules in addition to those already investigated are incorporated into HIV-1 particles to enhance or inhibit viral replication (e.g. envelope, clathrin, tetherin, APOBCE3G); how and when these molecules are packaged is only partly understood and could be illuminated by the imaging of individual particles. One key question that remains only partly resolved, and is probably best addressed by single particle imaging studies in live cells is whether and how HIV-1 selects specific locations on the plasma membrane at which to initiate assembly. Lipid rafts57; 58; 59, tetraspanin enriched microdomains60 and sites of cell-to-cell contact61; 62 have each been posited to be selected by HIV-1 as locations for particle assembly, but how these domains are selected remains unclear. In principle HIV-1 Gag may contain at least two different targeting activities. Specifically, Gag binds to PI(4,5)P2, a plasma-membrane associated phospholipid63; 64, and also likely binds to the cytoplasmic tail of the HIV-1 envelope protein65. Each of these interactions could strongly influence the location on the plasma membrane where the Gag:viral RNA complexes that initiate assembly are enriched prior to virion formation.
A number of imaging studies have indicated that HIV-1 Gag and Env proteins accumulate at sites of cell-to-cell contact, particularly in cultures of infected T-cells61; 62; 66. These accumulations have been termed a ‘virological synapse’ and posited to be sites at which virion particles are assembled and directly transferred from an infected cell to a target cell. Virological synapses have been primarily studied by light and electronic microscopic studies of fixed infected cells, where their formation appears to be dependent on the HIV-1 receptors in the target cell and the HIV-1 envelope in the infected cell66. Some live cell imaging of viriological synapses has been done using spinning disk confocal microscopy techniques and a replication competent HIV-1 molecular clone that carries a GFP between at the C-terminus of MA. The transfer of fluorescent signal from a donor to a target T-cell was tracked and, as was infection of neighboring cells62. While this approach has allowed the direct visualization of the translocation of large quantities of fluorescent Gag from a donor to a target cells, this technique currently lacks the resolution to allow the tracking of individual particles through the virological synapse, or to allow the conclusion that assembly is specifically directed to sites of cell-to-cell contact. However, the notion that assembly might be directed to specific regions of the plasma membrane that are advantageous for virus transfer to new target cells has been quite well developed using MLV37. In this instance, single particle analyses have been used to demonstrate that assembly events occur preferentially at zones of cell-to-cell contact. This apparently directed assembly of individual particles was shown to be dependent on the cytoplasmic tail of the envelope protein and on viral receptors in the neighboring target cell, suggesting that the viral envelope protein may recruit the Gag molecules that initiate assembly37.
Overall, the particular advantages of individual particle imaging in live cells have yielded findings that would have been difficult or impossible to obtain using macroscopic or fixed cell microscopic techniques. Moreover, these techniques should be applicable to many other viruses that assemble at the plasma membrane. With further technological and conceptual development, yet deeper understanding of virus particle assembly processes awaits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gottlinger HG. The HIV-1 assembly machine. Aids. 2001;15(Suppl 5):S13–20. doi: 10.1097/00002030-200100005-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bieniasz PD. The Cell Biology of HIV-1 Virion Genesis. Cell Host Microbe. 2009;5:550–8. doi: 10.1016/j.chom.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opella SJ, Zeri AC, Park SH. Structure, dynamics, and assembly of filamentous bacteriophages by nuclear magnetic resonance spectroscopy. Annu Rev Phys Chem. 2008;59:635–57. doi: 10.1146/annurev.physchem.58.032806.104640. [DOI] [PubMed] [Google Scholar]
- 4.Tritel M, Resh MD. Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates. J Virol. 2000;74:5845–55. doi: 10.1128/jvi.74.13.5845-5855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert U, Ott DE, Chertova EN, Welker R, Tessmer U, Princiotta MF, Bennink JR, Krausslich HG, Yewdell JW. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc Natl Acad Sci U S A. 2000;97:13057–62. doi: 10.1073/pnas.97.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finzi A, Orthwein A, Mercier J, Cohen EA. Productive human immunodeficiency virus type 1 assembly takes place at the plasma membrane. J Virol. 2007;81:7476–90. doi: 10.1128/JVI.00308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsien RY. Building and breeding molecules to spy on cells and tumors. FEBS Lett. 2005;579:927–32. doi: 10.1016/j.febslet.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Rappoport JZ, Simon SM. A functional GFP fusion for imaging clathrin-mediated endocytosis. Traffic. 2008;9:1250–5. doi: 10.1111/j.1600-0854.2008.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon SM. Partial internal reflections on total internal reflection fluorescent microscopy. Trends Cell Biol. 2009;19:661–8. doi: 10.1016/j.tcb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrin-Tricaud C, Davoust J, Jones IM. Tagging the human immunodeficiency virus gag protein with green fluorescent protein. Virology. 1999;255:20–5. doi: 10.1006/viro.1998.9573. [DOI] [PubMed] [Google Scholar]
- 11.Sandefur S, Smith RM, Varthakavi V, Spearman P. Mapping and characterization of the N-terminal I domain of human immunodeficiency virus type 1 Pr55(Gag) J Virol. 2000;74:7238–49. doi: 10.1128/jvi.74.16.7238-7249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermida-Matsumoto L, Resh MD. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J Virol. 2000;74:8670–9. doi: 10.1128/jvi.74.18.8670-8679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller B, Daecke J, Fackler OT, Dittmar MT, Zentgraf H, Krausslich HG. Construction and characterization of a fluorescently labeled infectious human immunodeficiency virus type 1 derivative. J Virol. 2004;78:10803–13. doi: 10.1128/JVI.78.19.10803-10813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudner L, Nydegger S, Coren LV, Nagashima K, Thali M, Ott DE. Dynamic fluorescent imaging of human immunodeficiency virus type 1 gag in live cells by biarsenical labeling. J Virol. 2005;79:4055–65. doi: 10.1128/JVI.79.7.4055-4065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gousset K, Ablan SD, Coren LV, Ono A, Soheilian F, Nagashima K, Ott DE, Freed EO. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 2008;4:e1000015. doi: 10.1371/journal.ppat.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briggs JA, Simon MN, Gross I, Krausslich HG, Fuller SD, Vogt VM, Johnson MC. The stoichiometry of Gag protein in HIV-1. Nat Struct Mol Biol. 2004;11:672–5. doi: 10.1038/nsmb785. [DOI] [PubMed] [Google Scholar]
- 17.Larson DR, Johnson MC, Webb WW, Vogt VM. Visualization of retrovirus budding with correlated light and electron microscopy. Proc Natl Acad Sci U S A. 2005;102:15453–8. doi: 10.1073/pnas.0504812102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jouvenet N, Simon SM, Bieniasz PD. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc Natl Acad Sci U S A. 2009;106:19114–9. doi: 10.1073/pnas.0907364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemler I, Meehan A, Poeschla EM. Live-cell coimaging of the genomic RNAs and Gag proteins of two lentiviruses. J Virol. 2010;84:6352–66. doi: 10.1128/JVI.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Nikolaitchik O, Singh J, Wright A, Bencsics CE, Coffin JM, Ni N, Lockett S, Pathak VK, Hu WS. High efficiency of HIV-1 genomic RNA packaging and heterozygote formation revealed by single virion analysis. Proc Natl Acad Sci U S A. 2009;106:13535–40. doi: 10.1073/pnas.0906822106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouvenet N, Neil SJ, Bess C, Johnson MC, Virgen CA, Simon SM, Bieniasz PD. Plasma membrane is the site of productive HIV-1 particle assembly. PLoS Biol. 2006;4:e435. doi: 10.1371/journal.pbio.0040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neil SJ, Eastman SW, Jouvenet N, Bieniasz PD. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006;2:e39. doi: 10.1371/journal.ppat.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harila K, Prior I, Sjoberg M, Salminen A, Hinkula J, Suomalainen M. Vpu and Tsg101 regulate intracellular targeting of the human immunodeficiency virus type 1 core protein precursor Pr55gag. J Virol. 2006;80:3765–72. doi: 10.1128/JVI.80.8.3765-3772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubner W, Chen P, Del Portillo A, Liu Y, Gordon RE, Chen BK. Sequence of human immunodeficiency virus type 1 (HIV-1) Gag localization and oligomerization monitored with live confocal imaging of a replication-competent, fluorescently tagged HIV-1. J Virol. 2007;81:12596–607. doi: 10.1128/JVI.01088-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez CY, Hope TJ. Mobility of human immunodeficiency virus type 1 Pr55Gag in living cells. J Virol. 2006;80:8796–806. doi: 10.1128/JVI.02159-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nydegger S, Foti M, Derdowski A, Spearman P, Thali M. HIV-1 egress is gated through late endosomal membranes. Traffic. 2003;4:902–10. doi: 10.1046/j.1600-0854.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 27.Pelchen-Matthews A, Kramer B, Marsh M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol. 2003;162:443–55. doi: 10.1083/jcb.200304008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutluay SB, Bieniasz PD. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog. 6:e1001200. doi: 10.1371/journal.ppat.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paillart JC, Gottlinger HG. Opposing effects of human immunodeficiency virus type 1 matrix mutations support a myristyl switch model of gag membrane targeting. J Virol. 1999;73:2604–12. doi: 10.1128/jvi.73.4.2604-2612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–30. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 31.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007;177:329–41. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welsch S, Keppler OT, Habermann A, Allespach I, Krijnse-Locker J, Krausslich HG. HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 2007;3:e36. doi: 10.1371/journal.ppat.0030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanchenko S, Godinez WJ, Lampe M, Krausslich HG, Eils R, Rohr K, Brauchle C, Muller B, Lamb DC. Dynamics of HIV-1 assembly and release. PLoS Pathog. 2009;5:e1000652. doi: 10.1371/journal.ppat.1000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jouvenet N, Bieniasz PD, Simon SM. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature. 2008;454:236–40. doi: 10.1038/nature06998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Caballero D, Hatziioannou T, Martin-Serrano J, Bieniasz PD. Human immunodeficiency virus type 1 matrix inhibits and confers cooperativity on gag precursor-membrane interactions. J Virol. 2004;78:9560–3. doi: 10.1128/JVI.78.17.9560-9563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jouvenet N, Zhadina M, Bieniasz P, Simon S. Dynamics of ESCRT proteins recruitment during retroviral assembly. Nature Cell Biology. 2011 doi: 10.1038/ncb2207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin J, Sherer NM, Heidecker G, Derse D, Mothes W. Assembly of the murine leukemia virus is directed towards sites of cell-cell contact. PLoS Biol. 2009;7:e1000163. doi: 10.1371/journal.pbio.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gladnikoff M, Rousso I. Directly monitoring individual retrovirus budding events using atomic force microscopy. Biophys J. 2008;94:320–6. doi: 10.1529/biophysj.107.114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derdowski A, Ding L, Spearman P. A novel fluorescence resonance energy transfer assay demonstrates that the human immunodeficiency virus type 1 Pr55Gag I domain mediates Gag-Gag interactions. J Virol. 2004;78:1230–42. doi: 10.1128/JVI.78.3.1230-1242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larson DR, Ma YM, Vogt VM, Webb WW. Direct measurement of Gag-Gag interaction during retrovirus assembly with FRET and fluorescence correlation spectroscopy. J Cell Biol. 2003;162:1233–44. doi: 10.1083/jcb.200303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fusco D, Accornero N, Lavoie B, Shenoy SM, Blanchard JM, Singer RH, Bertrand E. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr Biol. 2003;13:161–7. doi: 10.1016/s0960-9822(02)01436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–5. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 43.Baumgartel V, Ivanchenko S, Dupont A, Sergeev M, Wiseman PW, Krausslich HG, Brauchle C, Muller B, Lamb DC. Live-cell visualization of dynamics of HIV budding site interactions with an ESCRT component. Nat Cell Biol. 13:469–74. doi: 10.1038/ncb2215. [DOI] [PubMed] [Google Scholar]
- 44.Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–73. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Carlton JG, Martin-Serrano J. The ESCRT machinery: new functions in viral and cellular biology. Biochem Soc Trans. 2009;37:195–9. doi: 10.1042/BST0370195. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Serrano J, Yarovoy A, Perez-Caballero D, Bieniasz PD. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc Natl Acad Sci U S A. 2003;100:12414–9. doi: 10.1073/pnas.2133846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, Scott A, Krausslich HG, Kaplan J, Morham SG, Sundquist WI. The protein network of HIV budding. Cell. 2003;114:701–13. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 48.Strack B, Calistri A, Craig S, Popova E, Gottlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–99. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 49.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–68. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 50.Langelier C, von Schwedler UK, Fisher RD, De Domenico I, White PL, Hill CP, Kaplan J, Ward D, Sundquist WI. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J Virol. 2006;80:9465–80. doi: 10.1128/JVI.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–7. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VerPlank L, Bouamr F, LaGrassa TJ, Agresta B, Kikonyogo A, Leis J, Carter CA. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag) Proc Natl Acad Sci U S A. 2001;98:7724–9. doi: 10.1073/pnas.131059198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM, Cote M, Rich RL, Myszka DG, Sundquist WI. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 54.Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7:1313–9. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- 55.Fisher RD, Chung HY, Zhai Q, Robinson H, Sundquist WI, Hill CP. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell. 2007;128:841–52. doi: 10.1016/j.cell.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 56.Zamborlini A, Usami Y, Radoshitzky SR, Popova E, Palu G, Gottlinger H. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc Natl Acad Sci U S A. 2006;103:19140–5. doi: 10.1073/pnas.0603788103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen DH, Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–72. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A. 2001;98:13925–30. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindwasser OW, Resh MD. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J Virol. 2001;75:7913–24. doi: 10.1128/JVI.75.17.7913-7924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nydegger S, Khurana S, Krementsov DN, Foti M, Thali M. Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1. J Cell Biol. 2006;173:795–807. doi: 10.1083/jcb.200508165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jolly C, Sattentau QJ. Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J Virol. 2007;81:7873–84. doi: 10.1128/JVI.01845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–7. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:14889–94. doi: 10.1073/pnas.0405596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. From the Cover: Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci U S A. 2006;103:11364–9. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wyma DJ, Kotov A, Aiken C. Evidence for a stable interaction of gp41 with Pr55(Gag) in immature human immunodeficiency virus type 1 particles. J Virol. 2000;74:9381–7. doi: 10.1128/jvi.74.20.9381-9387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jolly C, Sattentau QJ. Retroviral spread by induction of virological synapses. Traffic. 2004;5:643–50. doi: 10.1111/j.1600-0854.2004.00209.x. [DOI] [PubMed] [Google Scholar]