Abstract
Introduction. Aberrant experience of agency is characteristic of schizophrenia. An understanding of the neurobiological basis of such experience is therefore of considerable importance for developing successful models of the disease. We aimed to characterise the effects of ketamine, a drug model for psychosis, on sense of agency (SoA). SoA is associated with a subjective compression of the temporal interval between an action and its effects: This is known as “intentional binding”. This action–effect binding provides an indirect measure of SoA. Previous research has found that the magnitude of binding is exaggerated in patients with schizophrenia. We therefore investigated whether ketamine administration to otherwise healthy adults induced a similar pattern of binding.
Methods. 14 right-handed healthy participants (8 female; mean age 22.4 years) were given low-dose ketamine (100 ng/mL plasma) and completed the binding task. They also underwent structured clinical interviews.
Results. Ketamine mimicked the performance of schizophrenia patients on the intentional binding task, significantly increasing binding relative to placebo. The size of this effect also correlated with aberrant bodily experiences engendered by the drug.
Conclusions. These data suggest that ketamine may be able to mimic certain aberrant agency experiences that characterise schizophrenia. The link to individual changes in bodily experience suggests that the fundamental change produced by the drug has wider consequences in terms of individuals’ experiences of their bodies and movements.
Keywords: Action-outcome binding, Ketamine, Schizophrenia, Sense of agency, Volition, Voluntary action
INTRODUCTION
Administration of the anaesthetic agent, ketamine, to healthy participants produces a state that resembles schizophrenia (Ghoneim, Hinrichs, Mewaldt, & Petersen, 1985; Krystal et al., 1994; Lahti, Weiler, Tamara Michaelidis, Parwani, & Tamminga, 2001). Although there are notable differences between the ketamine state and established schizophrenic illness (for example, ketamine does not reliably produce auditory hallucinations; Fletcher & Honey, 2006), ketamine does produce a range of symptoms associated with endogenous psychosis, including perceptual changes, ideas of reference, thought disorder, and some negative symptoms (Ghoneim et al., 1985; Krystal et al., 1994; Lahti et al., 2001; Mason, Morgan, Stefanovic, & Curran, 2008; Morgan, Mofeez, Brandner, Bromley, & Curran, 2004; Pomarol-Clotet et al., 2006). In addition, a number of cognitive changes produced by ketamine are comparable to those seen in schizophrenia (e.g., learning: Corlett, Murray, et al., 2007; memory: Fletcher & Honey, 2006; attention: Oranje et al., 2000; language: Covington et al., 2007). Overall, the effects of ketamine are most strikingly characteristic of the earliest stages of psychosis (Corlett, Honey, & Fletcher, 2007). Moreover, ketamine causes changes in brain activity that overlap with those reported in schizophrenia (Breier, Malhotra, Pinals, Weisenfeld, & Pickar, 1997; Corlett et al., 2006; Vollenweider, Leenders, Oye, Hell, & Angst, 1997; Vollenweider, Leenders, Scharfetter, et al., 1997). An important next step is to explore the effects of ketamine in greater detail and to exploit the potential that this approach offers for relating cognitive-behavioural function to subjective experiences in psychosis.
Schizophrenia is associated with important changes in the experience of voluntary action such as those that occur in delusions of control (Frith, 1992). Although it has received little formal documentation, ketamine also, in our experience, alters the way that participants experience their own actions. For example, participants sometimes report that they don't feel fully in control of their own actions (“I don't feel in control of my muscles …,” and “… as though someone else was controlling my movements;” Pomarol-Clotet et al., 2006). Given these observations, together with the perceptuomotor abnormalities in schizophrenia, the current study was set up to characterise the effects of ketamine on a task examining voluntary actions and their sensory consequences.
Sense of agency (SoA) refers to the experience of initiating and controlling voluntary action to achieve effects in the outside world. Sense of agency is a background feeling that accompanies most of our actions. Perhaps because of its ubiquity, it has proved difficult to isolate and measure experimentally. Recently, action-related changes in time perception have been proposed as a proxy for SoA (Haggard, Clark, & Kalogeras, 2002; Moore & Haggard, 2008; Moore, Lagnado, Deal, & Haggard, 2009).
Situations that elicit SoA are associated with systematic changes in the temporal experience of actions and outcomes: There is a subjective compression of the interval between the action and the outcome. This relation between SoA and subjective time is revealed in the intentional binding paradigm developed by Haggard et al. (2002). In an agency condition, in which participants’ actions produced outcome tones, participants judged the time of an action or the time of the subsequent tone, in separate blocks of trials. Actions were perceived as occurring later in time compared to a nonagency (baseline) condition in which participants’ actions did not produce tones. In addition, a tone that followed the action was perceived as occurring earlier in time compared to a nonagency (baseline) condition involving tones but no actions. Importantly, these shifts were only found for voluntary actions: When the outcome was caused by an involuntary movement the reverse pattern of results was observed (actions perceived earlier and outcomes perceived later than their respective baseline estimates).
Increased SoA is therefore associated with a later awareness of the action, and an earlier awareness of the outcome. This effect is robust and has been consistently replicated (see, for example, Engbert & Wohlschläger, 2007; Engbert, Wohlschläger, & Haggard, 2008; Moore, Wegner, & Haggard, 2009; Tsakiris & Haggard, 2003). It has also been shown that these changes in the subjective experience of time correlate with explicit higher order changes in the sense of agency, as measured using subjective rating scales (Ebert & Wegner, 2010; Moore & Haggard, 2010). In this way, intentional binding offers a precise, implicit measure of SoA.
Of primary interest to the present study is the fact that the binding effect, defined as the temporal attraction between voluntary action and outcome, is greater in people with schizophrenia (Haggard, Martin, Taylor-Clarke, Jeannerod, & Franck, 2003; Voss et al., 2010). That is, people with schizophrenia show increased intentional binding. Our principal aim here was to determine whether ketamine also induced increased binding, as previously reported in schizophrenia.
We also investigated the relationship between this implicit measure of SoA and subjective experiences of dissociation and psychotic-like phenomena produced by the drug as measured using the Clinician-Administered Dissociative States Scale (CADSS; Bremner et al., 1998). Here we focused our analysis on changes in the subjective experience of one's own body, since sense of ownership (SoO) over one's body and SoA may be related. For example, in healthy individuals SoA for a voluntary action may strongly depend on a SoO (Gallagher, 2000, 2007; Tsakiris, Schütz-Bosbach, & Gallagher, 2007). The reverse relationship may also hold, whereby the neurocognitive processes that give rise to sense of agency also contribute to Soo (Tsakiris, Prabhu, & Haggard, 2006).
Dissociative symptoms, such as depersonalisation, are a common effect of the ketamine challenge (Goff & Coyle, 2001). Furthermore, there is frequent co-occurrence of depersonalisation and abnormal bodily experience (Sierra, Baker, Medford, & David, 2005; Simeon et al., 2008). Although not typically associated with established schizophrenic illness, depersonalisation appears to be associated with the schizophrenia prodrome (Goff & Coyle, 2001; Krystal et al., 1994). Therefore, given the link between bodily experience and sense of agency, and the common disruption of bodily experience engendered by the ketamine challenge, the body perception subscale on the CADSS questionnaire was of primary interest.
MATERIALS AND METHODS
Participants
Eighteen right-handed healthy volunteers were recruited (eight female; mean age = 22.4, range = 19–26; mean NART IQ = 114 [± 7]). The study was approved by Addenbrooke's NHS Trust Research Ethics Committee. Participants provided written, informed consent.
One participant was excluded from the analysis on the basis of a preexisting history of psychiatric illness (although all participants were screened for the presence of psychiatric illness in themselves and relatives prior to taking part in the study, this participant only disclosed this information after testing). Three participants failed to complete the intentional binding task owing to nausea produced by the drug infusion. Therefore, 14 participants were included in the final analysis.
These same participants also completed other cognitive tasks, unrelated to SoA, during infusion. It is planned to publish those results elsewhere.
Experimental design
The study used a double-blind, placebo-controlled, randomised, within-subjects design.
Infusion protocol
Participants were administered placebo (saline) or racemic ketamine (2 mg/mL) as an intravenous infusion using a target-controlled infusion system comprising a computer which implemented Stanpump software (S Shafer; http://www.opentci.org/doku.php?id=code:code) to control a syringe driver infusion pump (Graseby 3500; Graseby Medical Ltd, Watford, UK). Stanpump was programmed to use a two-compartmental pharmacokinetic model (Rigby-Jones, Sneyd, & Absalom, 2006), to implement a complex infusion profile designed to achieve prespecified plasma ketamine concentrations.
During the drug session, participants received first low-dose ketamine (plasma target 100 ng/mL) and then higher dose (plasma target 200 ng/mL). The intentional binding task was completed at the low dose. Drug and placebo sessions were separated by at least 1 week. Participants also underwent a clinical rating (see later). The order of drug and placebo visits was counterbalanced across all 18 participants initially recruited. Of the 14 participants who were included in the final analysis, eight participants completed the ketamine session first.
Intentional binding
Participants watched a computer screen on which a hand rotated around a clock-face (marked at conventional “5-minute” intervals) (see Figure 1). Each full rotation lasted 2560 ms. In the agency condition, participants pressed a key with their right index finger at a time of their choosing. This keypress produced a tone after a delay of 250 ms. The clock-hand continued rotating for a random period of time (between 1500 ms and 2500 ms). This ensures that the finishing position of the clock-hand is not informative with respect to where it was when the action or tone occurred (see Libet, Gleason, Wright, & Pearl, 1983). When the hand stopped rotating, participants verbally reported the time of their keypress or the subsequent tone. These judgements were blocked, so participants only made a single type of estimate on each trial in each block. To make the time estimates, participants reported the position of the hand on the clock face when they either pressed the key or heard the tone. Participants completed a block of 20 action estimate trials and a block of 20 tone estimate trials.
Figure 1.
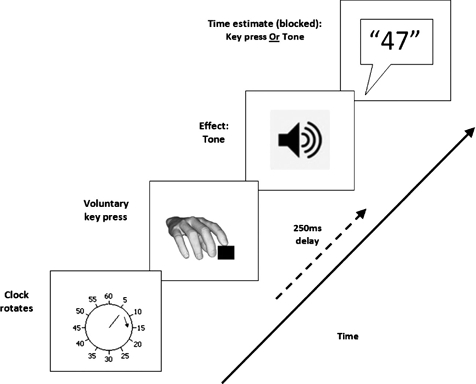
Trial structure in the agency condition (following Haggard et al., 2002). Participants pressed the key at a time of their choosing, which produced a tone after a delay of 250 ms. Participants judged where the clock hand was when they pressed the key or when they heard the tone, in separate blocks of trials.
They completed two further 20 trial baseline blocks of time estimates. In one block (baseline action) participants pressed the key at a time of their choosing. However, the keypress never produced a tone, and on each trial participants reported the time of the keypress. In the other block (baseline effect) participants made no keypresses. Instead, a tone would sound at a random time on each trial and participants reported the time of the tone. The order of agency and baseline blocks was randomised anew for each participant. All blocks (baseline and agency) were performed during the drug/saline infusion.
For our analysis we calculated an overall measure of intentional binding. We first calculated the binding effect for actions and tones individually. Action binding is found by subtracting the mean time estimate in the baseline action condition from the mean time estimate of actions in the agency condition. Tone binding is found by subtracting the mean time estimate in the baseline tone condition from the mean time estimate of tones in the agency condition. The overall measure of intentional binding was calculated by combining action and tone binding (i.e., action binding minus tone binding). To determine the effect of ketamine on intentional binding (and therefore SoA), this overall measure of intentional binding was compared within subjects (ketamine vs. placebo; paired-samples t-test).
Clinical assessment
The Clinician-Administered Dissociative States Scale (CADSS; Bremner et al., 1998) was administered at both 100 ng/mL and 200 ng/mL. There are five subscales, each of which consists of items (questions), and participants’ responses are coded on a 5-point scale (0 = “Not at all” through to 4 = “Extremely”). As discussed, our analysis focused primarily on the body perception category. We assessed the strength of correlation between scores on items relating to body perception at 100 ng/mL with binding on ketamine.
RESULTS
During the intentional binding task, the target plasma ketamine concentration was 100 ng/mL, and the mean ± SD) measured ketamine plasma concentration was 157 ± 36 ng/mL.
Ketamine effects on intentional binding
Table 1 presents the binding effects for keypresses and tones (mean shifts from baseline) for the 14 participants who completed the task. These data show that in the agency conditions on both placebo and ketamine, keypresses were bound towards tones and tones were bound back towards keypresses. This is consistent with the intentional binding effect, as previously reported (e.g., Engbert et al., 2008; Haggard et al., 2002; Moore & Haggard, 2008).
TABLE 1.
Mean judgement errors in ms (SD across subjects) and shifts relative to baseline conditions in ms
| Judged event | Mean (SD) judgement error (ms) | Mean shift from baseline (ms) (SD) | Overall binding measure (ms) (SD) | |
| Baseline conditions | ||||
| Placebo | Action | 4(42) | ||
| Tone | −14(55) | |||
| Ketamine | Action | −24 (52) | ||
| Tone | −8 (46) | |||
| Agency conditions | ||||
| Placebo | Action | 26 (55) | 22 (36) | |
| Tone | −37 (61) | −23 (51) | 45 (69) | |
| Ketamine | Action | 28 (63) | 52 (38) | |
| Tone | −28 (71) | −20 (59) | 72 (70) | |
Table 1 (final column) also presents the overall binding measure (keypress binding minus tone binding). These data show that overall binding was greater under ketamine compared with placebo. A paired-samples t-test revealed that this difference was significant, t(13) = 2.79, p = .008 (onetailed). Follow-up paired sample t-tests suggest that this difference is due to differences in binding for actions towards tones, t(13) = 2.35, p = .036 (two-tailed) rather than differences in binding for tones towards actions, t(13) = 0.242, p = .812 (two-tailed). Furthermore, this exaggerated binding appears to be driven by changes in baseline action judgements; isolated actions on ketamine were perceived as occurring significantly earlier than on placebo, t(13) = 2.59, p = .023 (two-tailed). Intentional binding is an implicit measure of SoA. These findings therefore suggest that SoA is exaggerated under ketamine, which is consistent with previous data on patients with schizophrenia (Haggard et al., 2003; Voss et al., 2010).
The relation between binding and body perception
We also examined the strength of correlation between binding on ketamine and scores on the CADSS assessment. The overall main effect of ketamine was generated by changes in action binding. Therefore, our correlations were based on this binding measure. There was no significant correlation between action binding and the overall CADSS score, r = .197, p = 499 (two-tailed). Further analyses focused on the body perception subscale. There was a significant positive correlation between action binding and Item 6 on the CADSS, which asks, “Do you feel disconnected from your own body?,” r = .549, p = .042 (two-tailed) (see Figure 2). This suggests that the more participants felt disconnected from their bodies on ketamine, the greater the intentional binding effect. There was no significant correlation between action binding and Item 7 on the CADSS which asks “Does your sense of your own body feel changed: for instance, does your own body feel unusually large or unusually small?,” r = .208, p = .476 (two-tailed).
Figure 2.
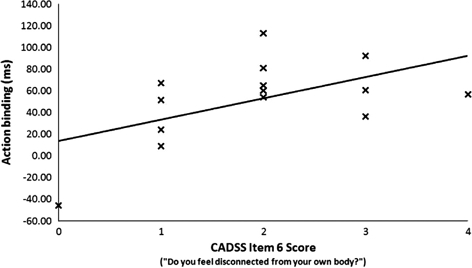
Scatter plot showing the significant correlation between action binding and CADSS Item 6 (“Do you feel disconnected from your own body?”) on ketamine (100 ng/mL).
Control analyses
The CADSS questionnaire also measures changes in time perception. Given the temporal nature of our SoA measure we investigated the putative relation between binding and general changes in time perception. There were no significant correlations between action binding and time perception items on the scale (Item 1 “Do things seem to be moving in slow motion?,” r = 198, p = .498; Item 12 “Does this experience seem to take much longer than you would have expected?,” r = −.337, p = .238; Item 13 “Do things seem to be happening very quickly, as if there is a lifetime in a moment?,” r = .265, p = .360). This suggests that changes in action binding were not related to general changes in the subjective experience of time.
To determine the presence of possible drug order effects in our data we compared mean overall binding on ketamine versus placebo, introducing “order” (ketamine first vs. placebo first) as a between-subjects variable. We found no significant main effect of “order,” F(1, 12) = 0.381, p = .548, and no significant interaction, F(1, 12) = 0.889, p = 364. This suggests that changes in binding were not linked to drug order.
We also compared standard deviations of time estimates across repeated trials. These provide a measure of perceptual timing variability, with higher standard deviations reflecting inconsistent timing performance. This may indicate difficulty in using the clock for timing judgements, erratic allocation of attention either to the action/tone or to the clock, or general confusion. The increase in binding on ketamine was driven by differences in the binding of actions towards tones, so we focus on standard deviation of action time estimates. On ketamine the mean standard deviation was 77 ms (SD = 32) while on placebo it was 67 ms (SD = 18). Despite the numerical increase, the difference in mean standard deviation was not significant, t(13) = 1.149, p = .271 (two-tailed). This suggests that changes in action binding were not related to general changes in timing ability.
In a final control analysis, we investigated whether there was a significant reduction in the speed of the self-paced response on ketamine, as it could be that changes in binding are related to changes in motor function. On ketamine the mean response latency was 3798 ms (SD = 1580), whereas on placebo it was 3538 ms (SD = 1160). Despite the numerical increase in response latency, this difference was not significant, t(13) = 0.945, p = .362 (two-tailed). This suggests that changes in action binding were not related to changes in motor function (as measured by the response latency).
DISCUSSION
We investigated whether the psychotomimetic effects of ketamine extend to producing aberrant agency experiences associated with schizophrenia. On the intentional binding task under placebo conditions, the expected binding effect (a compression of the subjective interval between action and outcome; Haggard et al., 2002) was observed. Under ketamine this effect was exaggerated, as has been previously reported in people with schizophrenia (Haggard et al., 2003; Voss et al., 2010).
The effect of ketamine on action–outcome binding is intriguing: The exaggerated effect was driven primarily by an increase in binding of actions towards the tone, rather than binding of tones back towards actions. Action binding represents the difference between action time estimates in the agency condition and action time estimates in the baseline condition. Previous studies have found that the experience of isolated action, as in the baseline condition, is anticipatory: On average, participants are aware of moving slightly before the actual onset of movement (Haggard, Newman, & Magno, 1999; Libet et al., 1983). This suggests that motor experience in this context is not based on feedback generated by the actual movement itself. If it were, one would expect a slightly delayed awareness of moving owing to inherent delays in the transmission of sensory information to the brain (Obhi, Planetta, & Scantlebury, 2009). Instead, it has been proposed that the experience of isolated action is linked to processes occurring prior to movement onset (Haggard, 2003). In our data, the baseline experience of action on ketamine was significantly earlier than on placebo, whereas the baseline action awareness on placebo was, unusually, slightly delayed relative to the actual keypress. This pattern of results suggests that the drug may have exaggerated the putative influence of action preparation on the experience of action.
However, although baseline action experience is generally anticipatory, the intentional binding effect in healthy adults shows that causing an external event through one's own actions (as in our agency condition) draws the temporal experience of action towards that event (Engbert & Wohlschläger, 2007; Haggard et al., 2002; Moore & Haggard, 2008; Moore, Lagnado, et al., 2009). It is this shift in action experience that represents the binding effect for actions, and it was present on both placebo and ketamine. However, the magnitude of the shift was significantly greater on ketamine. It appears, therefore, that the presence of the tone exerted a particularly strong influence on action experience. In short, although ketamine has a strong effect on action experience when the action occurs without a perceptual consequence, we cannot interpret the drug's effect merely in terms of this baseline action experience. Rather, the significantly greater subjective shift, on ketamine, in the experience of action towards the tone means that a full explanation of the effects of ketamine must take into account the experience of action in both the absence and the presence of the tone.
Thus, bringing together the key results from the intentional binding task, ketamine appears to boost the influence of action preparation on action awareness, but also to boost the influence of the effects of action (a tone) on action awareness. This combination may seem paradoxical. However, several results suggest that the action experience is in fact a synthesis of a range of different events occurring over an extended time period between preparation and consequence (Banks & Isham, 2009; Haggard, 2005; Haggard, Cartledge, Dafydd, & Oakley, 2004; Lau, Rogers, & Passingham, 2007; Moore & Haggard, 2008; Moore, Wegner, et al., 2009). In normal circumstances, action awareness is likely to be the result of integration of efferent and afferent processes in the sensorimotor system (Moore & Haggard, 2008; Moore, Wegner, et al., 2009; Synofzik, Vosgerau, & Newen, 2008). On ketamine, however, the processes underlying this normal process of integration may be compromised.
To this extent, our results are consistent with a ketamine-induced deficit in monitoring action signals. Participants appeared to feel dissociated from their own actions while on ketamine, since their representations of their own actions were susceptible to influences from other events, such as their original intentions and their subsequent effects. Confirmation of this dissociative interpretation comes from the correlations found between intentional binding and the specific CADSS item concerning the feeling of disconnection from the body. Taken together, these findings suggest that ketamine may preferentially influence a neural system for monitoring action. As a result of this deficit, actions on ketamine become mutable and vulnerable to capture by other events. However, given the apparently tight coupling of SoO and SoA, the fact that increased SoA was associated with an increase in the feeling of disconnection from one's body may be surprising. Dissociations between SoO and SoA are not uncommon in psychiatric illness such as schizophrenia. For example, a patient with passivity phenomena will recognise their actions as the movements of their own body (preserved SoO) but will experience their actions as produced by an external force (reduced SoA). However, these dissociations cannot explain our finding that an increase in SoA was associated with reduced SoO on ketamine. The mutability hypothesis discussed earlier may provide an explanation: If ketamine engenders mutability in the experience of action, then the more one's experience of action is “captured” by external sensory events the greater the externalisation of bodily experience may be, resulting in the feeling of “disconnection” from one's own body.
What might be the neurochemical and neuroanatomical basis of the hyperbinding effect we observed? One possibility is that hyperbinding is the product of aberrant prediction error signalling. Prediction error refers to the mismatch between expectation and occurrence, and is used as a teaching signal to drive causal associations between events (Dickinson, 2001). Although midbrain dopamine neurons may signal a reward prediction error (Schultz & Dickinson, 2000), others have argued that their activity profile may reflect a novelty, salience, or surprise signal used by organisms to judge whether or not they caused a surprising event to happen (Redgrave & Gurney, 2006). We have previously shown that ketamine induces prediction error responses to predictable events and thus increases the salience of those events (Corlett et al., 2006). Neurochemically, ketamine may increase dopamine and glutamate corelease, in the mesocortical pathway between the midbrain and prefrontal cortex (Corlett et al., 2006; Corlett, Honey, et al., 2007). Such signalling has been suggested to register surprise and permit its explanation (Lavin et al., 2005). Since associations between intention, action, and outcome are well learned, the ketamine induced hyperbinding effect we report presently may reflect inappropriate salience of action–outcome causal associations, via aberrant prediction error signalling. Our findings overall are compatible with the notion that the execution of action and SoA may be linked by a simple computational principle (minimising prediction error), which, when perturbed, could explain the varied phenomenology of psychosis (Corlett, Frith, & Fletcher, 2009; Fletcher & Frith, 2009).
The hyperbinding found previously in schizophrenia patients (Haggard et al., 2003; Voss et al., 2010), and here found also with ketamine, suggests an exaggerated SoA. A number of other studies, using different paradigms, have reported data that are consistent with this interpretation. For example, people with schizophrenia (including those experiencing passivity symptoms) are more likely than healthy controls to attribute the source of distorted or ambiguous visual feedback of an action to themselves (Daprati et al., 1997; Fourneret et al., 2002; Franck et al., 2001; Schnell et al., 2008). This suggests a tendency towards over-attribution of sensory consequences of movement to oneself (Synofzik et al., 2008). However, these data are at odds with the feeling of reduced SoA that is typically reported by patients. One solution to this paradox is offered by Franck et al. (2001), who have suggested that patients with passivity symptoms have a tendency towards self-attribution of extraneous events (see also Daprati et al., 1997). This could result in a feeling of being influenced when observing another action, and hyperassociation when observing action outcomes. In short, it may be possible to recognise strongly the outcomes of one's actions while at the same time feeling a diminished sense of agency for the actions themselves. This implies a distinction between feeling one is the author of action on the one hand, and feeling one is the author of an effect on the other. This putative distinction would be usefully explored in future studies.
It should also be noted that exaggerated SoA may be associated with certain schizophrenia subtypes, particularly those with self-referential symptoms. For example, patients with persecutory delusions feel a greater sense of control over action outcomes compared with healthy and patient controls (Kaney & Bentall, 1992). Therefore, the exaggerated agency effects shown in previous patient studies could be driven by the presence of patients with self-referential symptoms in these samples. Intriguingly, self-referential symptoms are also a common effect of the ketamine challenge (Corlett, Honey, & Fletcher, 2007; Honey et al., 2006). It may be, therefore, that the increased SoA found in the current study is associated with this specific effect of the drug.
Our study shows that ketamine can mimic aberrant agency experiences associated with schizophrenia, but certain limitations of the task used should be noted. Unlike previous intentional binding studies (Haggard et al., 2002), we did not include any involuntary movement conditions. Using transcranial magnetic stimulation to induce involuntary movements, Haggard et al. (2002) showed that the binding of actions and outcomes was specific to voluntary, self-generated movement. In fact, when involuntary transcranial magnetic stimulation (TMS)-induced movements were followed by tones, they found a temporal repulsion between involuntary movement and tone. We did not include this TMS condition because the focus of our investigation was whether ketamine increased the magnitude of intentional binding for voluntary actions. It would be interesting in the future to explore the effect of the ketamine challenge on this “repulsion” effect.
Limitations of the paradigm should also be noted. Intentional binding represents an implicit measure of agency experience. That is, participants are not required to make explicit agency judgements, such as the attribution of an observed movement to its correct origin (as in Farrer & Frith, 2002, for example). Implicit measures have certain advantages, such as the quantification of subjective experience, and the mitigation of demand effects. Also, such tasks may allow us to detect subtle perceptual and cognitive changes engendered by the drug and relate them to the early stages of psychosis. However, there are certain drawbacks. Primarily, implicit measures will fail to capture the broader phenomenology of SoA, in particular the highly complex phenomenology associated with delusions of agency in established psychosis. In the current study this limitation was mitigated somewhat by the observation that changes in these subtle implicit measures correlate with participants’ self-reports of drug-induced changes in body experience.
Finally, limitations of the ketamine model of schizophrenia should also be acknowledged. For example, ketamine produces a range of symptoms associated with endogenous psychosis (arguably a broader range than other drug models of the disease; Krystal et al., 1994), but there are notable exceptions (Fletcher & Honey, 2006). Furthermore, ketamine produces changes that are not necessarily associated with schizophrenia, such as euphoria (Fletcher & Honey, 2006). Although it is important to acknowledge limitations of the drug model, we do not feel they undermine our interpretation of the present data, given the fact that these data are consistent with schizophrenic psychopathology and replicate previous behavioural data from patients with the disease (Haggard et al., 2003; Voss et al., 2010).
Despite these caveats, this study shows that the psychotomimetic property of ketamine may extend to aberrant experiences of agency associated with schizophrenia. In particular, ketamine mimics the exaggerated intentional binding effect that has been found in association with the disease. The pattern of results suggested a mutable experience of action on ketamine, consistent with a deficit in the neural circuits for action monitoring. We believe that these findings may be explained in terms of changes in stimulus salience via aberrant prediction error signalling. Ketamine may be a valuable psychopharmacological model of aberrant agency experiences found in schizophrenia. To this extent, it could be used to elucidate the neurobiological and psychological basis of such aberrant experiences.
Acknowledgments
This work was supported by the Wellcome Trust and the Bernard Wolfe Health Neuroscience fund. It was carried out at the Wellcome Trust Clinical Research Facility (Addenbrooke's Hospital, Cambridge) and within the Behavioural and Clinical Neurosciences Institute, jointly supported by the Medical Research Council and the Wellcome Trust. JM and PCF had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- Banks W.P., Isham E.A. We infer rather than perceive the moment we decided to act. Psychological Science. 2009;20(1):17–21. doi: 10.1111/j.1467-9280.2008.02254.x. doi: 10.1111/j.1467-9280.2008.02254.x. [DOI] [PubMed] [Google Scholar]
- Breier A., Malhotra A.K., Pinals D.A., Weisenfeld N.I., Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. American Journal of Psychiatry. 1997;154(6):805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Krystal J.H., Putnam F.W., Southwick S.M., Marmar C., Charney D.S., Mazure C.M. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) Journal of Traumatic Stress. 1998;11(1):125–136. doi: 10.1023/A:1024465317902. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Corlett P.R., Frith C.D., Fletcher P.C. From drugs to deprivation: A Bayesian framework for understanding models of psychosis. Psychopharmacology. 2009;206(4):515–530. doi: 10.1007/s00213-009-1561-0. doi: 10.1007/s00213-009-1561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett P.R., Honey G.D., Aitken M. R.F., Dickinson A., Shanks D.R., Absalom A.R., et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: Linking cognition, brain activity, and psychosis. Archives of General Psychiatry. 2006;63(6):611–621. doi: 10.1001/archpsyc.63.6.611. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- Corlett P.R., Honey G.D., Fletcher P.C. From prediction error to psychosis: Ketamine as a pharmacological model of delusions. Journal of Psychopharmacology. 2007;21(3):238–252. doi: 10.1177/0269881107077716. doi: 10.1177/0269881107077716. [DOI] [PubMed] [Google Scholar]
- Corlett P.R., Murray G.K., Honey G.D., Aitken M. R.F., Shanks D.R., Robbins T.W., et al. Disrupted prediction-error signal in psychosis: Evidence for an associative account of delusions. Brain. 2007;130(9):2387–2400. doi: 10.1093/brain/awm173. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington M.A., Riedel W.J., Brown C., He C., Morris E., Weinstein S., et al. Does ketamine mimic aspects of schizophrenic speech? Journal of Psychopharmacology. 2007;21(3):338–346. doi: 10.1177/0269881107077729. doi: 10.1177/0269881107077729. [DOI] [PubMed] [Google Scholar]
- Daprati E., Franck N., Georgieff N., Proust J., Pacherie E., Dalery J., Jeannerod M. Looking for the agent: An investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition. 1997;65(1):71–86. doi: 10.1016/s0010-0277(97)00039-5. [DOI] [PubMed] [Google Scholar]
- Dickinson A. The 28th Bartlett Memorial Lecture. Causal learning: An associative analysis. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 2001;54B(1):3–25. doi: 10.1080/02724990042000010. [DOI] [PubMed] [Google Scholar]
- Ebert J.P., Wegner D.M. Time warp: Authorship shapes the perceived timing of actions and events. Consciousness and Cognition. 2010;19(1):481–489. doi: 10.1016/j.concog.2009.10.002. doi: 10.1016/j.concog.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert K., Wohlschläger A. Intentions and expectations in temporal binding. Consciousness and Cognition. 2007;16(2):255–264. doi: 10.1016/j.concog.2006.09.010. doi: 10.1016/j.concog.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Engbert K., Wohlschläger A., Haggard P. Who is causing what? The sense of agency is relational and efferent-triggered. Cognition. 2008;107(2):693–704. doi: 10.1016/j.cognition.2007.07.021. doi: 10.1016/j.cognition.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Farrer C., Frith C.D. Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. NeuroImage. 2002;15(3):596–603. doi: 10.1006/nimg.2001.1009. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Fletcher P.C., Frith C.D. Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nature Reviews Neuroscience. 2009;10(1):48–58. doi: 10.1038/nrn2536. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- Fletcher P.C., Honey G.D. Schizophrenia, ketamine and cannabis: Evidence of overlapping memory deficits. Trends in Cognitive Sciences. 2006;10(4):167–174. doi: 10.1016/j.tics.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Fourneret P., de Vignemont F., Franck N., Slachevsky A., Dubois B., Jeannerod M. Perception of self-generated action in schizophrenia. Cognitive Neuropsychiatry. 2002;7(2):139–156. doi: 10.1080/13546800143000212. doi: 10.1080/13546800143000212. [DOI] [PubMed] [Google Scholar]
- Franck N., Farrer C., Georgieff N., Marie-Cardine M., Daléry J., d'Amato T., Jeannerod M. Defective recognition of one's own actions in patients with schizophrenia. American Journal of Psychiatry. 2001;158(3):454–459. doi: 10.1176/appi.ajp.158.3.454. [DOI] [PubMed] [Google Scholar]
- Frith C.D. The cognitive neuropsychology of schizophrenia. Hove, UK: Psychology Press; 1992. [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Sciences. 2000;4(1):14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Sense of agency and higher-order cognition: Levels of explanation for schizophrenia. Cognitive Semiotics. 2007;0:32–48. [Google Scholar]
- Ghoneim M.M., Hinrichs J.V., Mewaldt S.P., Petersen R.C. Ketamine: Behavioral effects of subanesthetic doses. Journal of Clinical Psychopharmacology. 1985;5(2):70–77. [PubMed] [Google Scholar]
- Goff D.C., Coyle J.T. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. American Journal of Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Haggard P. Conscious awareness of intention and action. In: Roessler J., Eilan N., editors. Agency and self-awareness. Oxford, UK: Oxford University Press; 2003. pp. 111–127. [Google Scholar]
- Haggard P. Conscious intention and motor cognition. Trends in Cognitive Sciences. 2005;9(6):290–295. doi: 10.1016/j.tics.2005.04.012. doi: 10.1016/j.tics.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Haggard P., Cartledge P., Dafydd M., Oakley D.A. Anomalous control: When “freewill” is not conscious. Consciousness and Cognition. 2004;13(3):646–654. doi: 10.1016/j.concog.2004.06.001. doi: 10.1016/j.concog.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Haggard P., Clark S., Kalogeras J. Voluntary action and conscious awareness. Nature Neuroscience. 2002;5(4):382–385. doi: 10.1038/nn827. doi: 10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- Haggard P., Martin F., Taylor-Clarke M., Jeannerod M., Franck N. Awareness of action in schizophrenia. Neuroreport. 2003;14(7):1081–1085. doi: 10.1097/01.wnr.0000073684.00308.c0. doi: 10.1097/01.wnr.0000073684.00308.c0. [DOI] [PubMed] [Google Scholar]
- Haggard P., Newman C., Magno E. On the perceived time of voluntary actions. British Journal of Psychology. 1999;90:291–303. doi: 10.1348/000712699161413. [DOI] [PubMed] [Google Scholar]
- Honey G.D., O'Loughlin C., Turner D.C., Pomarol-Clotet E., Corlett P.R., Fletcher P.C. The effects of a subpsychotic dose of ketamine on recognition and source memory for agency: Implications for pharmacological modelling of core symptoms of schizophrenia. Neuropsychopharmacology. 2006;31(2):413–423. doi: 10.1038/sj.npp.1300846. doi: 10.1038/sj.npp.1300846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaney S., Bentall R.P. Persecutory delusions and the self-serving bias: Evidence from a contingency judgment task. Journal of Nervous and Mental Disease. 1992;180(12):773–780. doi: 10.1097/00005053-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Krystal J.H., Karper L.P., Seibyl J.P., Freeman G.K., Delaney R., Bremner J.D., et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti A.C., Weiler M.A., Tamara Michaelidis B.A., Parwani A., Tamminga C.A. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. doi: 10.1016/S0893-133X(01)00243-3. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lau H.C., Rogers R.D., Passingham R.E. Manipulating the experienced onset of intention after action execution. Journal of Cognitive Neuroscience. 2007;19(1):81–90. doi: 10.1162/jocn.2007.19.1.81. [DOI] [PubMed] [Google Scholar]
- Lavin A., Nogueira L., Lapish C.C., Wightman R.M., Phillips P. E.M., Seamans J.K. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. Journal of Neuroscience. 2005;25(20):5013–5023. doi: 10.1523/JNEUROSCI.0557-05.2005. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B., Gleason C.A., Wright E.W., Pearl D.K. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential): The unconscious initiation of a freely voluntary act. Brain. 1983;106(3):623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Mason O.J., Morgan C. J.M., Stefanovic A., Curran H.V. The Psychotomimetic States Inventory (PSI): Measuring psychotic-type experiences from ketamine and cannabis. Schizophrenia Research. 2008;103(1-3):138–142. doi: 10.1016/j.schres.2008.02.020. doi: 10.1016/j.schres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Moore J., Haggard P. Awareness of action: Inference and prediction. Consciousness and Cognition. 2008;17(1):136–144. doi: 10.1016/j.concog.2006.12.004. doi: 10.1016/j.concog.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Moore J.W., Haggard P. Intentional binding and higher order agency experience. Consciousness and Cognition. 2010;19(1):490–491. doi: 10.1016/j.concog.2009.11.007. doi: 10.1016/j.concog.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Moore J.W., Lagnado D., Deal D.C., Haggard P. Feelings of control: Contingency determines experience of action. Cognition. 2009;110(2):279–283. doi: 10.1016/j.cognition.2008.11.006. doi: 10.1016/j.cognition.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Moore J.W., Wegner D.M., Haggard P. Modulating the sense of agency with external cues. Consciousness and Cognition. 2009;18(4):1056–1064. doi: 10.1016/j.concog.2009.05.004. doi: 10.1016/j.concog.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Morgan C. J.A., Mofeez A., Brandner B., Bromley L., Curran H.V. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004;29(1):208–218. doi: 10.1038/sj.npp.1300342. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Obhi S.S., Planetta P.J., Scantlebury J. On the signals underlying conscious awareness of action. Cognition. 2009;110(1):65–73. doi: 10.1016/j.cognition.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Oranje B., van Berckel B.N., Kemner C., van Ree J.M., Kahn R.S., Verbaten M.N. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22(3):293–302. doi: 10.1016/S0893-133X(99)00118-9. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E., Honey G.D., Murray G.K., Corlett P.R., Absalom A.R., Lee M., et al. Psychological effects of ketamine in healthy volunteers: Phenomenological study. British Journal of Psychiatry. 2006;189(2):173–179. doi: 10.1192/bjp.bp.105.015263. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P., Gurney K. The short-latency dopamine signal: A role in discovering novel actions? Nature Reviews Neuroscience. 2006;7(12):967–975. doi: 10.1038/nrn2022. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Rigby-Jones A.E., Sneyed J.R., Absolom A.R. Ketamine pharmacokinetics in healthy volunteers: A pooled population analysis. 2006. Poster presented at the annual Scientific meeting of the UK Society for Intravenous Anaesthesia. [Google Scholar]
- Schnell K., Heekeren K., Daumann I., Schnell T., Schnitker R., Möller-Hartmann W., Gouzoulis-Mayfrank E. Correlation of passivity symptoms and dysfunctional visuomotor action monitoring in psychosis. Brain. 2008;131(10):2783–2797. doi: 10.1093/brain/awn184. doi: 10.1093/brain/awn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sierra M., Baker D., Medford N., David A.S. Unpacking the depersonalization syndrome: An exploratory factor analysis on the Cambridge Depersonalization Scale. Psychological Medicine. 2005;35(10):1523–1532. doi: 10.1017/S0033291705005325. doi: 10.1017/S0033291705005325. [DOI] [PubMed] [Google Scholar]
- Simeon D., Kozin D.S., Segal K., Lerch B., Dujour R., Giesbrecht T. De-constructing depersonalization: Further evidence for symptom clusters. Psychiatry Research. 2008;157(1-3):303–306. doi: 10.1016/j.psychres.2007.07.007. doi: 10.1016/j.psychres.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Synofzik M., Vosgerau G., Newen A. Beyond the comparator model: A multifactorial two-step account of agency. Consciousness and Cognition. 2008;17(1):219–239. doi: 10.1016/j.concog.2007.03.010. doi: 10.1016/j.concog.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Haggard P. Awareness of somatic events associated with a voluntary action. Experimental Brain Research. 2003;149(4):439–446. doi: 10.1007/s00221-003-1386-8. doi: 10.1007/s00221-003-1386-8. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Prabhu G., Haggard P. Having a body versus moving your body: How agency structures body-ownership. Consciousness and Cognition. 2006;15(2):423–432. doi: 10.1016/j.concog.2005.09.004. doi: 10.1016/j.concog.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Schütz-Bosbach S., Gallagher S. On agency and body-ownership: Phenomenological and neurocognitive reflections. Consciousness and Cognition. 2007;16(3):645–660. doi: 10.1016/j.concog.2007.05.012. doi: 10.1016/j.concog.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Vollenweider F.X., Leenders K.L., Oye I., Hell D., Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) European Neuropsychopharmacology. 1997;7(1):25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- Vollenweider F.X., Leenders K.L., Scharfetter C., Antonini A., Maguire P., Missimer J., Angst J. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG) European Neuropsychopharmacology. 1997;7(1):9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- Voss M., Moore J., Hauser M., Gallinat J., Heinz A., Haggard P. Altered awareness of action in schizophrenia: A specific deficit in predicting action consequences. Brain. 2010;133(10):3104–3112. doi: 10.1093/brain/awq152. doi: 10.1093/brain/awq152. [DOI] [PubMed] [Google Scholar]


