Abstract
Rationale
The response characteristics of the six-minute walk test (6MWT) in studies of idiopathic pulmonary fibrosis (IPF) are only poorly understood, and the change in walk distance that constitutes the minimum important difference (MID) over time is unknown.
Objectives
To examine changes over time in distance walked (i.e., 6MWD) during the 6MWT and to estimate the change in distance that constitutes the MID in patients with IPF.
Methods
We used data from a recently completed trial that included subjects with IPF who completed the 6MWT, Saint George’s Respiratory Questionnaire (SGRQ), and forced vital capacity (FVC) at six and twelve months to examine longitudinal changes in 6MWD. We used both anchor-and distribution-based approaches as well as linear regression analyses to determine the MID for 6MWD. The SGRQ Total score and FVC were used as clinical anchors.
Main results
Among 123 subjects alive and able to complete the 6MWT at both follow-up time points, 6MWD did not to change significantly over time (378.1m at baseline vs. 376.8m at six months vs. 361.3m at twelve months, p = 0.5). The point estimate for the 6MWD MID was 28m with a range of 10.8–58.5m.
Conclusion
In a group of IPF patients with moderate physiologic impairment, for those alive and able to complete a 6MWT, 6MWD does not change over twelve months. At the population level, the MID for 6MWD appears to be around 28 meters. Further investigation using other anchors and derivation methods is required to refine estimates of the MID for 6MWD in this patient population.
Keywords: interstitial lung disease, idiopathic pulmonary fibrosis, six-minute walk test, minimum important difference
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal fibrosing interstitial lung disease (ILD) that impairs functional status and quality of life (QOL). Although recent advances in our understanding of the underlying pathogenesis of IPF have led to the investigation of numerous pro-fibrotic pathways,1 no medical therapy has been shown to improve respiratory symptoms or functional status, or to prolong survival.
The six-minute walk test (6MWT) is a simple and easily performed assessment of a subject’s submaximal functional capacity. It measures the distance a patient can walk on a flat surface over a period of six minutes and is used to measure the response to medical interventions in patients with moderate to severe cardiac or pulmonary disease.2 Its reproducibility (with a coefficient of variation of approximately 8%) appears to be better than the one-second forced expiratory volume in patients with chronic obstructive pulmonary disease (COPD),3–6 and it has less short-term variability than questionnaire indices of functional status (22–33%).7
For IPF, 6MWD appears highly reproducible (test-retest reliability 0.98) over short time intervals (e.g., 1–2 weeks), and is highly correlated (r = 0.78) with maximal oxygen uptake derived from a maximal cardiopulmonary exercise test.8 In two relatively small studies, investigators have examined changes in distance walked during the 6MWT over longer time periods in patients with confidently-defined IPF. Tomioka and colleagues9 found that 6MWD declined to a non-significant degree (37.3±120.9m, p=0.2) from baseline among 32 IPF subjects who completed a 6MWT after a median 14 months of follow-up. In a recently completed placebo-controlled study investigating the efficacy of etanercept in the treatment of IPF, the 6MWT was used as a secondary endpoint. In terms of 6MWD, there was no statistically significant differences between the two treatment arms, and subjects in the placebo arm (n=41) experienced a mean decline of 14.7 (± 112.5) meters (p=0.5) at week 48, with changes of −2.2 (± 65.80), 2.1 (± 71.7), and −22.3 (±106.8) meters at weeks 12, 24 and 36 respectively.10
What is not known about the 6MWT in patients with IPF is what distance constitutes the minimum important difference (MID)—the smallest change in distance that patients can perceive as different from the previous test and that would mandate, in the absence of troublesome side effects and excessive costs, a change in management.11 Several methods can be used to determine the MID for an outcome measure, but there is much controversy over which ones are best.12 A common approach is to use multiple methods to derive MID estimates. One—the anchor-based method—calls for a different-but-related clinical variable to be a so-called anchor; the mean change in the outcome (e.g., 6MWD) is calculated for subjects who change “minimally” according to that anchor is the MID estimate. There are no restrictions on what variables can serve as anchors; however, any anchor must be clinically relevant and cut-off values for defining minimal change should be sensible. Distribution-based methods, including the effect size, standardized response mean, and standard error of measurement, use statistical calculations based solely on study sample data for the outcome variable to derive the MID.
The goals of this study were to determine the changes in 6MWD over time in a large, well-defined sample of IPF subjects with moderate physiologic impairment and to use multiple approaches to determine the MID for 6MWD in this population.
Methods
Patient Population
Data from the international, prospective, double-blinded, randomized, placebo-controlled, parallel group study investigating the use of Bosentan in the treatment of patients with IPF (BUILD-1) were analyzed.13 Its study design, inclusion and exclusion criteria, and primary results have been previously published.13 Briefly, patients were enrolled in BUILD-1 if their IPF diagnosis met American Thoracic Society (ATS)/European Respiratory Society consensus guidelines,14 if they were diagnosed with IPF between 3 and 36 months prior to enrollment, and if a screening 6MWD was between 150 and 499m. Patients were excluded if they had an FVC of < 50% or > 90% predicted,15 a diffusing capacity for carbon monoxide (DLCO) corrected for hemoglobin level < 30% predicted,16 a resting arterial oxygen pressure (PaO2) less than 55 mm Hg (sea level) or 50 mm Hg (above 1,400m), echocardiographic evidence of severe pulmonary hypertension (systolic pulmonary pressure > 50 mm Hg or tricuspid regurgitation velocity > 3.2 m/s), or severe congestive heart failure. The study was approved by the appropriate independent ethics committees or institutional review boards and conducted in accordance with the principles of the Declaration of Helsinki, local laws, and guidelines for good clinical practice. All patients provided written, informed consent. Our study sample was comprised of subjects with baseline, six- and twelvemonth 6MWD data.
Study Protocol
The 6MWD was measured without supplemental oxygen using a modified 6MWT protocol, conducted in accordance with ATS guidelines,2 but terminated prematurely if SpO2 fell below 80% (pulse oximeter; Nellcor N-595; Pleasanton, CA). Technicians conducting 6MWT were blinded to all questionnaire data.
Assessment tools
The SGRQ is a self-administered, obstructive lung disease-specific questionnaire with 50 items comprising three domains (Symptoms, Activity, Impact) and a Total score. Domain and Total scores range from 0–100, with higher scores corresponding to worse HRQL
Statistical Analysis
Descriptive statistics were used for baseline, six-month, and twelve-month data. Change over time in 6MWD was examined by using longitudinal data analytic methods. We used SAS PROC MIXED (SAS Institute Inc., Cary, NC) to model mean 6MWD at each study time point (baseline, six and twelve months) while handling the within-subject correlation of repeated measures of 6MWD. Thus, the model yielded least squared means estimates for 6MWD at each of the three time points, and the null hypothesis of equality among the three mean values for a given variable was tested. Even with mild deviations from normality and particularly for sample sizes over 100—the data from this sample of 123 subjects were mildly skewed—PROC MIXED will yield robust estimates.
Next, we stratified subjects according to anchor score changes (SGRQ Total score and FVC) over the two time intervals—from baseline to six months as well as six to twelve months. The SGRQ Total was chosen as an anchor because it captures patients’ perceptions about their own health status; thus it assesses certain effects of IPF on patient well-being. In a previous study, we determined the MID for the SGRQ Total to be 7 points, with a range of 5–10 points. Thus, for this analysis, we categorized subjects as “unchanged” if the difference in SGRQ Total was within 5 points (exclusive) of the value at the previous time point, as “changed minimally” if the difference in SGRQ Total was 5–10 points (exclusive) different from the previous time point, and as ”changed more than minimally” if the difference was ≥ 10 points. For each time interval, the mean change in 6MWD was calculated for subjects stratified according to categorical changes (i.e., unchanged, changed minimally, changed more than minimally) in SGRQ Total score. To show the ability of SGRQ Total to discriminate between subjects whose 6MWD changed over time, we used repeated measures analysis of variance (ANOVA) to compare linear contrasts in mean changes in 6MWD across categories of change in SGRQ Total score. We used this same approach for the FVC anchor: first, stratifying the sample on change in FVC, then calculating mean changes in 6MWD for categories of change in FVC, and finally using repeated measures ANOVA to compare changes in 6MWD across FVC categories. For FVC, we categorized subjects as “unchanged” if the difference in the raw FVC was within 7% (inclusive) of the value at the previous time point, as “changed minimally” if the difference in the raw FVC value was between 7 and 12% (exclusive) of the value at the previous time point, and as ”changed more than minimally” if the difference was ≥ 12%. We considered a 7–12% change in raw FVC as a minimally important change because this range covers 7% (a so-called marginal change that has been shown to carry prognostic significance in IPF17,18) as well as 10% (a commonly used endpoint in trials and in clinical practice14).
To estimate the MID for 6MWD, we calculated weighted averages for 6MWD for subjects with minimal change in the SGRQ Total (5–10 points) or FVC anchor (7–12% from the baseline raw value). We next used the within-patient anchor based method 19 to derive another MID estimate for 6MWD. For this method, linear regression is used to examine the relationship between changes in 6MWD (dependent variable) and changes in the anchor—SGRQ Total score or FVC% (independent variables). Lastly, we used distribution-based methods to generate MID estimates. The effect size (referred to as ES and calculated as the change in 6MWD divided by the standard deviation of the baseline 6MWD) is one such method.20 For ES, values of 0.2 are considered small effects, 0.5 medium effects, and 0.8 large effects. Although there is no consensus about how or even whether12 ES should be used in the estimation of MIDs, some investigators consider 0.5 to correspond to the MID.21,22 Thus, we derived the change in 6MWD that would correspond to an ES of 0.5 and used that as an MID estimate. All analyses were performed with SAS version 9.1.3 (SAS Institute Inc., Cary, NC), and p-values < .05 were considered statistically significant.
Results
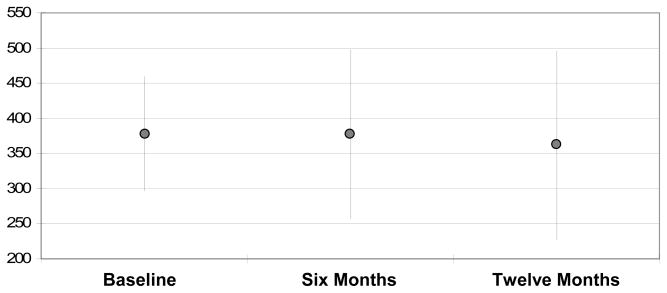
The mean age of the sample was 65 years, and most subjects were men (Table 1). Among all subjects able to complete a 6MWT at baseline and at both six- and twelve-month follow-up (n=123), there was no statistical difference in mean 6MWD between any time point (Figure 1). Further, Table 2 shows that baseline walk distance appeared not to influence subsequent measures of 6MWD.
Table 1.
Baseline characteristics of the entire study sample.
| All subjects N = 154 | |
|---|---|
| Age | 65.1 (8.74) |
|
| |
| M:F | 73:27 |
|
| |
| Height(cm) | 170.0 (8.4) |
|
| |
| Weight(kg) | 84.7 (15.87) |
|
| |
| FVC(L) | 2.6 (.71) |
|
| |
| FVC% | 67.8 (11.84) |
|
| |
| DLCO | 4.1 (1.18) |
|
| |
| DLCO% | 41.8 (9.48) |
|
| |
| Baseline 6MWD | 372.9 (82.63) |
| 150–249m | (n=15) 200.9 (29.3) |
| 250–349m | (n=37) 309.5 (28.7) |
| 350–450m | (n=102) 421.7 (40.4) |
Data are presented as mean (standard deviation) or percentages for gender
Figure 1.
Mean 6MWD in meters at baseline, six months, and twelve months for the subjects alive and able to complete the 6MWT at all three time points.
Dot=mean 6MWD in meters. Whiskers depict 95% confidence intervals. Mixed model p-value = 0.5 for difference between 6MWD at the three time points.
Table 2.
Longitudinal change in 6MWD for the subgroup alive and able to complete the 6MWT
| Baseline | Six Months | Twelve Months | P-value | |
|---|---|---|---|---|
| Baseline 6MWD | ||||
| 150–249m (n=11) | 198.3 (32) | 211.5 (127.5) | 172.5 (118.8) | 0.7 |
| 250–349m (n=28) | 311.3 (28.5) | 279.4 (106) | 262.9 (110.2) | 0.13 |
| 350–499m (N=84) | 424.5 (39.6) | 430.9 (77) | 419.5 (97.5) | 0.6 |
|
| ||||
| Baseline | Six Months | Twelve Months | P-value | |
|
| ||||
| Baseline 6MWD | ||||
| 150–249m (n=11) | 198.3 (32) | 211.5 (127.5) | 172.5 (118.8) | 0.7 |
| 250–349m (n=28) | 311.3 (28.5) | 279.4 (106) | 262.9 (110.2) | 0.13 |
| 350–499m (N=84) | 424.5 (39.6) | 430.9 (77) | 419.5 (97.5) | 0.6 |
Data are presented as mean (standard deviation). P-value from repeated measures ANOVA that compared within-group 6MWD between time points.
Statistically significant relationships were observed between change in 6MWD and change in SGRQ Total score or FVC (Table 3). Table 4 gives MID estimates from distribution and anchor-based approaches. The distribution-based approach (ES=0.5) yielded a higher estimate than the anchor-based approaches. The mean of all MID point estimates yielded a value of 28m.
Table 3.
Mean 6MWD for the subgroup alive and able to complete the 6MWT at all three time points stratified by the SGRQ and FVC anchors.
| SGRQ Total Score | |||||
|---|---|---|---|---|---|
| Worsened ≥ 10 | Worsened 5–10 | Within 4.9 Points | Improved 5–10 | Improved ≥ 10 | |
| n=16 | n=19 | n=36 | n=16 | n=34 | |
| Baseline-6mos | −39.0(128.2) | −6.6(77.3) | 8.0(58.6) | 15.8(47.1) | 2.9(76.7) |
| n=17 | n=20 | n=48 | n=14 | n=24 | |
| 6mos-12mos | −84.6(111.2) | −20.2(49.0) | −10.4(67.4) | 25.9(58.4) | 5.6(57.9) |
| FVC | |||||
| Declined ≥ 12% | Declined 7–12% | No Change | Improved 7–12% | Improved ≥ 12% | |
| n=17 | n=24 | n=65 | n=9 | n=8 | |
| Baseline-6mos | −56.1(111.9) | −20.6(105.4) | 11.7(49.6) | 8.8(35.6) | 56.8(43.1) |
| n=18 | n=23 | n=71 | n=9 | n=2 | |
| 6mos-12mos | −33.8(74.1) | −51.6(99.8) | −2.4(65.2) | 11.2(56.9) | 9.5(67.2) |
Data are presented as mean (standard deviation). Tests of significance for linear contrast in group effect yielded P=0.05 for baseline-6mos and P=0.0001 for 6mos-12mos for SGRQ Total and P=0.0003 for baseline-6mos and P=0.2 for 6mos-12mos for FVC.
Table 4.
MID estimates for 6MWD
| Time | ES=0.5 | ΔFVC=7–12% | ΔSGRQt=5–10 | Regression equation for FVC | Regression equation for SGRQt | Mean |
|---|---|---|---|---|---|---|
| Baseline to 6mos | 41.3m | 17.4m* | 10.8m | Δ6MWD=2.6 + 1.9(ΔFVC) Corresponding to 10% change in raw FVC, Δ6MWD=21.6(8.6–33.6) |
Δ6MWD=−4 + (−1.2)(ΔSGRQt) Corresponding to 7 unit change in SGRQt, Δ6MWD=12.4(5.4–19.4) |
20.7 |
| 6mos to 12mos | 58.5m | 40.2m* | 22.5m | Δ6MWD=−6.1 + 2.9(ΔFVC) Corresponding to 10% change in raw FVC, Δ6MWD=22.9(6.9–39.9) |
Δ6MWD=−1.9 + (−4.4)(ΔSGRQt) Corresponding to 7 unit change in SGRQt, Δ6MWD=32.7(25–41.1) |
35.4 |
| Grand Mean = 28, thus MID = 28 meters | ||||||
Weighted mean of subjects whose raw FVC changed by 7–12% over the indicated time interval; SGRQt=SGRQ Total;
Note: the 95% confidence intervals around the MID estimates from the regression equations are sample size dependent. The point estimates should be viewed as the best estimates of the MID.
Discussion
For patients with IPF, the 6MWT appears to be a valid reflection of global functional capacity;8 it is frequently used clinically to assess changes in IPF disease status over time but with few data to support this practice. More importantly, the 6MWT—either the 6MWT itself, variations thereof, or data collected during the 6MWT (e.g., measures of oxygenation) —has been used as an outcome measure in trials enrolling subjects with IPF.23–25 However, there are large knowledge gaps regarding certain important aspects of this test in IPF.
In this study, for a select group of well-defined subjects with IPF who were able to walk no less than 150m and no more than 499m during a baseline 6MWT, and who performed follow-up testing, we found no significant change from baseline in 6MWD at six or twelve months. These data are supported by studies from Tomioka and Raghu who also found no significant change over time frames ranging from about 12–14 months.9,10
Our study estimates the MID for 6MWD in patients with IPF, which we found to be in the range of 28 meters. To our knowledge, only two other groups of investigators have assessed the MID for the 6MWD in patients with IPF. In a study published only in abstract form, Mathai and colleagues divided into two subgroups 20 IPF patients who were participating in a one-day support group. Each patient performed a 6MWT, and then each subgroup of 10 patients spent the day together at a support group meeting (details not provided). At the end of the day, each patient was asked to rate his ability to walk relative to the other members in his subgroup. These investigators found that 6MWD needed to differ by a mean 17.9±103.6m for patients to stop rating themselves as “the same” as other members in their group. Redelmeier and colleagues developed this method, and used it to show that the MID for 6MWD in patients with COPD is 54m (95% confidence interval 37–71m); that is, the mean difference in 6MWD between subjects who rated themselves as being able to walk either “a little bit better” or “a little bit worse,” compared with those who rated themselves as being able to walk “about the same” as other patients, was 54m. In that study, patients who rated themselves as “a little worse” on average walked 80m less than other patients in their group, whereas subjects who rated themselves as “a little better” walked only 30m more than other patients in their group—yielding a weighted average of 54m among subjects who rated themselves as minimally (or “a little…”) different from other patients.
Another method commonly used to determine the MID for an outcome measure involves asking subjects to provide a so-called transition assessment at the time of follow-up testing. For example, at six months, subjects (blinded to baseline and follow-up data) might be asked to report whether they perceived their 6MWD to be “the same as,” “a little bit less,” “a lot less,” “a little bit greater,” or “a lot greater” than their baseline walk. The mean 6MWD for subjects reporting a minimal change in perceived 6MWD (i.e., “a little bit less” or “a little bit greater”) would be the estimate of the MID. Singh and co-investigators used this method to estimate the MID for the incremental shuttle walk test to be 47.5m in patients with COPD.26 In the only study of the 6MWD MID in IPF published in manuscript format, Holland and her colleagues used global change ratings, receiver operating characteristic (ROC) curves, and a distribution-based approach to derive their estimate.27 Among 24 subjects with IPF who completed a 6MWT before and after an 8-week exercise program, the MID was found to be between 29 and 34 meters
No transition assessment was used in the BUILD-1 trial, but we employed a related (and perhaps the most common) method of determining the MID—an anchor-based method. Although any variable can be an anchor, each should meet three criteria: (1) it should be related to the outcome variable, (2) it must possess face validity, and (3) it must be able to be divided into at least three categories: no change, minimal, and other.12 As one anchor, we selected the SGRQ Total for several reasons: (1) it is a patient-oriented or patient-assessed outcome measure, and as such, it asks specifically about patients’ perceptions28—a notion viewed by many investigators as paramount to deriving the MID for an outcome variable;12 (2) IPF impairs—and patients value—the quality of their lives, so the SGRQ and other health status questionnaires provide meaningful data in this population;29,30 and (3) we have shown previously that a 7-point change in SGRQ Total score is its MID among patients with IPF (paper accepted for publication: Swigris et al. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med 2009). As the second anchor we chose the FVC because it is perhaps the most widely used physiologic measurement to assess IPF severity, and cut-off values for its clinical and prognostic meaningfulness have been established in a number of studies.31–35 We elected to use FVC rather than DLCO because of the greater intrinsic variability in DLCO measures as well as our inability to confidently define the clinically significant range for its minimum change.
There are a number of limitations to this study. As is often the case, the distribution-based method yielded higher estimates for the MID than the anchor-based methods.36 The selected population included only IPF patients who could walk more than 150 but less than 499m during a 6MWT at baseline—and the overwhelming majority could walk at least 350m. Furthermore, data from subjects who died, or who were unable to complete the 6MWT for other reasons, was not included in our analyses; this could introduce bias. Thus, inferences drawn here may not be applicable to extremely debilitated IPF patients in the latter stages of the disease or to more fit IPF patients in the earlier stages of disease. However, the results of this study may have important implications for both IPF investigators who plan to power future IPF studies for change in 6MWD as well as clinicians who prognosticate future changes in functional status. While the pattern of decline in 6MWD over time is unclear, if a linear decline were assumed, one might expect a 30 meter decline in 6MWD to occur, on average, at 31 months of follow-up (data not shown). It has been recommended that multiple anchors (in multiple studies) be used to generate a range for the MID for any outcome variable.12 Although this is but one study, we derived the MID for 6MWD by using two clinically meaningful anchors as well as by employing distribution-based methods. Furthermore, our estimate is nearly identical to the estimate from the only other published study to examine the 6MWD MID in IPF. Finally, it must be recognized that the MID estimate here is to be used at the population level; that is, the mean change in 6MWD that is considered clinically important in a population is “often much less” than the change in 6MWD that would allow a practitioner to be confident that a change within an individual patient is outside of inter-test variability.36
In conclusion, data from this study provide the first systematic examination of 12-month longitudinal changes in 6MWD as a prospectively acquired, primary outcome variable in a well-defined subset of patients with IPF, as well as an estimate of the MID for 6MWD in patients with this disease. It appears as though the MID for 6MWD is smaller for IPF than for COPD, but future confirmatory studies should be performed to estimate the MID for 6MWD in IPF.
Acknowledgments
The authors would like to acknowledge all of the investigators involved in the BUILD-1 trial: Ishaar Ben-Dov, Charles Chan, Jean-Francois Cordier, James Dauber, Joao De Andrade, Adaani Frost, Thomas Geiser, Marilyn Glassberg, Jeffrey Golden, Gary Hunninghake, Sanjay Kalra, Lisa Lancaster, Robert Levy, Keith Meyer, Joachim Mueller-Quernheim, Paul Noble, Christophe Pison, Charles Poirier, Milton Rossman, Paola Rottoli, Gerd Staehler, Athol Wells, Gordon Yung and David Zisman. The authors also wish to acknowledge the study coordinators and nurses for their hard work and all of the study subjects for their generous contributions.
Details of funding:
Actelion Pharmaceuticals funded the performance of the underlying BUILD-1 trial that investigated the efficacy of Bosentan in the treatment of idiopathic pulmonary fibrosis. No additional external funding was obtained. All authors had full access to all the data in this study. The corresponding author had final responsibility for the decision to submit the paper for publication.
Footnotes
The work in this manuscript is the original work of the stated authors.
Competing interest:
Drs Behr, du Bois, King, Raghu, and Brown served on the steering committee for the BUILD-1 trial, sponsored by Actelion Pharmaceuticals. Dr. Swigris has served as a paid consultant to Actelion Pharmaceuticals. Dr. Wamboldt has no competing interests to declare.
License statement:
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Thorax and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://thorax.bmj.com/ifora/licence.pdf).
References
- 1.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–51. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 2.ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 3.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 1982;284:1607–8. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knox AJ, Morrison JF, Muers MF. Reproducibility of walking test results in chronic obstructive airways disease. Thorax. 1988;43:388–92. doi: 10.1136/thx.43.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyatt GH, Pugsley SO, Sullivan MJ, et al. Effect of encouragement on walking test performance. Thorax. 1984;39:818–22. doi: 10.1136/thx.39.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leger LA, Lambert J. A maximal multistage 20-m shuttle run test to predict VO2 max. Eur J Appl Physiol Occup Physiol. 1982;49:1–12. doi: 10.1007/BF00428958. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt G, Sullivan M, Thompson P, et al. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005;171:1150–7. doi: 10.1164/rccm.200405-578OC. [DOI] [PubMed] [Google Scholar]
- 9.Tomioka H, Imanaka K, Hashimoto K, Iwasaki H. Health-related quality of life in patients with idiopathic pulmonary fibrosis--cross-sectional and longitudinal study. Intern Med. 2007;46:1533–42. doi: 10.2169/internalmedicine.46.6218. [DOI] [PubMed] [Google Scholar]
- 10.Raghu G, Brown KK, Costabel U, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–55. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 11.Juniper E, Guyatt G, Willan A, Griffith L. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 12.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. Copd. 2005;2:63–7. doi: 10.1081/copd-200050663. [DOI] [PubMed] [Google Scholar]
- 13.King TE, Jr, Behr J, Brown KK, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 14.Joint Statement of the American Thoracic Society and the European Respiratory Society: Idiopathic pulmonary fibrosis: diagnosis and treatment. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 15.Morris J, Koski A, Johnson L. Spirometric standards for the healthy non-smoking adults. Am Rev Respir Dis. 1971;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Crapo R, Morris A. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 17.Zappala C, Latsi P, Nicholson AC, Wells AU. Marginal declines in FVC levels are associated with increased mortality in idiopathic pulmonary fibrosis. Thorax. 2007;175:A143. [Google Scholar]
- 18.Du Bois RM, Albera C, Costabel U, et al. Categorical declines in percent predicted forced vital capacity are associated with a graded risk of death in patients with idiopathic pulmonary fibrosis. Chest. 2008;134:S20003. [Google Scholar]
- 19.Puhan MA, Frey M, Buchi S, Schunemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6:46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical analysis for the behavioral sciences. 2. New Jersey: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 21.Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Qual Life Outcomes. 2003;1:4. doi: 10.1186/1477-7525-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 23.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama O, Kondoh Y, Kimura T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. 2008;13:394–9. doi: 10.1111/j.1440-1843.2007.01205.x. [DOI] [PubMed] [Google Scholar]
- 25.Nishiyama O, Taniguchi H, Kondoh Y, et al. Dyspnoea at 6-min walk test in idiopathic pulmonary fibrosis: comparison with COPD. Respir Med. 2007;101:833–8. doi: 10.1016/j.rmed.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Clark M, Cooper B, Singh S, Cooper M, Carr A, Hubbard R. A survey of nocturnal hypoxaemia and health related quality of life in patients with cryptogenic fibrosing alveolitis. Thorax. 2001;56:482–486. doi: 10.1136/thorax.56.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Small changes in six-minute walk distance are important in diffuse parenchymal lung disease. Respir Med. 2009 doi: 10.1016/j.rmed.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Abrams D. Analysis of a life-satisfaction index. J Gerontol. 1976;24:470. doi: 10.1093/geronj/24.4.470. [DOI] [PubMed] [Google Scholar]
- 29.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax. 2005;60:588–94. doi: 10.1136/thx.2004.035220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swigris JJ, Stewart AL, Gould MK, Wilson SR. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61. doi: 10.1186/1477-7525-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–42. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 32.Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174:803–9. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–8. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 34.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–81. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 35.Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–7. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 36.Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD. 2005;2:125–9. doi: 10.1081/copd-200050527. [DOI] [PubMed] [Google Scholar]