Abstract
Background
Iron deficiency (ID) during early development impairs myelination and basal ganglia function in animal models.
Aims
To examine the effects of iron deficiency anemia (IDA) and iron deficiency (ID) without anemia on infant motor skills that are likely related to myelination and basal ganglia function.
Study design
Observational study.
Subjects
Full-term inner-city African-American 9- to 10-month-old infants who were free of acute or chronic health problems with iron status indicators ranging from IDA to iron sufficiency (n = 106). Criteria for final iron status classification were met by 77 of these infants: 28 IDA, 28 non-anemic iron-deficient (NA ID), and 21 iron-sufficient (IS).
Outcome measures
Gross motor developmental milestones, Peabody Developmental Motor Scale, Infant Neurological International Battery (INFANIB), motor quality factor of the Bayley Behavioral Rating Scale, and a sequential/bi-manual coordination toy retrieval task. General linear model analyses tested for linear effects of iron status group and thresholds for effects.
Results
There were linear effects of iron status on developmental milestones, Peabody gross motor (suggestive trend), INFANIB standing item, motor quality, and toy retrieval. The threshold for effects was ID with or without anemia for developmental milestones, INFANIB standing item, and motor quality and IDA for toy retrieval.
Conclusions
Using a comprehensive and sensitive assessment of motor development, this study found poorer motor function in ID infants with and without anemia. Poorer motor function among non-anemic ID infants is particularly concerning, since ID without anemia is not detected by common screening procedures and is more widespread than IDA.
Motor development in infancy interacts with and affects several other domains, including perceptual, cognitive, and social-emotional development (1–3). One widespread condition that appears to impede the normal course of early motor development is iron deficiency (ID). In developing countries, over 50% of pregnant women are anemic (4), as are 46–66% of children under 4 years, with half attributed to ID (5).
Nine of 12 previous studies that assessed infant motor development using global tests reported lower motor scores in infants with iron deficiency anemia (IDA) compared to nonanemic infants (6). A few studies showed motor improvement after iron therapy, but all available follow-up studies reported long-lasting motor differences (see review (7)). Among randomized controlled trials of across-the-board iron supplementation (in contrast to iron treatment for infants with IDA/ID), 7 of 9 that assessed motor function reported benefits of iron (6). These global test results, though consistent, give little indication of specific central nervous system (CNS) effects of ID that are relevant to motor development.
Iron is involved in many CNS processes. Most relevant to motor function are documented effects of ID on myelination and basal ganglia function, especially related to the dopamine system (8). Based on such brain-behavior findings in rodent models and using a more comprehensive motor assessment than previous studies, this study tested the hypothesis that motor functions specifically related to myelination and/or basal ganglia would be affected by IDA in infancy. We further hypothesized that motor function would vary depending on the severity of ID, predicting that non-anemic iron-deficient (NA ID) infants would be intermediate between IDA and iron-sufficient (IS) infants. The final aim was to assess reversibility of effects with 3 months of oral iron therapy. We were not able to achieve this final aim, however, because there was uncertainty about adherence to treatment and more than 40% of the sample did not return for a post-treatment blood test (9). Given these uncertain and incomplete data on treatment, this report focuses on pretreatment findings. The study was part of an integrated cross-species (human, non-human primate, and rodent) program project on the brain-behavior effects of early ID.
METHODS
Sample
The study was approved by the Institutional Review Boards at Wayne State University and the University of Michigan, and signed informed consent was obtained from the infants’ mothers. Infants were recruited from the General Pediatric Clinic of the Children’s Hospital of Michigan, which serves an economically-stressed inner-city population. Participation was restricted to healthy, full-term singleton infants, born to mothers ≥ 18 years, with birth weight > 5th percentile, no perinatal complications, no maternal diabetes, emergency C-section, heavy alcohol use, or other incapacitating condition, not in foster care, and no chronic health problem or hospitalization more than once or for > 5 days. Since the clinic population was > 90% African-American, infants who were not African-American were not recruited. A total of 113 9- to 10-month-old infants participated in the neurobehavioral study; 106 met criteria for initial iron status classification and 77 did so for the final classification (see below). Details of recruitment and attrition have been described previously (9;10).
Sample characteristics are described in Table 1. Infants weighed over 3.2 kg at birth on average, and slightly more than half were male. All infants received iron-fortified formula, with 40% initially breast-fed. Most mothers were single; 77% completed high school. The economically stressed nature of the sample is indicated by almost universal participation in the Women, Infants, and Children program, health care coverage primarily by Medicaid insurance, and relatively low scores for socioeconomic status.
Table 1.
Sample characteristics1
| N2 | 106 |
|---|---|
| Infant | |
| Age, months | 9.7 ±0.4 |
| Gender, % male (n) | 55 (58) |
| Birth weight, kg | 3.25 ±0.4 |
| Gestational age, weeks | 39.7 ± 1.2 |
| Breast-fed, % yes (n) | 40(42) |
| Mother and family | |
| Maternal age, years | 24.7 ±5.9 |
| Maternal marital status (married), % (n) | 12(13) |
| Maternal education, years | 12.4 ± 1.4 |
| Socioeconomic status3 | 29.1 ±8.0 |
| Maternal depressive symptoms4 | 6.5 ±5.7 |
| Maternal anxiety5 | 34.4 ±9.6 |
| Maternal alcohol intake | 0.02 ±0.08 |
| (oz absolute alcohol/day) | |
| HOME score6 | 31.3 ±5.7 |
| Life events7 | 6.3 ±4.6 |
| Social support8 | 3.3 ±0.5 |
Values are means ± SD or % (n) for categorical variables.
N varies slightly due to occasional missing data for some measures.
Hollingshead Scale for Socioeconomic Status (33)
Beck Depression Inventory (34)
Spielberger State-Trait Anxiety Scale (Trait) (35)
Home Observation for Measurement of the Environment-Revised (36)
Life Experiences Survey (37)
Social Support (Crnic's adaptation of a scale by Henderson) (38)
Iron status
Initial venous blood tests included a complete blood count, lead, and zinc protoporphyrin/heme ratio (ZPP/H), performed at the Detroit Medical Center. Remaining blood was separated and frozen for subsequent determination of serum iron, total iron binding capacity, transferrin saturation, and ferritin. Assays were performed using standard techniques and strict quality control (11). To classify iron status, we used cutoffs from NHANES II (12), NHANES III (13), and CDC publications (14;15). Initial hematologic criteria were based on measures that were available for all infants within a few days: hemoglobin (HB), mean corpuscular volume (MCV), red cell distribution width (RDW), lead, and ZPP/H (missing for 4 infants). Infants were considered provisionally qualified based on the following criteria: at least one abnormal value among MCV < 74 fl (15), RDW > 14.0% (14), and ZPP/H > 69 µmol/mol heme [corresponding to free erythrocyte protoporphyrin > 80 µg/dl] (12), with or without anemia (HB < 110 g/L), or clearly nonanemic (HB ≥ 115 g/L) with normal MCV, RDW, and ZPP/H. There were 39 IDA, 45 NA ID, and 22 IS infants provisionally classified (total n = 106). For final iron status classification, ID was defined as 2 or more abnormal iron measures, with transferrin saturation < 12% (12) and ferritin < 12 µg/L as additional abnormalities (13;14). IS was defined as HB ≥ 115 g/L and no more than one abnormal iron measure. 77 infants met criteria for final classification (28 IDA, 28 NA ID, and 21 IS). Missing data were due to insufficient blood or technical problems. Table 2 shows iron status measures for provisional and final iron status classifications.
Table 2.
Iron status of study groups
| Group | IDA | NA ID | IS | |||
|---|---|---|---|---|---|---|
| Provisional | Final | Provisional | Final | Provisional | Final | |
| n | 39 | 28 | 45 | 28 | 22 | 21 |
| Hemoglobin (g/L)a,b,c | 101.5(5.1) | 101.9(5.3) | 119.2(5.8) | 119.0(5.3) | 123.7(5.1) | 123.2(5.1) |
| MCV (fl)a,c | 73.1 (4.7) | 72.1 (5.0) | 75.0 (4.7) | 73.8 (4.3) | 78.5 (2.6) | 78.8(3.3) |
| RDW (%)a,c | 14.3(1.5) | 14.8(1.4) | 13.8(1.0) | 14.2 (0.9) | 13.0(0.7) | 12.9 (0.8) |
| ZPP/H (µmol/mol heme)a,b,c | 110.9(40.9) | 119.0(38.2) | 99.6 (54.0) | 96.4(51.9) | 57.8 (8.2) | 70.0(23.4) |
| Transferrin saturation (%)b | 21.6(8.2) | 20.2 (9.0) | 26.0 (10.2) | 26.3(11.6) | 24.8 (10.4) | 24.8 (8.9) |
| Ferritin (µg/L) | 38.5 (29.2) | 34.2 (29.3) | 35.4 (29.9) | 34.6(32.3) | 35.5 (24.7) | 32.1 (21.5) |
| Lead ((µg/dL) | 2.3 (1.6) | 2.4(1.5) | 2.7 (2.3) | 2.7(2.5) | 2.4(1.1) | 2.5(1.4) |
Values are means (SD). Significant group differences based on the final iron status classification (p < .05) are indicated by superscripts as follows:
IDA differs from IS,
IDA differs from NA ID,
NA ID differs from IS. The significant differences based on the provisional iron status classification were the same as for the final classification, with one exception: NA ID and IDA groups did not differ in ZPP/H.
Assessment of motor development
The infants were assessed at the Child Development Research Laboratory, Department of Psychiatry and Behavioral Neurosciences, Wayne State University, by experienced examiners who were unaware of infant iron status. To consider effects of ID on myelination, we used several tests that are sensitive to overall motor delays (i.e., gross motor developmental milestones (16), Peabody Developmental Motor Scales (PDMS-2) (17), and Infant Neurological International Battery (INFANIB) (18). We also used a toy retrieval task to assess bi-manual coordination and sequencing of movements (19), skills developing during this age period that may depend on basal ganglia function (20–22).
Project personnel directly assessed gross motor milestones based on a pictorial milestone chart, ordered by age of expected achievement and ranging from sitting with support to running (16). The highest (most advanced) milestone was the major outcome analyzed.
Overall motor development was also assessed by the PDMS-2, a standardized test that measures gross and fine motor abilities from birth through 5 years of age. Gross, fine, and overall motor scores were the major outcomes. The examiner’s general evaluation of motor performance was recorded on the motor quality factor of the Bayley Behavioral Rating Scale (BRS) (23).
Early neurological function was evaluated by the INFANIB (18), a neuromotor examination of reflexes, joints angles, and posture. Eight age-appropriate items were administered: scarf sign, heel to ear, popliteal angle, leg abduction, foot grasp, sideways parachute, backwards parachute, and weight-bearing during standing. Items were analyzed individually and as a sum.
A toy retrieval task specifically assessed bi-manual and sequential movements (19). Retrieving a toy from a translucent plastic box (20x12x6 cm) required a sequence of movements in which the infant’s two hands were differentially activated: opening the lid with one hand and maintaining it in an open position against light pressure by the examiner, while reaching for the toy inside the box with the other hand and taking it out. Infants were videotaped during five 30-sec trials, with subsequent scoring by trained coders who reached reliability levels of 90–96%. The major outcomes were retrieval of the toy and performance with good bi-manual coordination. In good bi-manual coordination, the hand that retrieves the toy moves towards the body, while the other hand pushes the lid away from the body, so that the two hands act in opposite directions in a coordinated fashion.
Statistical analysis
All statistical analyses were performed using SAS 9.1 (24). For most motor outcomes, general linear model (GLM) analyses were used to test for linear effects (dose-response) of iron status group. To determine whether the threshold for effects was IDA or ID (with or without anemia), GLM analyses tested two preplanned contrasts: a) IDA vs. non-anemic infants (NA ID + IS) and b) ID (IDA + NA ID) vs. IS infants. The toy retrieval task, with its categorical outcomes, was analyzed by logistic regression utilizing generalized estimated equation (GEE) methodology (25).
Regarding covariates, there were no statistically significant group differences in birth or family background characteristics, regardless of whether the provisional or final iron status groups were compared. However, we controlled for any background factor that was even weakly correlated (p < 0.10) with a given outcome. Infant age and gender were initially considered in all models, as they may affect motor performance. Additional covariates were on conceptual grounds. Weight-for-height z-score was considered as a covariate for gross motor tasks, since it is possible that infants who are heavier for their length might have less muscle force relative to their size for gross motor movements. Hand size (the product of width X length) was considered as a covariate for fine motor tasks, since skills such as grasping could be influenced by object size in relation to hand size. Covariates that significantly contributed to the R2 were retained.
RESULTS
Overall motor function
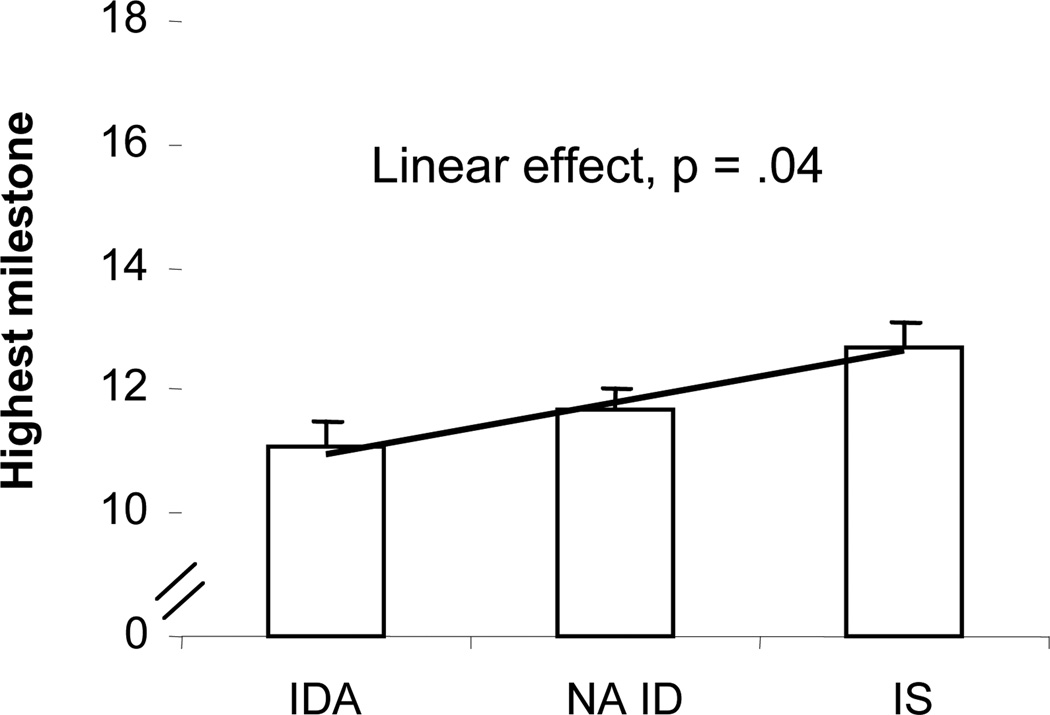
For gross motor milestones, there was a significant linear effect of iron status (controlling for weight-for-height z-score, which was the only significant covariate): IDA lowest, NA ID intermediate, and IS highest (F(1, 67) = 4.6, p = .04) (Fig. 1). The threshold was ID (with or without anemia) v. IS (F(1, 67) = 4.33, p = .04); the difference between NA ID and IS in pairwise comparisons was suggestive (p = .06). Only 19% of the IDA + NA ID infants could stand alone, whereas 34% of IS infants could do so and 19% were already walking alone.
Figure 1.
Effect of iron status (final classification) on gross motor developmental milestones scores at 9 months. There was a significant threshold between ID (with or without anemia) and IS (p = .04), but the linear effect better represents the results.
On the Peabody Developmental Motor Scale (PDMS-2), there was a suggestive linear effect for gross motor scores. Scores for the NA ID group seemed to be intermediate between the IDA and IS groups (F(1, 73) = 3.49, p = .07), but the difference between NA ID and IS was not statistically significant. Age was the only significant covariate.
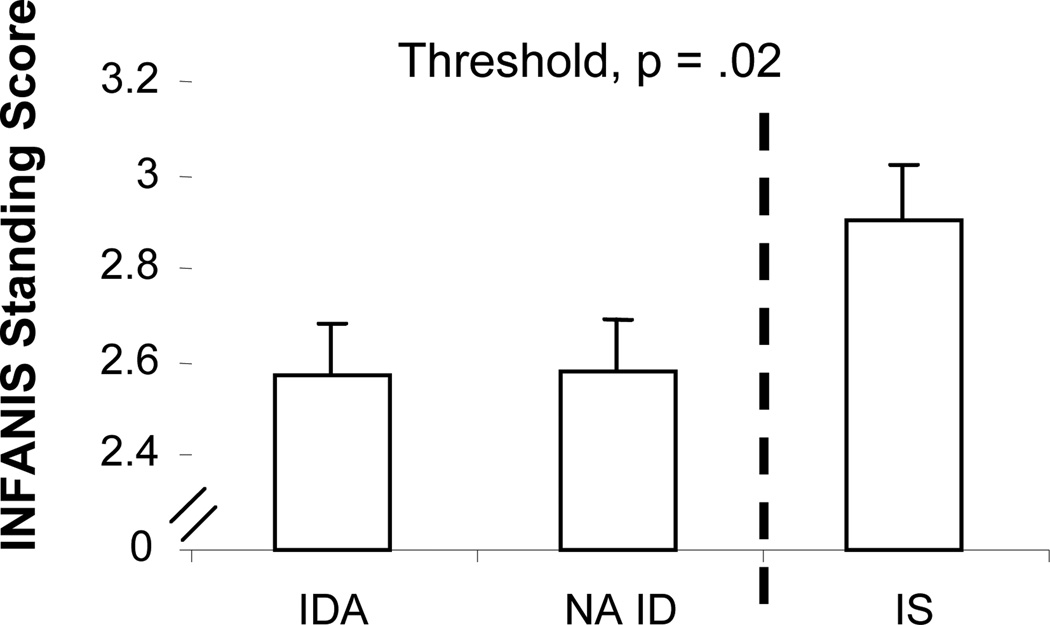
On the INFANIB, there was a linear effect for the standing item. More of the NA ID infants, compared to IDA infants, had normal adult-like weight bearing while standing, and the IS group had the highest percentage of such infants (F(1, 70) = 4.80, p = .03). The threshold was ID with or without anemia. ID infants, regardless of anemia, performed more poorly on the standing item compared to IS infants (F(1, 70) = 5.30, p = .02) (Fig. 2). NA ID was significantly lower than IS in pairwise comparisons (p = .02). Infant age and mother’s age were the only significant covariates.
Figure 2.
Effect of iron status (final classification) on INFANIB standing item at 9 months. The standing item of the INFANIB showed a significant linear effect (p = .03), but a threshold between the ID (with or without anemia) and IS groups better represents the results.
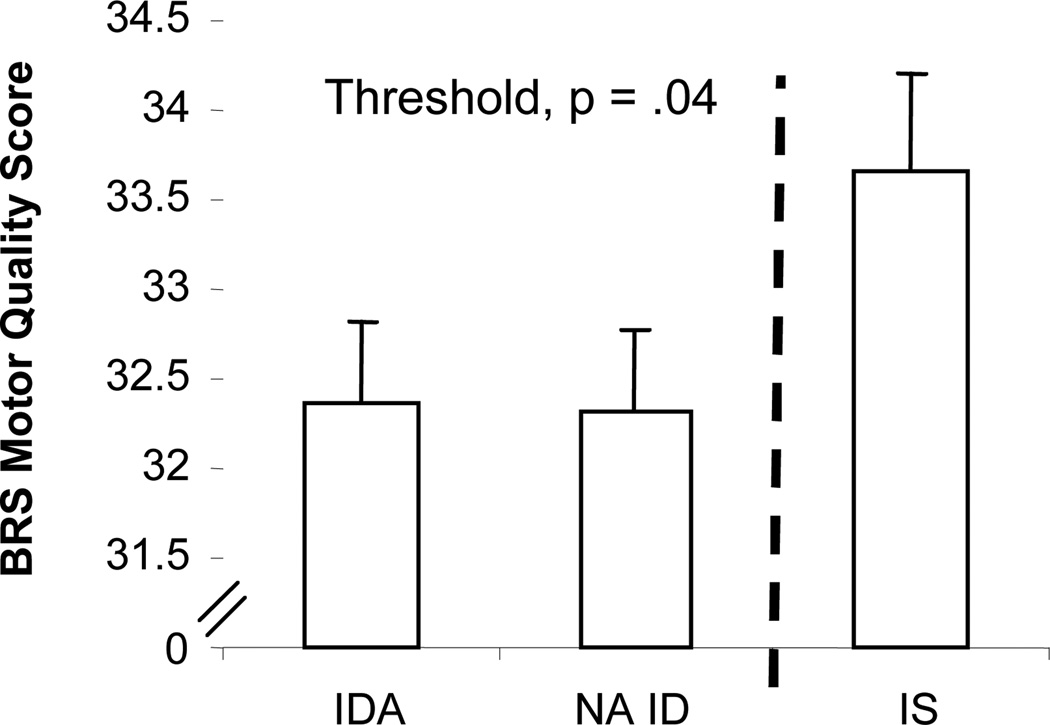
There was a significant linear effect of iron status on examiners’ assessment of the general quality of motor performance, using the BRS Motor Quality factor (F(1, 65) = 5.90, p = .02). The threshold was ID (with or without anemia) v. IS; IDA and NA ID infants were rated as having poorer motor quality than IS infants (F(1, 65) = 6.31, p = .01) (Fig. 3). NA ID was significantly lower than IS in pairwise comparisons (p = .01). Infant age, mother’s age, and maternal depression were the significant covariates.
Figure 3.
Effect of iron status (final classification) on BRS Motor Quality at 9 months. There was a significant linear effect (p = .05), but a threshold between the ID (with or without anemia) and IS groups better represents the results.
Bi-manual coordination and motor sequencing
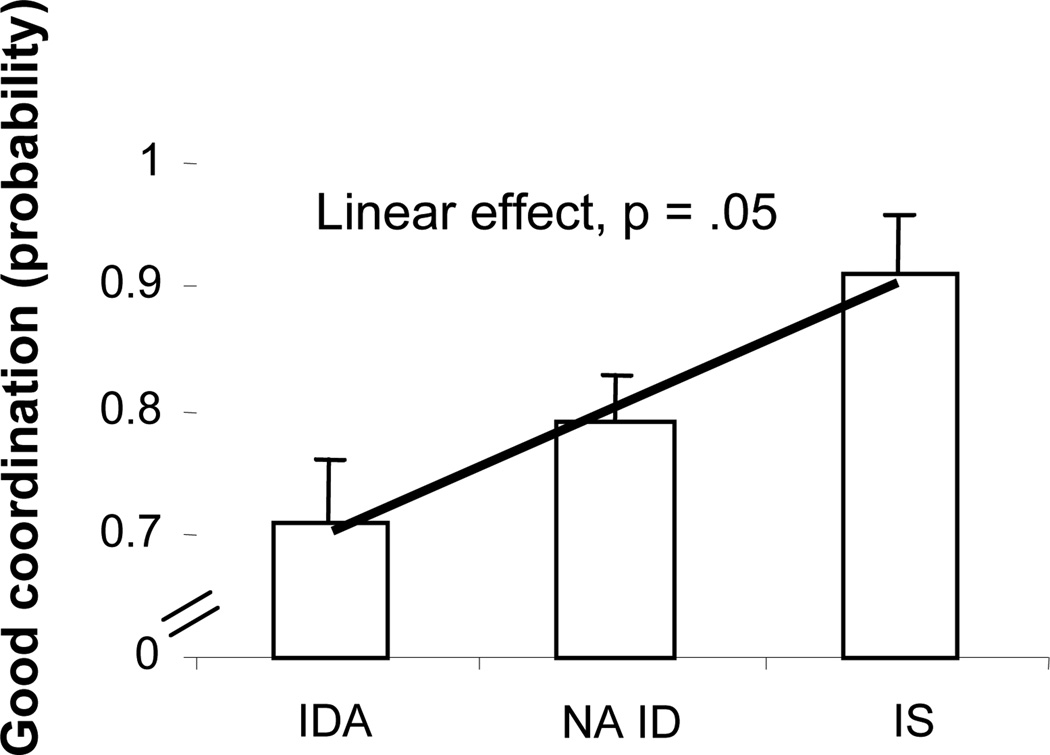
On the toy retrieval task, there was a significant linear effect of iron status on the quality of bi-manual coordination with the provisional iron classification and its larger sample size (χ2(1, N = 105) = 3.83, p = .05). The NA ID group was intermediate between the IDA and IS groups (Fig. 4). The threshold was IDA v. non-anemic; IDA infants were less able to retrieve the toy from the box with good bi-manual coordination (χ2(1, N = 105) = 3.93, p = .05), compared to NA ID + IS. Gender and age were the significant covariates, with boys doing better than girls.
Figure 4.
Effects of iron status (provisional classification) on the quality of bi-manual coordination during the toy retrieval task at 9 months. There was a significant threshold between IDA and non-anemic infants (p = .05), but the linear effect better represents the results.
DISCUSSION
This study examined the effects of IDA and ID without anemia on infant motor development by assessing motor skills that relate to brain areas and/or processes known to be altered by early ID, namely myelination and basal ganglia function, especially related to the dopamine system. Several tests that are sensitive to overall motor delays, which may be affected by impaired myelination, indicated linear effects of iron status, specifically, developmental milestones, Peabody gross motor (suggestive trend), INFANIB standing item, and the BRS Motor Quality factor. The threshold for the effects on developmental milestones, INFANIB standing item, and motor quality was ID with or without anemia, and scores for the NA ID group were significantly lower than the IS group. The toy retrieval task, where performance may depend on basal ganglia function, also showed linear effects of iron status, and the threshold for this effect was IDA. These results controlled for background factors that correlated with outcome. A few previous studies found motor effects of ID without anemia (e.g., (26;27)), but most did not (see review (7)). Our detection of such effects may be due to using a more comprehensive and more sensitive motor assessment.
We will first discuss how ID effects on impaired myelination and poor function of the basal ganglia could affect and impair motor performance. We will point to some limitations of our study and end with a suggestion for a future intervention, aimed at overcoming these effects.
Impaired myelination is one potential mechanism for ID effects on overall motor development. Iron is essential for oligodendrocyte function, which is crucial for myelin production. ID during gestation and lactation in the rat induces changes in myelin components (protein, cholesterol, phospholipids and galactolipids) and compaction in adulthood, despite an iron-sufficient diet beginning at weaning (8). In the human IDA infant, slower transmission in the auditory and/or visual systems, both short-term and years after iron therapy (28), suggests that effects of ID in infancy on myelination may be widespread and long-lasting. The corticospinal tract, in which motor commands traverse from the motor and sensory regions of the cerebral cortex to the spinal cord, is not completely myelinated at birth and thus may be particularly vulnerable to early ID effects. Impaired myelination in the entire brain and especially the corticospinal tract may delay and/or alter the normal development and refinement of motor skills.
Another potential mechanism for the effects of early ID on motor development is through altered basal ganglia function. In rodent models of diet-induced ID during gestation and lactation, severe IDA reduced D1 and D2 dopamine receptor densities in the striatum (8), along with poor growth. To be more relevant to IDA in human infants, the rodent model in our program project produced ID with a more moderate level of brain ID than previous studies and avoided marked growth restriction (29;30). Even in the more moderate ID model, deficits in a natural grooming sequence that depends on striatal dopamine function were observed in adulthood, despite correction of IDA and normalization of brain iron content in all areas but the thalamus (29).
The basal ganglia play important roles in learning and execution of sequential movements (20) and also control of bi-manual coordination, through motor inhibition of contralateral movements (21). Thus, the difficulty ID infants showed on the toy retrieval task, which requires sequential movements during bi-manual coordination, may relate to IDinduced changes in striatal dopamine function.
Although postural control has not been traditionally considered a major function of the basal ganglia, a recent review emphasizes their important contribution to regulation of postural control (31). Other outcomes with a threshold of ID with or without anemia (developmental milestones and INFANIB standing) depend on postural control at this age. Postural control may be sensitive even to ID without anemia because it involves more than one CNS process impaired by ID (i.e., myelination and basal ganglia function).
The study is limited by small sample size, and results may not generalize to other populations. Uncertainty concerning compliance with iron intake and the high proportion of missing hematology data at 12 months mean that the study could not assess the ability of iron therapy to improve infant motor development.
In sum, using a comprehensive and sensitive assessment of motor development, this study found poorer motor function in ID infants with and without anemia. These findings were independent of family background, suggesting that poorer development was not due to family factors, such as lack of maternal stimulation and encouragement/attention. The observed effects of ID without anemia are particularly concerning, since ID without anemia is not detected by common screening procedures and is more widespread than IDA. The observed motor deficits in early ID are consistent with impaired myelination and oligodendrocyte function, both of which appear to be sensitive to developmental experience (32). Thus, future interventions for ID in infancy that include motor training or other environmental enrichment might help reduce long-term effects.
ACKNOWLEDGEMENTS
This research was funded by grants from the National Institutes of Health (P01 HD39386, Brain and Behavior in Early Iron Deficiency, Betsy Lozoff, Principal Investigator) and the Joseph Young, Sr., Fund, in Michigan (Sandra W. Jacobson, Principal Investigator). We are grateful to the study families; to Sheila Gahagan for training in administration of the INFANIB; to Joseph L. Jacobson for consultation regarding research design and data analysis; to Rinat Armony-Sivan, Renee Sun, Margo Laskowski, Jigna Zatakia, Brenda Tuttle, and Douglas Fuller for their contributions in recruitment, infant assessment, and data management and analysis; to William Neeley (Director, Detroit Medical Center University Laboratories), John Beard (Pennsylvania State University) and the laboratory staff at both institutions for performing the hematologic and biochemical assays; and to student coders for their help in video coding. The entire group of investigators participating in the Brain and Behavior in Early Iron Deficiency Program Project contributed to our thinking and understanding of effects of ID on early motor development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
No author has a conflict of interest.
Contributor Information
Tal Shafir, Center for Human Growth and Development, University of Michigan, Ann Arbor, Michigan
Rosa Angulo-Barroso, Center for Human Growth and Development and Division of Kinesiology, University of Michigan, Ann Arbor, Michigan
Yuezhou Jing, Center for Human Growth and Development, University of Michigan, Ann Arbor, Michigan
Mary Lu Angelilli, Carman and Ann Adams Department of Pediatrics, Children's Hospital of Michigan, Wayne State University School of Medicine, Detroit, Michigan
Sandra W. Jacobson, Department of Psychiatry and Behavioral Neuroscience, Wayne State University School of Medicine, Detroit, Michigan
Betsy Lozoff, Center for Human Growth and Development and Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor, Michigan
REFERENCES
- 1.Bushnell EW, Boudreau JP. Motor development and the mind: the potential role of motor abilities as a determinant of aspects of perceptual development. Child Dev. 1993;64(4):1005–1021. [PubMed] [Google Scholar]
- 2.Biringen Z, Emde RN, Campos JJ, Appelbaum MI. Affective reorganization in the infant, the mother, and the dyad: the role of upright locomotion and its timing. Child Dev. 1995;66(2):499–514. [PubMed] [Google Scholar]
- 3.Shafir T, Angulo-Barroso R, Calatroni A, Jimenez E, Lozoff B. Effects of iron deficiency on patterns of motor development over time. Hum Mov Sci. 2006;25:821–838. doi: 10.1016/j.humov.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fourth Report on the World Nutrition Situation. Geneva: ACC/SCN in collaboration with IFPRI; 2000. Administrative Committee on Coordination Sub-Committee on Nutrition (ACC/SCN) [Google Scholar]
- 5.Stoltzfus RJ, Mullany L, Black RE, Ezzati M, Lopez AD, Rodgers A, Murray CJL. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization; 2004. Iron deficiency anaemia; pp. 163–209. [Google Scholar]
- 6.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28(4):S560–S571. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 8.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, et al. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120:e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2007 doi: 10.1016/j.jpeds.2007.09.048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozoff B, Angelilli ML, Zatakia J, Jacobson SW, Calatroni A, Beard JL. Iron status of inner-city African-American infants. Am J Hematol. 2007;82:112–121. doi: 10.1002/ajh.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Life Sciences Research Office. Bethesda: Federation of American Societies for Experimental Biology; 1984. Assessment of the Iron Nutrition Status of the U.S. Population Based on Data Collected in the Second National Health and Nutrition Survey, 1976–1980. [Google Scholar]
- 13.Looker AC, Dallman P, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR. 1998;47:1–29. [PubMed] [Google Scholar]
- 15.Centers for Disease Control. Hyattsville, MD: Department of Health and Human Services; 2001. Healthy People - 2000 National Health Promotion and Disease Prevention Objectives Final Review. [Google Scholar]
- 16.Pollitt E, Husaini MA, Harahap H, Halati S, Nugraheni A, Sherlock AO. Stunting and delayed motor development in rural West Java. Am J Human Biol. 1994;6:627–636. doi: 10.1002/ajhb.1310060511. [DOI] [PubMed] [Google Scholar]
- 17.Folio MR, Fewell RR. Second ed. Austin, TX: 2000. Peabody Developmental Motor Scale. Pro-Ed. [Google Scholar]
- 18.Ellison PH. A Reliable Method for the Neuromotor Assessment of Infants. San Antonio, TX: Therapy Skill Builders; 1994. The INFANIB. [Google Scholar]
- 19.Bojczyk KE, Corbetta D. Object retrieval in the 1st year of life: learning effects of task exposure and box transparency. Dev Psychol. 2004;40(1):54–66. doi: 10.1037/0012-1649.40.1.54. [DOI] [PubMed] [Google Scholar]
- 20.Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. PNAS. 2005;102(35):12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholz VH, Flaherty AW, Kraft E, Keltner JR, Kwong KK, Chen YI, et al. Laterality, somatotopy and reproducibility of the basal ganglia and motor cortex during motor tasks. Brain Res. 2000;879(1–2):204–215. doi: 10.1016/s0006-8993(00)02749-9. [DOI] [PubMed] [Google Scholar]
- 22.Vink M, Kahn RS, Raemaekers M, van den HM, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp. 2005;25(3):336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayley N. Bayley Scales of Infant Development. 2nd ed. San Antonio: The Psychological Corporation; 1993. [Google Scholar]
- 24.SAS 9.1. Cary, NC: SAS Institute Inc; 2003. [Google Scholar]
- 25.Liang KY, Zeger SL. Longitudinal data analysis using general linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 26.Oski FA, Honig AS, Helu B, Howanitz P. Effect of iron therapy on behavior performance in nonanemic, iron-deficient infants. Pediatrics. 1983;71:877–880. [PubMed] [Google Scholar]
- 27.Gunnarsson BS, Thorsdottir I, Palsson G, Gretarsson SJ. Iron status at 1 and 6 years versus developmental scores at 6 years in a well-nourished affluent population. Acta Paediatr. 2007;96(3):391–395. doi: 10.1111/j.1651-2227.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 28.Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: Long-lasting effects on auditory and visual systems functioning. Pediatr Res. 2003;53(2):217–223. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- 29.Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–270. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: Biology and behavior in young rats. Behav Brain Res. 2006;170:224–232. doi: 10.1016/j.bbr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Visser JE, Bloem BR. Role of the basal ganglia in balance control. Neural Plast. 2005;12(2–3):161–174. doi: 10.1155/NP.2005.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markham JA, Greenough WT. Experienced-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. Beck Depression inventory -- II. San Antonio: The Psych Corp.; 1996. [Google Scholar]
- 35.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 36.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment (Revised Edition) Little Rock: University of Arkansas; 1984. [Google Scholar]
- 37.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 38.Crnic KA, Greenberg MT, Ragozin AS, Robinson NM, Basham RB. Effects of stress and social support on mothers and premature and full-term infants. Child Dev. 1983;54(1):209–217. [PubMed] [Google Scholar]