Abstract
The neural link between ostensibly aversive stress experiences and intensely rewarding drug taking remains to be delineated. Epidemiological data associate stress and the abuse of various drugs, and experimental data identify the conditions that determine how episodic social stress intensifies the motivation for cocaine and the actual self-administration of cocaine. Two types of social stress have been the focus of experimental study in Long–Evans rats, since they engender divergent changes in drug- or sugar-rewarded behavior and in neuroadaptation. Episodic social defeat stress consists of four brief confrontations between the experimental rat and an aggressive resident rat of the Long–Evans strain over the course of 10 d. Subordination stress involves the continuous exposure to an aggressive resident for 5 weeks, while living in a protective cage within the resident's home cage with brief daily confrontations. These stress experiences result in (1) increased intravenous cocaine self-administration under a fixed ratio schedule with prolonged binge-like access in episodically defeated intruder rats but suppressed cocaine intake by continuously subordinate rats; (2) deteriorated sugar preference and intake and decreased exploratory behavior in subordinate, but not intermittently defeated, rats; and (3) a sensitized dopamine (DA) response in the nucleus accumbens via in vivo microdialysis and increased tegmental brain-derived neural growth factor (BDNF) in episodically defeated rats, whereas the continuously subordinate rats show suppression of the DA and BDNF responses. These divergent neuroadaptations to social stress may represent the substrates for the intensification of cocaine “bingeing” relative to the anhedonia-like deterioration of reward processes during subordination stress.
Introduction
Stressful life experiences can promote drug abuse and trigger relapse (Shaham et al., 2000; Brady and Sinha, 2005), which contrasts with the impairing effects of stress in the genesis of affective disorders (Arborelius et al., 1999; De Kloet et al., 2005). In animal models, brief episodes of social defeat stress engender neural, physiological, and behavioral effects opposite to those caused by continuous subordination stress (Tornatzky and Miczek, 1993; Miczek et al., 1999a; Sgoifo et al., 2001; Fuchs et al., 2004; Kozorovitskiy and Gould, 2004; Covington and Miczek, 2005; Razzoli et al., 2007). Repeated intermittent stress exposures augment the motor-stimulating effects of psychostimulant drugs and reinstate previously extinguished cocaine seeking (Antelman et al., 1980; Shaham et al., 2003), but when animals experience prolonged uncontrollable stress, several indices of dysfunctional reward become evident (Katz, 1981; Willner et al., 1992); this blunting of reward, known as anhedonia, is a cardinal sign of stress disorders. Here, we begin to resolve this paradox by focusing on dopamine (DA) and brain-derived neural growth factor (BDNF) as neural mediators of divergent adaptations to different types of social stress.
Brief episodes of social defeat stress produce long-lasting sensitized neural responses to psychomotor stimulant challenge, particularly in the ventral tegmental area (VTA), and decrease activation in medial prefrontal cortex (mPFC) (Nikulina et al., 2004; Covington et al., 2005). These effects parallel stimulant-induced behavioral sensitization, and cross-sensitization between these brief stressors and stimulant drugs suggests shared neural mechanisms in rodents (Covington and Miczek, 2001; Pacchioni et al., 2007; Yap and Miczek, 2007). Intermittent exposure to either social defeat stress or psychomotor stimulants activates dopaminergic cells that originate in the VTA and project to the nucleus accumbens (NAC) and mPFC (Tidey and Miczek, 1996; Vanderschuren and Kalivas, 2000; Covington et al., 2008; Anstrom et al., 2009).
An intracellular cascade of molecular events in the VTA–NAC–mPFC–amygdala/hippocampus circuit includes several candidate mechanisms for the persistent neuroplastic adaptation to social defeat stress and other types of stress, foremost BDNF. As a result of its high expression levels in the hippocampus, the inhibition of BDNF is readily seen, especially after stress, although it remains unclear how different stimuli regulate distinct BDNF transcripts (Duman and Monteggia, 2006; Nair and Vaidya, 2006). Whereas the suppression of both the message and protein of BDNF in hippocampal cells of animals experiencing various types of environmental stress is long documented (Smith et al., 1995; Duman and Monteggia, 2006; Tsankova et al., 2006), some types of stress can increase the mRNA for BDNF in hypothalamic and amygdaloid cells (Aguilar-Valles et al., 2005). The current work focuses on tonic and phasic changes in dopaminergic cells in the VTA in rats that had experienced either brief episodes of social defeat stress or, alternatively, prolonged subordination stress. We contrasted four intermittent social defeat episodes over the course of 10 d with continuous social subordination stress for 5 weeks, as these parameters were found to model the activational versus impairing consequences of stress.
Materials and Methods
Animals
Adult male Long–Evans rats from Charles River Laboratories, weighing 225–250 g on arrival in the laboratory, were singly housed in standard polycarbonate cages (45 × 24 × 20 cm) with water bottles in the stainless steel lids and wood chip-covered bottoms, or in custom-built polycarbonate cages (25 × 30 × 30 cm) that were fitted with wire mesh screens as side walls, water bottle and food tray, and floors lined with Cellu-Dri pellet bedding. Separate male rats (500–700 g) with a previous history of reliable aggressive behavior in confrontations with an intruder, termed stimulus resident rats, were housed in male–female pairs in large stainless steel cages (71 × 46 × 46 cm), as described previously (Miczek, 1979). All cages were located in an environmentally controlled suite of a vivarium and procedure rooms that were kept at 21 ± 1°C, 35–40% humidity with an inverted 12 h light/dark cycle (lights on 8:00 P.M. to 8:00 A.M.). The experimental facilities and procedures were supervised and approved by the Tufts University Institutional Animal Care and Use Committee following the NIH guide (National Research Council, 1996).
Experimental design
The sequence of experimental procedures and the number of animals per group are summarized in Tables 1 and 2.
Table 1.
Sequence of experimental procedures
| Experimental days | Experimental events | Number of subjects |
|---|---|---|
| Episodic social defeat stress protocol and cocaine self-administration | ||
| Days 1–10 | Episodic social defeat stress | |
| Days 1, 4, 7, and 10: aggressive encounter with resident male | ||
| Days 1–4 and 7–10: body weight | 24 Stressed, 24 control | |
| Days 2 and 8: sucrose preference | 24 Stressed, 24 control | |
| Day 21 | Cocaine challenge (locomotor sensitization) | 24 Stressed, 24 control |
| Day 22 | Intravenous catheter implantation | |
| Days 27–48 | Cocaine self-administration | |
| Days 27–35: acquisition and training | ||
| Days 36–40: FR 5 maintenance sessions | ||
| Days 41–45: progressive ratio (3 trials) | 14 Stressed, 14 control | |
| Days 47–48: 24 h unlimited access binge | 14 Stressed, 14 control | |
| Continuous subordination stress protocol | ||
| Days 1–36 | Continuous subordination stress | |
| Daily: housed in home cage of aggressive resident male, brief daily unprotected defeat encounter | ||
| Daily M–F: body weight | 22 Stressed, 22 control | |
| Once weekly: sucrose preference | 22 Stressed, 22 control | |
| Once weekly: motor activity | 9 Stressed, 9 control | |
| Twice weekly: saccharin-reinforced PR responding | 7 Stressed, 7 control | |
| Day 47 | Cocaine challenge (locomotor sensitization) | 22 Stressed, 22 control |
| Day 48 | Intravenous catheter implantation | |
| Days 53–74 | Cocaine self-administration | |
| Days 53–61: acquisition and training | ||
| Days 62–66: FR 5 maintenance sessions | ||
| Days 67–71: progressive ratio (3 trials) | 9 Stressed, 8 control | |
| Days 73–74: 24 h unlimited access binge | 9 Stressed, 8 control | |
M–F, Monday through Friday.
Table 2.
Immunohistochemistry and microdialysis experiments
| Experimental days | BDNF immunohistochemistry |
Microdialysis |
||
|---|---|---|---|---|
| Stressed | Controls | Stressed | Controls | |
| Episodic social defeat stress | ||||
| 5 d before first social defeat | Guide cannula implanted in NAC | |||
| Days 1, 4, 7, and 10 | Defeated in resident encounters | Handled | Defeated in resident encounters (n = 7) | Handled (n = 6) |
| Day 21 | Injected with cocaine or saline 60 min before perfusion | Injected with saline and then cocaine during microdialysis | ||
| Continuous subordination stress | ||||
| Days 1–36 | Housed in home cage of aggressive male | Handled | Housed in home cage of aggressive male (n = 6) | Handled (n = 6) |
| Day 37 | Guide cannula implanted in NAC | |||
| Day 47 | Injected with cocaine or saline 60 min before perfusion | Injected with saline and then cocaine during microdialysis | ||
Social stress procedures
Based on previous experiments (Covington and Miczek, 2001; Covington et al., 2005; Rygula et al., 2005; Abumaria et al., 2006), the maximally effective parameters for divergent outcomes of two types of social stress were selected for study. Rats were either subjected to four brief intermittent social defeat episodes over the course of 10 d or to continuous social subordination stress for 36 d, or they served as contemporary control groups. The experimental animals were weighed and monitored for health conditions daily.
Intermittent brief episodes of defeat stress.
In this social stress condition, male rats, termed “intruders,” confronted a different aggressive resident on four occasions at 72 h intervals (Tornatzky and Miczek, 1993). First, the intruder's home cage, fitted with wire mesh walls, was placed into the larger home cage of the aggressive resident stimulus rat for 10 min; the female partner of the male resident was removed for the duration of the confrontation. The intruder was investigated and threatened by the resident through the protective wire mesh. Second, the intruder was removed from its protective home cage and confronted directly by the aggressive resident. After a latency of ∼30 s, the resident delivered the first attack bite directed at the neck and back, and the intruder evaded or escaped, assuming a defensive upright posture and eventually a submissive supine posture (Miczek, 1974; Miczek and de Boer, 2005). The fight was terminated once the intruder maintained a supine posture for at least 6 s, or after 5 min. Third, the intruder was placed back into its protective home cage within the resident's cage for another 10 min. During the intervening 72 h, the intruder's home cage was placed in a room of the vivarium separate from that housing the resident rats.
Continuous subordination stress.
In a second type of social stress, male rats, termed “subordinates,” confronted an aggressive male resident daily for a brief flurry of attacks that resulted in clear and unambiguous signs of submission (supine posture, immobile crouch posture, ultrasonic vocalizations in the 22 kHz range) as end points (van der Poel and Miczek, 1991; Blanchard et al., 1993). The encounter typically lasted <2 min and was terminated after a maximum of 5 min. The resident female rat was removed during the daily encounter. After the confrontation, the intruder was placed into a protective wire mesh cage (20 × 30 × 20 cm) within the resident's large home cage for the remainder of the 24 h. The subordinate confronted a different resident every day for 5 d of the week and was housed in a resident's home cage 7 d per week for 36 d, allowing continuous contact with the aggressive residents. The subordinate had unrestricted access to standard rodent chow and water in the protective cage.
Sucrose preference
Once per week, the rats were tested for 0.8% sucrose intake and preference for 1 h while removed from their respective stress exposure. In subgroups of experimental animals that were exposed to either intermittent brief episodes of social defeat stress (n = 24) or continuous subordination stress (n = 22) and their contemporary controls (n = 24 and n = 22, respectively), baseline sucrose preference was assessed in the week before the stress procedures. After first exposing the rats to sucrose by replacing their water bottle with 0.8% sucrose overnight, the rats were given a daily two-bottle choice test to determine baseline preference and intake measures. During this 1 h test, the rats had access to one bottle containing tap water and another containing 0.8% sucrose. Based on multiple baseline intake measurements, the rats were assigned to evenly matched treatment groups, either exposure to stress or control. Once per week, the rats were tested for sucrose intake and preference for 1 h while removed from their respective stress condition.
Motor activity
Once per week during the continuous subordination stress procedure, a subgroup of continuously stressed rats (n = 9; n = 9 controls) was removed from its home cage during the dark period and placed in the center of a large red-illuminated open field (71 × 45 × 45 cm) with wood chip lining. After a 30 min acclimation period, two 5 min video samples were recorded, and the frequency and duration of grooming, rearing, walking, and inactivity were encoded and analyzed using The Observer software (version 5.0; Noldus Information Technology).
Saccharin-reinforced nose-poke responding
Saccharin-reinforced nose-poke responding on a progressive ratio (PR) schedule was examined in another subgroup of experimental rats that experienced continuous subordination stress (n = 7) and their contemporary controls (n = 7). Specifically, without any restriction in food or water, the rats' responses were studied in a familiar polycarbonate cage (45 × 24 × 20 cm) fitted with an aluminum panel as illustrated and described previously (Miczek and de Almeida, 2001). These panels contained two operanda for detecting nose pokes and delivering fluid into a receptacle (MED Associates). Each response in one nose poke hole was reinforced with the delivery of 0.05 ml of 0.02% saccharin solution, whereas responding in the other hole was not. The position of the active nose poke hole was counterbalanced across rats. After four to seven daily 60 min sessions of saccharin-reinforced responding, the rats were exposed to a PR schedule of saccharin reinforcement, with progressively increasing response requirements for fluid delivery (Hodos, 1961). The break point was defined as the total number of saccharin reinforcements achieved before the maximum 60 min inter-reinforcement interval was reached, terminating the session.
After completing the social stress procedure, subgroups from each condition were assigned to further neurochemical or behavioral studies. The intermittently defeated rats and continuously subordinate rats as well as their respective controls were used for cocaine challenge and intravenous cocaine self-administration studies, or for BDNF immunohistochemistry, or for in vivo microdialysis, as described below.
Cocaine challenge
Ten days after the last defeat in the intermittent and continuous social stress procedures, the expression of behavioral cross-sensitization was assessed (Table 1), as described previously (Covington and Miczek, 2001). Initially, experimental and control rats (n = 22 intermittent defeat and 22 corresponding controls, n = 24 continuous stress and 24 corresponding controls) were given a saline injection and returned to their home cages; 5 min later, they were videotaped for 5 min. Then they were given a challenge injection of cocaine (10 mg/kg, i.p.), and additional 5 min samples were recorded 5 and 25 min later. The frequency and duration of rearing, walking, grooming, digging, and inactivity were recorded by an observer who was unfamiliar with the experimental treatment and whose reliability had been established, using The Observer software (Noldus Information Technology).
Intravenous cocaine self-administration
After the cocaine challenge, one subset of rats (n = 14 episodically stressed, n = 8 continuously subordinate, and their matched controls) was prepared for cocaine self-administration (Tables 1 and 2). Indwelling catheters (SILASTIC silicon tubing; inner diameter, 0.63 mm; outer diameter, 1.17 mm) were implanted into the right jugular vein (Remie et al., 1990; Covington and Miczek, 2001) under ketamine (100 mg/kg) and xylazine (6 mg/kg) anesthesia. The catheter was passed subcutaneously to the rat's back where it exited through a small incision and was affixed to a plastic pedestal (Plastics One) mounted inside a harness system (Instech Laboratories). Throughout the experiment, catheters were flushed with sterile heparinized saline (20 IU/ml) each morning, and pulses of 0.17 ml of saline were delivered every 30 min overnight between daily self-administration sessions.
Acquisition.
After a 5 d recovery period, the rats were given the opportunity to self-administer cocaine (0.75 mg/kg per infusion) without a priming infusion during daily sessions that were signaled by a stimulus light. One wall of the home cage contained two retractable levers, and pressing the left one was reinforced by an intravenous infusion followed by a 30 s time out, during which the stimulus light was turned off. Each daily session terminated after the delivery of 15 infusions or after 5 h of access. After the rat self-administered 15 cocaine infusions on 2 consecutive days, the response requirement was gradually increased over the course of 3–5 d so that eventually every fifth lever press was reinforced by an infusion [fixed ratio 5 (FR 5)].
FR 5 limited access.
The rats were maintained on an FR 5 schedule for 15 infusions per day within, maximally, 5 h for 5 consecutive days. Each infusion was followed by a 30 s time out, during which responses were recorded but did not count toward the ratio requirement.
PR schedule of cocaine reinforcement.
The response demand for each successive cocaine infusion was progressively escalated so that the average rat would extinguish responding within 5 h. The ratios between required responses and cocaine infusions were as follows: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95… (Richardson and Roberts, 1996). The session terminated when no cocaine infusion was delivered within a 60 min interval. The median number of completed infusions for each rat was used as the dependent measure. Three sessions with the PR schedule alternated with sessions controlled by the FR 5 schedule.
Twenty-four-hour FR 5 binge.
As the final assessment of cocaine self-administration, each rat was given continuous unlimited access to cocaine self-administration for 24 h. Every fifth lever press was reinforced with an infusion of 0.31 mg/kg cocaine, followed by a 30 s time out. During the 24 h session, rats were considered to have stopped self-administering once 60 min elapsed without an infusion.
Immunohistochemistry
The effect of intermittent and continuous social defeat stress as well as a cellular response to cocaine challenge was estimated in the VTA where we previously observed a sensitized Fos response during psychostimulant challenge after intermittent social defeat stress (Nikulina et al., 2004). BDNF immunohistochemistry was performed in brains collected from animals that had received intraperitoneal saline injections. In the cross-sensitization experiment, neural activation after cocaine challenge was measured by double immunostaining for Fos-BDNF using a sequential procedure that involved first staining of nuclear Fos, then applying antisera for BDNF.
Tissue preparation.
In the cross-sensitization experiment, the rats received injections of cocaine (10 mg/kg, i.p.) or saline 60 min before being deeply anesthetized with an overdose of sodium pentobarbital and perfused transcardially with saline followed by 4% paraformaledhyde in 0.1 m phosphate buffer. Brains were removed, postfixed for 1.5 h in the same fixative at 4°C, and placed in graded concentrations of sucrose (12.5 and 25%) at 4°C for 3–4 d. Coronal sections (20 μm) from the anterior VTA [−4.9 to −5.2 mm from bregma (Paxinos and Watson, 1997)] were cut on a sliding microtome and collected in a chilled 0.1 m phosphate buffer, pH 7.4. The sections were mounted onto slides and stored at −80°C before immunohistochemistry.
BDNF immunohistochemistry.
Brain sections were washed three times in 0.05 m potassium PBS (KPBS), pH 7.4. Afterward, the sections were incubated for 1 h in 5% normal goat serum (NGS) in 0.05 m KPBS/0.4% Triton X-100, followed by incubation with a primary antibody. The sections were then incubated for 48 h at 4°C in humid chambers with rabbit polyclonal antisera for BDNF in 5% NGS/0.4% Triton X-100/0.05 m KPBS. BDNF immunohistochemistry used a rabbit anti-BDNF polyclonal antibody specific for BDNF over other neurotrophins (AB1779; Millipore). The sections were then triple washed in 0.05 m KPBS and incubated for 1 h at room temperature with biotin-conjugated goat anti-rabbit serum (Vector Laboratories). Immobilized antigen within the cells was visualized by incubation with the ABC complex of the Vectastain Elite ABC system (Vector Laboratories) for 45 min, after which sections were washed twice in 0.05 m KPBS and BDNF staining was developed by incubation with the VIP kit (Vector Laboratories), which provides purple cytoplasmic staining.
Fos-BDNF immunohistochemistry.
Double immunostaining for Fos-BDNF was performed using a sequential procedure that involved first staining of nuclear Fos. Rabbit polyclonal antisera directed against amino acid residues 4–17 of human c-Fos (AB-5; Calbiochem) was then applied for 48 h at 4°C (1:7500 dilution in 5% normal goat serum/0.05 m KPBS/0.4% Triton X-100). Sections were then washed three times in 0.05 m KPBS, and Fos was developed using the avidin–biotin–peroxidase method (Vectastain Elite ABC kit; Vector Laboratories). The sections were again washed in 0.05 m KPBS, and Fos was developed using the DAB kit (Vector Laboratories), which provides dark gray staining. After rinsing in KPBS and incubating with 0.001 m biotin for 45 min, antisera for BDNF were applied as described previously. Sections from stressed and control groups were processed simultaneously throughout all stages of the immunohistochemical procedures.
Counts of immunostained profiles were determined using ImageJ 1.33 (Wayne Raband, NIH), as in prior studies (Nikulina et al., 2004; Covington et al., 2005). Selected areas were captured and digitized using a video camera interfaced to the microscope using a 20× objective. The mean density of nonspecific background labeling was measured in a cell-free area of each experimental region and digitally subtracted so that any background staining was eliminated. Specific threshold values were then used to detect only those labeled objects of appropriate size and staining density. Double-labeled Fos-BDNF cells were counted when neuronal Fos was present in purple BDNF cytoplasmic stained cells. Raw counts of the number of Fos- or BDNF-positive cells in selected brain regions were made in sections from the same level in both stressed and control animals.
In vivo DA microdialysis
As described in our previous work (Van Erp and Miczek, 2000; Ferrari et al., 2003), rats (n = 25) were implanted with a guide cannula (MD-2250; Bioanalytical Systems) dorsal to the NAC shell (anteroposterior, +2.1 mm; mediolateral, ±1.1 mm from skull; dorsoventral, −5.8 mm from dura). The head mount was adapted for aggressive confrontations, allowing for a strong connection between the head mount and the protective wire spring around the microdialysis tubing. After at least 2 d of recovery, a microdialysis probe (MD-2200; Bioanalytical Systems) was lowered into the NAC under isoflurane inhalation anesthesia. The probe was perfused with artificial CSF (147 mm NaCl, 1.2 mm CaCl2, 0.85 mm MgCl2, 2.7 mm KCl; CMA/Microdialysis) at a rate of 0.5 μl/min overnight using a CMA/102 pump. A swivel arm (MED Associates), dual-channel swivel (Instech Laboratories), and 45 cm spring wire protecting the microdialysis tubing (FEP tubing; CMA/Microdialysis) allowed free movement of the animal.
On the experimental day, the flow rate was increased to 1.5 μl/min for sample collections. After a 30 min stabilization period, 15 μl dialysate samples were collected every 10 min before the animal was subjected to an intraperitoneal 10 mg/kg cocaine challenge after saline injection. Samples were collected into vials containing 5 μl of an antioxidizing agent (0.1 m phosphate buffer including 25 mm EDTA and 0.5 mm ascorbic acid) and were stored at −20°C until HPLC analysis. At the conclusion of the experiment, the animal was killed, and the brain was removed and fixed for histological confirmation of the probe placement.
Microdialysis samples (15 μl) were analyzed for DA using an LC-10ADVP pump (Shimadzu), a manual injector model LC1445 system organizer (GBC Scientific Equipment) with a 20 μl sample loop, a CAPCELL PAK SCX column (1.5 × 250 mm, 5 μm; Shiseido), an electrochemical detector (Decade II; Antec LLC), and a data collection and analysis software package (ALEXYS; Antec LLC). Mobile phase consisting of 150 mm ammonium acetate, 50 mm citric acid, 10% methanol, and 1% acetonitrile with pH adjusted to 4.6 was pumped at a flow rate of 200 μl/min. Retention times for these neurotransmitters were verified daily using standard solutions.
Statistical analysis
Two-way repeated-measures ANOVAs were used to analyze repeated observations of body weight, sucrose preference, locomotor behavior in the neutral cage, PR responding for sucrose, motor behavior after cocaine challenge, and microdialysis DA concentrations within the stress and control groups; when indicated by a significant main effect, post hoc comparisons to the nonstressed control group or the prestress baseline were performed using the Holm-Sidak method for multiple comparisons to control. In the cocaine self-administration experiments, t tests were used to compare stressed animals to corresponding controls on each dependent measure. The immunohistochemistry data were analyzed using t tests to compare stressed animals to their matched controls, and one-way ANOVA was used to compare BDNF levels in the four treatment groups corresponding to the various stress manipulations; all pairwise post hoc mean comparisons were performed using the Holm-Sidak method.
Results
Behavioral effects of episodic and continuous social stress
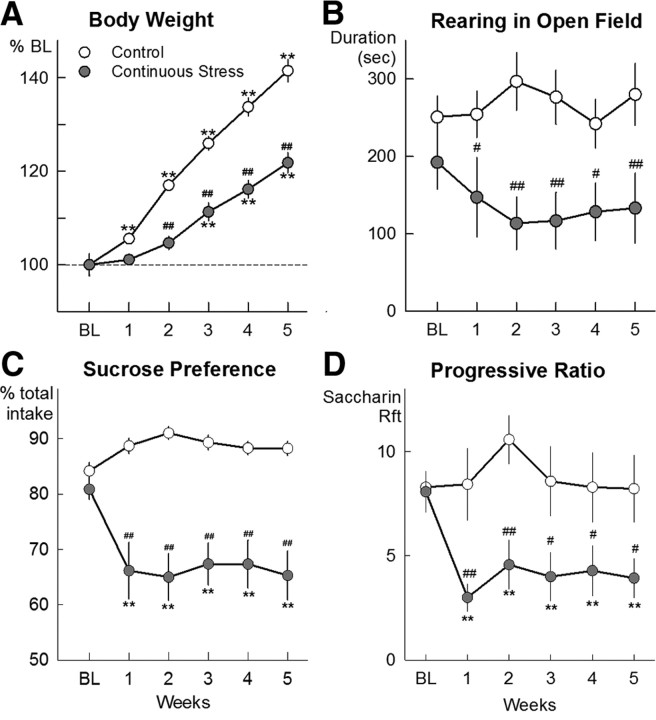
Chronic subordination stress reduced weight gain for the duration of the 5 week continuous exposure to an aggressive resident opponent, although the intruder was provisioned without restriction in a protected environment. The reduced weight gain was detected in the very first week of subordination stress and continued to extend for at least 10 d after termination of the 5 week subordination stress (stress × week interaction during subordination stress; F(5,210) = 40.0, p < 0.001) (Fig. 1A). In contrast, intermittently stressed rats gained weight at the same rate as contemporary controls (Table 3). The intermittent type of stress had no deleterious effects on the amount of autogrooming or the fur condition.
Figure 1.
A, Body weights of rats exposed to 36 d of continuous social stress (n = 24; filled circles) and corresponding controls (n = 24; open circles). B, Duration of rearing in an open field by continuously stressed rats (n = 9) and controls (n = 9). C, Preference for 0.8% sucrose solution versus water in a 60 min two-bottle preference test by continuously stressed rats (n = 24) and controls (n = 24). D, Number of ratios completed (“break point”) in a PR schedule of saccharin-reinforced nose poke responding by continuously stressed rats (n = 7) and controls (n = 7). *p < 0.05, **p < 0.01 versus baseline; #p < 0.05, ##p < 0.01 versus control group. % BL, Percentage of prestress baseline.
Table 3.
Effect of episodic social stress on body weight and sucrose preference
| Controls (n = 24) | Episodic Stress (n = 24) | |
|---|---|---|
| Body weight (g) | ||
| BL | 341.9 ± 3.2 | 342.0 ± 3.2 |
| Day 4 | 357.4 ± 3.2* | 359.6 ± 4.0* |
| Day 7 | 368.8 ± 3.3* | 370.7 ± 4.2* |
| Day 10 | 387.8 ± 3.8* | 385.8 ± 4.4* |
| Sucrose preference in 2-bottle test (% of total intake) | ||
| BL | 82.9 ± 1.9 | 84.4 ± 2.1 |
| Day 2 | 79.1 ± 2.6 | 77.0 ± 3.4 |
| Day 8 | 77.6 ± 3.4 | 81.5 ± 3.9 |
All values are means ± SEM.
*p < 0.01, compared with prestress baseline.
Exploratory behavior in the open field remained unaltered by exposure to intermittent episodes of social defeat stress. In contrast, continuous subordination stress significantly suppressed exploratory behavior over the course of the 60 min test starting in the first week of the 5 week period without evidence of habituation (Fig. 1B). Relative to measurements in control animals, exploratory rearing and walking remained significantly lower and inactivity was higher in rats that experienced continuous subordination stress (walking duration: F(1,16) = 16.88, p < 0.001; rearing duration: F(1,16) = 13.98, p = 0.002; duration of inactivity: F(1,16) = 16.95, p < 0.001).
Sucrose preference as a percentage of total 60 min fluid intake was significantly suppressed for the entire 36 d period of continuous subordination stress (stress: F(1,42) = 71.05, p < 0.001; stress × weeks interaction: F(5,210) = 4.24, p = 0.001) (Fig. 1C) and did not differ from control measurements in the intermittently stressed rats (Table 3). Similar effects were observed on total sucrose intake (stress: F(1,42) = 21.6, p < 0.001; stress × weeks interaction, F(5,210) = 3.71, p = 0.003). In another subgroup of animals, break points during a PR schedule of saccharin reinforcement showed a significant decline in rats that experienced continuous subordination stress for the duration of the stress procedure (Fig. 1D) (F(1,12) = 7.35, p = 0.019).
Behavioral cross-sensitization and self-administration of cocaine
A major effect of four intermittent episodes of social defeat stress over the course of 10 d was the induction of behavioral sensitization as evidenced by significantly augmented walking in response to a 10 mg/kg cocaine challenge 10 d after the last stress episode (stress: F(1,48) = 13.92, p < 0.001; stress × time: F(2,90) = 3.85, p = 0.025) (Fig. 2A). In contrast, a cocaine challenge (10 mg/kg, i.p.) 10 d after termination of the 5 week continuous subordination stress failed to increase walking (Fig. 2A). There was no significant main effect of stress on rearing or grooming frequency or duration in either the episodic stress or control groups.
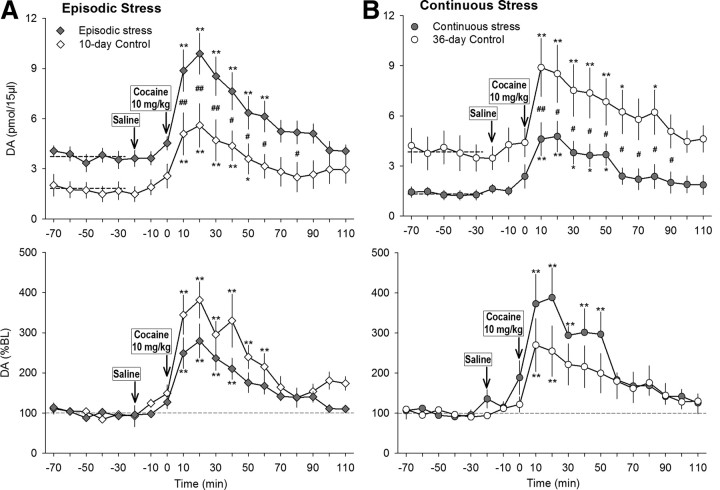
Figure 2.
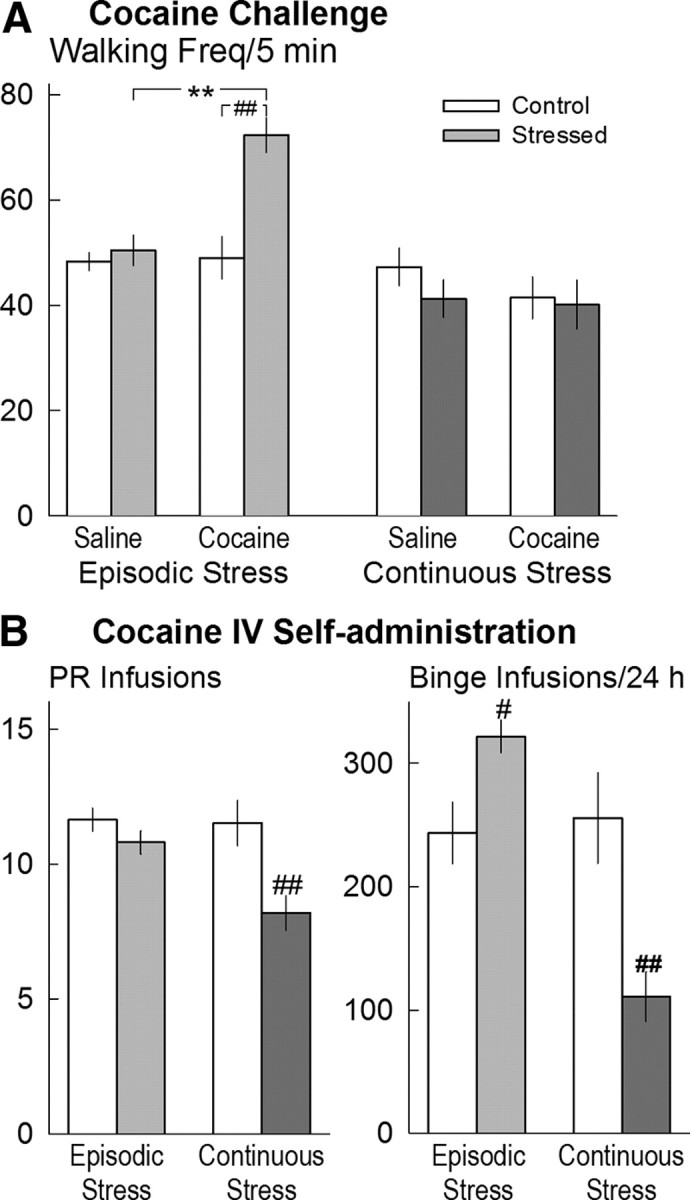
A, Frequency (Freq) of walking during 5 min samples after successive injections of saline and cocaine (10 mg/kg, i.p.) by rats exposed to 10 d of episodic stress (n = 24; light gray bar) and corresponding controls (n = 24; open bar) or to 36 d of continuous social stress (n = 22; dark gray bar) and corresponding controls (n = 22; open bar). B, Intravenous cocaine self-administration by rats exposed to 10 d of episodic stress (n = 14; light gray bars) and corresponding controls (n = 14; open bars) or exposed to 36 d of continuous social stress (n = 9; dark gray bars) and corresponding controls (n = 8; open bars). Left, Median number of ratios completed (break point) over three sessions of responding according to a PR schedule. Right, Total number of infusions accumulated during a 24 h unrestricted-access binge. All values are means ± SEM. Filled symbols and bars, Stressed animals; open symbols and bars, controls. **p < 0.01, compared with saline; #p < 0.05, ##p < 0.01, compared with the relevant control group.
Neither social stress procedure produced significant changes in the rate of acquiring or maintaining intravenous cocaine self-administration during daily limited access sessions. Subsequent studies revealed that rats that had previously been subjected to four brief episodes of social defeat stress accumulated significantly more cocaine reinforcements and responded at a higher rate during a 24 h continuous access session (“binge”) than their contemporary controls (intake: t = 2.71, df = 26, p = 0.012; rate: t = 3.07, df = 26, p = 0.005) (Fig. 2B). In contrast, the rats that were previously exposed to continuous subordination stress accumulated significantly less cocaine during the 24 h continuous access session than their contemporary controls (t = 3.29, df = 14, p = 0.005) and stopped self-administering cocaine sooner (t = 3.29, df = 14, p = 0.005). The previously subordinate rats also responded less on a PR schedule of cocaine reinforcement, accumulating fewer cocaine infusions than their contemporary controls (t = 3.13, df = 14, p = 0.007) (Fig. 2B).
Extracellular dopamine in NAC
After performing the two different types of social stress procedures, another set of animals (n = 7 for episodic stress with n = 6 contemporary 10 d controls; n = 6 for continuous stress with n = 6 contemporary 36 d controls) was implanted with a cannula in the shell of the NAC to measure DA in response to a cocaine challenge. There was no significant effect of sample time across five baseline measurements in either episodically or continuously stressed rats and their contemporary controls, so average baseline values were calculated for each rat and used for all subsequent analyses.
In episodically stressed rats, the average tonic DA level was significantly higher than that of controls (F(1,11) = 9.94, p = 0.009) (Fig. 3A, top). There was a significant stress effect on DA levels (F(1,14) = 5.71, p = 0.036) and a significant effect of sample time (F(12,132) = 29.35, p < 0.001) (Fig. 3A, top). Moreover, there was a significant interaction between episodic stress exposure and response to cocaine challenge; the stress group had significantly higher DA levels than controls from 5 to 65 min after injection (stress × sample time interaction: F(12,132) = 2.71, p = 0.003). When DA levels were expressed as a percentage of each rat's average baseline level, there was a significant time effect (F(12,132) = 21.43, p < 0.001) but no difference between episodically stressed rats and controls (Fig. 3A, bottom). Saline injection did not change NAC DA levels.
Figure 3.
Top, Extracellular concentrations of dopamine (fmol/15 μl) in NAC before and after injections of saline (−20 min on the time scale) and cocaine (10 mg/kg, i.p., at 0 min) in rats previously exposed to 10 d of episodic stress (n = 7; filled diamonds) or controls (n = 6; open diamonds) (A) or 36 d of continuous social stress (n = 6; filled circles) or controls (n = 6; open circles) (B). Bottom, DA levels expressed as percentage of baseline level in the same animals. All values are means ± SEM. *p < 0.05, **p < 0.01, compared with within-group baseline; #p < 0.05, ##p < 0.01, compared with controls.
On the other hand, 36 d of continuous subordination stress significantly reduced baseline DA levels compared with controls (F(1,10) = 6.44, p = 0.029). There were significant effects of stress (F(1,10) = 7.23, p = 0.023) and sample time (F(12,120) = 13.89, p < 0.001) (Fig. 3B); however, no interaction between continuous subordination stress and response to cocaine challenge was observed. When DA levels were expressed as percentage baseline, there was a significant sample time effect (F(12,120) = 12.39, p < 0.001), but chronically stressed rats did not differ significantly from controls (Fig. 3B, bottom). Saline injection did not change NAC DA levels.
BDNF
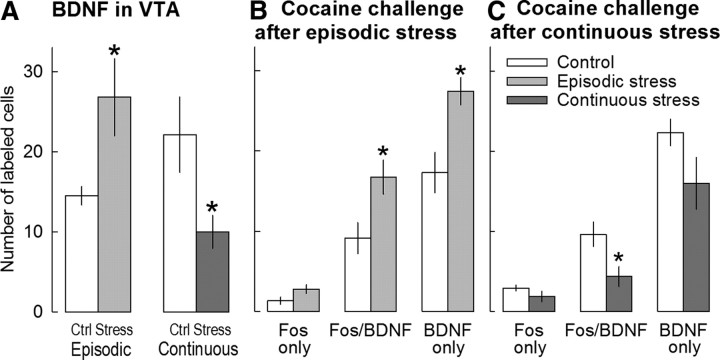
In another set of animals, BDNF expression in the VTA was determined by immunohistochemistry. Both types of social stress had a significant effect on BDNF levels in the VTA. BDNF levels were significantly elevated in the VTA of episodically stressed rats relative to handled controls (t = 2.20, df = 14, p = 0.045) (Fig. 4A). In contrast, BDNF levels were significantly lower in the VTA of animals that experienced continuous subordination stress (t = 2.49, df = 11, p = 0.030) (Fig. 4A).
Figure 4.
A, Expression of BDNF in the VTA after saline injection in rats previously exposed to episodic (n = 9 samples; left) or continuous (n = 7; right) social stress or corresponding controls (n = 7 and n = 6, respectively). B, Expression of Fos, Fos/BDNF, and BDNF in the VTA after a challenge injection of 10 mg/kg cocaine in rats previously exposed to episodic stress (n = 6) and nonstressed controls (n = 5). C, Expression of Fos, Fos/BDNF, and BDNF in the VTA after a challenge injection of cocaine in rats previously exposed to continuous stress (n = 6) and nonstressed controls (n = 5). All values are means ± SEM. Filled bars, Stressed rats; open bars, controls. *p < 0.05; **p < 0.01, compared to the corresponding controls.
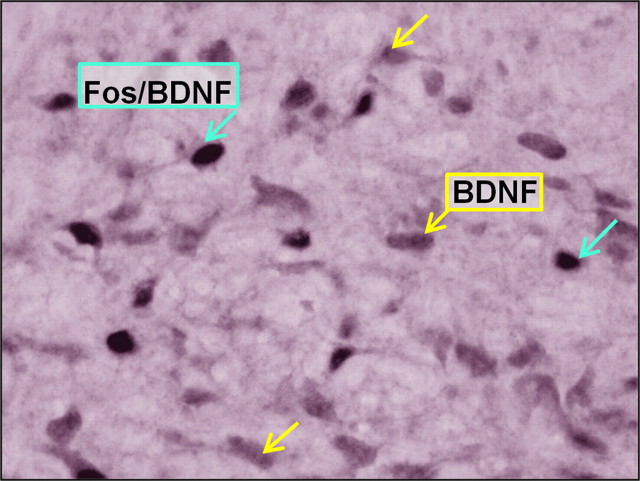
The challenge with 10 mg/kg cocaine 10 d after either the last episode of social defeat stress or after the last day of continuous subordination stress revealed that the type of defeat stress also had a significant effect on the response to the psychostimulant. In episodically and continuously stressed animals, the number of single-labeled Fos cells in the VTA after a cocaine challenge did not differ from paired handled controls. More BDNF-labeled cells were observed in the VTA of episodically stressed rats after cocaine challenge than in controls that had been handled but not exposed to social stress (t = 3.19, df = 9, p = 0.011) (Fig. 4B). Double-labeled Fos-BDNF cells were compared between stressed and handled control rats after cocaine challenge, to reveal the effects of different types of stress on neuronal activation in BDNF cells. Indeed, BDNF cells in the VTA of episodically stressed rats were characterized by significantly higher neuronal activation after cocaine challenge compared with saline controls, as assessed by the number of double-labeled Fos-BDNF cells (t = 2.66, df = 9, p = 0.026). Sixty-one percent of BDNF cells were activated by Fos expression in episodically stressed rats (vs 51% in the control group); this points to preferential activation of BDNF cells after cocaine administration (Fig. 5).
Figure 5.
Photomicrograph of Fos/BDNF-labeled cells in rat VTA after an intraperitoneal 10 mg/kg cocaine challenge 10 d after episodic social stress. Yellow arrows, BDNF-labeled cells; blue arrows, Fos/BDNF-labeled cells.
At the same time, the cocaine challenge induced a considerably smaller neuronal activation in the BDNF cells of the VTA after continuous subordination stress, as assessed by Fos-BDNF coexpression, and fewer activated cells (27%) were observed compared with the corresponding nondefeated control rats (43%). There was a significant reduction in the number of double-labeled Fos/BDNF cells in the VTA after cocaine challenge in the continuously stressed rats compared with nonstressed controls (t = 2.59, df = 9, p = 0.029).
Discussion
The current results begin to resolve some of the apparently contradictory links between social stress and disorders involving reward mechanisms. After study of the consequences of two types of social stress that differ primarily in their temporal characteristics, brief episodes of defeat versus continuous social subordination stress, it was found that psychomotor stimulation and binge-like cocaine self-administration either intensified or deteriorated. These behavioral changes were paralleled by those in BDNF and DA in the VTA–NAC pathway, suggesting candidate mechanisms for these divergent behavioral and neural adaptations.
Validity of social stress and learned helplessness
The appeal of challenging individuals with salient social confrontations is the validity of this type of stress for the species under study (Von Holst, 1998), although it is difficult to achieve the degree of precision and accuracy characteristic of other experimental stressors. In a socially organized species such as rats (Berdoy and Drickamer, 2007; Barnett, 1975), individuals cope with social stress chiefly by escape and, if that is not possible, by subordination. The current methodological approach attempted to capture the brief confrontation that activates physiological and behavioral coping responses within minutes (Koolhaas et al., 1999, 2011). In contrast, the prolonged exposure to a potential aggressor for several weeks shifted from activation to a passive, debilitating stress response. The currently implemented 5 week subordination stress avoided the development of a morbid course that often requires rescues in rats and tree shrews (Blanchard et al., 1985; Von Holst, 1985). Individuals differ in their propensity to submit passively, and these individually differentiated coping strategies may be shaped by repeated experiences in interaction with predispositions. It will be important to delineate these epigenetic mechanisms in future work.
A critical determinant of social stress is whether or not the individual perceives to have control over the delivery of the stress (Koolhaas et al., 2011). In fact, uncontrollable (i.e., inescapable) stress experiences engender a transient pattern of learned helplessness in the rodent laboratory and in the human clinic (Seligman and Maier, 1967). The perception of controllability of stress has been proposed to depend critically on serotonergic innervations of medial prefrontal cortical cells that are regulated via glutamatergic feedback (Amat et al., 2005, 2006). It is possible that individuals are inherently predisposed to exert control in stressful social encounters or, alternatively, learn to implement active or passive coping strategies. Clearly, information on these genetic predispositions or epigenetic influences is a matter of considerable interest at present (Fish et al., 2004; Tsankova et al., 2007), and resilience to social stress has begun to be characterized (Krishnan et al., 2007).
Social stress and behavioral cross-sensitization: role of accumbal DA
The increased locomotor activity in rats that were challenged with a cocaine injection confirmed the behavioral cross-sensitization that is seen after repeated stimulation with dopaminergic agonists and mild environmental and social stressors (Antelman et al., 1980; Miczek et al., 1999a,b). In contrast, psychomotor activation in response to a cocaine challenge appeared to be attenuated in subordinate rats, but the source for the substantial individual differences in this effect requires further study.
Sensitization at the cellular level is readily evident by the increased extracellular dopamine levels in the terminal region of the mesolimbic pathway in response to a cocaine challenge in individuals who experienced repeated stimulant injections or intermittent episodes of social defeat stress (Pierce et al., 1996; Vanderschuren and Kalivas, 2000). Intermittent social stress induces two changes in accumbal DA, elevating the tonic level of DA and also increasing the phasic response to cocaine challenge. This pattern may imply that acute episodes of social defeat stress belong to the class of salient events that prompt phasic dopamine release caused by burst firing, whereas chronic subordination stress may reduce tonic dopamine release, possibly via glutamatergic feedback regulation from the prefrontal cortex (Grace, 1991, 1995; Grace et al., 2007; Cao et al., 2010). Although behavioral and neural sensitization have been proposed to serve important roles in the processes leading to cocaine self-administration (Robinson and Berridge, 1993), both the sensitizing and reinforcing effects of psychomotor stimulants can be dissociated (Ahmed and Cador, 2006).
Cocaine binge
One of the most serious forms of intravenous cocaine self-administration that can be captured in an experimental model is the so-called binge. The currently implemented protocol of 24 h continuous access comprises a phase of regularly timed cocaine infusions for ∼12 h, followed by either an irregular “burst-and-run” pattern or complete cessation (Tornatzky and Miczek, 2000; Koob and Kreek, 2007). It is this latter phase of cocaine bingeing that is intensified, since rats that were previously sensitized by intermittent episodes of social defeat stress persisted in cocaine self-administration, each accumulating >300 infusions (Covington and Miczek, 2005; Quadros and Miczek, 2009). In contrast, rats ceased to engage in cocaine self-administration much sooner during the 24 h continuous access if they were subjected to chronic subordination stress. This severe reduction in the effectiveness of cocaine reinforcement extended also to the impaired saccharin preference and reinforced responding, indicative of an anhedonia-like profile in chronically subordinate animals (Rygula et al., 2005).
Social stress and the modulation of BDNF
BDNF in dopamine cells in the VTA appears to be a candidate mechanism for the contrasting neuroadaptive changes after either intermittent brief episodes of social defeat stress or persistent chronic subordination stress (Duman and Monteggia, 2006). The suppression of BDNF mRNA and protein was demonstrated in hippocampus and cortex after exposure to an intense, prolonged stressor (Smith et al., 1995). In mice, a single severe social defeat significantly decreased BDNF mRNA expression in hippocampus, piriform cortex, and basolateral amygdala 24 h later (Pizarro et al., 2004). BDNF is colocalized in aminergic neurons in the brainstem, also encompassing dopaminergic neurons in the VTA (Seroogy et al. 1994). Recent studies of severe chronic social defeat in mice suggest an essential role for BDNF in the VTA in mediating long-term neural plasticity in response to aversive social stimuli (Berton et al., 2006; Krishnan et al., 2007). Intermittent social defeat stress induces both transient activation of BDNF protein and mRNA expression in the prefrontal cortex and persistent enhancement of BDNF expression in the medial amygdala and VTA for up to 4 weeks after the stress termination (Fanous et al., 2010). BDNF can enhance dopamine release and turnover (Altar et al., 1992), and the present experiments found that episodic and continuous social stress had opposite effects on VTA BDNF and DA tonic activity in the NAC. Moreover, DA levels in the NAC were significantly lower after continuous subordination stress, relative to episodically stressed animals. Thus, postcocaine differences in BDNF and DA levels across social stress conditions likely reflect differences that existed before the cocaine injection. Intermittent episodes of social defeat stress activate the VTA neurons after cocaine challenge as indicated by the Fos/BDNF double-labeled cells, whereas chronic subordination stress engendered less activity in these cells. The cocaine challenge induced significant Fos expression in BDNF cells, and cocaine administration also increased the number of single-labeled BDNF cells in episodically stressed rats. Enhanced BDNF signaling in the DA neurons might be a sign of glutamatergic activity in the VTA that is BDNF dependent during cocaine sensitization (Pu et al., 2006). Moreover, the cocaine-induced DA response in the NAC was significantly lower after continuous subordination stress relative to episodically stressed animals. The temporal pattern of the stress stimuli might strongly influence the cocaine-induced DA release, which is accompanied by low Fos activation in BDNF-labeled cells. Our results are consistent with recent observations that deletion of Bdnf in the VTA of mice was accompanied by deficits in DA secretion in the NAC (Cordeira et al., 2010). The pattern of results from the intermittently stressed rats agrees also with previous observations that BDNF in VTA–NAC DA cells is part of a stress-induced signaling cascade that promotes behavioral and neural sensitization and contributes to the escalation of cocaine and heroin reward, even weeks after the termination of the drug exposure (Grimm et al., 2003; Narita et al., 2003; Graham et al., 2007).
One consequence of BDNF activation and subsequent TrkB receptor stimulation is the phosphorylation of MEK, ERK, and CREB, and this cascade has been implicated in the rewarding actions of cocaine (Carlezon et al., 1998). The stress-induced signaling cascade may include the activated glucocorticoid receptors that interact with TrkB receptors for BDNF, and we speculate that this interaction stimulates BDNF-dependent glutamate release. However, excessive stimulation of glucocorticoid receptors disturbs BDNF signaling for glutamate release via a glutamate transporter (Numakawa et al., 2009). Pharmacological blockade of MEK in the VTA before each of four intermittent social stress episodes can, in fact, prevent the ensuing locomotor sensitization in response to a cocaine challenge and also attenuates stress-induced escalation of cocaine self-administration during a 24 h binge (J. J. Yap, E. H. Chartoff, W. A. Carlezon Jr., and K.A. Miczek, unpublished results).
The current experiments reveal that intermittency of social stress results in a pattern of neuroadaptation opposite to that seen in continuously subordinate animals. The latter type of social stress, possibly attributable to its uncontrollability, profoundly decreases BDNF in the VTA. Functionally, these changes are related to the persistent suppression in cocaine and saccharine reward, and they can be considered consistent with the proposed role of BDNF in cardinal features of depressive-like anhedonia (Duman and Monteggia, 2006). The current focus on the low levels in BDNF in the VTA suggests regionally specific BDNF activity that may be linked to species-specific adaptations in a socially organized rodent species. Mechanistically, the decreases in BDNF result in reduced tyrosine kinase-regulated signal transduction, although it remains to be determined which BDNF transcript in which type of aminergic pathway is regulated by a specific social stress.
Footnotes
This work was supported by NIDA Grants DA02632 (K.A.M.) and DA024817 and DA026451 (E.M.N.).
References
- Abumaria N, Rygula R, Havemann-Reinecke U, Ruther E, Bodemer W, Roos C, Flügge G. Identification of genes regulated by chronic social stress in the rat dorsal raphe nucleus. Cell Mol Neurobiol. 2006;26:145–162. doi: 10.1007/s10571-006-9024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A, Sánchez E, de Gortari P, Balderas I, Ramrez-Amaya V, Bermúdez-Rattoni F, Joseph-Bravo P. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005;82:306–319. doi: 10.1159/000093129. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Barnett SA. The rat. A study in behavior. Chicago: University of Chicago; 1975. [Google Scholar]
- Berdoy M, Drickamer LC. Comparative social organization and life history of Rattus and Mus. In: Wolff JO, Sherman PW, editors. Rodent societies: an ecological and evolutionary perspective. Chicago: University of Chicago; 2007. pp. 380–392. [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress—behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Flannelly KJ. Social stress, mortality and aggression in colonies and burrowing habitats. Behav Processes. 1985;11:209–213. doi: 10.1016/0376-6357(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Cao JL, Covington HE, III, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology. 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Fanous S, Hammer RP, Jr, Nikulina EM. Short- and long-term effects of intermittent social defeat stress on BDNF expression in mesocorticolimbic brain regions. Neuroscience. 2010;167:598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Van Erp AMM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Czeh B, Flugge G. Examining novel concepts of the pathophysiology of depression in the chronic psychosocial stress paradigm in tree shrews. Behav Pharmacol. 2004;15:315–325. doi: 10.1097/00008877-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Katz RJ. Possible muscarinic-cholinergic mediation of patterned aggressive reflexes in the cat. Prog Neuropsychopharmacol. 1981;5:49–56. doi: 10.1016/0364-7722(81)90004-7. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, de Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Intraspecies aggression in rats: effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia. 1974;39:275–301. doi: 10.1007/BF00422968. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM. Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology. 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Boer SF. Aggressive, defensive, and submissive behavior. In: Whishaw IQ, Kolb B, editors. The behavior of the laboratory rat: a handbook with tests. New York: Oxford UP; 2005. pp. 344–352. [Google Scholar]
- Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. Behavioral sensitization to cocaine after a brief social defeat stress: c-fos expression in the PAG. Psychopharmacology. 1999a;141:225–234. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, Van Erp AMM, Blank AD, McInerney SC. d-Amphetamine “cue” generalizes to social defeat stress: sensitization and role of accumbens dopamine. Psychopharmacology. 1999b;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Nair A, Vaidya VA. Cyclic AMP response element binding protein and brain-derived neurotrophic factor: molecules that modulate our mood? J Biosci. 2006;31:423–434. doi: 10.1007/BF02704114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neuroscience. 2003;119:767–775. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington DC: National Academy; 1996. [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Kumamaru E, Adachi N, Yagasaki Y, Izumi A, Kunugi H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-γ signaling for glutamate release via a glutamate transporter. Proc Natl Acad Sci U S A. 2009;106:647–652. doi: 10.1073/pnas.0800888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–692. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 3. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Pu L, Liu QS, Poo MM. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci. 2006;9:605–607. doi: 10.1038/nn1687. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology. 2009;206:109–121. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Carboni L, Guidi A, Gerrard P, Arban R. Social defeat-induced contextual conditioning differentially imprints behavioral and adrenal reactivity: a time-course study in the rat. Physiol Behav. 2007;92:734–740. doi: 10.1016/j.physbeh.2007.05.063. [DOI] [PubMed] [Google Scholar]
- Remie R, van Dongen JJ, Rensema JW. Permanent cannulation of the jugular vein (acc. to Steffens) In: van Dongen JJ, editor. Manual of microsurgery on the laboratory rat. Amsterdam: Elsevier; 1990. pp. 159–169. [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Pozzato C, Costoli T, Manghi M, Stilli D, Ferrari PF, Ceresini G, Musso E. Cardiac autonomic responses to intermittent social conflict in rats. Physiol Behav. 2001;73:343–349. doi: 10.1016/s0031-9384(01)00455-3. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology. 2000;148:289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- van der Poel AM, Miczek KA. Long ultrasonic calls in male rats following mating, defeat and aversive stimulation: frequency modulation and bout structure. Behaviour. 1991;119:127–142. [Google Scholar]
- Van Erp AMM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Von Holst D. Coping behaviour and stress physiology in male tree shrews (Tupaia belangeri) In: Hölldobler B, Lindberg I, editors. Experimental behavioral ecology and sociobiology. Sunderland, MA: Sinauer Associates; 1985. pp. 461–470. [Google Scholar]
- Von Holst D. The concept of stress and its relevance for animal behavior. In: Møller AP, Milinski M, Slater PJB, editors. Advances in the study of behavior, Vol 27, Stress and behavior. New York: Academic; 1998. pp. 1–131. [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia—a realistic animal-model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology. 2007;192:261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]