Abstract
With over thirty different hormones identified as being produced in the gastrointestinal (GI) tract, the gut has been described as ‘the largest endocrine organ in the body’ (Ann. Oncol., 12, 2003, S63). The classification of these hormones and the cells that produce them, the enteroendocrine cells (EECs), has provided the foundation for digestive physiology. Furthermore, alterations in the composition and function of EEC may influence digestive physiology and thereby associate with GI pathologies. Whilst there is a rapidly increasing body of data on the role and function of EEC in the upper GI tract, there is a less clear-cut understanding of the function of EEC in the lower GI. Nonetheless, their presence and diversity are indicative of a role. This review focuses on the EECs of the lower GI where new evidence also suggests a possible relationship with the development and progression of primary adenocarcinoma.
Keywords: colon, enteroendocrine, neuroendocrine, rectum
Development of enteroendocrine cells of gastrointestinal tract
The alternative terms, ‘neuroendocrine’ and ‘APUD’ cells, used to describe EECs reflect how these cells were once thought to originate from the neural crest. Although this was originally suggested by similarities the cells share with neurons, Andrew et al. (1998) chronicle a robust body of evidence demonstrating that EECs of the gut are derived from the endoderm and not the neural crest. Indeed, enteroendocrine cells (EECs) have been shown to develop from the same pluripotent stem cells as the other three cell lineages of the intestinal epithelium: absorptive enterocytes, goblet cells and paneth cells (Gordon 1993).
The process of differentiation of EECs begins as proliferating pluripotent stem cells at the base of the intestinal crypt and progresses as daughter cells migrate, in an upward linear fashion, towards the epithelial cuff at the luminal surface (Figure 1). It is thought that pluripotent cells commit to one of the four cellular lineages in the transitional area, located in the lower and middle thirds of the crypt (Gordon & Hermiston 1994). The same, upward, direction of migration is shared by differentiating cells of the enteroendocrine, absorptive and goblet cell lineages albeit it more rapidly in the latter two. Conversely, differentiating cells of the Paneth cell lineage mature as they migrate along the same axis but in the opposite direction (Hocker & Wiedenmann 1998).
Figure 1.
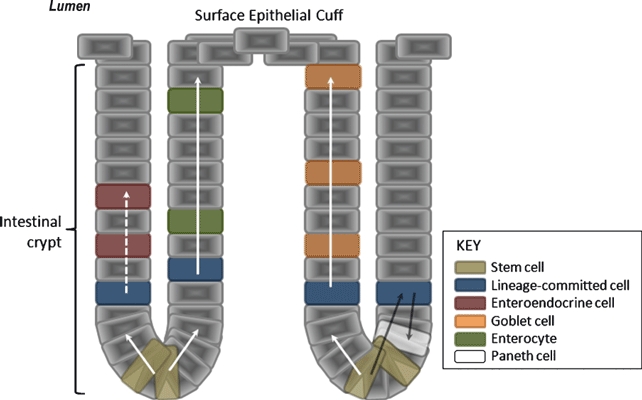
Development of enteroendocrine cells of the gastrointestinal tract. As daughter cells of the pluripotent stem cells migrate from the base of the crypt towards the surface epithelial cuff, they commit to one of the four cellular lineages. Enteroendocrine cell-committed differentiating cells migrate slowly in comparison with differentiating cells of the goblet and enterocyte lineages. Paneth cell-committed differentiating cells migrate towards the base of the crypts as they mature.
In a study by Roth et al. on the proximal colon of mice, colocalization patterns of peptide markers for different EECs were examined. It was found that serotonin and substance P failed to colocalize with peptide YY, GLP-1, cholecystokinin or neurotensin and that the latter four peptide markers often colocalized with each other. The investigators presented these findings as evidence of two branches of cellular differentiation once cells have committed to the EEC lineage (Roth et al. 1992). In their review, Schonhoff et al. present evidence from gain and loss of function studies in transgenic mice for a cascade of basic helix-loop-helix (bHLH) factors that are expressed sequentially during EEC differentiation. Neurogenin 3, a downstream factor of Math1, is thought to be necessary for commitment to the EEC lineage, and expression of further downstream factors including Pax 4 and Pax 6 results in specific hormone-producing EECs (Schonhoff et al. 2004).
Roth et al. also demonstrated characteristic distributions along the crypt-to-surface epithelial cuff axis for different EEC subtypes. The location of EECS and their terminal differentiation produces replicable patterns not only in the crypt-surface epithelial cuff axis, but also in the proximal–distal axis of the gastrointestinal (GI) tract. Holle et al. (2003) examined how these patterns come about and demonstrated that denervation of small intestine in Wistar rats by myenteric ablation produced alterations in the distribution of EECs within the targeted intestinal mucosa. This suggests that intramural innervation of the small intestine has some influence on the differentiation of EECs, although similar findings have yet to be reported in the large intestine.
General features of enteroendocrine cells
Intestinal EECs are restricted to the mucosa, predominately located within its deeper half, and comprise only a small minority (<1%) of the overall epithelial cell population, often lying isolated from one another interspersed by non-endocrine epithelial cells (Buffa et al. 1978; Sternini et al. 2008). A mechanism underlying this scattered distribution is described by Schonhoff et al. (2004) whereby the signalling pathway of cell surface protein ‘Notch’ prevents adjacent cells from differentiating into EECs by lateral inhibition.
The EEC population of the large bowel is generally less diverse than in the small intestine (Buffa et al. 1978). For instance, cholecystokinin-secreting cells, secretin-secreting S cells, gastric inhibitory polypeptide-secreting cells, motilin-secreting M cells and neurotensin-secreting N cells are found in the small intestine but are absent from the large (Rindi et al. 2004). From duodenum to rectum, the frequency of EECs is highest proximally and falls steadily to reach a trough at the colon before rising again within the rectum. After proximal small bowel, the rectum is the location with the next greatest frequency of EECs and the only location in the GI tract where EECs are occasionally seen adjacent to each other or in clusters (Cristina et al. 1978; Shamsuddin et al. 1982; Sjolund et al. 1983).
Although cellular morphology has been demonstrated to vary with EEC cell subtype, there are some general features common to most of them. For instance, EECs often have an apical cytoplasmic process with microvilli that extend towards the luminal surface, and a unique morphological feature of EECs of the large intestine is the presence of basal processes that extend towards adjacent epithelial cells, which is not seen in small intestinal EECs (Capella et al. 1976; Sjolund et al. 1983).
Enteroendocrine cells are characterized by the presence of secretary vesicles, which are either large dense-core vesicles (LDCVs) or the smaller synaptic-like microvesicles (SLMVs) similar to those found in neurones. Components of these vesicles can be exploited as general markers for EECs using immunohistochemistry (IHC) and include chromogranin A, which is a matrix-soluble glycoprotein commonly found in LDCVs, and synaptophysin, a membrane glycoprotein of SLMVs (Varndell et al. 1985; Weidenmann et al. 1986). Other general markers for EECs include neuron-specific enolase and protein gene product 9.5, both of which are found within the cellular cytoplasm (Rindi et al. 2004).
Biogenic amine transporters allow the passage of amines across the vesicular membrane against their concentration gradient acting to compartmentalize them within the secretory vesicles. The vesicular mono-amine transporter, VMAT1, carries out this process for serotonin (5-HT) in 5-HT-secreting EECs (Weihe & Eiden 2000). In a recent review by Borges et al. (2010) based on a series of experiments with knockout mice, chromogranin A has been implicated as a transporter of catecholamines in adrenal chromaffin cell LDCVs and may serve a similar function, as a biogenic amine transporter, in EECs.
In a striking similarity with neuronal postsynaptic vesicles, it has been shown that the contents of these SLMVs are exocytosed in response to cellular plasma membrane depolarization, and by in vitro study of mouse cells, Uehara et al. have specifically demonstrated this in intestinal EECs (Ashcroft et al. 1994; Franklin & Wollheim 2004; Uehara et al. 2006). This offers a mechanism by which EEC secretion may be under neural control, a hypothesis strengthened by the finding of true synapses between neurones and EECs in rat ileal mucosa at what have since been termed ‘neuroendocrine complexes’ (Ahlman & Dahlstrom 1983; Ahlman & Nilsson 2003).
Neuroendocrine tumours (NETs) have been classified by the World Health Organisation into well-differentiated NET, well-differentiated neuroendocrine carcinoma and poorly differentiated neuroendocrine carcinoma (Kloppel et al. 2004). The incidence of these rare tumours has been cited as two per 100,000 and is relatively more common in the rectum than in the colon, rectal NETs comprising 27% of all GI NETs (Kloppel & Anlauf 2005; Lowell et al. 2010). Whilst poorly differentiated tumours can be identified by the expression of general enteroendocrine markers such as synaptophysin and chromogranin A, more differentiated tumours also express the peptide products of their constituent EEC subtype. In the most recent WHO classification of NETs, released in 2004, NETs are further subclassified according to size, functionality, site and invasion. Of note, EEC cell subtype is not included in the classification and the College of American Pathologists does not recommend routine staining of NETs to identify the specific EEC cell subtype because of a lack of evidence of its prognostic significance (Washington et al. 2010). The ontology around NETs is in flux: in the WHO classification of NETs, they are still referred to as ‘NETs’, although in a consensus report of the TNM staging of NETs they use the terms ‘NETs’, ‘(neuro)endocrine tumours’ and ‘endocrine tumours’ interchangeably (Rindi et al. 2007).
As the most specific feature of EEC subtypes, the peptide/amine(s) contained within a cell's secretory vesicles has formed the basis for the classification of EECs (Solcia et al. 1978); prior to this, EEC subtypes were distinguished by differences in the appearance of their secretory vesicles on electron microscopy. Investigations utilizing a combination of immunohistochemical and ultrastructural techniques have demonstrated three main cell types prevalent within the lower GI tract. These cell types are summarized in Table 1.
Table 1.
Features of EECs of colon and rectum
| Cell type | Distribution | Ultrastructural features | Secretory granules | IHC | Secretory products |
|---|---|---|---|---|---|
| EC cell | Most common EEC type; throughout GIT | Pyramid shaped; often have slender apical process reaching luminal surface | 150–500 nm; pleomorphic | CgA, Syn, 5-HT, TPH | 5-HT |
| D cell | Throughout GIT. Least common EEC type in colon and rectum | Spindle shaped; often have slender apical process and one shorter wider basal extension | 150–300 nm; round | (Syn), somatostatin | Somatostatin |
| L cell | Found in duodenum to rectum; rare before terminal ileum; greatest frequency in rectum | Bottle shaped; often have apical process reaching luminal surface; sometimes have basal process along basement membrane | 200–400 nm; round | (CgA), (Syn), PYY, GLP-1, GLP-2, oxyntomodulin, glicentin | Peptide YY, GLP-1, GLP-2, glicentin, oxyntomodulin |
IHC, immunohistochemistry; EEC, enteroendocrine cell; GIT, gastrointestinal tract; CgA, chromogranin A; Syn, synaptophysin; 5-HT, serotonin; TPH, tryptophan 5-hydroxylase; PYY, peptide YY; GLP-1, glucagon-like peptide 1; GLP-2, glucagon-like peptide 2.
Enterochromaffin cells
Enterochromaffin (EC) cells are the most abundant EEC of the GI tract and are distributed widely, populating the gastric antrum, duodenum, jejunum, ileum and appendix as well as the colon and rectum (see Figure 2). EC cells have been shown to make up over 70% of the EEC population in the proximal large bowel, which falls to around 40% in the rectum. As the absolute numbers of EC cells are fairly constant throughout the large intestine, this fall in proportion reflects an increase in the incidence of other EEC cell types in the distal colon and, especially, rectum (Cristina et al. 1978; Sjolund et al. 1983).
Figure 2.
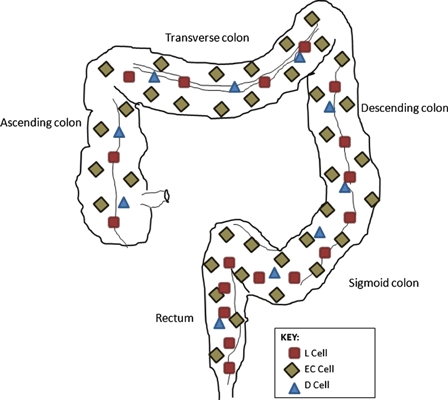
Distribution of enteroendocrine cell subtypes. Enterochromaffin cells are the most abundant enteroendocrine cell subtype of the colon and rectum. D cells are uncommon although scattered evenly throughout the gastrointestinal tract. L cells are uncommon proximal to the terminal ileum, and their frequency increases from proximal to distal being most concentrated in the rectum.
Originally named because of their affinity to bind chromium salts, EC cells’ main secretory product is serotonin (5-HT, Table 2), which is synthesized by the hydroxylation and decarboxylation of tryptophan (Ahlman & Nilsson 2003). EC cells are approximately 8 micrometres in size and triangular or pyramidal in shape, displaying an apical process that often extends to the luminal surface (Buffa et al. 1978; Modlin et al. 2006). LDCVs are found apically and basally (Spiller 2008), and the diameter of the pleomorphic EC secretory granules has been reported to be approximately 150–500 nm (Solcia et al. 1998). Some regional differences in EC cell morphology have also been noted. For instance, occasional EC cells with basal extensions containing secretory vesicles have been observed in the colon or rectum (but not in the small intestine), which Gustafsson et al. (2006) postulate may communicate with neurones. Gastric ECs also differ slightly in that their secretory vesicles are often smaller, approximately 100–150 nm (Cristina et al. 1978).
Table 2.
Secretory products of enteroendocrine cells of the colon and rectum and their actions
| Peptide | Actions |
|---|---|
| 5-HT | Intestinal motility; intestinal secretion; visceral sensation; appetite |
| Somatostatin | Major inhibitory hormone for digestive endocrine and exocrine function; stimulates colonic peristalsis |
| PYY | Inhibits gastric emptying and intestinal motility; inhibits gastric acid secretion and pancreatic exocrine function; suppresses appetite; stimulates mucosal enterocyte proliferation |
| GLP-1 | Incretin effect; delays gastric emptying; postprandial satiety |
| GLP-2 | Stimulates mucosal enterocyte proliferation |
| Glicentin | Stimulates mucosal enterocyte proliferation; inhibits gastric emptying |
| Oxyntomodulin | Inhibits gastric emptying |
PYY, peptide YY; GLP-1, glucagon-like peptide 1; GLP-2, glucagon-like peptide 2.
Immunohistochemical studies demonstrate EC cells stain positively for chromogranin A in all regions of the GI tract (Schmid et al. 1989; Portela-Gomes et al. 1997). That staining intensity is strong in all regions except the gastric antrum explains the popular use of chromogranin A as a general marker for EECs. A series of colocalization studies of chromogranins and various hormones of EEC origin in colonic mucosa demonstrated that whilst chromogranin A colocalizes with 5-HT, it also colocalizes to some extent with peptide YY and enteroglucagon that are not EC cell products (Portela-Gomes et al. 1997), limiting its use as a specific marker of EC cells.
Antibodies directed against tryptophan 5-hydroxylase (TPH), the initial enzyme in 5-HT biosynthesis, have recently shown promise as specific markers for EC cells (Modlin et al. 2006). Whereas 5-HT is present in neurones within the intestinal mucosa as well as with EC cells, differences have been reported between neural and non-neural TPH isotypes (Kuhn et al. 1980; Hasegawa et al. 1987). Difficulties in developing antibodies specific to non-neural TPH have been overcome by the use of dual staining immunofluorescence techniques for acridine orange (AO) and TPH, where EC cell morphology can be confirmed ultrastructurally by the localization of AO within the vesicles and TPH within the cytoplasm (Yu et al. 1999; Modlin et al. 2006). It is noteworthy that 5-HT-containing neurones of the gut are likely to be intrinsic as they are present after 3 weeks in tissue culture, by which time extrinsic neurones have degenerated (Dreyfus et al. 1977).
Ninety-five per cent of the body's 5-HT content is within the GI tract, the majority of which is contained within the secretory granules of EC cells. Furthermore, 5-HT content is said to be highest in the rectum (Spiller 2008). In a study on EC cells isolated from human ileal mucosa, Modlin et al. profiled the receptors that are expressed by EC cells and assessed the receptors’ downstream effects by applying specific agonists. The results implicated β-adrenergic and PACAP receptors in the secretory stimulation pathway and GABAA and cholinergic receptors in the secretory inhibition pathway (Modlin et al. 2006). These findings suggest that 5-HT release by EC cells is, at least in part, neurally regulated, a hypothesis supported by experimental evidence from animal studies (Ferrara et al. 1987).
Mucosal stroking, an ex vivo technique that simulates the passage of local luminal content matter, was utilized by Kellum et al. (1999) to demonstrate its potent effect on 5-HT secretion in human jejunum. Mechanisms that have been suggested for this effect include direct mechanically evoked 5-HT release from EC cells and indirect mechanisms such as neural reflexes.
The possibility that chemical stimulants within the bowel lumen act as regulators of 5-HT release from EC cells has been raised by studies that have demonstrated the expression of a variety of tastant and olfactant receptors by EC cells (Modlin et al. 2006; Braun et al. 2007). This hypothesis is further strengthened by the observation that 5-HT release is observed in response to adding various tastants to cell cultures (Kidd et al. 2008). Pertinent to the lower GI tract, this effect has been shown for short-chain fatty acids (SCFAs), which are the products of fermentation of undigested carbohydrates by anaerobic bacteria in the large bowel (Fukumoto et al. 2003). Animal studies demonstrate an interaction between T lymphocytes and EC cells, with a reduction in EC cell number in mice lacking the T-cell receptor and reduced 5-HT in severe combined immunodeficiency (SCID) mice (Rubin et al. 2005; Wang et al. 2007; Spiller 2008). These findings may suggest EC cells play a role in mediating effects as part of the immune response.
Seven families of receptor subtypes of 5-HT have been identified and are expressed by a variety of cell types within the GI tract including enteric neurones, EC cells, smooth muscle myocytes, absorptive enterocytes and interstitial cells (Tonini 2005; Van Lelyveld et al. 2007; Hasler 2009; Holbrook et al. 2009). As well as giving us an idea of the range of target effects of 5-HT within the GI tract, this suggests that EC-derived 5-HT acts not only in an endocrine manner but by autocrine, paracrine and neurocrine means also.
Information regarding the functions of 5-HT can be drawn from both experimental data and lessons learned from pathological conditions affecting EC cells (Gershon & Tack 2007; Hasler 2009). Animal and human studies have demonstrated that 5-HT causes increased gut motility and accelerated intestinal transit (Lordal et al. 1998; Chen et al. 2001; Coleman et al. 2003). It has been suggested that bidirectional interaction between EC cells and the enteric nervous system is partly responsible for this effect whereby 5-HT released from EC cells binds to 5-HT4 receptors on the nerve terminals of intrinsic primary afferent neurones (IPANs), which in turn results in the activation of excitatory cholinergic neurones that innervate smooth muscle (Costedio et al. 2007; Gershon & Tack 2007). 5-HT also contributes to gut motility by its direct effect on smooth muscle 5-HT receptor subtypes, although stimulation of these receptors mainly produces relaxation (Bjornssen et al. 2002). The simultaneous relaxation of local smooth muscle by direct action of EC cell-derived 5-HT and contraction of neighbouring smooth muscle via the neural pathway may explain a mechanism by which EC cells produce a pattern of smooth muscle contraction compatible with the peristaltic waveform.
Borman and Burleigh's experiments on human intestinal mucosa demonstrated that local application of 5-HT resulted in the secretion of an electrogenic fluid. The greatest secretory response was found in the mucosa of terminal ileum, followed by that of the sigmoid colon and finally by that of the ascending colon. Symptoms relating to colonic hypersecretion and increased colonic motility commonly seen in inflammatory bowel disease (IBD) may at least partly be attributable to a raised 5-HT release. A mechanism by which this occurs is offered by Wang et al. who demonstrated an increase in EC cell number and 5-HT production in SCID mice when reconstituted with helper T cells (Rubin et al. 2005; Wang et al. 2007). A similar effect of helper T cells may occur in IBD. Additionally, Dunlop et al. (2005) observed a reduced number of EC cells in constipation-predominant irritable bowel syndrome (C-IBS), suggesting that EC cells may have a role in the pathophysiology of this condition also. Through the use of specific 5-HT receptor antagonists, the 5-HT4 and 5-HT2A receptor subtypes have been implicated in mediating ileal and colonic secretion, respectively, and may represent avenues of future research exploring the symptomatic treatment of the these conditions (Borman & Burleigh 1997; Spiller 2008).
5-HT is involved in the perception of colorectal distension as evidenced by the presence of 5-HT receptors on ascending neurones and the efficacy of 5-HT3 antagonists (Gershon & Tack 2007) in relieving visceral pain. Unlike colonic motility and intestinal secretion, where effects have been shown to persist after in vitro application of neurotoxin, it is much more difficult to demonstrate that visceral sensation is mediated by EC cell-derived 5-HT rather than neurally derived 5-HT (Kuemmerle et al. 1987). In their review, Garfield and Heisler (2009) summarize the evidence from experimental studies on rodents of 5-HT causing a reduction in appetite and evaluate the therapeutic effect of 5-HT receptor agonists in the treatment of obesity in humans. This is thought to occur by the action of 5-HT in the hypothalamus, particularly the arcuate, paraventricular and ventromedial nuclei. Again, whether this is an effect mediated primarily by neurally derived 5-HT or EC cell-derived 5-HT that reaches the hypothalamus via the bloodstream has yet to be determined.
D cells
D cells, like EC cells, are found throughout the GI tract but contrastingly are found in much lower numbers, making up around 3–5% of the EEC population in the lower GI tract (Buffa et al. 1978; Cristina et al. 1978; Sjolund et al. 1983). Penman et al. (1987) report the highest frequency of D cells in the duodenum and pancreas, with lower levels in the ileum and colon compared with the upper GI tract (see Figure 2). Within the mucosa, D cells have a propensity towards the lower third of the crypt and their main secretory product is somatostatin (Low 2004).
Hauso et al. describe the ultrastructure of D cells as being relatively homogenous in the large intestine with one elongated apical extension and one shorter, wider basal extension. This differs from the overall appearance of D cells in the stomach which were described as being much more varied, often with two or three cytoplasmic extensions that appeared to end in bulbous swellings connected to other epithelial cells (Hauso et al. 2007). Studies have demonstrated the secretory vesicles of D cells to be approximately 200–400 nm in diameter (Solcia et al. 1998; Seretis et al. 2004).
D cells are identified by IHC as being somatostatin immunoreactive. Somatostatin (known as SST14 in its tetradecapeptide form) is located in the hypothalamus (where it is involved in the regulation of pituitary hormones), as well as in the pancreas and GI tract where it is also produced. Within the GI tract, somatostatin immunoreactivity occurs within the muscular layer, where it has been localized to neurones of the myenteric plexus, and within the mucosa, where it localizes to D cells (Penman et al. 1987). Patel et al. (1981) discuss a number of high molecular forms of SST-14 including the 28 amino acid form, SST-28, which they found also to be immunoreactive for antibodies directed against SST-14. Through the use of gel chromatography, whereby the difference in molecular weights between SST-14 and SST-28 were taken advantage of, SST-14 was found to be the predominant form of somatostatin in neural tissue and gastric D cells, whereas SST-28 was the predominant form in D cells throughout mucosa of the GI tract distal to the stomach (Patel et al. 1981; Penman et al. 1987).
In contrast to the postprandial rise in the circulating levels of somatostatin which remains raised for up to 4 h and is likely to be released by D cells of the upper GI tract, Kido et al. found that somatostatin concentration and mRNA expression within the rat colon increased after 48 h of fasting (Ensinck et al. 1990; Ensinck et al. 2002, 2003; Kido et al. 2003). Studies on patients with H. pylori-related chronic gastritis and on patients with IBD have demonstrated a decrease in both the number of D cells and circulating somatostatin levels in the stomach and colon, respectively, during these chronic inflammatory processes, providing further evidence of a role of the immune system in EEC regulation (Watanabe et al. 1992; Milutinovic et al. 2003). Stanisz et al. (1986) found that somatostatin has inhibitory effects on the immune system including the proliferation of T lymphocytes and production of immunoglobulins. D cell-derived SST has also been demonstrated to inhibit the secretion of pro-inflammatory cytokines from both T lymphocytes and intestinal epithelial cells, indicating that the regulatory influence between D cells and immunity is two way (Blum et al. 1992; Chowers et al. 2000).
Somatostatin is the major inhibitory hormone of the digestive system, acting to decrease the release of all known GI hormones including itself, as well as exocrine functions of the GI tract and pancreas (Reichlin 1987; Table 2). Saras et al. (2007) demonstrated that within 2 min, somatostatin causes the contraction of EECs through rearrangement of the actin filament system and a translocation of their secretory vesicles from the cell periphery to the perinuclear region.
Five somatostatin receptor (SSTR) subtypes have been identified, of which SSTR2 is especially relevant to the effects of somatostatin within the large intestine, where it is the most prevalent of the receptor subtypes, having been demonstrated to stimulate peristalsis and ion secretion in the colon upon activation (Grider 2003; Abdu et al. 2002; Hope et al. 2001). In the colon, somatostatin also acts on SSTRs of myenteric neurones to mediate descending relaxation of the smooth muscle (Low 2004).
L cells
L cells occur from the duodenum to the rectum although are rare proximal to the terminal ileum. Within the large intestine however, they constitute the second largest population of EECs, their frequency rising from proximal to distal and make up approximately 14% of the EEC population in the rectum (Figure 2; Sjolund et al. 1983; Cristina et al. 1978). L cells have been observed to occur in all parts of the crypts but with some predominance in the basal portion and their secretory products are proglucagon-derived peptides (PGDPs, see Figure 3) and peptide YY (Bottcher et al. 1984; Table 2).
Figure 3.
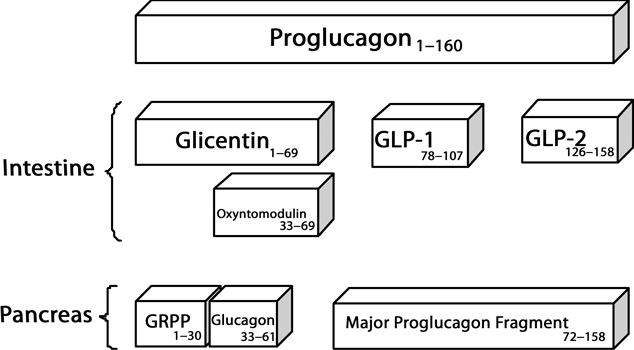
Processing of proglucagon. Alternative post-transcriptional splicing of the proglucagon precursor molecule is into glicentin, GLP-1, GLP-2 and oxyntomodulin in intestinal L cells and into pancreatic glucagon in pancreatic alpha cells. GLP-1, glucagon-like peptide 1; GLP-2, glucagon-like peptide 2. Adapted from Holst (2007).
L cells have been described by Buffa et al. (1978) as being bottle or flask shaped. They often display an open morphology whereby an apical protrusion of cytoplasm extends to the luminal surface and exhibits microvilli. Bottcher et al. also report basal processes that run along the basement membrane, and their secretory vesicles have been observed to occur adjacent to the basolateral membranes and are approximately 150–300 nm in diameter (Bottcher et al. 1984; Solcia et al. 1998).
A series of immunohistochemical investigations have demonstrated the localization of peptide YY and PGDPs to intestinal L cells (Bottcher et al. 1984; Fiocca et al. 1987; Nilsson et al. 1991). Pro-pancreatic polypeptide (Pro-PP), the precursor molecule of PYY, and proglucagon, precursor to the PGDP, have been shown to be expressed both in pancreatic alpha cells and in intestinal L cells. The profile of precursor-derived peptides, however, differs between the two cell types. For instance, pancreatic glucagon, a product of proglucagon processing, is found in the pancreas but not in the gut, and the reverse is true for GLP-1, which is not present in pancreatic alpha cells (Figure 3). These findings demonstrate that alternative post-transcriptional splicing of the same precursor molecules occurs in distinct patterns between pancreatic alpha cells and intestinal L cells to yield different bioactive peptides (Rehfeld 1998).
A study on rectal carcinoid tumours and the unaffected adjacent rectal mucosa in humans showed immunoreactivity of intestinal L cells to a range of PGDPs including glicentin (1–69), GLP-1 (72–108), GLP-2 (126–159) and oxyntomodulin (33–69) – so-called glucagon-like immunoreactivity – in addition to PYY (Fiocca et al. 1987). In a study by Nilsson et al. (1991) utilizing double immunogold labelling in rabbit colon, PYY and PGDP were both found in all L cells examined, colocalizing to 85% of secretory vesicles, the remaining vesicles containing PYY only. Despite colocalization of these two hormones to the same cells, a number of studies have demonstrated the selective secretion of one hormone, suggesting discrete intracellular mechanisms underlying their release (Pironi et al. 1993; Plaisancie et al. 1995; Anini et al. 1999).
In a study on 151 healthy human volunteers, circulating PYY and GLP-1 levels peaked 20 min after having an oral glucose load and remained elevated for 2 h (Kim et al. 2005). Although this could represent direct stimulation of duodenal L cells, the majority of L cells are found in the distal colon and rectum, and so Anini et al. (1999) hypothesized this early response to be neurally regulated. They tested their hypothesis on Wistar rats and demonstrated that PYY and GLP-1 release was suppressed or inhibited by techniques including bilateral vagotomy and administration of hexamethonium (an anticholinergic agent), supporting their hypothesis. They also demonstrated a later phase of PYY release in response to intraluminal SCFAs that was replicated by direct intracolonic infusion and unaffected by hexamethonium, making this later response much more likely to be as a direct effect of intraluminal nutrients acting directly on L cells (Anini et al. 1999). In addition to glucose and SCFAs, studies have also demonstrated the release of PYY in response to intraluminal bile salts and amino acids (Adrian et al. 1993; Zhang et al. 1993). PYY release has been shown to be influenced by regulatory peptides including cholecystokinin, vasoactive intestinal peptide and GLP-1, and Chisholm and Greenberg demonstrated the inhibitory effect of somatostatin, particularly in its SST-28 form, on PYY release from foetal rat intestinal cell cultures (Greeley et al. 1989; Rudnicki et al. 1992; Näslund et al. 1999; Chisholm & Greenberg 2000).
Studies involving intravenous infusion of PYY in healthy human volunteers have demonstrated reduced intestinal and colonic transit, reduced gastric emptying and reduced gastric acid and pancreatic exocrine secretion (Ballantyne 2006). This illustrates PYY as an important mediator of the ‘ileo-colonic brake’ whereby unabsorbed nutrients in the distal intestine and colon trigger the inhibition of motility and secretion upstream. A recent review by Karaki and Kuwahara (2010) outlines the evidence linking SCFAs within the colonic lumen to the inhibition of proximal intestinal motility, which is thought to be through the release of PYY from intestinal L cells. In their review, El-Salhy et al. (2002) discuss the evidence of reduced tissue and circulating levels of peptide YY in patients with IBD, which may contribute to diarrhoea in these patients owing to increased gastric emptying, intestinal motility and intestinal secretions. A further inhibitory action of PYY is outlined in a review by Dietrich and Horvath who discuss the role of PYY in the anorexigenic response and present evidence for the peptide reducing food intake when administered peripherally. This suggests an interaction between L cells and the central nervous system in producing postprandial satiety (Dietrich & Horvath 2009). Additionally, following observations in animal studies whereby raised circulating levels of PYY were seen following intestinal resection suggesting an adaptive response, in vitro investigations provide evidence that PYY acts on Y1 receptors in the human colon epithelium to stimulate cell proliferation, suggesting a role of L cells in maintaining intestinal mucosal integrity (Mannon 2002).
Elliott et al. (1993) found that in healthy human subjects, GLP-1 release increases after ingestion of carbohydrate, fat and protein meals. This effect has been demonstrated on the GLUTag cell line, a cellular model for intestinal L cells derived from the colonic tumour of transgenic mice, and further studies on the GLUTag cell model have implicated GABA, a neurotransmitter of the enteric nervous system, in the regulatory mechanism of GLP-1 release (Gameiro et al. 2005; Reimann et al. 2006).
One of the most important functions of GLP-1 is its major contributory role in the incretin effect. This is whereby gut hormones stimulate pancreatic insulin release in response to ingested glucose in a dose-dependent manner. Evidence of the effect comes from studies demonstrating a much greater insulin release observed after an oral glucose load in comparison with intravenous administration (Mari et al. 2002). By interaction between GLP-1 and its receptor on beta cells of the pancreatic islets, insulin transcription, biosynthesis and release are stimulated as is beta cell differentiation and proliferation. This has attracted interest in the role of GLP-1 in the pathogenesis of type 2 diabetes mellitus, as studies show a reduced GLP-1 response in sufferers of this condition (Holst & Gromada 2004). Glucose-dependent insuliotropic peptide (GIP) is secreted by K cells in the stomach and proximal small intestine and also potentiates insulin release by pancreatic beta cells. Mortensen et al. demonstrated that GIP and GLP-1 colocalize in the mid-small intestine of human, rats and pigs and these cells have been called K/L cells (Wang et al. 2002; Mortensen et al. 2003; Cho & Kieffer 2010).
The study by Wettergren et al. (1993) on humans, whereby synthetic GLP-1 was administered intravenously to physiological levels, found an inhibitory effect on gastric acid secretion and exocrine function of the pancreas. The feedback mechanism between the distal large bowel and proximal stomach is directly demonstrated by Jian et al. (1981) who carried out a study involving 16 healthy volunteers in whom gastric acid secretion was reduced in response to intracolonic glucose infusion. Given that in further studies, truncal vagotomy abolishes the inhibitory effect of GLP-1 on gastric acid secretion, and that the half-life of circulating GLP-1 is very short, it has been proposed that this mechanism is neurally mediated (Holst 2007). GLP-1 has been shown to have an additive inhibitory effect on gastric acid secretion with PYY (Wettergren et al. 1997). Furthermore, animal models have demonstrated the inhibitory effect of GLP-1, oxynomodulin and glicentin on gastric outflow (Schjoldager et al. 1989; Otani et al. 1994; Tolessa et al. 1998).
Verdich et al.'s (2001) meta-analysis of nine randomized cross-over trials examining the effect of intravenous GLP-1 on energy intake demonstrated the effect was found to be dose dependent and inhibitory. The mechanism for this is likely to involve the arcuate nucleus of the hypothalamus (Tang-Christensen et al. 1998). Given that some studies demonstrate postprandial secretion of GLP-1 to be impaired in morbidly obese patients, but that the satiety response to intravenously administered GLP-1 remains intact, this PGDP may offer potential in the future treatment of obesity (Naslund et al. 1998).
GLP-2 is cosecreted into the bloodstream with GLP-1 shortly following a meal but has been shown to have no effect on the regulation of glucose homoeostasis. Its main action is as a potent stimulator of mucosal epithelial proliferation (Drucker 2002). Animal studies have demonstrated an increase in both large and small bowel mucosa following GLP-2 injection, although the small bowel seems to be more sensitive to the effects of GLP-2 (Tsai et al. 1997; Ghatei et al. 2002). Glicentin has been shown to exhibit a similar but less potent effect on intestinal epithelial cell proliferation and, together with GLP-2, has been shown to be part of the adaptive response in short bowel syndrome, offering a promising therapeutic avenue in this condition as well as TPN-associated gut atrophy (Drucker 2002; Chiba et al. 2007). Independent of its effect in epithelial cell proliferation, glicentin has also been implicated as a protective mucosal agent, preventing bacterial translocation of enteric bacteria when added to cell lines in vitro (Chiba et al. 2007).
Enteroendocrine cells and colorectal carcinoma
Differentiated EECs have been detected in approximately 35% of colorectal carcinomas (Swatek & Chibowski 2000; Gulubova & Vlaykova 2008). Given that EEC products serotonin, PYY, glicentin and GLP-2 have been proposed to enhance cellular proliferation within the colonic epithelium, the hypothesis arises that colorectal carcinomas with an enteroendocrine component are endowed with a proliferative advantage. In addition to this, some EECs in colorectal adenocarcinomas have been demonstrated to express pro-angiogenic factors including vascular endothelial growth factor (VEGF), which is known to be an important factor in the development and progression of colorectal adenocarcinoma (La Rosa et al. 1998; Gulubova & Vlaykova 2008). This being the case, this subgroup of carcinomas would be expected to be associated with a worse prognosis as is seen in primary colorectal neuroendocrine carcinomas compared with pure adenocarcinoma (Saclarides et al. 1994).
Studies utilizing both general and specific markers for EECs have examined the possible relationship between the presence of EECs within colorectal carcinomas and prognosis. Overall, the findings of these studies have been equivocal as some studies have identified a significant relationship between the presence of EECs within the carcinoma and prognosis (Hamada et al. 1991; De Bruine et al. 1993; Grabowski et al. 2006; Gulubova & Vlaykova 2008), whereas others have not (Smith & Haggitt 1984; Mori et al. 1995; Swatek & Chibowski 2000). Of note, a study by Lloyd et al. examining 289 cases of moderately differentiated (grades II and III) colorectal carcinomas found that EECs identified by IHC for chromogranin A and by in situ hybridization analysis for chromogranin A and B were present in 26% of cases. With a mean follow-up period of 7 years, it was concluded that the number of chromogranin-positive cells did not correlate significantly with a number of factors including histological grade and survival (Lloyd et al. 1998).
It is well established that the incidence of colorectal cancer is lower in populations with a high dietary fibre intake. Given their location within the intestinal crypts and their ability to respond to chemical stimulants within the lumen, EECs are a potential candidate for being contributors to this effect (Bingham et al. 2003). Indeed, free fatty acid receptors FFA 2 and 3 have been demonstrated to be expressed by L cells and act as receptors to SCFAs (Karaki & Kuwahara 2010). Cani et al. fed rats a diet supplemented with non-digestible carbohydrate oligofructose for 4 weeks and observed an increased number of L cells in the ascending colon compared with rats fed with a standard diet. EEC differentiation factors NGN3 and NeuroD were also found to be increased, suggesting a relationship between dietary carbohydrates and enteroendocrine differentiation (Cani et al. 2007).
In a study on 42 cases of primary colorectal carcinoma whereby tissues were examined with immunohistochemical markers for the cellular lineages of colonic epithelial stem cells, 9% were found to be pluripotent, expressing markers for all four cell lineages: secretory component for absorptive enterocytes, mucin for goblet cells, chromogranin A for EECs and lysozyme for paneth cells (Ho et al. 1989). In accordance with the shared ancestry model of colonic epithelial cell lineages, this finding can be attributed to neoplastic transformation occurring at an earlier stage of stem cell differentiation. This hypothesis is strengthened by the work of Yeung et al. (2010) who have demonstrated, with colorectal cancer cell lines, that cancer stem cells continue to differentiate in a similar pattern to that observed in normal stem cells. With this in mind, adenocarcinomas and NETs may be viewed as opposite ends of a spectrum, adenocarcinomas with an enteroendocrine component being an intermediary. Lessons regarding therapeutic intervention may therefore be transferrable from gastroenteropancreatic NETs. This hypothesis could be tested by directly comparing the survival outcomes of colorectal adenocarcinomas with an endocrine component with poorly differentiated NETs of the same site.
One particularly promising field in this regard is the use of somatostatin analogues. Although the mainstay of symptomatic treatment of hypersecretion syndromes is associated with NETs, recent data also provide substantial evidence that administration of long-acting somatostatin analogues inhibits tumour growth and increased time to tumour progression; however, this effect was most pronounced in the more differentiated NETs (Rinke et al. 2009). The mechanism underlying the antiproliferative effects of somatostatin and related analogues on tumour cells is incompletely understood. It has been proposed that the effect is because of an inhibition of release of various growth factors including VEGF. This hypothesis is supported by the finding that VEGF release is inhibited by SSTR-1 agonists in human dermal microvascular endothelial cells (HMEC) (Bocci et al. 2007). As high levels of SSTR subtypes have been found to be expressed in colorectal adenocarcinoma as well as in NETs, there may be potential for the therapeutic application of somatostatin analogues in the treatment of SSTR-positive colorectal adenocarcinoma (Reubi 2004).
Conclusion
Normal GI physiology involves complex interactions between the central nervous system, the enteric nervous system and the endocrine system whereby information is transferred by feedback and feedforward mechanisms to regulate GI function. EECs of the lower GI tract play an important role in this complex process by acting as sensors to luminal contents and mechanical distension as well as mediators of function. Their peptide secretory products act both in a paracrine fashion to exert local effects such as on colonic motility and secretion and in an endocrine fashion to exert effects at distant sites in the GI tract, such as gastric emptying.
As yet data exploring the role of EECs in pathological conditions affecting the GI tract arepreliminary, yet suggest that EEC perturbation may have a role in the pathology of conditions including irritable bowel syndrome and colorectal adenocarcinoma. Even more preliminary data suggest that colorectal tumours themselves cause perturbations in the balance of EEC (Nitta et al. 2001) and by extension in GI function. This may explain why lesions of modest size, such as small adenoma, may lead to altered bowel function as an indicator of early colorectal carcinogenesis. Future investigations should address the two-way interaction between EEC and lesion as a route to new chemopreventive and diagnostic targets.
References
- Abdu F, Hicks GA, Hennig G, Allen JP, Grundy D. Somatostatin sst(2) receptors inhibit peristalsis in the rat and mouse jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G624–G633. doi: 10.1152/ajpgi.00354.2001. [DOI] [PubMed] [Google Scholar]
- Adrian TE, Ballantyne GH, Longo WE, et al. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34:1219–1224. doi: 10.1136/gut.34.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlman H, Dahlstrom A. Vagal mechanisms controlling serotonin release from the gastrointestinal tract and pyloric motor function. J. Auton. Nerv. Syst. 1983;9:119–140. doi: 10.1016/0165-1838(83)90136-4. [DOI] [PubMed] [Google Scholar]
- Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2003;12(Suppl. 2):S63–S68. doi: 10.1093/annonc/12.suppl_2.s63. [DOI] [PubMed] [Google Scholar]
- Andrew A, Kramer B, Rawdon BB. The origin of gut and pancreatic neuroendocrine (APUD) cells – the last word? J. Pathol. 1998;186:117–118. doi: 10.1002/(SICI)1096-9896(1998100)186:2<117::AID-PATH152>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Anini Y, Fu-Cheng X, Cuber JCC, Kervran JC, Roze C. Comparison of the post-prandial release of peptide YY and proglucagon-derived peptides in the rat. Eur. J. Physiol. 1999;438:299–306. doi: 10.1007/s004240050913. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Proks P, Smith PA, Ammala C, Bokvist K, Rorsman P. Stimulus-secretion coupling in pancreatic beta cells. J. Cell. Biochem. 1994;55:54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- Ballantyne GH. Peptide YY(1–36) and peptide YY (3–36): past I. Distribution, release and actions. Obes. Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- Bingham SA, Day NE, Luben R, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- Bjornssen ES, Chen WD, Hooper F, Woods ML, Owyang C, Hasler W. Impaired gastrocolonic response and peristaltic reflex in slow transit constipation: role of 5-HT3 pathways. Am. J. Physiol. 2002;283:G400–G407. doi: 10.1152/ajpgi.00082.2001. [DOI] [PubMed] [Google Scholar]
- Blum AM, Metwali A, Mathew RC, Cook MG, Elliott D, Weinstock JV. Granuloma T lymphocytes in murine schistosomiasis mansoni have somatostatin receptors and respond to somatostatin with decreased IFN-gamma secretion. J. Immunol. 1992;149:3621. [PubMed] [Google Scholar]
- Bocci G, Culler MD, Fioravanti A, et al. In vitro antiangiogenic activity of selective somatostatin subtype-1 receptor agonists. Eur. J. Clin. Invest. 2007;37:700–708. doi: 10.1111/j.1365-2362.2007.01848.x. [DOI] [PubMed] [Google Scholar]
- Borges R, Diaz-Vera J, Dominguez N, Arnau MR, Machado JD. Chromogranins as regulators of exocytosis. J. Neurochem. 2010;114:335–343. doi: 10.1111/j.1471-4159.2010.06786.x. [DOI] [PubMed] [Google Scholar]
- Borman RA, Burleigh DE. Heterogeneity of 5-HT receptors mediating secretion in the human intestine. Ann. N Y Acad. Sci. 1997;812:224–225. doi: 10.1111/j.1749-6632.1997.tb48183.x. [DOI] [PubMed] [Google Scholar]
- Bottcher G, Sjolund K, Ekblad R, Hakanson R, Schwartz TW, Sundler F. Coexistence of peptide YY and glicentin immunoreactivity in endocrine cells of the gut. Regul. Pept. 1984;8:261–266. doi: 10.1016/0167-0115(84)90034-x. [DOI] [PubMed] [Google Scholar]
- Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Buffa R, Capella C, Fontana P, Usellini L, Solcia E. Types of endocrine cells in the human colon and rectum. Cell Tissue Res. 1978;192:227–240. doi: 10.1007/BF00220741. [DOI] [PubMed] [Google Scholar]
- Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br. J. Nutr. 2007;98:32–37. doi: 10.1017/S0007114507691648. [DOI] [PubMed] [Google Scholar]
- Capella C, Solcia E, Frigerio B, Buffa R. Endocrine cells of the human intestine. An ultrastructural study. In: Fujita T, editor. Endocrine Gut and Pancreas. Amsterdam: Elsevier; 1976. pp. 42–59. [Google Scholar]
- Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J. Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba M, Sanada Y, Kawano S, et al. Glicentin inhibits internalization of enteric bacteria by cultured INT-407 enterocytes. Pediatr. Surg. Int. 2007;23:551–554. doi: 10.1007/s00383-007-1895-9. [DOI] [PubMed] [Google Scholar]
- Chisholm C, Greenberg GR. Somatostatin receptor subtype-5 mediates inhibition of peptide YY secretion from rat intestinal cultures. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G983–G989. doi: 10.1152/ajpgi.2000.279.5.G983. [DOI] [PubMed] [Google Scholar]
- Cho YM, Kieffer TJ. K-cells and glucose-dependent insulinoptropic polypeptide in health and disease. In: Litwack G, editor. Incretins and Insulin Secretion. Vitamins and Hormones. Vol. 84. Oxford: Elsevier; 2010. pp. 111–113. [DOI] [PubMed] [Google Scholar]
- Chowers Y, Cahalon L, Lahav M, et al. Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha and bacteria-induced IL-8 and IL-1b secretion from intestinal epithelial cells. J. Immunol. 2000;165:2955–2961. doi: 10.4049/jimmunol.165.6.2955. [DOI] [PubMed] [Google Scholar]
- Coleman NS, Marciani L, Blackshaw E, et al. Effect of a novel 5-HT3 receptor agonist MKC-733 on upper gastrointestinal motility in humans. Aliment. Pharmacol. Ther. 2003;18:1039–1048. doi: 10.1046/j.1365-2036.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis. Colon Rectum. 2007;50:376–388. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- Cristina ML, Lehy T, Zeitoun CR, Dufougheray F. Fine Structural classification and comparative distribution of endocrine cells in normal human large intestine. Gastroenterology. 1978;75:20–28. [PubMed] [Google Scholar]
- De Bruine AP, Wiggers T, Beek C, et al. Endocrine cells in colorectal adenocarcinomas: incidence, hormone profile and prognostic relevance. Int. J. Cancer. 1993;54:765–771. doi: 10.1002/ijc.2910540510. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur. J. Neurosci. 2009;30:1688–1696. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- Dreyfus CF, Sherman DL, Gershon MD. Uptake of serotonin by intrinsic neurons of the myenteric plexus grown in organotypic tissue culture. Brain Res. 1977;128:109–123. doi: 10.1016/0006-8993(77)90239-6. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531–544. doi: 10.1053/gast.2002.31068. [DOI] [PubMed] [Google Scholar]
- Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Suhr O, Danielsson A. Peptide YY in gastrointestinal disorders. Peptides. 2002;23:397–402. doi: 10.1016/s0196-9781(01)00617-9. [DOI] [PubMed] [Google Scholar]
- Ensinck JW, Vogel RE, Laschansky EC, Francis BH. Effect of ingested carbohydrate, fat, and protein on the release of somatostatin-28 in humans. Gastroenterology. 1990;98:633–638. doi: 10.1016/0016-5085(90)90282-6. [DOI] [PubMed] [Google Scholar]
- Ensinck JW, Baskin DG, Vahl TP, et al. Thrittene, homologous with somatostatin-28((1–13)), is a novel peptide in mammalian gut and circulation. Endocrinology. 2002;143:2599–2609. doi: 10.1210/endo.143.7.8904. [DOI] [PubMed] [Google Scholar]
- Ensinck JW, Laschansky EC, Vogel RE, D'Alessio DA. Effect of ingested nutrients on release of thrittene into the human circulation. J. Clin. Endocrinol. Metab. 2003;88:4798–4804. doi: 10.1210/jc.2003-030063. [DOI] [PubMed] [Google Scholar]
- Ferrara A, Zinner MJ, Jaffe BM. Intraluminal release of serotonin, substance P, and gastrin in the canine small intestine. Dig. Dis. Sci. 1987;32:289–294. doi: 10.1007/BF01297056. [DOI] [PubMed] [Google Scholar]
- Fiocca R, Rindi G, Capella C, et al. Glucagon, glicentin, proglucagon, PYY, PP and prop-icosapeptide immunoreactivities of rectal carcinoid tumours and related non-tumor cells. Regul. Pept. 1987;17:9–29. doi: 10.1016/0167-0115(87)90029-2. [DOI] [PubMed] [Google Scholar]
- Franklin IK, Wollheim CB. GABA in the endocrine pancreas: its putative role as an islet cell paracrine-signalling molecule. J. Gen. Physiol. 2004;123:185–190. doi: 10.1085/jgp.200409016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- Gameiro A, Reimann F, Habib AM, et al. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005;569:761–772. doi: 10.1113/jphysiol.2005.098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J. Physiol. 2009;587:49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signalling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Ghatei MA, Goodlad RA, Taheri S, et al. Proglucagon-derived peptides in intestinal epithelial proliferation: glucagon-like peptide-2 is a major mediator of intestinal epithelial proliferation in rats. Dig. Dis. Sci. 2002;46:1255–1263. doi: 10.1023/a:1010615429639. [DOI] [PubMed] [Google Scholar]
- Gordon JI. Understanding gastrointestinal epithelial cell biology: lessons from mice with help from worms and flies. Gastroenterology. 1993;104:315–324. doi: 10.1016/0016-5085(93)90703-f. [DOI] [PubMed] [Google Scholar]
- Gordon JI, Hermiston ML. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr. Opin. Cell Biol. 1994;6:795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Grabowski P, Sturm I, Schelwies K, et al. Analysis of neuroendocrine differentiation and the p53/BAX pathway in UICC stage III colorectal carcinoma identifies patients with good prognosis. Int. J. Colorectal Dis. 2006;21:221–230. doi: 10.1007/s00384-005-0779-5. [DOI] [PubMed] [Google Scholar]
- Greeley GH, Jeng YJ, Gomez G, et al. Evidence for regulation of peptide-YY release by the proximal gut. Endocrinology. 1989;124:1438–1443. doi: 10.1210/endo-124-3-1438. [DOI] [PubMed] [Google Scholar]
- Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J. Pharmacol. Exp. Ther. 2003;307:460–467. doi: 10.1124/jpet.103.053512. [DOI] [PubMed] [Google Scholar]
- Gulubova M, Vlaykova T. Chromogranin A-, serotonin-, synaptophysin- and vascular endothelial growth factor-positive endocrine cells and the prognosis of colorectal cancer: an immunohistochemical and ultrastructural study. J. Gastroenterol. Hepatol. 2008;23:1574–1585. doi: 10.1111/j.1440-1746.2008.05560.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson BI, Bakke I, Tommeras K, Waldrum HL. A new method for visualisation of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scand. J. Gastroenterol. 2006;41:390–395. doi: 10.1080/00365520500331281. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Oishi A, Shoji T, et al. Endocrine cells and prognosis in patients with colorectal carcinoma. Cancer. 1991;69:2641–2646. doi: 10.1002/1097-0142(19920601)69:11<2641::aid-cncr2820691104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Yanagisawa M, Inoue F, Yanaihara N, Ichiyama A. Demonstration of non-neural tryptophan 5-monooxygenase in mouse intestinal mucosa. Biochem. J. 1987;248:501–509. doi: 10.1042/bj2480501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler WL. Serotonin and the GI Tract. Curr. Gastroenterol. Rep. 2009;11:383–391. doi: 10.1007/s11894-009-0058-7. [DOI] [PubMed] [Google Scholar]
- Hauso O, Gustafsson BI, Waldum HL. Long slender cytoplasmic extensions: a common feature of neuroendocrine cells? J. Neuroendocrinol. 2007;19:739–742. doi: 10.1111/j.1365-2826.2007.01578.x. [DOI] [PubMed] [Google Scholar]
- Ho SB, Itzkowitz SH, Friera AM, Jiang S-H, Kim YS. Cell lineage markers in premalignant and malignant colonic mucosa. Gastroenterology. 1989;97:392–404. doi: 10.1016/0016-5085(89)90075-9. [DOI] [PubMed] [Google Scholar]
- Hocker M, Wiedenmann B. Molecular mechanisms of enteroendocrine differentiation. Ann. N Y Acad. Sci. 1998;859:160–174. doi: 10.1111/j.1749-6632.1998.tb11120.x. [DOI] [PubMed] [Google Scholar]
- Holbrook JD, Gill CH, Zebda N, et al. Characterisation of 5-HTc, 5-HT3d and 5-HT3e receptor subunits: evolution, distribution and function. J. Neurochem. 2009;108:384–396. doi: 10.1111/j.1471-4159.2008.05775.x. [DOI] [PubMed] [Google Scholar]
- Holle GE, Dietl J, Demir I. Influence of the intramural innervation on the morphogenesis of the enteroendocrine cells and genetic construct involved (review) Int. J. Mol. Med. 2003;11:275–285. doi: 10.3892/ijmm.11.3.275. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and non diabetic humans. Am. J. Physiol. Endocrinol. Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- Hope N, Butt G, Ross I, et al. Somatostatin enhances cAMP-dependant short-circuit current in human colon via somatostain receptor subtype 2. Dig. Dis. Sci. 2001;46:2499–2503. doi: 10.1023/a:1012392307462. [DOI] [PubMed] [Google Scholar]
- Jian R, Besterman HS, Sarson DL, et al. Colonic inhibition of gastric secretion in man. Dig. Dis. Sci. 1981;26:195–201. doi: 10.1007/BF01391629. [DOI] [PubMed] [Google Scholar]
- Karaki S, Kuwahara A. Roles of short-chain fatty acids and their receptors in colonic motility. Biosci. Microflora. 2010;29:31–40. [Google Scholar]
- Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5HT4 receptors. Am. J. Physiol. 1999;277:G515–G520. doi: 10.1152/ajpgi.1999.277.3.G515. [DOI] [PubMed] [Google Scholar]
- Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants and olfactants. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:260–272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- Kido T, Sumii K, Kawano M, Sumii M, Yoshihara M, Chayami K. Food deprivation enhances somatostatin and somatostatin receptor subtype expression in the rat colon. Regul. Pept. 2003;114:167–173. doi: 10.1016/s0167-0115(03)00122-8. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Carlson OD, Jang HJ, Elahai D, Barry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J. Clin. Endocrinol. Metab. 2005;90:6665–6671. doi: 10.1210/jc.2005-0409. [DOI] [PubMed] [Google Scholar]
- Kloppel G, Anlauf M. Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2005;19:507–517. doi: 10.1016/j.bpg.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kloppel G, Perren A, Hetz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors. The WHO classification. Ann. N Y Acad. Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- Kuemmerle JF, Kraus H, Kellum JM. Serotonin release is mediated by muscarinic receptors on duodenal mucosal cells. J. Surg. Res. 1987;43:139–142. doi: 10.1016/0022-4804(87)90156-9. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Meyer MA, Lovenburg W. Comparisons of tryptophan hydroxylase from a malignant murine mast cell tumor rat mesencephalic tegmentum. Arch. Biochem. Biophys. 1980;199:355–361. doi: 10.1016/0003-9861(80)90291-x. [DOI] [PubMed] [Google Scholar]
- La Rosa S, Uccella S, Capella C, Chiaravalli AM, Sessa F. Localization of acidic fibroblast growth factor, fibroblast growth factor receptor-4, transforming growth factor-alpha, and epidermal growth factor receptor in human endocrine cells of the gut and related tumours: an immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. 1998;6:199–208. [Google Scholar]
- Lloyd RV, Schroeder G, Bauman MD, et al. Prevalence and prognostic significance of neuroendocrine differentiation in colorectal carcinomas. Endocr. Pathol. 1998;9:35–42. doi: 10.1007/BF02739950. [DOI] [PubMed] [Google Scholar]
- Lordal M, Wallen H, Hjemdahl P, Beck O, Hellstrom PM. Concentration-dependent stimulation of intestinal phase III of migrating motor complex by circulating serotonin in humans. Clin. Sci. (Colch) 1998;94:663–670. doi: 10.1042/cs0940663. [DOI] [PubMed] [Google Scholar]
- Low MJ. The somatostatin neuroendocrine system: physiology and clinical relevance in gastrointestinal and pancreatic disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:607–622. doi: 10.1016/j.beem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Lowell AB, Strosberg JR, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (NETs). Well-differentiated NETs of the distal colon and rectum. Pancreas. 2010;39:767–774. doi: 10.1097/MPA.0b013e3181ec1261. [DOI] [PubMed] [Google Scholar]
- Mannon P. Peptide YY as a growth factor for intestinal epithelium. Peptides. 2002;23:383–388. doi: 10.1016/s0196-9781(01)00615-5. [DOI] [PubMed] [Google Scholar]
- Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of beta-cell function: modelling analysis in normal subjects. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1159–E1166. doi: 10.1152/ajpendo.00093.2002. [DOI] [PubMed] [Google Scholar]
- Milutinovic AS, Todorovic V, Milosavljevic T, Micev M, Spuran M, Drndarevic N. Somatostatin and D cells in patients with gastritis in the course of Helicobacter pylori eradication: a six-month, follow-up study. Eur. J. Gastroenterol. Hepatol. 2003;15:755–756. doi: 10.1097/01.meg.0000059153.68845.1a. [DOI] [PubMed] [Google Scholar]
- Modlin I, Kidd M, Pfragner R, Eick GN, Champaneria MC. The functional characterization of normal and neoplastic human enterochromaffin cells. J. Clin. Endocrinol. Metab. 2006;91:2340–2348. doi: 10.1210/jc.2006-0110. [DOI] [PubMed] [Google Scholar]
- Mori M, Mimori K, Kamakura T, Adachi Y, Ikeda Y, Sugimachi K. Chromogranin positive cells in colorectal carcinoma and transitional mucosa. J. Clin. Pathol. 1995;48:754–758. doi: 10.1136/jcp.48.8.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen K, Christensen LL, Holst JJ, Orskov K. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003;114:189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- Naslund E, Gryback P, Backman L, et al. Distal small bowel hormones: correlation with fasting antroduodenal motility and gastric emptying. Dig. Dis. Sci. 1998;43:945–952. doi: 10.1023/a:1018806129102. [DOI] [PubMed] [Google Scholar]
- Näslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am. J. Physiol. 1999;277(3 Pt 2):R910–R916. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- Nilsson O, Bilchik AJ, Goldenring JR, Ballantyne GH, Adrian TE, Modlin IM. Distribution and immunocytochemical colocalization of peptide YY and enteroglucagon in endocrine cells of the gut. Endocrinology. 1991;129:139–148. doi: 10.1210/endo-129-1-139. [DOI] [PubMed] [Google Scholar]
- Nitta Y, Nishibori M, Iwagaki H, et al. Changes in serotonin dynamics in the gastrointestinal tract of Colon-26 tumour-bearing mice: effects of cisplatin treatment. Nuanyn-Schiedeberg's Arch. Pharmacol. 2001;364:329–334. doi: 10.1007/s002100100461. [DOI] [PubMed] [Google Scholar]
- Otani N, Sasaki I, Naito H, et al. Inhibitory effect of peptide YY, neurotensin and glicentin on upper gastrointestinal motility in dogs. Biomed. Res. 1994;15:299–302. [Google Scholar]
- Patel YC, Wheatley T, Ning C. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Endocrinology. 1981;109:1943–1949. doi: 10.1210/endo-109-6-1943. [DOI] [PubMed] [Google Scholar]
- Penman E, Wass JAH, Butler MG, et al. Distribution and characterisation of immunoreactive somatostatin in human gastrointestinal tract. Reg. Peptides. 1987;7:53–65. doi: 10.1016/0167-0115(83)90281-1. [DOI] [PubMed] [Google Scholar]
- Pironi L, Stanghellini V, Mignoli M, et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to plasma level of peptide YY. Gastroenterology. 1993;105:733–739. doi: 10.1016/0016-5085(93)90890-o. [DOI] [PubMed] [Google Scholar]
- Plaisancie P, Dumoulin V, Chayvialle JA, Cuber JC. Luminal glucagon-like peptide-1-(7–36) amide-releasing factors in the isolated vascularly perfused rat colon. J. Endocrinol. 1995;145:521–526. doi: 10.1677/joe.0.1450521. [DOI] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L. Complex co-localisation of chromogranins and neurohormones in the human gastrointestinal tract. J. Histochem. Cytochem. 1997;45:815–822. doi: 10.1177/002215549704500606. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. The new biology of gastrointestinal hormones. Physiol. Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Secretion of somatostatin and its physiological effect. J. Lab. Clin. Med. 1987;109:320–326. [PubMed] [Google Scholar]
- Reimann F, Ward PS, Gribble FM. Signaling mechanisms underlying the release of glucagon-like peptide 1. Diabetes. 2006;55(Suppl. 2):S78–S85. [Google Scholar]
- Reubi JC. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80(Suppl. 1):51–56. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut. Changing concepts and new evidences. Ann. N Y Acad. Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- Rindi G, Efrat S, Ghatei MA, Bloom SR, Solcia E, Polak JM. Glucagonomas of transgenic mice express a wide range of general neuroendocrine markers and bioactive peptides. Virchows Arch. 2007;419:115–129. doi: 10.1007/BF01600225. [DOI] [PubMed] [Google Scholar]
- Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J. Clin. Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- Roth KA, Kim S, Gordon JI. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am. J. Physiol. 1992;263:G174–G180. doi: 10.1152/ajpgi.1992.263.2.G174. [DOI] [PubMed] [Google Scholar]
- Rubin G, de Wit N, Meineche-Schmidt V, Seifert B, Hall NP, Hungin AP. Identification and diagnosis of patients with irritable bowel syndrome in primary care: nominal group technique. Gut. 2005;54:A3. [Google Scholar]
- Rudnicki M, Kuvshinoff BW, McFadden DW. Extrinsic neural contribution to ileal peptide YY (PYY) release. J. Surg. Res. 1992;52:591–595. doi: 10.1016/0022-4804(92)90134-l. [DOI] [PubMed] [Google Scholar]
- Saclarides TJ, Szeluga D, Staren ED. Neuroendocrine cancers of the colon and rectum: results of a ten year experience. Dis. Colon Rectum. 1994;37:635–642. doi: 10.1007/BF02054405. [DOI] [PubMed] [Google Scholar]
- Saras J, Gronberg M, Stridsberg M, Oberg KE, Janson ET. Somatostatin induces rapid contraction of neuroendocrine cells. FEBS Lett. 2007;581:1957–1962. doi: 10.1016/j.febslet.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Oxyntomodulin from the distal gut: role in the regulation of gastric and pancreatic functions. Dig. Dis. Sci. 1989;34:1411–1420. doi: 10.1007/BF01538078. [DOI] [PubMed] [Google Scholar]
- Schmid KW, Weiler R, Xu RW, Hogue-Angeletti R, Fischer-Colbrie R, Winkler H. An immunological study on chromogranin A and B in human endocrine and nervous tissues. Histochem. J. 1989;21:365–373. doi: 10.1007/BF01798500. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter A. Minireview: development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- Seretis EC, Agnantis NJ, Golematis VC, Voloudakis-Balatzis IE. Electron immunocytochemical demonstration of serotonin, vasoactive intestinal polypeptide, bombesin, somatostatin and glucagon in mirror biopsies from primary colorectal adenocarcinoma. J. Exp. Clin. Cancer Res. 2004;23:477–484. [PubMed] [Google Scholar]
- Shamsuddin AM, Phelps PC, Trump BF. Human large intestinal epithelium: light microscopy, histochemistry and ultrastructure. Human Pathol. 1982;13:790–803. doi: 10.1016/s0046-8177(82)80075-0. [DOI] [PubMed] [Google Scholar]
- Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- Smith DM, Haggitt RC. The prevalence and prognostic significance of argyrophil cells in colorectal carcinomas. Am. J. Surg. Pathol. 1984;8:123–128. doi: 10.1097/00000478-198402000-00006. [DOI] [PubMed] [Google Scholar]
- Solcia E, Polak JM, Pearse AGE, et al. Lausanne 1977 classification of gastroenteropancreatic endocrine cells. In: Bloom SR, editor. Gut Hormones. Edinburgh: Churchill Livingstone; 1978. pp. 40–48. [Google Scholar]
- Solcia E, Capella C, Fiocca R, Sessa F, La Rosa S, Rindi G. Disorders of the Endocrine System. In: Ming SC, Goldman H, editors. Pathology of the Gastrointestinal Tract. Philadelphia, PA: Williams & Wilkins; 1998. pp. 295–322. [Google Scholar]
- Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072–1080. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Stanisz AM, Befus D, Bienenstock J. Differential effects of vasoactive intestinal polypeptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J. Immunol. 1986;136:152–156. [PubMed] [Google Scholar]
- Sternini C, Anselm L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatek J, Chibowski D. Endocrine cells in colorectal carcinomas. Immunohistochemical study. Pol. J. Pathol. 2000;51:127–136. [PubMed] [Google Scholar]
- Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide 1 (7–36) amide's central inhibition of feeding and peripheral inhibition of drinking are abolished by neonatal monosodium glutamate treatment. Diabetes. 1998;47:530–537. doi: 10.2337/diabetes.47.4.530. [DOI] [PubMed] [Google Scholar]
- Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellstrom PM. Glucagon-like peptide 1 retards gastric emptying and small bowel transit in the rat: effect mediated through central or enteric nervous mechanism. Dig. Dis. Sci. 1998;43:2284–2290. doi: 10.1023/a:1026678925120. [DOI] [PubMed] [Google Scholar]
- Tonini M. 5-Hydroxytryptamine effect in the gut: the 3,4 and 7 receptors. Neurogastroenterol. Motil. 2005;17:637–642. doi: 10.1111/j.1365-2982.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- Tsai C-H, Hill M, Asa SL, Brubaker PL, Drucker DJ. Intestinal growth-promoting properties of glucagon-like peptide 2 in mice. Am. J. Physiol. 1997;273:E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- Uehara S, Jung S, Morimoto R, et al. Vesicular storage and secretion of l-glutamate from glucagon-like peptide 1-secreting clonal intestinal L cells. J. Neurochem. 2006;96:550–560. doi: 10.1111/j.1471-4159.2005.03575.x. [DOI] [PubMed] [Google Scholar]
- Van Lelyveld N, Ter Linde J, Schipper ME, Samsom M. Regional differences in expression of TPH-1, SERT, 5-HT3, and 5-HT4 receptors in the human stomach and duodenum. Neurogastroenterol. Motil. 2007;19:342–348. doi: 10.1111/j.1365-2982.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- Varndell IM, Lloyd RV, Wilson BS, Polak JM. Ultrastructural localization of chromogranin: a potential marker for the electron microscopical recognition of endocrine cell secretory granules. Histochem. J. 1985;17:981–992. doi: 10.1007/BF01417947. [DOI] [PubMed] [Google Scholar]
- Verdich C, Flint A, Gutzwiller J-P, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- Wang SY, Chi MM-Y, Li L, Moley KH, Wice BM. Studies with GIP/Ins cells indicate secretion by gut K cells is KATP channel independent. Am. J. Physiol. Endocrinol. Metab. 2002;284:E988–E1000. doi: 10.1152/ajpendo.00398.2002. [DOI] [PubMed] [Google Scholar]
- Wang H, Steeds J, Motomura Y, et al. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–957. doi: 10.1136/gut.2006.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington MK, Tang LH, Berlin J, et al. Protocol for the examination of specimens from patients with neuroendocrine tumours (carcinoid tumours) of the colon and rectum. Arch. Pathol. Lab. Med. 2010;134:176–180. doi: 10.5858/134.2.176. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kubota Y, Sawada T, Muto T. Distribution and quantification of somatostatin in inflammatory disease. Dis. Colon Rectum. 1992;35:488–494. doi: 10.1007/BF02049408. [DOI] [PubMed] [Google Scholar]
- Weidenmann B, Frank WW, Kuhn C, Moll R, Gould VE. Synaptophysin: a novel marker protein for neuroendocrine cells and neoplasms. Proc. Natl Acad. Sci. USA. 1986;83:3500–3504. doi: 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. FASEB J. 2000;14:2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- Wettergren A, Scjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (Proglucagon 78-107-Amide) inhibits gastric and pancreatic functions in man. Dig. Dis. Sci. 1993;38:665–673. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]
- Wettergren A, Maina P, Boesby S, Holst JJ. Glucagon-like peptide-1 7-36 amide and peptide YY have additive inhibitory effect on gastric acid secretion in man. Scand. J. Gastroenterol. 1997;32:552–555. doi: 10.3109/00365529709025098. [DOI] [PubMed] [Google Scholar]
- Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. Proc. Natl Acad. Sci. USA. 2010;107:3722–3727. doi: 10.1073/pnas.0915135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PL, Okumiya K, Kinoshita M, Hasegawa H, Fujimiya M. Immunohistochemical localization of tryptophan hydroxylase in the human and rat gastrointestinal tracts. J. Comp. Neurol. 1999;411:654–665. doi: 10.1002/(sici)1096-9861(19990906)411:4<654::aid-cne9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Zhang T, Brubaker PL, Thompson JC, Greeley GH., Jr Characterization of peptide-YY release in response to intracolonic infusion of amino acids. Endocrinology. 1993;132:553–557. doi: 10.1210/endo.132.2.8093875. [DOI] [PubMed] [Google Scholar]


