Abstract
Endoplasmic reticulum (ER) stress has been shown to participate in many disease pathologies. Although recent reports have demonstrated that ER stress in chondrocytes is present in human osteoarthritis (OA), its role in the pathology of cartilage degeneration, such as chondrocyte apoptosis, remains unclear. In the present study, we investigated the expression of phosphorylated PERK (pPERK), ubiquitin (Ub), GRP78, CHOP, phosphorylated JNK (pJNK) and cleaved caspase-3 (C-CASP3) and the mRNA splicing of XBP1 (XBP1 splicing) in human OA cartilage by immunohistochemistry and RT-PCR. Additionally, human chondrocytes were treated with several concentrations of tunicamycin, an ER stress inducer, to assess the impact of ER stress on the mRNA expression of CHOP, XBP1 splicing and apoptosis, as determined by real-time PCR, RT-PCR and ELISA analyses respectively. In human OA cartilage, the number of chondrocytes expressing pPERK, Ub, CHOP and pJNK positively correlated with cartilage degeneration and the number of C-CASP3-positive chondrocytes. XBP1 splicing and GRP78 expression in severe OA containing the greatest number of C-CASP3-positive chondrocytes were similar to the levels in mild OA, however, XBP1 splicing was higher in moderate OA than in mild and severe OA. Tunicamycin dose dependently increased CHOP expression and apoptosis of cultured chondrocytes. Although tunicamycin upregulated XBP1 splicing in cultured chondrocytes, its impact on XBP1 splicing was weakened at higher concentrations. In conclusion, the present results indicate that ER stress may contribute to chondrocyte apoptosis along with OA progression, which was closely associated with an enhanced apoptotic response and a reduced protective response by the cells.
Keywords: apoptosis, cartilage, endoplasmic reticulum stress, osteoarthritis
Endoplasmic reticulum (ER) stress, which is provoked by an imbalance between the load of unfolded proteins in the ER and the capacity of the ER, leads to the accumulation of unfolded or misfolded proteins in the ER (Ron & Walter 2007). Three ER transmembrane proteins, such as protein kinase RNA-like ER kinase (PERK), inositol-requiring protein-1 (IRE1α) and activating transcription factor-6 (ATF6), sense ER stress and induce specialized responses to recover or maintain ER function (Ron & Walter 2007). Activated PERK leads to the general inhibition of translation to reduce the load of newly synthesized proteins that are translocated to the ER. Activated IRE1α splices X-box binding protein 1 (XBP1) mRNA. The XBP1 protein encoded by the spliced XBP1 mRNA enhances the capacity of the ER by upregulating the expression of ER chaperon proteins, such as the 78-kDa glucose-regulated protein (GRP78), and reduces unfolded or misfolded proteins in the ER by the endoplasmic reticulum-associated protein degradation system (ERAD). The ERAD regulates the degradation of unfolded or misfolded proteins in the ER through the ubiquitin (Ub)-proteasome system. However, if these protective responses fail and ER stress persists, specialized apoptotic pathways are activated to eliminate the damaged cells. At least three pathways are involved in the induction of cell apoptosis by ER stress (Gotoh & Mori 2006). The first pathway is the enhanced expression of C/EBP-homologous protein (CHOP) by activated PERK. The second acts through the activation of c-Jun N-terminal kinase (JNK), a mitogen-activated protein kinase, through the recruitment of TNF receptor-associated factor 2 by activated IRE1α. The last pathway is the activation of caspase-12, which is not functional in humans (Fischer et al. 2002).
Recent reports have demonstrated that ER stress in chondrocytes is present in human osteoarthritis (OA) cartilage (Horton et al. 2006; Ruiz-Romero et al. 2008; Nugent et al. 2009) and that chondrocytes are sensitive to ER stress (Boot-Handford & Briggs 2010). In vitro experiments using rat chondrocytes showed that ER stress induced apoptosis and decreased the mRNA expression of extracellular matrix (ECM) proteins in articular cartilage, such as aggrecan and type II collagen (Yang et al. 2005, 2007). Additionally, human chondrocytes increase the mRNA level of matrix metalloproteinase 13 (MMP 13), an enzyme involved in cartilage degeneration, after surviving ER stress (Hamamura et al. 2009). Although apoptosis, a decrease in ECM production and an increase in MMP-13 production by chondrocytes are well-known signs of cartilage degeneration (Blanco et al. 1998; Kim et al. 2000; Sandell & Aigner 2001), it remains unclear whether ER stress is involved in the pathology of OA.
The purpose of the present study was to investigate the association between ER stress and chondrocyte apoptosis or ECM gene expression in degenerative cartilage, and to clarify the involvement of ER stress in the pathology of OA. We assessed the expression of pPERK and Ub as markers of ER stress, the expression of CHOP and pJNK as markers of the apoptotic ER stress response, and the expression of GRP78 and splicing of XBP mRNA as markers of the protective ER stress response, in cartilage samples that were representative of different degrees of human degenerative OA. Thereafter, we examined the relationship between the expression of these markers and apoptosis and the mRNA expression of aggrecan and type II collagen in OA cartilage. Furthermore, we verified the impact of ER stress on the ER stress response, apoptosis, and the mRNA expression of aggrecan and type II collagen using human articular chondrocytes.
Materials and methods
Cartilage samples
The present study was approved by the institutional ethics committee of Kumamoto University and was performed in accordance with the Helsinki Declaration of 1975, as revised in 2000. Articular cartilage samples were obtained at total knee arthroplasty from the tibial plateaus of 11 patients suffering from knee OA (71.7 ± 6.5 years old; eight female subjects and three male subjects). Taking care not to sample from the cartilage of joint margins or osteophytes, 1–3 samples, which differed from each other in the degree of cartilage degeneration defined by their International Cartilage Repair Society (ICRS) grade (0 = normal, I = nearly normal, II = abnormal, III = severely abnormal) (Kleemann et al. 2005), were obtained from each knee. Ultimately, 20 cartilage samples were obtained (Table 1). All tissues were divided into two osteochondral sections. Half of each divided tissue was fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 24 h, decalcified in 10% EDTA for several days and embedded in paraffin for histochemical evaluation. The other half of the samples were immediately frozen in liquid nitrogen and then stored at −80 °C until total RNA was extracted.
Table 1.
Background information of patients
| Patient | Age (years) | Sex | ICRS grade (Mankin score) |
|---|---|---|---|
| 1 | 71 | Female | 0 (1) |
| 2 | 76 | Female | I (3) |
| 3 | 74 | Female | I (6) |
| 4 | 76 | Female | I (6), II (8), III (9) |
| 5 | 76 | Female | I (3), III (4) |
| 6 | 59 | Female | I (5), III (10) |
| 7 | 73 | Female | II (7), III (9) |
| 8 | 58 | Female | III (9) |
| 9 | 70 | Male | 0 (2), I (4), III (7) |
| 10 | 77 | Male | I (3), II (5) |
| 11 | 79 | Male | I (6), III (11) |
ICRS, International Cartilage Repair Society.
Chondrocyte culture
Human articular cartilage specimens were obtained during total knee arthroplasty from the tibial plateaus and the fermoral condyle of two patients (two women, 71–75 years old) with knee OA. Therefore, four separate cartilage pieces were obtained from four separate sites and were processed separately. Primary human chondrocytes were isolated from these cartilage specimens by a sequential enzyme digestion method described previously (Hirose et al. 2002). Chondrocytes were plated in high-density monolayers and cultured in DMEM/Ham's F-12 medium containing 10% FBS. To avoid the de-differentiation of isolated chondrocytes, we performed the stimulation experiment within a short period, namely 4–5 days, after digestion. Twelve hours after the culture media were replaced with serum-free Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 (Nacalai, Kyoto, Japan), chondrocytes were incubated in DMEM/Ham's F-12 medium containing 0.5% FBS (Invitrogen, Carlsbad, CA, USA) with tunicamycin (Calbiochem, San Diego, CA, USA) at various concentrations (0, 0.5, 1, 5 or 10 μg/ml) for 24 h. The stimulation procedures were performed using cells grown on 24-well plates for the analysis of mRNA expression or 96-well plates for the apoptosis assays.
Safranin-O staining
The paraffin-embedded samples were cut into 4-μm sections and stained with safranin-O. The histological severity of cartilage degeneration of each sample was evaluated by the Mankin scoring system (Mankin et al. 1971), and samples were classified into 3 grades: mild (0–3 points), moderate (4–7 points) and severe (8–14 points) (Lippiello et al. 2000). Mild OA cartilage was constituted by a superficial zone where the top area of cartilage had a disposition of flattened cells parallel to the cartilage surface, the middle zone had a random disposition of round cells and the deep zone had columns of round cells perpendicular to the cartilage surface. Moderate and severe OA cartilage had lost the superficial zone. Therefore, areas near the surface of these cartilage samples corresponded to the upper middle zone, and the lower areas corresponded to the lower middle and deep zones.
Immunohistochemistry
The expression levels of phosphorylated PERK (pPERK), Ub, GRP78, CHOP, phosphorylated JNK (pJNK) and cleaved caspase-3 (C-CASP3) in cartilage samples were analysed by immunohistochemistry. The rabbit anti-pPERK polyclonal antibody, goat anti-GRP78 antibody, rabbit anti-CHOP polyclonal antibody and mouse anti-pJNK monoclonal antibody were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The mouse anti-Ub monoclonal antibody and the rabbit anti-C-CASP3 antibody were purchased from MBL (Nagoya, Japan) and Cell Signaling Technology Inc. (Bervely, MA, USA) respectively. The availabilities of these antibodies for the immunohistochemical analyses of human tissue were described previously (Dil Kuazi et al. 2003; Bek et al. 2006; Li et al. 2008; Ruiz-Romero et al. 2008; Hoozemans et al. 2009). Histofine MAX-PO (R), Histofine MAX-PO (M) and Histofine MAX-PO (G) were obtained from Nichirei Co. Ltd (Tokyo, Japan) as secondary antibodies. Sections (4 μm) were deparaffinized and rehydrated. During the analyses for pPERK, Ub, CHOP and C-CASP3, sections were treated with proteinase K (Roche Diagnostics, Mannheim, Germany)/PBS (20 μg/ml) for 12 min at room temperature for antigen retrieval and samples were washed with water. To block endogenous peroxidase activities, the sections were incubated in 0.3% hydrogen peroxide/methanol for 30 min and washed in water. Non-specific binding sites were blocked with normal rabbit serum (Nichirei Co. Ltd) prior to anti-GRP78 antibody incubation or with normal goat serum (Nichirei Co. Ltd) prior to incubation with the other antibodies, at room temperature. After 30 min, the sections were incubated with primary antibodies diluted in PBS (pPERK; 1:200, Ub; 1:500, GRP78; 1:300, CHOP; 1:200, pJNK; 1:200 and C-CASP3; 1:500) for 18 h at 4 °C. After washing, sections were incubated with the appropriate secondary antibodies for 30 min at room temperature. Staining was visualized with 3,3-diaminobenzide tetrahydrochloride (pPERK, Ub, GRP78 and pJNK) or 3-amino-9-ethylcarbazole (CHOP and C-CASP3), followed by counterstaining with hematoxylin. The sections incubated without a primary antibody, with a negative control mouse IgG, or with a negative control rabbit immunoglobulin fraction instead of a primary antibody served as the negative control samples. A histological evaluation was performed for one section per cartilage sample using light microscopy. Two full-thickness areas of cartilage separated from each other by at least 1 mm were randomly selected from each section. The digital photographs of these two selected areas were obtained in 24–40 parts (400 × 300 μm) per section at a magnification of 200×. The positive and negative cells in all photographs were counted in a blinded manner by an evaluator not informed about the sections, and the total percentage of positive cells in the section was calculated.
Extraction and reverse transcription of RNA
Total RNA of the cartilage sample stored at −80 °C was extracted using the TRIzol reagent (Invitrogen) and further purified using the RNeasy mini kit (Qiagen, Valencia, CA, USA) in combination with DNA digestion using DNase (Qiagen). The extraction of total RNA from cultured chondrocytes was performed using the RNeasy mini kit and DNase. Purified RNA was reverse-transcribed using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster, CA, USA). All procedures were performed according to the manufacturer's protocols.
Polymerase chain reaction (PCR)
PCR amplification of XBP1 mRNA was performed using TaKaRa LA Taq (Takara, Kyoto, Japan) according to the manufacturer's protocol. The following primer pair was used for XBP1: 5′-GCCTTGTAGTTGAGAACCAG-3′ (sense) and 5′-TTAATGGCTTCCAGCTTGGC-3′ (antisense). This primer pair was designed so that the PCR products contained both the spliced and unspliced forms of XBP1 mRNA, encompassing the 26-bp region excised by IRE1α. Because this 26-bp region contains a Pst-I restriction site, electrophoresis of PCR products after Pst-I treatment could separate the spliced form of XBP1 from the unspliced form (Uehara et al. 2006). The thermal cycling was carried out with denaturation at 94 °C, followed by 33 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s and extension at 72 °C for 30 s. PCR products were incubated with the Pst-I restriction enzyme for 2 h at 37 °C and were visualized on a 2% agarose gel using ethidium bromide. The ratio of the spliced form to the unspliced form (spliced/unspliced XBP1) was calculated by the densitometric measurement of each band using a densitometer (AE-6920-MF; ATTO, Tokyo, Japan) and the CS analyzer software program (ATTO). The value of the spliced/unspliced XBP1 was the average of three separate assays for each sample.
Real-time PCR
A quantitative real-time RT-PCR analysis was performed on an Applied Biosystems 7300/7500 Real-Time PCR system (Applied Biosystems). TaqMan Gene Expression Master Mix and TaqMan Gene Expression Assays for CHOP (Hs00358796), aggrecan (ACAN) (Hs00202971), α-1 type II collagen (COL2A1) (Hs00156568) and GAPDH (Hs99999905) were purchased from Applied Biosystems. Reactions were carried out with the following conditions: 2 min at 50 °C and 10 min at 95 °C; 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The calibration of real-time PCR was performed by the relative standard curve method (User Bulletin #2, Applied Biosystems; Horii et al. 2002). For a construct standard curve, a standard sample cDNA was prepared from cultured human chondrocytes and used as a stock solution. In every PCR assay, a construct standard curve was made using the same stock standard sample. The relative concentration of the target gene of cartilage samples was calculated from a construct standard curve, and the ratio of the relative concentration of the target gene to GAPDH of cartilage samples was calculated. This ratio represented the relative expression of the target gene normalized to GAPDH of cartilage samples compared to the standard sample.
ELISA for apoptosis
The extent of apoptosis of cultured chondrocytes was analysed with the Cell Death Detection ELISA Plus (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer's protocol. For each experiment, the amount of protein in the cell lysate was assessed in separate wells using the Quick Start Bradford protein assay (Bio-Rad Laboratories, Richmond, CA, USA) to normalize the extent of cellular apoptosis.
Statistical analysis
Differences in the expression of the various markers in the three grades of cartilage degeneration and the following exposure to various concentration of tunicamycin were analysed using the Kruskal–Wallis test. The Mann–Whitney U-test was performed for post hoc comparisons. Correlations between each term were analysed using Spearman's rank correlation coefficient (rs). Data were expressed as the means ± standard error of the mean (SEM). Differences were considered to be statistically significant for P-values of <0.05.
Results
ER stress-associated molecules in OA cartilage
In mild OA cartilage, chondrocytes positive for pPERK, Ub, GRP78, CHOP and pJNK were present in the superficial zone, but were rarely located in the middle and deep zones (Figures 1b–d, 2a–c, 3a–c, 4a–c and 5a–c). In moderate OA cartilage, chondrocytes expressing pPERK, Ub and pJNK were found in the upper middle zone, with particularly high expression of Ub (Figures 1e, 2d, and 5d). In the lower middle and deep zones of moderate OA cartilage, chondrocytes occasionally weakly expressed pPERK, Ub and pJNK (Figures 1f,g, 2e,f and 5e,f). The expression of GRP78 and CHOP was detectable in chondrocytes within the upper middle zone of moderate OA cartilage, but not in chondrocytes within the lower middle and deep zones (Figures 3d–f and 4d–f). In severe OA, a large population of chondrocytes was positively stained for pPERK, Ub, CHOP and pJNK throughout all zones, frequently accompanied by strong staining for pPERK and Ub in the upper middle zone (Figures 1j, 2g, 4g and 5g). However, chondrocytes expressing GRP78 were restricted to the upper middle zone at a similar localization as in moderate OA cartilage (Figure 3g–i). The mRNA expression of XBP1 was detected in all cartilage samples (Figure 6a). Moderate OA cartilage showed higher expression of the spliced form of XBP1 mRNA than mild and severe OA cartilage (Figure 6a,b). The mRNA expression of CHOP was significantly increased in severe OA cartilage compared to mild OA cartilage (Figure 6c).
Figure 1.
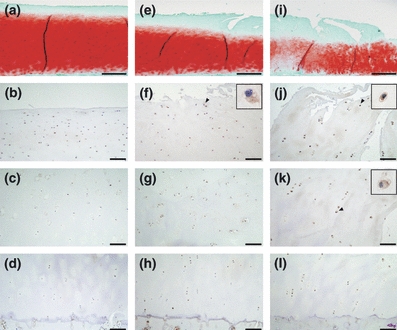
Safranin-O staining and immunohistochemistry of pPERK (a–d, f–h and j–l) in representative sections of osteoarthritis (OA) cartilage. (a, e and i) Safranin-O stained sections of mild (Mankin score = 1), moderate (Mankin score = 5) and severe (Mankin score = 9) OA cartilage respectively. (b–d) The superficial, middle and deep zones of mild OA cartilage respectively. (f–h) The upper middle, lower middle and deep zones of moderate OA cartilage respectively. (j–l) The upper middle, lower middle and deep zones of severe OA cartilage respectively. The scale bar is 500 μm in a, e and i, and 100 μm in b–d, f–h and j–l. Insets are magnifications of the arrowheads. In severe cartilage, cells positive for pPERK are extensively detected from the surface to the deep zone.
Figure 2.
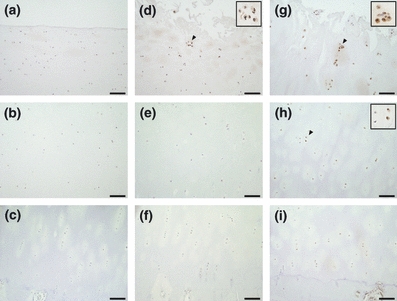
Immunohistochemical staining of Ub in osteoarthritis (OA) cartilage. The superficial, middle and deep zones of mild OA cartilage are shown in a–c respectively. The upper middle, lower middle and deep zone of moderate or severe cartilage are shown in d–f and g–i respectively. The scale bar is 100 μm. Insets are magnifications of arrowheads. The number of cells positive for Ub was increased in all zones of severe cartilage.
Figure 3.
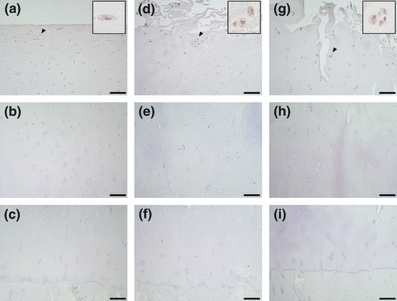
Immunohistochemical staining of GRP78 in osteoarthritis (OA) cartilage. The superficial, middle and deep zones of mild OA cartilage are shown in a–c respectively. The upper middle, lower middle and deep zones of moderate or severe cartilage are shown in d–f and g–i respectively. The scale bar is 100 μm. Insets are magnifications of arrowheads. Cells stained for GRP78 were detected in the superficial zone of mild cartilage and the upper middle zone of moderate and severe cartilage.
Figure 4.
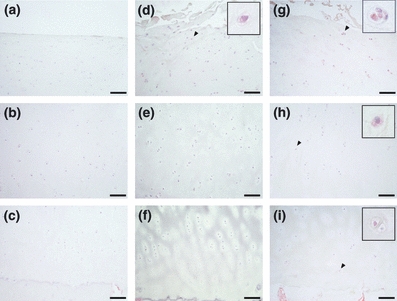
Immunohistochemical staining of CHOP in osteoarthritis (OA) cartilage. The superficial, middle and deep zones of mild OA cartilage are shown in a–c respectively. The upper middle, lower middle and deep zones of moderate or severe cartilage are shown in d–f and g–i respectively. The scale bar is 100 μm. Insets are magnifications of arrowheads. The number of cells expressing CHOP was increased in middle zones of severe cartilage.
Figure 5.
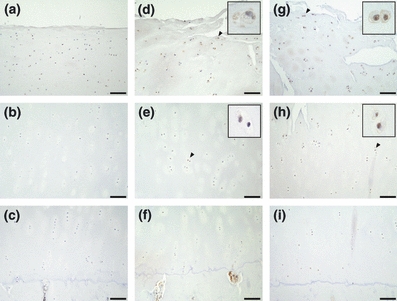
Immunohistochemical staining of pJNK in osteoarthritis (OA) cartilage. The superficial, middle and deep zones of mild OA cartilage are shown in a–c respectively. The upper middle, lower middle and deep zones of moderate or severe cartilage are shown in d–f, and g–i respectively. The scale bar is 100 μm. Insets are magnifications of arrowheads. Chondrocytes stained for pJNK were detectable in the superficial zone of mild cartilage and the middle zones of moderate and severe cartilage.
Figure 6.

Endoplasmic reticulum stress-associated mRNA expression in osteoarthritis (OA) cartilage. (a) XBP1 mRNA in four representative samples of each grade was shown. The upper bands indicate the total expression of XBP1 mRNA. The middle and lower bands are spliced and unspliced XBP1 mRNA respectively, loaded after Pst-I restriction enzyme treatment of the PCR products. (b, c) The ratio of spliced/unspliced XBP1 (b) and CHOP/GAPDH (c) in each grade (mild: n = 5, moderate: n = 7, severe: n = 8) was shown. The spliced/unspliced XBP1 and CHOP/GADPH ratios were normalized to averaged values of mild OA. *P < 0.05 compared with mild; †P < 0.05 compared with moderate.
Table 2 shows the relationship between the percentages of cells positive for ER stress-associated molecules, spliced/unspliced XBP1 and the mRNA expression of CHOP to the severity of cartilage degeneration. The percentage of cells positive for pPERK, Ub and CHOP was significantly greater in the severe OA cartilage samples compared to both mild and moderate OA cartilage samples, and the percentage of cells positively correlated with the Mankin score of the sample. The percentage of cells positive for pJNK was significantly higher in both moderate and severe OA cartilage than in mild OA cartilage, but there were no significant differences between moderate and severe OA cartilage. There was also a positive correlation between the percentage of cells positive for pJNK and the Mankin score of the sample. However, the percentage of cells positive for GRP78 was not significantly different among the samples of the three degrees of OA cartilage degeneration, showing a weak correlation with the Mankin score.
Table 2.
The relationship between endoplasmic reticulum stress-associated molecules and cartilage degeneration
| Mild | Moderate | Severe | Mankin score | ||
|---|---|---|---|---|---|
| (n = 5) | (n = 7) | (n = 8) | rs | P-value | |
| pPERK (%) | 23.0 ± 10.4 | 37.0 ± 5.0 | 49.0 ± 7.0*† | 0.86 | 0.001 |
| Ub (%) | 19.1 ± 2.8 | 29.6 ± 6.6 | 59.3 ± 3.7*† | 0.81 | 0.001 |
| GRP78 (%) | 16.2 ± 2.9 | 21.2 ± 1.9 | 24.2 ± 2.8 | 0.51 | 0.028 |
| CHOP (%) | 8.2 ± 5.2 | 15.9 ± 13.7 | 35.4 ± 9.2*† | 0.74 | 0.002 |
| pJNK (%) | 8.6 ± 7.5 | 32.7 ± 14.9* | 48.6 ± 13.8* | 0.81 | 0.001 |
rs, Spearman's rank correlation coefficient.
The values are expressed as mean ± SEM.
P < 0.05 vs. mild
P < 0.05 vs. moderate.
In paired comparisons of samples from 6 of 11 patients (data not shown), all paired samples showed that the expression of pPERK and Ub was increased in more degenerated samples. Furthermore, five of six paired samples showed that the percentage of cells positive for CHOP and pJNK was also increased in the more degenerated cartilage, and the spliced/unspliced XBP1 ratio was higher in moderate OA cartilage than mild and severe OA cartilage.
Apoptosis and ECM gene expression by chondrocytes in OA cartilage
C-CASP3-positive chondrocytes were detectable in the superficial zone of mild OA cartilage and the upper middle zone of moderate and severe OA cartilage (Figure 7a,d and g). The number of positive cells in these areas increased with the degree of cartilage degeneration. However, the number of C-CASP3-positive cells increased in the lower middle of severe OA cartilage compared to moderate cartilage. (Figure 7b,c, e–f and h,i). The percentage of C-CASP3-positive cells was significantly higher in severe OA cartilage than in mild OA cartilage, and the staining positively correlated with the Mankin score (Table 3). ACAN expression in OA cartilage showed a negative correlation with the Mankin score, but COL2A1 expression did not have any significant correlation with the Mankin score (Table 3). During the paired comparisons of the samples from 6 of 11 patients (data not shown), five of six paired samples showed that C-CASP3-positive chondrocytes were more common in the more degenerated cartilage than in the less degenerated cartilage. ACAN expression was decreased in the more degenerated cartilage in three of six paired samples in comparison with the less degenerated cartilage of the same joints. The COL2A1 expression in the more degenerated cartilage was increased in two of six paired samples and decreased in one of six paired sample compared to the less degenerated cartilage of the same joints. However, the differences in the ACAN and COL2A1 expression between the more and less degenerated cartilage from the six paired samples were not significant.
Figure 7.
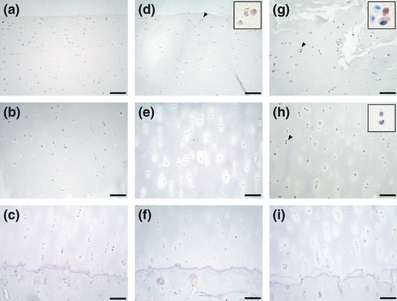
Immunohistochemical staining of C-CASP3 in osteoarthritis (OA) cartilage. The superficial, middle and deep zones of mild OA cartilage are shown in a–c respectively. The upper middle, lower middle and deep zones of moderate or severe cartilage are shown in d–f and g–i respectively. The scale bar is 100 μm. Insets are magnifications of arrowheads. C-CASP3-positive cells were particularly increased in the upper middle zone of severe cartilage.
Table 3.
The relationship between cartilage degeneration and C-CASP3 and matrix gene expression levels of chondrocytes
| Mild | Moderate | Severe | Mankin score | ||
|---|---|---|---|---|---|
| (n = 5) | (n = 7) | (n = 8) | rs | P-value | |
| C-CASP3 (%) | 5.4 ± 1.6 | 9.2 ± 1.2 | 13.7 ± 1.9* | 0.58 | 0.013 |
| ACAN/GAPDH | 1.00 ± 0.22 | 0.39 ± 0.07* | 0.20 ± 0.04*,† | −0.77 | 0.001 |
| COL2A1/GAPDH | 1.00 ± 0.32 | 1.51 ± 0.54 | 1.47 ± 0.47 | 0.12 | 0.684 |
rs, Spearman's rank correlation coefficient; C-CASP3, cleaved caspase-3.
The values are expressed as mean ± SEM. ACAN/GAPDH and COL2A1/GAPDH were normalized by averaged values of mild.
P < 0.05 vs. mild
P < 0.05 vs. moderate.
The relationship between the expression of ER stress-associated molecules and apoptosis and ECM gene expression by chondrocyte in OA cartilage
The percentage of C-CASP3-positive cells in OA cartilage significantly correlated with the percentage of cells positive for pPERK, Ub and CHOP, and the mRNA expression of CHOP (Table 4). However, there was no relationship between the percentage of C-CASP3-positive cells and the expression of GRP78 and pJNK and the ratio of spliced/unspliced XBP1 (Table 4). ACAN expression negatively correlated with the percentage of cells positive for pPERK, Ub, CHOP and pJNK in OA cartilage (Table 4). However, there was no correlation between COL2A1 expression and any of the ER stress-associated molecules that were examined (Table 4).
Table 4.
The relationship between the endoplasmic reticulum stress-associated molecules, C-CASP3 and matrix gene expressions by chondrocytes in human osteoarthritic cartilage
| C-CASP3 | ACAN/GAPDH | COL2A1/GAPDH | ||||
|---|---|---|---|---|---|---|
| rs | P-value | rs | P-value | rs | P-value | |
| Immunohistochemistry | ||||||
| pPERK (%) | 0.52 | 0.031 | −0.73 | 0.001 | 0.06 | 0.822 |
| Ub (%) | 0.49 | 0.039 | −0.57 | 0.015 | −0.01 | 0.953 |
| GRP78 (%) | 0.30 | 0.195 | −0.14 | 0.392 | 0.38 | 0.124 |
| CHOP (%) | 0.68 | 0.003 | −0.70 | 0.003 | −0.11 | 0.629 |
| pJNK (%) | 0.24 | 0.308 | −0.66 | 0.005 | 0.16 | 0.489 |
| mRNA analysis | ||||||
| Spliced/unspliced XBP1 | 0.34 | 0.147 | −0.25 | 0.282 | 0.01 | 0.969 |
| CHOP/GAPDH | 0.49 | 0.034 | −0.278 | 0.225 | 0.026 | 0.911 |
rs, Spearman's rank correlation coefficient; C-CASP3, cleaved caspase-3.
ER stress response, apoptosis and ECM gene expression by cultured chondrocytes under ER stress
The chondrocytes dose dependently increased their mRNA expression of CHOP following a 24-h exposure to tunicamycin (Figure 8a). Tunicamycin increased XBP1 mRNA splicing of chondrocytes, while the levels of the splicing in chondrocytes stimulated with 10 μg/ml concentration of tunicamycin were lower than the splicing levels stimulated by 1 μg/ml of tunicamycin (Figure 9a and b). Chondrocyte apoptosis was induced by 5 and 10 μg/ml of tunicamycin and was dose dependently increased by tunicamycin treatment (Figure 8b), and was closely associated with both the increased expression of CHOP and a decrease in XBP1 mRNA splicing. Furthermore, tunicamycin significantly suppressed the expression of ACAN and COL2A1 by the chondrocytes, even at a concentration of 0.5 μg/ml (Figure 8c and d), and the suppressive effect on ACAN and COL2A1 paralleled the increase in the ER stress responses.
Figure 8.
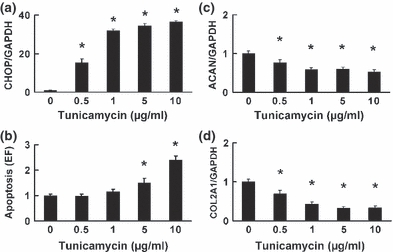
The impact of endoplasmic reticulum stress on cultured chondrocytes. The mRNA expression of CHOP (a), ACAN (c) and COL2A1 (d), and cellular apoptosis (b) were analysed 24 h after stimulation with tunicamycin (0, 0.5, 1, 5 or 10 μg/ml). (a) The expression of CHOP was dose dependently upregulated. (b) Chondrocyte apoptosis was significantly increased by exposure to tunicamycin at 5 and 10 μg/ml. (c, d) The expression of ACAN (c) and COL2A1 (d) was suppressed by tunicamycin treatment. Data are expressed relative to the mean value of cells treated without tunicamycin in each experiment. The values represented in the graph are expressed as the means ± SEM of four independent experiments. *P < 0.05 compared with treatment without tunicamycin.
Figure 9.
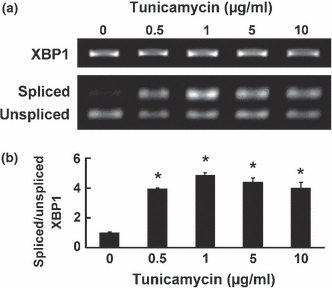
The impact of endoplasmic reticulum stress on splicing of XBP1 mRNA in cultured chondrocytes. XBP1 mRNA splicing was analysed 24 h after stimulation with tunicamycin (0, 0.5, 1, 5 or 10 μg/ml). The ratio of spliced/unspliced XBP1 was examined following Pst-I treatment, as described in the Materials and Methods. (a) Representative result of the experiments is shown. The upper, middle and lower bands are total, spliced and unspliced XBP1 mRNA respectively. (b) The ratio of spliced/unspliced XBP1 was increased by tunicamycin treatment, while the ratio of spliced/unspliced XBP1 in chondrocytes stimulated with 10 μg/ml of tunicamycin was lower than the ratio stimulated with 1 μg/ml of tunicamycin. Data are expressed relative to the mean value of cells treated without tunicamycin in each experiment. The value of the graph is expressed as the mean ± SEM of four independent experiments. *P < 0.05 compared with treatment without tunicamycin.
Discussion
The present study investigated the relationship between ER stress-associated molecules and both chondrocyte apoptosis and ECM gene expression of chondrocyte in OA cartilage. Our results demonstrated that the expression of ER stress-associated molecules, such as pPERK, Ub and CHOP, and chondrocyte apoptosis analysed by the expression of C-CASP3 in OA cartilage were increased with the progression of cartilage degeneration. Moreover, pPERK, Ub, CHOP and pJNK negatively correlated with ACAN expression in OA cartilage. However, XBP1 mRNA splicing, which represented a protective ER stress response, increased in moderate OA cartilage, but not in mild or severe cartilage. In vitro, ER stress induced the apoptosis of cultured human chondrocytes and also suppressed their mRNA expression of aggrecan and type II collagen. Furthermore, the induction of chondrocyte apoptosis by ER stress occurred in parallel with increased CHOP expression and a reduction in XBP1 mRNA splicing. These results indicated that ER stress might be involved in the pathology of OA, including the increased apoptosis and suppressed ECM production by the chondrocytes.
Our observation that the expression of pPERK and Ub was detectable in human OA and correlated with the severity of cartilage degeneration indicated that ER stress occurs in OA cartilage and that it increased with cartilage degeneration. PERK is a sensor protein that directly detects unfolded proteins in the ER lumen and is activated by autophosphorylation (Ron & Walter 2007). Ub is a marker of the accumulation of unfolded proteins (Hara et al. 2006). Previous studies concerning ER stress in cartilage assessed the expression of GRP78 (Horton et al. 2006; Nugent et al. 2009). GRP78 is consistently expressed, assists in the maturation of newly synthesized proteins and is upregulated by ER stress through the activation of transcription factors such as ATF6 and XBP1. Therefore, in contrast to GRP78, the analysis of pPERK expression is a more direct method for evaluating ER stress. To the best of our knowledge, this report is the first to conclusively demonstrate the occurrence of ER stress in human OA cartilage and its association with the severity of cartilage damage.
In our immunohistochemical examinations, we observed that pPERK and Ub were strongly expressed in chondrocytes in the upper zone of the OA cartilage. Pro-inflammatory cytokines, such as IL-1β and TNFα, which induce ER stress in rat pancreatic β cells (Shkoda et al. 2007; Akerfeldt et al. 2008) and rat chondrocytes (Oliver et al. 2005), have been reported to be increased in this area of OA cartilage in comparison with the deep zone of OA cartilage and normal cartilage (Tetlow et al. 2001). We have confirmed that IL-1β and TNFα can induce ER stress in human articular chondrocytes (unpublished data), suggesting that these proinflammatory cytokines may be the inducers of ER stress that occur at the upper zone of OA cartilage. The articular cartilage degeneration during OA typically advances at the site centred on the load bearing areas damaged by excessive mechanical stress (Hashimoto et al. 2008). In the paired comparisons of the present study, the more degenerated cartilage, which was considered to be more damaged by mechanical loading stress, showed an increased number of chondrocytes expressing pPERK and Ub in comparison with the less degenerated cartilage of the same joint. Chondrocytes in injured cartilage markedly enhance the synthesis of proteins, which may increase the load on the ER (Mankin 1982), and chondrocytes also increase their production of nitric oxide, an inducer of ER stress (Gotoh & Mori 2006), in response to excessive mechanical stress (Guilak et al. 2004). Therefore, our results from the paired samples imply that the degeneration of articular cartilage because of an excessive mechanical load might also cause ER stress.
In the current study, we investigated the expression of cleaved caspase-3 in cartilage to identify cells undergoing apoptosis. Caspase-3 is a crucial enzyme in the apoptotic process. Because activation of caspase-3 requires proteolytic processing (Green 2000), detection of cleaved (activated) caspase-3 is a valuable and specific method to identify cells that fell to apoptosis, including those in the pre-apoptotic state (Kühn et al. 2004; Takagi et al. 2006). Therefore, the correlation between caspase-3 activation and cartilage degeneration observed in this study supported the conception that chondrocyte apoptosis is involved in the pathology of cartilage degeneration. We also showed that the expression of pPERK, Ub and CHOP correlated with the proportion of apoptotic chondrocytes, as indicated by caspase-3 activation in OA cartilage. Previous studies have revealed that the upregulation of CHOP is a pivotal event in the ER stress response and that its upregulation induces apoptosis (Zinszner et al. 1998; McCullough et al. 2001; Gotoh et al. 2004). We confirmed that ER stress-associated apoptosis in cultured chondrocytes was accompanied by the increased expression of CHOP. These results therefore indicate that ER stress might contribute to the chondrocyte apoptosis during OA progression.
JNK is another molecule central to ER stress-associated apoptosis (Nishitoh et al. 2002). The activation of JNK has been shown to be specifically elevated in the upper zone of human OA cartilage, compared to the normal cartilage (Clancy et al. 2001; Fan et al. 2007). Although our result was similar to previous reports, the expression of activated JNK (pJNK) showed only a weak correlation with chondrocyte apoptosis in OA cartilage, unlike CHOP expression. This finding may be because of the fact that JNK also has pleiotropic roles in the pathology of cartilage degeneration. For example, in addition to chondrocyte apoptosis, JNK is also involved in the chondrocyte synthesis of cartilage degradation enzymes, such as MMP-3, MMP-13 and ADAM-TS4 (Mengshol et al. 2000; Sylvester et al. 2004).
Under conditions of ER stress, accompanied by the upregulation of these apoptotic responses, cells induced specialized protective responses, such as the splicing of XBP1 mRNA. It has been suggested that the downregulation of IRE1 and XBP1 mRNA splicing, coupled with PERK signalling, leading to the enhanced expression of CHOP, may be responsible for driving cell death under conditions of ER stress (Lin et al. 2007). In the present study, the impact of tunicamycin on XBP1 mRNA splicing in cultured chondrocytes was reduced following intense stimulation resulting in extensive apoptosis, while the CHOP expression plateaued. These results indicated that the reduced protective ER stress responses, accompanied by enhanced apoptotic ER stress responses, played an important role in ER stress-associated apoptosis of human chondrocytes. Our observation that XBP1 mRNA splicing was reduced in severe OA cartilage, in contrast to the increased CHOP expression and apoptosis of chondrocytes, compared to moderate OA cartilage, which confirmed the initial protective role of the ER stress responses in chondrocytes.
Chondrocytes synthesize the components of articular cartilage, such as aggrecan and type II collagen, to maintain cartilage integrity, and are regulated by numerous factors such as proinflammatory cytokines and mechanical injury (Goldring et al. 1994; Dai et al. 2006). Therefore, chondrocyte dysfunction and apoptosis are crucial elements of the development and progression of OA. We have demonstrated that ER stress significantly suppressed the mRNA expression of aggrecan and type II collagen by cultured human chondrocytes. This result is consistent with previous report on rat chondrocytes (Yang et al. 2005). Similar to the present in vitro studies, in the overall OA cartilage of our study, the mRNA expression of aggrecan negatively correlated with the expression of ER stress-associated molecules, such as pPERK, Ub, CHOP and pJNK, and the severity of cartilage degeneration. However, the mRNA expression of type II collagen showed no correlation with these ER stress-associated molecules and cartilage degeneration. Furthermore, in our paired samples, although the chondrocytes expressing ER stress-associated molecules increased in the more degenerated cartilage, the mRNA expression of aggrecan and type II collagen was not significantly different between the more and less degenerated cartilage specimens from the same OA joint. Recently, Brew et al.(2010) also reported that there does not appear to be a significant relationship between cartilage degeneration and the mRNA expression of aggrecan and type II collagen in paired samples. Thus, we speculated that ER stress might have a weaker association with the expression of these ECM genes during the progression of cartilage degeneration. In this regard, however, the number of samples in the paired comparison of the present study was too small to evaluate the association between ECM gene expression, cartilage degeneration and ER stress. Additionally, it was previously reported that the expression of type II collagen and aggrecan significantly decreased in the upper zone of OA cartilage in comparison with normal cartilage and that the mRNA expression type II collagen, and to a lesser extent aggrecan, was found to increase in the deeper zone of OA cartilage (Aigner & Dudhia 1997; Aigner et al. 1997). These zonal differences in gene expression were presumed to influence the present analysis of full-thickness cartilage. Therefore, further studies that investigate a large number of cartilage samples and consider the zonal differences in cartilage are required to determine whether ER stress is associated with the regulation of the expression of these ECM genes during the progression of OA.
The main limitation of this study was the lack of analysis of normal cartilage from the tibial plateau of human subjects. Nugent et al. (2009) suggested that ER stress might occur in OA cartilage based on the immunohistochemical observation of increased expression of GRP78 in human degenerative cartilage from the tibial plateau compared to normal cartilage from the femoral condyle, femoral head and ankle. In our preliminary study, we immunohistochemically confirmed that GRP78 expression was higher in moderate and severe OA cartilage than in normal cartilage from the femoral head. Therefore, we presumed that ER stress was increased in moderately or severely degenerated cartilage in comparison with normal cartilage. However, Brew et al. (2010) reported that all cartilage in an OA joint was abnormal with regard to the expression of various genes, and thus, the assessment of normal cartilage is required to clarify the role of ER stress in cartilage degeneration. Another considerable limitation of this study was the lack of experiments using normal human chondrocytes, because normal human cartilage could not be obtained. Therefore, we were unable to determine whether normal human chondrocytes respond to ER stress in the same manner as chondrocytes from OA cartilage.
ER stress has been shown to participate in many disease pathologies, including Parkinson's disease, diabetes mellitus and arterial sclerosis (Malhotra & Kaufman 2007). Recently, it was demonstrated that ER stress is also responsible for other cartilage diseases, such as chodrodysplasia (Tsang et al. 2007; Rajpar et al. 2009). The present study revealed that ER stress was increased in cartilage during the progression of OA and might contribute to chondrocyte apoptosis, most likely as a result of an enhanced apoptotic response and a reduced protective response. In addition, ER stress could suppress ECM gene expression in cultured chondrocytes. These results suggested that ER stress has an important role in the pathology of cartilage degeneration. Further studies focused on ER stress will be beneficial to understanding the pathology of cartilage degeneration and to develop new treatments for patients with cartilage diseases.
Acknowledgments
The authors thank Drs Kensuke Yonemura, Tateki Segata (Kumamoto Saishunso National Hospital), Nobutake Nakane, Shuichiro Takahashi, Katsuhiko Kiyota, Tomoki Takahashi (Kumamoto Kinoh Hospital), Hiroaki Sakata, Suguru Oshima (Minamikumamoto Hospital), Koichi Kai (Inoue Orthopedic Hospital) and Toshihisa Anraku (Saiseikai Kumamoto Hospital) for providing the human cartilage specimens.
References
- Aigner T, Dudhia J. Phenotypic modulation of chondrocytes as a potential therapeutic target in osteoarthritis: a hypothesis. Ann. Rheum. Dis. 1997;56:287–291. doi: 10.1136/ard.56.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner T, Vornehm S, Zeiler G, Dudhia J, von der Mark K, Bayliss M. Suppression of cartilage matrix gene expression in upper zone chondrocytes of osteoarthritic cartilage. Arthritis Rheum. 1997;40:562–569. doi: 10.1002/art.1780400323. [DOI] [PubMed] [Google Scholar]
- Akerfeldt M, Howes J, Chan J, et al. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes. 2008;57:3034–3044. doi: 10.2337/db07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bek M, Bayer M, Müller B, et al. Expression and function of C/EBP homology protein (GADD153) in podocytes. Am. J. Pathol. 2006;168:20–32. doi: 10.2353/ajpath.2006.040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco F, Guitian R, Vázquez-Martul E, de Toro F, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Boot-Handford R, Briggs M. The unfolded protein response and its relevance to connective tissue diseases. Cell Tissue Res. 2010;339:197–211. doi: 10.1007/s00441-009-0877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew C, Clegg P, Boot-Handford R, Andrew J, Hardingham T. Gene expression in human chondrocytes in late osteoarthritis is changed in both fibrillated and intact cartilage without evidence of generalised chondrocyte hypertrophy. Ann. Rheum. Dis. 2010;69:234–240. doi: 10.1136/ard.2008.097139. [DOI] [PubMed] [Google Scholar]
- Clancy R, Rediske J, Koehne C, et al. Activation of stress-activated protein kinase in osteoarthritic cartilage: evidence for nitric oxide dependence. Osteoarthritis Cartilage. 2001;9:294–299. doi: 10.1053/joca.2000.0388. [DOI] [PubMed] [Google Scholar]
- Dai S, Shan Z, Nakamura H, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54:818–831. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- Dil Kuazi A, Kito K, Abe Y, Shin R, Kamitani T, Ueda N. NEDD8 protein is involved in ubiquitinated inclusion bodies. J. Pathol. 2003;199:259–266. doi: 10.1002/path.1283. [DOI] [PubMed] [Google Scholar]
- Fan Z, Söder S, Oehler S, Fundel K, Aigner T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am. J. Pathol. 2007;171:938–946. doi: 10.2353/ajpath.2007.061083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem. Biophys. Res. Commun. 2002;293:722–726. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- Goldring M, Fukuo K, Birkhead J, Dudek E, Sandell L. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J. Cell. Biochem. 1994;54:85–99. doi: 10.1002/jcb.240540110. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Terada K, Oyadomari S, Mori M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- Green D. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Guilak F, Fermor B, Keefe F, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin. Orthop. Relat. Res. 2004;423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- Hamamura K, Goldring M, Yokota H. Involvement of p38 MAPK in regulation of MMP13 mRNA in chondrocytes in response to surviving stress to endoplasmic reticulum. Arch. Oral Biol. 2009;54:279–286. doi: 10.1016/j.archoralbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Nakasa T, Hikata T, Asahara H. Molecular network of cartilage homeostasis and osteoarthritis. Med. Res. Rev. 2008;28:464–481. doi: 10.1002/med.20113. [DOI] [PubMed] [Google Scholar]
- Hirose J, Ryan L, Masuda I. Up-regulated expression of cartilage intermediate-layer protein and ANK in articular hyaline cartilage from patients with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 2002;46:3218–3229. doi: 10.1002/art.10632. [DOI] [PubMed] [Google Scholar]
- Hoozemans J, van Haastert E, Nijholt D, Rozemuller A, Eikelenboom P, Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am. J. Pathol. 2009;174:1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii A, Smith P, Darlington C. Application of real-time quantitative polymerase chain reaction to quantification of glutamate receptor gene expression in the vestibular brainstem and cerebellum. Brain Res. Brain Res. Protoc. 2002;9:77–83. doi: 10.1016/s1385-299x(01)00139-8. [DOI] [PubMed] [Google Scholar]
- Horton WJ, Bennion P, Yang L. Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J. Musculoskelet. Neuronal Interact. 2006;6:379–381. [PubMed] [Google Scholar]
- Kim H, Lee Y, Seong S, Choe K, Song Y. Apoptotic chondrocyte death in human osteoarthritis. J. Rheumatol. 2000;27:455–462. [PubMed] [Google Scholar]
- Kleemann R, Krocker D, Cedraro A, Tuischer J, Duda G. Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade) Osteoarthritis Cartilage. 2005;13:958–963. doi: 10.1016/j.joca.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kühn K, D'Lima D, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Li J, Wang H, May S, Song X, Fueyo J, Fuller G. Constitutive activation of c-Jun N-terminal kinase correlates with histologic grade and EGFR expression in diffuse gliomas. J. Neurooncol. 2008;88:11–17. doi: 10.1007/s11060-008-9529-1. [DOI] [PubMed] [Google Scholar]
- Lin J, Li H, Yasumura D, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiello L, Woodward J, Karpman R, Hammad T. In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin. Orthop. Relat. Res. 2000;381:229–240. doi: 10.1097/00003086-200012000-00027. [DOI] [PubMed] [Google Scholar]
- Malhotra J, Kaufman R. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin H. The response of articular cartilage to mechanical injury. J. Bone Joint Surg. Am. 1982;64:460–466. [PubMed] [Google Scholar]
- Mankin H, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 1971;53:523–537. [PubMed] [Google Scholar]
- McCullough K, Martindale J, Klotz L, Aw T, Holbrook N. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengshol J, Vincenti M, Coon C, Barchowsky A, Brinckerhoff C. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent A, Speicher D, Gradisar I, et al. Advanced osteoarthritis in humans is associated with altered collagen VI expression and upregulation of ER-stress markers Grp78 and bag-1. J. Histochem. Cytochem. 2009;57:923–931. doi: 10.1369/jhc.2009.953893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver B, Cronin C, Zhang-Benoit Y, Goldring M, Tanzer M. Divergent stress responses to IL-1beta, nitric oxide, and tunicamycin by chondrocytes. J. Cell. Physiol. 2005;204:45–50. doi: 10.1002/jcp.20261. [DOI] [PubMed] [Google Scholar]
- Rajpar M, McDermott B, Kung L, et al. Targeted induction of endoplasmic reticulum stress induces cartilage pathology. PLoS Genet. 2009;5:e1000691. doi: 10.1371/journal.pgen.1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Ruiz-Romero C, Carreira V, Rego I, Remeseiro S, López-Armada M, Blanco F. Proteomic analysis of human osteoarthritic chondrocytes reveals protein changes in stress and glycolysis. Proteomics. 2008;8:495–507. doi: 10.1002/pmic.200700249. [DOI] [PubMed] [Google Scholar]
- Sandell L, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkoda A, Ruiz P, Daniel H, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Sylvester J, Liacini A, Li W, Zafarullah M. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, -13 and aggrecanase-1 genes in articular chondrocytes. Cell. Signal. 2004;16:469–476. doi: 10.1016/j.cellsig.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Kikuta K, Sadamasa N, Nozaki K, Hashimoto N. Caspase-3-dependent apoptosis in middle cerebral arteries in patients with moyamoya disease. Neurosurgery. 2006;59:894–900. doi: 10.1227/01.NEU.0000232771.80339.15. discussion 900–891. [DOI] [PubMed] [Google Scholar]
- Tetlow L, Adlam D, Woolley D. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tsang K, Chan D, Cheslett D, et al. Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol. 2007;5:e44. doi: 10.1371/journal.pbio.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Nakamura T, Yao D, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- Yang L, Carlson S, McBurney D, Horton WJ. Multiple signals induce endoplasmic reticulum stress in both primary and immortalized chondrocytes resulting in loss of differentiation, impaired cell growth, and apoptosis. J. Biol. Chem. 2005;280:31156–31165. doi: 10.1074/jbc.M501069200. [DOI] [PubMed] [Google Scholar]
- Yang L, McBurney D, Tang S, Carlson S, Horton WJ. A novel role for Bcl-2 associated-athanogene-1 (Bag-1) in regulation of the endoplasmic reticulum stress response in mammalian chondrocytes. J. Cell. Biochem. 2007;102:786–800. doi: 10.1002/jcb.21328. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]


