Abstract
p23 is a cochaperone of heat shock protein 90 and also interacts functionally with numerous steroid receptors and kinases. However, the in vivo roles of p23 remain unclear. To explore its in vivo function, we generated the transgenic (TG) mice ubiquitously overexpressing p23. The p23 TG mice spontaneously developed kidney abnormalities closely resembling human hydronephrosis. Consistently, kidney functions deteriorate significantly in the p23 TG mice compared to their wild-type (WT) littermates. Furthermore, the expression of target genes for aryl hydrocarbon receptor (AhR), such as cytochrome P450, family 1, subfamily A, polypeptide 1 (Cyp1A1) and cytochrome P450, family 1, subfamily B, polypeptide 1 (Cyp1B1), were induced in the kidneys of the p23 TG mice. These results indicate that the overexpression of p23 contributes to the development of hydronephrosis through the upregulation of the AhR pathway in vivo.
Keywords: aryl hydrocarbon receptor, hydronephrosis, kidney, p23, TG mouse
p23 was first identified as a cochaperone of heat shock protein 90 (Hsp90) and has important roles in mediating various cellular signalling events, especially those triggered by nuclear hormone receptors (Hutchison et al. 1995; Johnson & Toft 1995; Freeman et al. 1996, 2000). The p23 protein is expressed ubiquitously and is highly conserved from yeast to human, and its deficiency is linked to perinatal lethality with retarded lung development in mice (Bohen 1998; Muñoz et al. 1999; Grad et al. 2006). Moreover, the Hsp90 chaperone complex affects a number of cellular targets and is upregulated in cancer cells; this heterocomplex consisting of Hsp90 and p23 is one of the potential targets for cancer therapy (Neckers et al. 1999). In vitro experiments further revealed that p23 regulates various cellular signal transduction pathways through the regulation of client proteins such as estrogen receptor, Fes tyrosine kinase, heat shock factor 1 (Hsf1), aryl hydrocarbon receptor and telomerase (Nair et al. 1996; Akalin et al. 2001).
Hydronephrosis, the pathological condition where there is dilation of the kidney caused by abnormal urine discharge, often results in irreversible parenchymal damage leading to severe renal failure and death (Gulmi et al. 2002; Kumar et al. 2005). It is a common and serious disease in human, occurring approximately 1 in 100 live births (Belarmino & Kogan 2006). Genetic studies suggest that abnormalities of kidney functions including injury to the electrolyte transporter, the water channel and pump, the renin–angiotensin system and renal cell differentiation and proliferation, can all induce hydronephrosis (McDill et al. 2006). In rodent models, treatment with 2,3,7,8-tetrachlorodibenzo-(p)-dioxin (TCDD) is known to induce hydronephrosis through the activation of aryl hydrocarbon receptor (AhR) signalling, leading to progressive atrophy of the kidney (Abbott et al. 1987; Mimura et al. 1997).
Although recent studies indicate that p23 is important for the potency and efficacy of ligand-induced AhR signalling (Kazlauskas et al. 1999, 2001; Marc et al. 2002; Cox & Miller 2004), the in vivo functions of p23 remained to be elucidated. We hypothesized that the dysregulation of p23 may induce hydronephrosis through the activation of the AhR signalling pathway. In this study, therefore we used TG mice overexpressing p23 as our model to test whether p23 overexpression promotes the hydronephrotic kidney via the AhR signalling.
Methods
Animals and genotyping
To generate p23 TG mice, we inserted the cDNA encoding hemagglutinin (HA)-tagged p23 downstream of the chicken (Ck) β-actin promoter and cytomegalovirus (CMV)-IE enhancer of the pCAGGS mammalian expression vector (Figure 1a). The plasmid was linearized with restriction enzymes SalI and NotI and then microinjected into the fertilized mouse eggs of a FVB/N mouse strain (Taconic Farms, Germantown, NY, USA). The genotype of the mice was determined by PCR, and gene expression was confirmed by Western blot and immunohistochemical analyses. The primers designed from the TG construct (Figure 1a) for genotyping were 5′-CGCCACCATGCAGCCTGCTTCTGCAAAG-3′ (forward for HA-p23), 5′-GTATTTGTGAGCCAGCGC ATTG-3′ (reverse for HA-p23), 5′-GCTGTGTATACATC TACCACT-3′ (forward for endogenous p23) and 5′-AAGGTGTTAGTCATCCCAGAC-3′ (reverse for endogenous p23). All mice were housed in groups in the specific pathogen-free (SPF) facility of the Laboratory Animal Research Center (LARC) in Yonsei University, which is operated in accordance with the Institutional Animal Care and Use Committee (IACUC) and international guidelines on the ethical use of animals. All animals were maintained on a 12:12-h light:dark cycle with access to food and water ad libitum.
Figure 1.
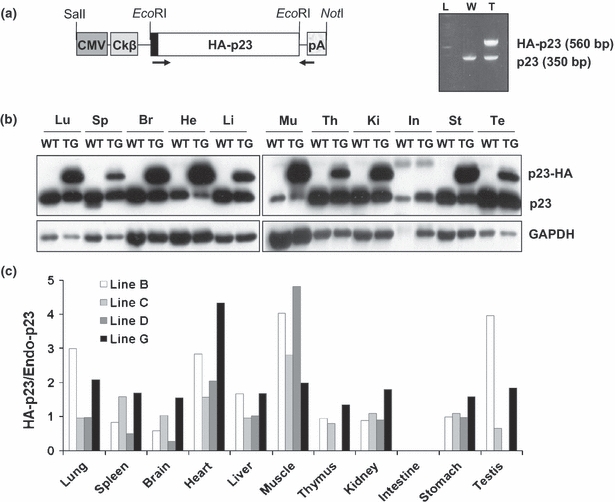
Generation of mice overexpressing p23. (a) Schematic representation of the p23 transgenic (TG) construct and a result of PCR genotyping are shown. The positions of restriction sites for cloning and linearization are indicated. Primers used for genotyping are represented by black arrows. Cytomegalovirus (CMV); CMV-IE enhancer; Ckβ, chicken β-actin promoter; pA, poly A signal. (b) Western blot analysis with p23 antibody shows a representative expression of endogenous and transgenic p23 in mouse tissues (p23 TG line G). Proteins were isolated from the lung (Lu), spleen (Sp), brain (Br), heart (He), liver (Li), muscle (Mu), thymus (Th), kidney (Ki), intestine (In), stomach (St) and testis (Te) of WT and p23 transgenic (TG) mice. GAPDH is used as a loading control. The representative data shown were obtained from the line G mice. (c) The p23 transgene expression patterns of four TG lines (lines B, C, D and G) were compared in various organs as indicated. The relative expression of transgene hemagglutinin (HA)-p23 was normalized to endogenous p23.
Ink injection experiment
To observe possible anatomical obstructions of the urinary tract under anaesthesia, dark blue ink was injected into the pyelocaliceal space of mice using a 30-gauge needle as described previously (Nishimura et al. 2008). All photographs were taken at the same magnification immediately after ink injection.
Microcomputed tomography imaging
Mice were anaesthetized by intraperitoneal (i.p.) injection of avertin solution. Computed tomography (CT) images were obtained with 16-section CT scanners (Somatom Sensation 16; Siemens Healthcare, Marburg, Germany). After obtaining unenhanced CT images, 1 ml of iopromide (Ultravist 370; Schering, Berlin, Germany) was administered via the tail vein and 15-min delayed images were obtained. All scanned images were sent to the Picture Archiving and Communication System (PACS; GE Healthcare, Freiburg, Germany) and reconstructed.
Haematoxylin and eosin (H&E) staining and immunohistochemistry
Kidneys were fixed in 10% formalin (Sigma, St. Louis, MO, USA), embedded in paraffin and sectioned at 4-μm intervals. For histological examination, the sections were stained with H&E as described previously (Cheong et al. 2006). To perform the immunohistochemical study, the paraffin sections were incubated with 0.3% H2O2 for 30 min to eliminate endogenous peroxidases, followed by several PBS rinses. The sections were blocked by normal goat serum for 20 min and incubated in a humid chamber with primary antibody against HA (Rockland, Gilbertsville, PA, USA) for 12 h at 4°C and then for 1 h with secondary antibody (biotinylated anti-rabbit antibody, Vector Laboratories, Burlingame, CA, USA). To amplify signals, the sections were incubated with an avidin–biotin complex (ABC, Vector Laboratories) and tissues were visualized by DAB (Vector Laboratories).
Blood urea nitrogen and creatinine test
Serum was obtained from wild-type (WT) and p23 TG mice at 9 months of age (males, n = 17 for WT and n = 18 for TG; females, n = 7 for WT and n = 10 for TG). Analysis of blood urea nitrogen (BUN) and creatinine concentration was performed in the Eone Reference Laboratory (http://www.eonelab.co.kr, Republic of Korea).
Reverse transcription (RT)-PCR
Kidney mRNA was isolated using 1 ml of Trizol solution according to manufacturer's procedures (Invitrogen, Carlsbad, CA, USA). Reverse transcription (RT) was performed with Superscript III (Invitrogen) for 60 min at 37 °C using an oligo-dT primer. All RT reactions were performed using 1 μg mRNA. The PCR cycling conditions were 94 °C for 2 min, followed by 30 cycles of 94 °C for 20 s, 55–60 °C for 30 s, 72 °C for 30 s. All RT reactions and PCRs were performed on a MyCycler™ Themal Cycler (Bio-Rad, Hercules, CA, USA). All signals were normalized to Gapdh. The primer sequences are listed in Table S2.
Western blot analysis
Whole cell lysates were obtained from each organ using CHAPs buffer [30 mM Tris–Cl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% CHAPS, 1 mM PMSF and proteinase inhibitor cocktail (Calbiochem)], and Western blot analyses were performed using antibodies against p23 (originally from Dr. Toft; Becton Dickinson, Cockeysville, MD, USA), cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) (Santa Cruz, Santa Cruz, CA, USA), cytochrome P450, family 1, subfamily B, polypeptide 1 (CYP1B1) (Lifespan Science, Seattle, WA, USA), cyclooxygenase-2 (COX-2) (BD Bioscience), β-actin (Sigma) and GAPDH (Labfrontier, Seoul, Republic of Korea).
Results
p23 TG mice develop spontaneous hydronephrosis
To investigate the in vivo effect of p23 overexpression, we designed a TG mouse strategy ubiquitously overexpressing HA-p23 (Figure 1a). Four independent TG lines were established, and their transgene expression in various tissues was examined by Western blot (Figure 1b, c). Marked expression of HA-p23 protein was ubiquitously detected from all TG mouse lines with variable degrees (Figure 1b, c). All TG mice were always maintained the hemizygous for p23 transgene and did not show developmental defects and reproductive problems. The transgenic mouse lines C and G showing relatively higher renal expression of HA-p23 than others were used for the subsequent experiments. In addition to the kidney, HA-p23 was highly expressed in ureter and bladder (Figure S1). Even though the p23 transgene was highly expressed, the phenotypic manifestation was not clear at an early age except for slight loss of body weight (data not shown). However, the adult p23 TG mice frequently showed spontaneous distension and dilatation of the renal pelvis and calyces (Table 1 and Figure 2a), closely resembling the pathological conditions of human hydronephrosis. The expanded pelvic region in the kidney had been filled with the urine. In its extreme case, the kidneys of p23 TG mice showed a fluid-filled cavity (perinephric pseudocyst) between the renal capsule and remnant kidney, as well as the dilated renal pelvis (Figure 2b). These mice showed no aberrant blood vessels and the dilated ureter connected correctly to the lower pole of the kidney (Figure 2b). There was no evidence of kidney stone or mass inducing ureteral obstruction in the urinary tract (data not shown). However, the pathological condition progressively deteriorate with age, and the incidence was higher in the males (90.6%) than in the females (20%, Table 1). The incidence and symptoms were more severe in p23 TG mice of line G than in those of line C, possibly reflecting the higher level of transgene expression in the line G than in the line C (Figure 1c and Table S1). Although total p23 expression in the kidney was the same in both sexes (Figure S1), the female TG mice were resistant to hydronephrosis (Tables 1 and S1), which is similar to human disease influenced by oestrogen (Wright & Lacy 1988; Weide & Lacy 1991; Beischlag & Perdew 2005). These results indicate that overexpression of p23 spontaneously induces hydronephrosis, and that both prevalence and severity are progressively increased with age.
Table 1.
Incidence of spontaneous hydronephrosis in WT and p23 TG mice (Line G). Similar to the epidemiological conditions observed in human hydronephrosis
| WT | TG | |||||||
|---|---|---|---|---|---|---|---|---|
| Month | 1–3 | 4–8 | 9–22 | Total | 1–3 | 4–8 | 9–22 | Total |
| Male | 0/2 | 0/9 | 0/19 | 0/30 | 1/2 | 9/10 | 19/20 | 29/32 |
| Female | 0/5 | 0/4 | 0/9 | 0/18 | 1/3 | 0/7 | 4/15 | 5/25 |
WT, wild type; TG, transgenic.
Figure 2.
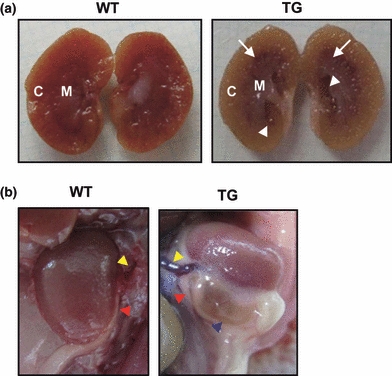
Transgenic (TG) mice overexpressing p23 exhibit hydronephrosis. (a) Kidney of p23 TG mice showed distension and dilatation of renal pelvis (white arrowheads) and calyces (white arrows). (b) p23 TG mice exhibited swollen kidneys with perinephric pseudocysts (blue arrowhead). yellow arrowhead, blood vessel; red arrowhead, ureter.
Histological analysis of hydronephrosis
As hydronephrosis leads to progressive atrophy of the kidney, the hydronephrotic kidneys of p23 TG mice were histologically analysed. Conspicuous dilatation of the pelvis and calyces was observed, and the renal parenchyma in the medullary region was thinner compared with that of WT mice (Figure 3a). However, there were no apparent morphological changes in the cortex, the outer part of the kidney containing the glomerulus, proximal and distal convoluted tubules (Figure 3a). The glomerulus and renal tubules of these regions were histologically intact (Figure 3b), even though prominent HA-p23 expression was immunohistochemically detected in the glomerulus and distal convoluted tubules (Figure 3c). Expression pattern of the HA-p23 is similar to that of endogenous p23 in the kidney of mice (Figures 3c and S2). These results indicate that hydronephrosis in p23 TG mice can result from the functional processes interrupting the flow of the urine.
Figure 3.
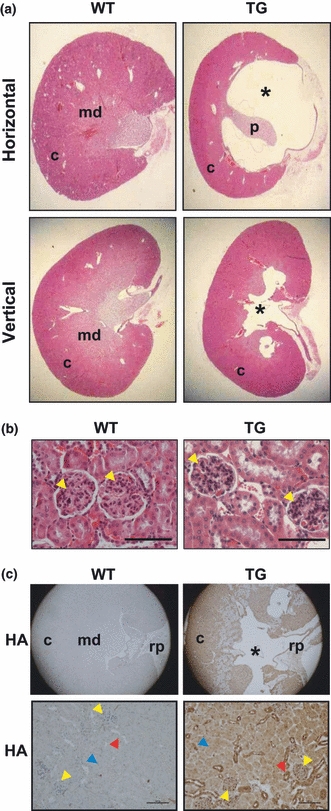
Histological analysis of hydronephrotic kidney of p23 transgenic (TG) mice. (a) Normal and hydronephrotic kidney sections from WT and p23 TG mice. The renal pelvis was grossly dilated in p23 TG mice. *The dilatation regions of the renal pelvis in hydronephrotic kidney of p23 TG mice. (b) Kidney sections from 10-month-old WT and p23 TG mice were stained with H&E. The cortex regions from normal and hydronephrotic kidneys of WT and p23 TG mice, respectively, are shown. Detailed images of sections from WT and hydronephrotic p23 TG kidney do not show detectable structural changes in the glomeruli (yellow arrowheads). (c) Immunohistochemical staining using an anti-HA antibody showing representative expression of the p23 protein in the kidney (Scale bar = 200 μm). yellow arrows, glomeruli; red arrows, distal convoluted tubules; blue arrows, proximal tubules; c, cortex; md, medulla; rp, renal pelvis; p, papilla.
Functional analysis of hydronephrosis
Because obstruction of the free urine flow from the kidney to the bladder usually causes hydronephrosis (McDill et al. 2006), dark blue ink was infused into the pyelocaliceal space of the kidney to diagnose the possible anatomical obstructions of the urinary track. The injected ink ran to the bladder and made the ureter visible in WT mice (Figure 4a). However, the ureter of p23 TG mice displayed the thinning of urethral lining and stenosis of the ureter with dilation and/or distortion (Figure 4a). These results were confirmed by microcomputed tomography using a conventional extracellular contrast agent, iopromide that is readily eliminated by functionally normal glomerular filtration (Hackstein et al. 2004). In WT mice, the contrast agent was detected in the renal pelvis 30 s after contrast injection and clearly disappeared 180 s after contrast injection (Figure 4b, a–c). Although the contrast agent similarly appeared 30 s after contrast injection in the p23 TG mouse, the renal pelvis revealed much brighter signal, indicating concentration of the contrast agent and distension of the renal pelvis (Figure 4b,e). In contrast to WT mice, the strong signal was still detected 600 s after contrast injection in the p23 TG mice (Figure 4b,f). These results suggest that the defect in the urine flow elevating the internal pressure on the kidney may cause the distention of kidney, hydronephrosis, in p23 TG mice.
Figure 4.
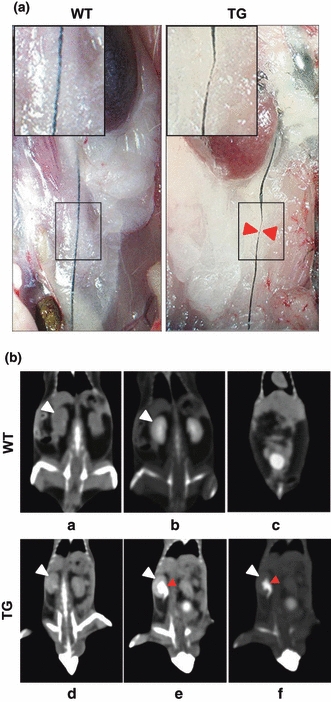
Histological analysis of kidney and dynamic contrast computed tomography (CT) in WT (a–c) and p23 transgenic (TG) (d–f) mice. (a) Representative images after ink injection into the pyelocaliceal space of kidney. Higher magnification images of the ureter are shown as insets. (b) Precontrast image (a, d). Coronal CT image 30 s after contrast injection via tail vein showed collection of the contrast dye in the renal pelvis without significant dilatation (b). At 180 s after contrast injection, most of the contrast dye was washed out in the renal pelvis and filled in the bladder (c). Coronal CT images 30 s after contrast injection showed dye accumulation along the marked dilated renal pelvis (red arrowheads) (e). CT image 600 sec after contrast injection showed that the contrast dye still remained in the renal pelvis (f). Contrast emptying in the bladder was incomplete even though it occurred over a prolonged time. White arrowhead, kidney.
As hydronephrosis frequently causes progressive atrophy of the kidney and irreversible parenchymal damage associated with severe renal failure (Gulmi et al. 2002; Kumar et al. 2005), we evaluated whether p23 transgene expression results in renal failure by measuring the BUN and serum creatinine levels as markers of kidney function (Gagnon & Duguid 1983; McDill et al. 2006). The levels of BUN (P < 0.015) and serum creatinine (P < 0.016) were significantly increased in male p23 TG mice, but not in females, compared with those of their WT littermate controls (Figure 5), further supporting its similarity to the gender disparity observed in human hydronephrosis. Therefore, the hydronephrosis of p23 TG mice is potentially harmful to normal kidney function.
Figure 5.
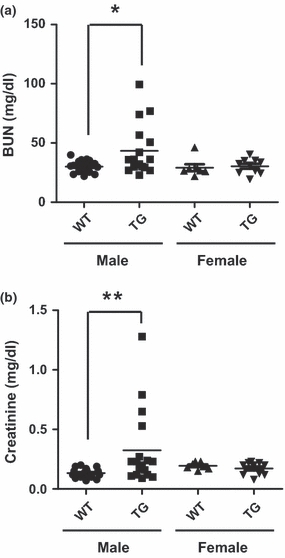
Hydronephrotic kidneys of p23 transgenic (TG) mice show renal failure. Blood urea nitrogen (BUN) and creatinine levels in serum from WT and p23 TG mice are shown. (a) BUN level in male p23 TG mice (43 ± 21 mg/dl, n = 18) was significantly higher than that of controls (30 ± 5 mg/dl, n = 17). This difference was not observed in female mice. (b) The creatinine level in male p23 TG mice (0.32 ± 0.31 mg/dl, n = 18) was markedly higher than that of controls (0.13 ± 0.03 mg/dl, n = 17). This difference was not observed in female mice. *P < 0.015; **P < 0.016.
Altered gene expression in hydronephrotic kidneys of p23 TG mice
A number of genes are involved in generating hydronephrosis in mice. Hydronephrosis is observed in mice lacking aquaporin 2 (Aqp2; McDill et al. 2006), inhibitor of DNA binding 2 (Id2; Aoki et al. 2004), arginine vasopressin receptor 2 (Avpr2; Yun et al. 2000) and angiotensin type 2 receptor (Agtr2; Nishimura et al. 1999). In addition, TCDD-induced hydronephrosis mouse models suggested the importance of AhR and its well-known target genes, cyclooxygenase-2 (Cox-2), Cyp1A1 and cytochrome P450, family 1, subfamily B, polypeptide 1 (Cyp1B1) in hydronephrosis (Nishimura et al. 1999; Nebert et al. 2004). The expression of these markers was assessed in the kidneys from WT and p23 TG mice. While expressions of Avpr2 and Id-2 were not changed, Aqp2 transcript was unexpectedly increased (Figure 6a). The level of Cyp1B1 mRNA was upregulated in the hydronephrotic kidneys of p23 TG mice compared with those of WT mice (Figure 6a). As Cyp1B1 is a well-known AhR target gene and its activation is implicated in TCDD-induced hydronephrosis, we examined the expression of AhR target genes including Cyp1A1, Cyp1B1 and Cox-2 by Western blot analyses. As these expressions were significantly upregulated in p23-TG kidneys (Figures 6b and S3B), hydronephrosis in the p23-TG kidneys may require AhR signalling. Taken together, the overexpression of p23 in vivo contributes to the development of hydronephrosis by regulating AhR activity and its target genes including Cyp1A1, Cyp1B1 and Cox-2. Moreover, this result shows significant correlations between the expression of Cyp1a1 and Cox-2 among each individual p23 TG mouse.
Figure 6.
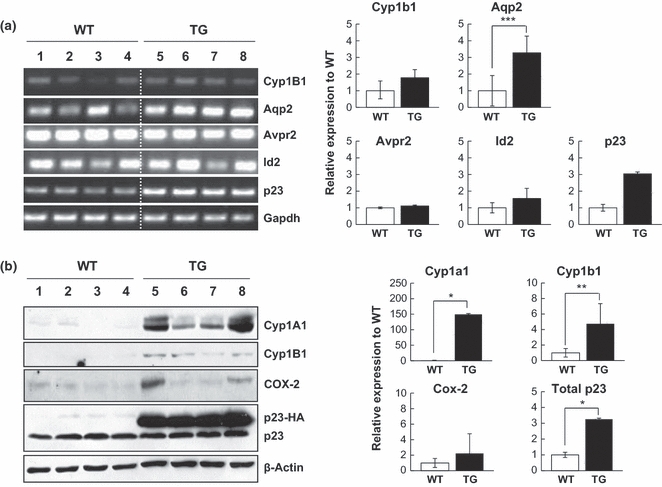
Altered expression of hydronephrosis-related genes in kidneys of WT and p23 transgenic (TG) mice at 9 months of age. (a) mRNA transcripts of Cyp1B1, Aqp2, Avpr2, Id-2 and p23 in kidneys from WT (lanes 1–4) and p23 TG (lanes 5–8) mice were quantified by RT-PCR. Each lane represents transcripts from an individual mouse. The transcript level was normalized to Gapdh values. A representative graph is shown (WT, n = 4; TG, n = 4). (b) The protein expression of cytochrome P450, family 1, subfamily A, polypeptide 1 (Cyp1A1), cytochrome P450, family 1, subfamily B, polypeptide 1 (Cyp1B1) and cyclooxygenase-2 (Cox-2) in kidneys from WT (lanes 1–4) and p23 TG (lanes 5–8) mice was analysed by Western blotting using the indicated antibodies. The expression level was normalized to β-actin values. A representative graph is shown (WT, n = 4; TG, n = 4). *P < 0.01; **P = 0.0117; ***P = 0.0131.
Discussion
Here, we have shown that two independent lines of p23 TG mice develop spontaneous hydronephrosis. Our results show that the prevalence and severity of hydronephrosis in p23 TG mice leads to progressive deterioration with age. Previous mouse models have shown that hydronephrosis is caused by mutations in the genes involved in normal kidney homoeostasis. However, there have been no reports linking p23 to the development of hydronephrosis. For example, the mice deficient for arginine vasopressin receptor 2 (Avpr2) or angiotensin type 2 receptor (Agtr2), which belongs to the subfamily of G protein-coupled receptors in the renin–angiotensin system, develop hydronephrosis, hypoplastic and multicystic kidneys and/or megaureter (Nishimura et al. 1999). The mice deficient for the integral membrane protein aquaporin 2 (Aqp2), which forms pores in the membrane and plays a vasopressin (VP)-regulated role in water reabsorption by the kidney, have been reported to develop hydronephrosis because of the obstruction in the ureteropelvic junction (McDill et al. 2006). The mice deficient for NaK2Cl cotransporter (NKCC2) (Takahashi et al. 2000) or renal outer medullary potassium channel (ROMK) (Lorenz et al. 2002; Lu et al. 2002) were shown to have the symptoms of polyuria and electrolyte imbalance, resulting in hydronephrosis of varying severities. In addition, the xenobiotic chemical TCDD has been used to induce hydronephrosis in rodents, and it upregulates Cox-2 and prostaglandin E2 (PGE2) in an AhR-dependent manner (Abbott et al. 1987; Mimura et al. 1997; Nishimura et al. 2008). These lines of evidence suggest that the disruption of normal kidney function contributes to the induction of hydronephrosis; however, the evidence regarding hydronephrosis including the effect of p23 remained equivocal.
Here, we have shown that p23 overexpression can induce spontaneous hydronephrosis. AhR target genes, Cyp1A1, Cyp1B1 and Cox-2, were upregulated in the kidneys of p23 TG mice (Figures 6 and S3), consistent with the TCDD-induced hydronephrosis model. Although the exact molecular mechanism is unknown, these evidences suggest that AhR activation is required for the induction of hydronephrosis in the kidneys of p23 TG mice. In addition, p23 is crucial for PGE2 production (Grad et al. 2006) and efficient AhR signalling through the stabilization of AhR (Kazlauskas et al. 1999, 2001; Marc et al. 2002; Cox & Miller 2004). Therefore, the molecular mechanism underlying the phenotype of p23 TG mice appears to be linked to AhR activation or to increase the sensitivity of AhR binding to unknown endogenous ligands (Seidel et al. 2001). As the expression of AhR target genes is known to be repressed by oestrogen signalling (Beischlag & Perdew 2005), the resistance of p23 TG females to hydronephrosis is expected to be a potential output of the estrogen signalling.
Hydronephrosis is induced not only by anatomical obstruction in the ureter, but also by abnormal peristaltic movement of the ureter (Aoki et al. 2004; Zeidel & Pirtskhalaishvili 2004; Mahoney et al. 2006). For example, hydronephrosis is caused by the deficiency of peristaltic contractions of the smooth muscle cells surrounding the ureter (Zeidel & Pirtskhalaishvili 2004). Furthermore, prostaglandins affect the smooth muscle contractility of the upper urinary tract (Cole et al. 1998), and TCDD-exposed hydronephrotic mice showed an increased production of PGE2 (Nishimura et al. 2008). Although further study is still required, the present findings suggest that p23 overexpression can lead to hydronephrosis in anatomical obstruction and/or abnormal peristaltic movement of the ureter. In addition, fibrotic lesions associated with hydronephrosis are considered to be caused secondarily by the severe lesions seen in the kidney (Yao et al. 2010). The absence of fibrotic lesions in the p23 TG kidneys (data not shown) indicates that p23 overexpression may not induce the type of severe lesions which result in renal fibrosis.
Apart from the individual genotypic differences, the genetic background can be an important factor affecting the incidence of hydronephrosis. Spontaneous hydronephrosis was observed in >60% of male C57BL/KsJ mice by 15 weeks of age (Wright & Lacy 1988; Weide & Lacy 1991) and in 6% of male and 9% of female C57BL/6 mice (Zurcher et al. 1982). In the present study, p23 TG mice were generated and maintained in a FVB/NJ genetic background, and the hydronephrotic kidneys were not found in WT littermates of this background (Table 1). Therefore, our mouse model also provides the first evidence for the effect of p23 overexpression in vivo on the development of congenital and spontaneous hydronephrosis.
Taken together, it is likely that p23 is important for the normal kidney function and that p23 overexpression may induce interruption in the urine flow through the AhR signalling events, finally leading to hydronephrosis and loss of kidney function that is reflected by the elevated BUN and creatinine levels in the blood. Although more detailed studies are required to elucidate the exact mechanism governing these phenomena, our TG mouse model will provide increased comprehensions of the role of p23 in the normal kidney function and will also provide insights into the diagnosis and therapy of congenital hydronephrosis in the future.
Acknowledgments
We greatly appreciate the gift of p23 antibody from Dr. David Toft (Department of Biochemistry and Molecular Biology, Mayo Clinic College of Medicine, Rochester, USA). The work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MEST) (2009-0083365, 2009-0081177, 2008-2005805, 2009K001284) and National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs (MoHWFA), Republic of Korea (0520220).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. The p23 transgene expression patterns in male and female mice. Western blot analysis showed the representative expression of endogenous and transgenic p23 in various organs as indicated. Proteins were isolated from indicated organs of p23 TG and WT male and female mice. GAPDH was used as a loading control. The representative data were obtained from the line G mice. M, male; F, female.
Figure S2. Expression patterns of endogenous p23 in the kidney. Immunohistochemical staining using an anti-p23 antibody showed the representative expression of endogenous p23 protein in the kidney of WT mice (scale bar = 200 µm).
Figure S3. Altered expression of hydronephrosis-related genes in the kidneys of TG line C mice. (A) mRNA transcripts of Aqp2, Avpr2, ROMK, NKCC2 and p23 in the kidneys from WT (lanes 1–4) and p23 TG (lanes 5–8) mice were quantified by RT-PCR. The transcript level was normalized to Gapdh. (B) The protein expression of Cyp1A1, Cyp1B1 and Cox-2 in the kidneys from WT (lanes 1–4) and p23 TG (lanes 5–8) mice was analysed by Western blotting using the indicated antibodies. The expression level was normalized to β-actin.
Table S1. Incidence of spontaneous hydronephrosis in WT and p23 TG mouse (line C). Similar to the epidemiological conditions observed in human hydronephrosis.
Table S2. Primer pairs used for RT-PCR analysis.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abbott BD, Birnbaum LS, Pratt RM. TCDD-induced hyperplasia of the ureteral epithelium produces hydronephrosis in murine fetuses. Teratology. 1987;35:329–334. doi: 10.1002/tera.1420350307. [DOI] [PubMed] [Google Scholar]
- Akalin A, Elmore LW, Forsythe HL, et al. Holt a novel mechanism for chaperone-mediated telomerase regulation during prostate cancer progression. Cancer Res. 2001;61:4791–4796. [PubMed] [Google Scholar]
- Aoki Y, Mori S, Kitajima K, et al. Id2 haploinsufficiency in mice leads to congenital hydronephrosis resembling that in humans. Genes Cells. 2004;9:1287–1296. doi: 10.1111/j.1365-2443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Beischlag TV, Perdew GH. ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J. Biol. Chem. 2005;280:21607–21611. doi: 10.1074/jbc.C500090200. [DOI] [PubMed] [Google Scholar]
- Belarmino JM, Kogan BA. Management of neonatal hydronephrosis. Early Hum. Dev. 2006;82:9–14. doi: 10.1016/j.earlhumdev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Bohen SP. Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of Hsp90-dependent signaling proteins. Mol. Cell. Biol. 1998;18:3330–3339. doi: 10.1128/mcb.18.6.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C, Sung YH, Lee J, et al. Role of INK4a locus in normal eye development and cataract genesis. Mech. Ageing Dev. 2006;127:633–638. doi: 10.1016/j.mad.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Cole RS, Fry CH, Shuttleworth KED. The action of the prostaglandins on isolated human ureteric smooth muscle. British J Urol. 1998;61:19–26. doi: 10.1111/j.1464-410x.1988.tb09155.x. [DOI] [PubMed] [Google Scholar]
- Cox MB, Miller CA., III Cooperation of heat shock protein 90 and p23 in aryl hydrocarbon receptor signaling. Cell Stress Chaperones. 2004;9:4–20. doi: 10.1379/460.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cylophilin Cyp-40 and the steroid aporeceptor-associated protein p 23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Felts SJ, Toft DO, Yamamoto KR. The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev. 2000;14:422–434. [PMC free article] [PubMed] [Google Scholar]
- Gagnon RF, Duguid WP. A reproducible model for chronic renal failure in the mouse. Urol Res. 1983;11:1–48. doi: 10.1007/BF00272702. [DOI] [PubMed] [Google Scholar]
- Grad I, McKee TA, Ludwig SM, et al. The Hsp90 cochaperone p23 is essential for perinatal survival. Mol. Cell. Biol. 2006;26:8976–8983. doi: 10.1128/MCB.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulmi FA, Felsen D, Vaughan ED. Pathophysiology of urinary tract obstruction in Campbell's Urology. Philadelphia, PA: Elsevier Saunders; 2002. [Google Scholar]
- Hackstein N, Wiegand C, Rau WS, Langheinrich AC. Glomerular filtration rate measured by using triphasic helical CT with a two-point Patlak plot technique. Radiology. 2004;230:221–226. doi: 10.1148/radiol.2301021266. [DOI] [PubMed] [Google Scholar]
- Hutchison KA, Stancato LF, Owens-Grillo JK, et al. The 23-kDa acidic protein in reticulocyte lysate is the weakly bound component of the HSP foldosome that is required for assembly of the glucocorticoid receptor into a functional heterocomplex with Hsp90. J. Biol. Chem. 1995;270:18841–18847. doi: 10.1074/jbc.270.32.18841. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Toft DO. Binding of p23 and Hsp90 during assembly with the progesterone receptor. Mol. Endocrinol. 1995;9:670–678. doi: 10.1210/mend.9.6.8592513. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. J. Biol. Chem. 1999;274:13519–13524. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Sundstrom S, Poellinger L, Pongratz I. The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol. Cell. Biol. 2001;21:2594–2607. doi: 10.1128/MCB.21.7.2594-2607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Fausto N, Abbas A. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier Saunders; 2005. [Google Scholar]
- Lorenz JN, Baird NR, Judd LM, et al. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter's syndrome. J. Biol. Chem. 2002;277:37871–37880. doi: 10.1074/jbc.M205627200. [DOI] [PubMed] [Google Scholar]
- Lu M, Wang T, Yan Q, et al. Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter's) knockout mice. J. Biol. Chem. 2002;277:37881–37887. doi: 10.1074/jbc.M206644200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney ZX, Sammut B, Xavier RJ, et al. Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proc. Natl Acad. Sci. USA. 2006;103:19872–19877. doi: 10.1073/pnas.0609326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc BC, Charles A, Miller CA., III The p23 co-chaperone facilitates dioxin receptor signaling in a yeast model system. Toxicol. Lett. 2002;129:13–21. doi: 10.1016/s0378-4274(01)00465-9. [DOI] [PubMed] [Google Scholar]
- McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc. Natl Acad. Sci. USA. 2006;103:6952–6957. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- Muñoz MJ, Bejarano ER, Daga RR, Jimenez J. The identification of Wos2, a p23 homologue that interacts with Wee1 and Cdc2 in the mitotic control of fission yeasts. Genetics. 1999;153:1561–1572. doi: 10.1093/genetics/153.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SC, Toran EJ, Rimerman RA, Hjermstad S, Smithgal TE, Smith DF. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Neckers L, Mimnaugh E, Schulte TW. Hsp90 as an anti-cancer target. Drug Resist. Updates. 1999;2:165–172. doi: 10.1054/drup.1999.0082. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Yerkes E, Hohenfellner K, et al. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol. Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Matsumura F, Vogel CF, et al. Critical role of cyclooxygenase-2 activation in pathogenesis of hydronephrosis caused by lactational exposure of mice to dioxin. Toxicol. Appl. Pharmacol. 2008;231:374–383. doi: 10.1016/j.taap.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Seidel SD, Winters GM, Rogers WJ, et al. Activation of the Ah receptor signaling pathway by prostaglandins. J. Biochem. Mol. Toxicol. 2001;15:187–196. doi: 10.1002/jbt.16. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc. Natl Acad. Sci. USA. 2000;97:5434–5439. doi: 10.1073/pnas.090091297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide LG, Lacy PE. Hereditary hydronephrosis in C57BL/KsJ mice. Lab. Anim. Sci. 1991;41:415–418. [PubMed] [Google Scholar]
- Wright JR, Lacy PE. Spontaneous hydronephrosis in C57BL/KsJ mice. J. Comp. Pathol. 1988;99:449–454. doi: 10.1016/0021-9975(88)90063-1. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhang J, Ye DF, et al. Left-right determination factor is down-regulated in fibrotic renal tissue of human hydronephrosis. BJU International. 2010 doi: 10.1111/j.1464-410X.2010.09520.x. DOI: 10.1111/j.1464-410X.2010.09520.x. [DOI] [PubMed] [Google Scholar]
- Yun J, Schöneberg T, Liu J, et al. Generation and phenotype of mice harboring a nonsense mutation in the V2 vasopressin receptor gene. J. Clin. Invest. 2000;106:1361–1371. doi: 10.1172/JCI9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel ML, Pirtskhalaishvili G. Urinary tract obstruction. In: Brenner BM, editor. Brenner and Rector's The Kidney. Philadelphia: Saunders; 2004. pp. 1867–1894. [Google Scholar]
- Zurcher C, Van Zwieten MJ, Solleveld HA, Hollander CF. Aging research in the mouse in biomedical research: volume IV. In: Foster HL, Small JD, Fox JG, editors. Experimental Biology and Oncology. New York, NY: Academic Press; 1982. pp. 11–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


