Abstract
The aim of this study was evaluate the effect of prolonged use of high dose of tibolone on the vagina of ovariectomized rats. Bilateral ovariectomy was performed on 14 rats weighing 250 g. Thirty days later, vaginal smears were collected verifying the menopause status by anoestrus cytology. Rats were divided randomly into groups: experimental rats (n = 9) received 1 mg tibolone/day orally and control rats (n = 6) received placebo (carboxymethylcellulose). After 150 days, all rats were sedated and euthanized by cervical displacement. The vagina was removed, fixed in 10% buffered formalin, sampled and processed for paraffin embedding. Histological sections were stained with haematoxylin and eosin, picrosirius red, periodic acid Schiff (PAS) and PAS-diastase, and Weigert's resorcin–fuchsin. Cell proliferation was analysed by immunohistochemistry to detect Ki67. Histomorphometric analyses were performed for epithelial thickness, per cent area of collagen fibres and blood vessels, mast cells and Ki67-positive nuclei per mm of basal membrane. Means and standard error of means were calculated, and data were compared using the Mann–Whitney test, with significance level at P < 0.05. In the vagina, epithelial thickness, number of Ki67-positive nuclei per mm of basal membrane, number of vessels and number of mast cells were significantly higher in the tibolone group when compared with the control group. Furthermore, the content of glycogen and glycoproteins in the vaginal epithelium was modified by tibolone. Tibolone administered in high dose and for a long period has a trophic effect, reversing vaginal atrophy, and has no dysplastic or neoplastic effect in the vagina of ovariectomized rats.
Keywords: Ki67, menopause, ovariectomy, rats, tibolone, vagina
The decrease in circulating oestrogen observed in climacterium is responsible for a variety of symptoms such as hot flushes, night sweats and vaginal atrophy. Approximately 75–80% of women experience menopausal symptoms, and among them, 20–30% have severe symptoms (Palacios 2008).
There is substantial evidence that the benefits of hormonal replacement therapy (HRT) in menopause include the reduction in distressing symptoms as well as the reduction in the risk of osteoporotic fractures, dementia and colorectal cancer, which improve well-being, quality of life, sexual enjoyment and bladder capacity (Wren 2009).
Despite benefits in relief of symptoms of menopause conventional HRT has serious adverse effects such as increased risk of breast cancer and endometrial cancer. Therefore, new drugs like tibolone are attractive options for the treatment (Jacobsen et al. 2008).
Tibolone is a synthetic steroid used in HRT (Blom et al. 2006). In the intestine and liver, tibolone is bioconverted into metabolites that have tissue-specific agonistic oestrogenic (3-alpha and 3-beta-hydroxytibolone) and progestogenic/androgenic (delta-4 tibolone) properties (Notelovitz 2007). Tibolone has favourable effects on bone, vagina, climacteric symptoms, mood and sexual well-being in postmenopausal women, without having an oestrogen-like stimulating effect on the endometrium or breast (Jacobsen et al. 2008).
In the vagina of postmenopausal women, tibolone normalizes the maturation index, alleviates the atrophic vaginitis symptoms (Notelovitz 2007) and increases the vaginal elasticity (Zárate et al. 2004), the blood flow and the vaginal lubrication (Modelska & Cummings 2002). In women, vaginal biopsies are rarely performed and most studies use symptoms and the maturation index to evaluate the effects of tibolone (Rymer et al. 1994; Swanson et al. 2006; Indhavivadhana et al. 2010). Thus, it is necessary to conduct detailed histological studies in other species such as the rat. Furthermore, the effect of tibolone on the rat vaginal tissue is controversial and few studies have examined this. In a modified Allen–Doisy assay, with the evaluation of vaginal cytology, it was shown previously that tibolone exerted lower oestrogenic activity than ethinyloestradiol (de Visser et al. 1984; de Gooyer et al. 2003), but possible effects of tibolone on the vagina tissue morphology in the rat are unknown. The aim of the present study was to evaluate the effect of prolonged use of high dose of tibolone in the vagina of ovariectomized rats by detailed histological examination.
Material and methods
Animals
Fourteen Wistar rats, aged 8 weeks and weighing in average 250 g, were used. All rats were produced and maintained in the Laboratory of Experimental Nutrition (LABNE) of the Fluminense Federal University (UFF). Rats were housed in individual plastic cages, with controlled temperature (24 ± 2 °C) and artificial illumination alternated in cycles of 12/12 h. Filtered water and commercial food (Fri-Lab Ratos II, Fri-Ribe) were supplied ad libitum.
Ovariectomy
Bilateral ovariectomy was performed in all rats 30 days before the beginning of the experiment, following the norms of vivisection of animals recommended by the Brazilian School of Animal Experimentation (COBEA). The work was approved by the Committee of Ethics in Research of the College of Medicine/Antônio Pedro Universitary Hospital/Fluminense Federal University. Anaesthesia was intramuscular with 100 mg/kg of ketamine (Crystal) and 20 mg/kg of xylazine (Anasedan Vetbrands) (Piovesan et al. 2005).
Chemicals
Tibolone was diluted at 0.2% in a solution of 0.5% carboxymethylcellulose (CMC).
Experimental design
After surgery, the rats did not receive medication for 30 days and received only food and water ad libitum to reduce sex hormone levels and arrive at surgical menopause (Jaita et al. 2005). The rats were randomly distributed into two groups: The experimental group (n = 9) received 0.5 ml per rat of tibolone, given 1 mg/day per rat. The control group (n = 5) received daily doses of 0.5 ml per rat of CMC. Each group received their treatment by gavage administration for 150 consecutive days.
Vaginal smears were obtained immediately before the ovariectomy (day 30) to ensure that the rats were in normal oestrus cycle. Thirty days after surgery (day 0), new vaginal cytology was performed to verify the menopause status. After starting the administration of tibolone and CMC, vaginal smears were collected on days 1 to 6, 30, 60, 90, 120 and 150 of the experiment to evaluate the vaginal tropism, which is classified in oestrus, pro-oestrus, metoestrus and dioestrus. Smears in oestrus, pro-oestrus and metoestrus indicate hormonal influence, and the vaginal cytology in anoestrus points to a lack of hormonal influence and to vaginal atrophy. Smears were immediately fixed in 95% alcohol and stained by the Papanicolaou method.
At the end of the experiment, all rats were anaesthetized with ketamine and xylazine and euthanized by cervical dislocation. Vaginal tissues were removed, immediately fixed in 10% buffered formalin, cleaved after 48 h and processed for paraffin embedding.
Histopathology
Histological sections of 4 μm obtained from the vagina were stained with haematoxylin and eosin (HE). The histochemical reactions of periodic acid Schiff (PAS) and PAS-diastase were performed to detect glycogen and glycoprotein respectively. Elastic fibres were analysed by the Weigert's resorcin–fuchsin stain; muscle fibres and collagen were detected by the picrosirius red (PR) stain. The collagen fibres were analysed using bright-field microscopy and circularly polarized light microscopy to identify collagen fibres type I (orange birefringence) and collagen type III (green birefringence).
Immunohistochemistry (IHC) for Ki67
Paraffin sections of 4 μm were deparaffinized in xylene and hydrated through graded alcohol concentrations. The sections were pretreated with hydrogen peroxide to abolish endogenous peroxidase activity and boiled in 10 mM citrate buffer at pH 6.0 in DakoCytomation Pascal pressure chamber (DAKO, Carpinteria, CA, USA) pot for 45 min. Unspecific binding was blocked in 1% milk (Molico®, Nestlé Brasil Ltda., Barra Mansa, RJ, Brazil) and 1% bovine albumin for 15 min at room temperature. The sections were incubated successively first with rabbit monoclonal anti-ki67 antibody [diluted 1:100; (SP6) ab1667 Abcam®, Cambridge, MA, USA] for 1 h at room temperature and later with secondary antibody and Streptavidin-Avidin-Biotin [LSAB®, 2 System-HRP DakoCytomation K0609 (DAKO)] for 30 min at room temperature. The immunoreaction was developed with 3,3′-diaminobenzidine [DAB; DAKO liquid DAB + substrate chromogen system K3467, (DAKO)], and the sections were lightly counterstained with haematoxylin. Sections of human mammary carcinoma were used as positive controls.
Histomorphometry
Digitized images of histological sections obtained under final magnification of ×400 were analysed using the Image-Pro Plus 4.5 software (Media Cybernetics, Silver Spring, MD, USA).
Epithelial thickness
The epithelial thickness was performed in slides stained with PR using the drag-line tool in the image analysis program. Four images were taken from each animal, and the average thickness was calculated from five measurements of each image.
Per cent area of collagen fibres and blood vessels
Eight images from each animal were obtained, and a 100-point grid mask was applied on each one. The manual count of structures was performed, and the average per cent area of collagen fibres and blood vessels was determined per group.
Mast Cells
The mast cells were counted under light microscopy at a final magnification of ×400 in the vaginal submucosa in slides stained with PR. The average of mast cells per field was obtained from the count in 10 microscopic fields for each animal.
Ki67-positive cells
Ten images of vaginal epithelium were obtained from each animal for the analysis of Ki67 immunohistochemistry positivity. A line was traced on the basal membrane to determine its length (μm) in every image. The Ki67-positive nuclei were counted manually, and the results were expressed as number of Ki67-positive nuclei per mm of basal membrane.
Statistical analysis
Results are presented as mean and standard error of the mean (SEM). The Shapiro–Wilk test was used to asses the normal distribution. Comparison between the groups was made by the Mann–Whitney test. The Graph-Pad Prism 5.0 statistical package (GraphPad Software Inc., San Diego, CA, USA) was used for the analysis, and P < 0.05 was regarded as statistically significant.
Results
At the beginning of the experiment, all the rats were in menopause confirmed by the vaginal cytology similar to anoestrus. This pattern remained until the end of the experiment in the control group. The oestrogenic effect of tibolone in the vagina was already observed at day 3 in the tibolone group, because all rats had cytology similar to oestrus with cornified epithelial cells.
Epithelial morphology and histomorphometry
In control animals, there was a significant thinning of the squamous epithelium consisting of approximately one to four layers of cells. On the other hand, in tibolone-treated animals, the squamous epithelium presented approximately 10 cellular layers (Figure 1a,b). These findings were confirmed by histomorphometric analysis showing a significant increase in tibolone epithelial thickness when compared with control group (75.06 ± 4.40 μm vs. 7.32 ± 0.64 μm, P < 0.001; Figure 2a). Epithelial infoldings into the lamina propria were observed in the tibolone group. The basal cells were columnar and there was a progressive flattening of cells towards the luminal surface. Therefore, keratohyalin granules could be seen in the most superficial layers, and cornification was noted in all tibolone-treated animals. Only sparse epithelial infoldings into lamina propria were observed in control animals, and the cells showed very little surrounding cytoplasm. Consistent with the decreased number of cell layers, there was no gradual transition to anucleated squamous cells at the epithelial surface; the basal cells were cuboidal and when more than a single epithelial cell layer was present, the superficial layer contained flattened cells without keratohyalin granules.
Figure 1.
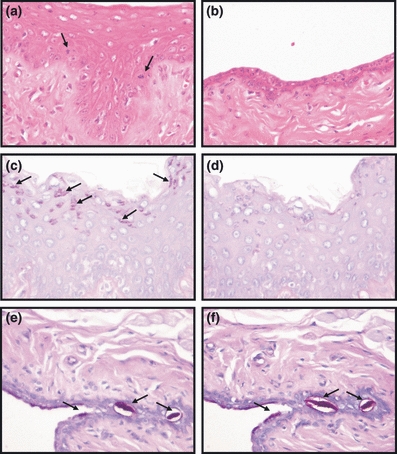
Vaginal morphology in ovariectomized rats. (a, c, d) Tibolone group. (b, e, f) Control group. (a and b) Haematoxylin and eosin ×400. (a) Vaginal epithelium showing hyperplasia and mitotic figures (arrows). (b) Atrophic vaginal epithelium. (c) Periodic acid Schiff (PAS) positive epithelial cells (arrows). (d) PAS-diastase negative reaction; ×400. (e) PAS positive substance (arrows). (f) PAS-diastase positive substance (arrows); ×400.
Figure 2.
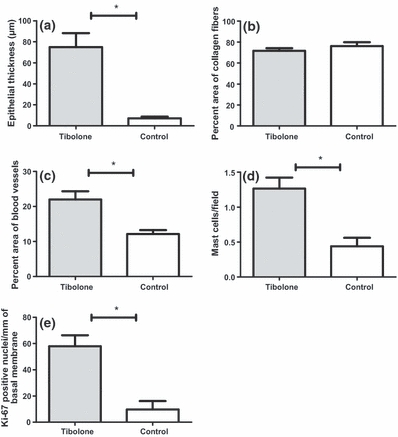
Vaginal histomorphometry in ovariectomized rats. Mann–Whitney test. (a) P < 0.001; (c) P < 0.01; (d) P < 0.05; (e) P < 0.01.
Submucosal morphology and histomorphometry
No difference in morphology of the collagen and elastic fibres was registered between the groups. Predominance of type I over type III collagen fibres was observed in both tibolone and control groups. Moreover, similar dense disposition of collagen fibres was seen. This result was confirmed by histomorphometry, which showed no significant difference in the per cent area of collagen fibres between the tibolone and control groups (71.63 ± 2.47 vs. 76.20 ± 3.69; Figure 2b). Sparse and thin elastic fibres in both groups were seen, corresponding to oxytalan fibres responsible for tissue resistance. The per cent area of blood vessels and the mast cells count was significantly higher in the tibolone group when compared with the control group (22.05 ± 2.33 vs. 12.15 ± 1.12, P < 0.01 and 1.27 ± 0.16 vs. 0.44 ± 0.12, P < 0.05 respectively; Figure 2c,d).
Cellular proliferation
Frequent mitotic figures were present in the basal layer of tibolone group. On the other hand, these figures were not seen in the control group. Consistent with these findings, the number of Ki67-positive nuclei per mm of basal membrane was significantly higher in the tibolone group when compared with the control group (57.95 ± 8.47 vs. 9.77 ± 6.32, P < 0.01; Figure 2e).
Glycogen and glycoprotein detection
Glycogen was detected in all animals from tibolone group by the PAS-positive and PAS-diastase-negative reaction. PAS positivity was seen in intraepithelial leucocytes and superficial epithelial cells with keratohyalin granules (Figure 1c,d). Glycoprotein was observed in all rats from the control group by the positive reactions of PAS and PAS-diastase (Figure 1e,f).
Discussion
The high dose of tibolone used in this experiment was similar to other studies (van Bezooijen et al. 1998; Ederveen & Kloosterboer 1999, 2001) studying the effects of the drug on the bone tissue in short-term (4 weeks) and long-term (64 weeks) treatments. One hundred and fifty days of treatment represent a prolonged period as it corresponds approximately to one sixth of an expected lifetime of a rat. The extension of our experimental protocol according to the ICH and OECD guidelines for chronic toxicity and carcinogenicity studies (Mutai 2000) was between subacute (2–13 weeks) and chronic (24–104 weeks) studies and was carried out to clarify possible toxicity the liver (data not shown) and potential deleterious effects on target organs by continuous exposure to tibolone (Pantaleão et al. 2009; Carvalho et al. 2010; Henriques et al. 2010a,b;).
The initial data clearly show the oestrogen effect of tibolone on the vagina evidenced by vaginal cytology showing only cornified epithelial cells similar to the oestrus phase. On the other hand, the vaginal smears of control group confirmed the lack of hormonal action because of surgical menopause. The Allen–Doisy vaginal cornification assay is used almost exclusively for the biological assay of oestrogenic compounds in vaginal smears assessed according to their cellular composition, the simplest method of scoring being to record those with cornified or nucleated epithelial cells but no leucocytes as positive and the rest as negative (Emmens 1939; Thayer et al. 1944). We used other validated and frequently used methods in which the various stages of the oestrus cycle were recorded.
In this study, we investigated the effects of tibolone treatment on the vaginal tissue architecture of ovariectomized rats. Our results showed that ovariectomy exerted a powerful effect on the vaginal epithelium leading to intense atrophy. This drastic structural alteration is related to changes in the functional properties of the epithelium, which becomes more susceptible to abrasion, supporting clinical findings in which the epithelium is reported to be more fragile after menopause (Rechberger & Skorupski 2007).
A lower protection against bacterial infection in women during menopause may be owing to thinner epithelium and loss of its ability to produce glycogen. This can lead to changes in the vaginal flora composition, increasing the risk of infections on the lower urinary tract (Rechberger & Skorupski 2007). The tibolone treatment reversed most changes in tissue rat morphology noted in this study after ovariectomy. The epithelium was remarkably hyperplasic and recovered its ability to produce glycogen. The glycogen present in the vaginal epithelium of tibolone group probably leads to vaginal flora recovery as well as occurs with women using HRT (Cauci et al. 2002). In fact, the vaginal flora of laboratory animals is influenced by the oestrus cycle (Larsen et al. 1977; Noguchi et al. 2003) and probably by mucous secretion (Noguchi et al. 2003). Ovarian hormones seem to be regulatory factors that favour the presence of a broad variety of bacteria, which are members of the normal genital tract flora. On the other hand, ovariectomy modifies the vaginal microbial profile, and hormone replacement therapy alleviates this disturbance (Bezirtzoglou et al. 2008).
After the tibolone treatment, our results were similar to the effect of hormone administration (oestradiol, oestradiol + testosterone and oestradiol + progesterone), which restored the epithelial thickness to what was seen before the ovariectomy in rats; the vaginal stratified squamous epithelium consisted of approximately the same number of layers seen in the non-ovariectomized rats (Pessina et al. 2006).
Tibolone had no influence on per cent area of collagen fibres in rats as well as in collagen type I/III ratio and the per cent area of elastic fibres. The oestrogen treatment in women leads to a decrease in the collagen content when compared with placebo treatment; however, there is no effect in the collagen type I/III ratio (Jackson et al. 2002). Thus, tibolone appears to exert a weak oestrogen effect on the vaginal collagen content of ovariectomized rats. The reduction of plasma oestradiol observed in diabetic mice leads to changes consistent with the menopause condition in the vagina, such as reduced epithelial thickness, reduced muscle layer and change in the arrangement of elastic fibres. Oestradiol administration significantly increases the volume of the muscle layer and the lamina propria, but induces less marked changes in elastic fibres (Cushman et al. 2009). Also, no remarkable changes were noted in the vaginal elastic fibres by the tibolone treatment.
The per cent area of blood vessels on the vaginal submucosa was higher in the tibolone group when compared with the control group. The oestrogen replacement in rats leads to an increase in the blood flow (Kim et al. 2006), which could be explained by the increase in per cent area of blood vessels found in this study.
The cellular proliferation – regenerative hyperplasia – was higher in the tibolone group when compared with the control rats. This result was evidenced by histopathology and histomorphometry. Data were comparable to a study in women showing that the Ki67 expression was higher in the oestrogen-treated group when compared with non-treated postmenopausal women (Blakeman et al. 2001).
Mast cells express high affinity for oestrogen receptors, and their granule secretion is increased by oestradiol (Vasiadi et al. 2006). This fact was proven by the study performed in possums (Trichosurus vulpecula), which showed an inverse relation between the mast cells number in the vagina and oestrogen levels (Mahoney et al. 2002). However, in the present study, an increase in non-degranulating mast cells was observed in the vaginal submucosa of the tibolone group when compared with the control group. In contrast, tibolone metabolites appeared not to be able to trigger degranulation of mast cells, which could explain why an increased number of these cells was observed in the vagina of tibolone-treated rats.
Tibolone administered in high dosages and for a long period of time has proliferative and trophic effects, regenerative hyperplasia, reversing vaginal atrophy, and does not have dysplastic or neoplastic effects in the vagina of ovariectomized rats. However, a very careful extrapolation of these results to humans is necessary as the background clinical history may exert influence of the response to tibolone hormonal therapy.
Acknowledgments
The authors thank OFFICILAB for the donation of tibolone.
References
- Bezirtzoglou E, Voidarou C, Papadaki A, Tsiotsias A, Kotsovolou O, Konstandi M. Hormone therapy alters the composition of the vaginal microflora in ovariectomized rats. Microb. Ecol. 2008;55:751–759. doi: 10.1007/s00248-007-9317-z. [DOI] [PubMed] [Google Scholar]
- van Bezooijen RL, Que I, Ederveen AG, Kloosterboer HJ, Papapoulos SE, Löwik CW. Plasma nitrate+nitrite levels are regulated by ovarian steroids but do not correlate with trabecular bone mineral density in rats. J. Endocrinol. 1998;159:27–34. doi: 10.1677/joe.0.1590027. [DOI] [PubMed] [Google Scholar]
- Blakeman PJ, Hilton P, Bulmer JN. Cellular proliferation in the female lower urinary tract with reference to oestrogen status. BJOG. 2001;108:813–816. doi: 10.1111/j.1471-0528.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- Blom MJ, Wassink MG, de Gooyer ME, et al. Metabolism of tibolone and its metabolites in uterine and vaginal tissue of rat and human origin. J. Steroid Biochem. Mol. Biol. 2006;101:42–49. doi: 10.1016/j.jsbmb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Carvalho ACB, Henriques HN, Pantaleão JAS, et al. Histomorfometria do tecido ósseo em ratas castradas tratadas com tibolona. J. Bras. Patol. Med. Lab. 2010;46:235–246. [Google Scholar]
- Cauci S, Driussi S, de Santo D, et al. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J. Clin. Microbiol. 2002;40:2147–2152. doi: 10.1128/JCM.40.6.2147-2152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman TT, Kim N, Hoyt R, Traish AM. Estradiol ameliorates diabetes-induced changes in vaginal structure of db/db mouse model. J. Sex. Med. 2009;6:2467–2479. doi: 10.1111/j.1743-6109.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- Ederveen AG, Kloosterboer HJ. Tibolone, a steroid with a tissue-specific hormonal profile, completely prevents ovariectomy-induced bone loss in sexually mature rats. J. Bone Miner. Res. 1999;14:1963–1970. doi: 10.1359/jbmr.1999.14.11.1963. [DOI] [PubMed] [Google Scholar]
- Ederveen AG, Kloosterboer HJ. Tibolone exerts its protective effect on trabecular bone loss through the estrogen receptor. J. Bone Miner. Res. 2001;16:1651–1657. doi: 10.1359/jbmr.2001.16.9.1651. [DOI] [PubMed] [Google Scholar]
- Emmens CW. The effect of prolonged dosage with oestrogens on the adult brown leghorn cock. J. Physiol. 1939;14:379–392. doi: 10.1113/jphysiol.1939.sp003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gooyer ME, Deckers GH, Schoonen WG, Verheul HA, Kloosterboer HJ. Receptor profiling and endocrine interactions of tibolone. Steroids. 2003;68:21–30. doi: 10.1016/s0039-128x(02)00112-5. [DOI] [PubMed] [Google Scholar]
- Henriques HN, Câmara NR, de Carvalho AC, Pantaleão JAS, Guzmán-Silva MA. Effect of high doses of tibolone in body weight and lipid profile of ovariectomized rats. Rev. Bras. Ginecol. Obstet. 2010a;32:88–93. doi: 10.1590/s0100-72032010000200007. [DOI] [PubMed] [Google Scholar]
- Henriques HN, de Carvalho AC, Pantaleão JAS, Guzmán-Silva MA. Effect of long-time administration of tibolone on vaginal cytology of castrated rats. Clin. Exp. Obstet. Gynecol. 2010b;37:123–126. [PubMed] [Google Scholar]
- Indhavivadhana S, Leerasiri P, Rattanachaivanont M, et al. Vaginal atrophy and sexual dysfunction in current users of systemic postmenopausal hormone therapy. J. Med. Assoc. Thai. 2010;93:667–675. [PubMed] [Google Scholar]
- Jackson S, James M, Abrams P. The effect of oestradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. BJOG. 2002;109:339–344. doi: 10.1111/j.1471-0528.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen DE, Samson MM, van der Schouw YT, Grobbee DE, Verhaar HJ. Efficacy of tibolone and raloxifene for the maintenance of skeletal muscle strength, bone mineral density, balance, body composition, cognitive function, mood/depression, anxiety and quality of life/well-being in late postmenopausal women >/= 70 years: study design of a randomized, double-blind, double-dummy, placebo-controlled, single-center trial. Trials. 2008;9:32. doi: 10.1186/1745-6215-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaita G, Candolfi M, Zaldivar V, et al. Estrogens up regulate the faz/fasl apoptotic pathway in lactotopes. Endocrinology. 2005;146:4737–4744. doi: 10.1210/en.2005-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NN, Stankovic M, Armagan A, Cushman TT, Goldstein I, Traish AM. Effects of tamoxifen on vaginal blood flow and epithelial morphology in the rat. BMC Womens Health. 2006;6:14. doi: 10.1186/1472-6874-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Markovetz AJ, Galask RP. Relationship of vaginal cytology to alterations of the vaginal microflora of rats during the estrous cycle. Appl. Environ. Microbiol. 1977;33:556–562. doi: 10.1128/aem.33.3.556-562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney PM, Hurst PR, McLeod BJ, McConnel MA. Quantification of mast cell and microflora in the vaginal cul-de-sac of the brushtail possum (Trichosurus vulpecula) Reproduction. 2002;124:199–408. doi: 10.1530/rep.0.1240399. [DOI] [PubMed] [Google Scholar]
- Modelska K, Cummings S. Tibolone for postmenopausal women: systematic review of randomized trials. J. Clin. Endocrinol. Metab. 2002;87:16–23. doi: 10.1210/jcem.87.1.8141. [DOI] [PubMed] [Google Scholar]
- Mutai M. National and International Guidelines for the conduct of chemical safety studies: choice of strains. In: Krinke GJ, editor. The Handbook of Experimental Animals – The Laboratory Rat. London: Academic Press; 2000. pp. 17–30. [Google Scholar]
- Noguchi K, Tsukumi K, Urano T. Qualitative and quantitative differences in normal vaginal flora of conventionally reared mice, rats, hamsters, rabbits, and dogs. Comp. Med. 2003;53:404–412. [PubMed] [Google Scholar]
- Notelovitz M. Postmenopausal tibolone therapy: biologic principles and applied clinical practice. MedGenMed. 2007;9:2. [PMC free article] [PubMed] [Google Scholar]
- Palacios S. Advances in hormone replacement therapy: making the menopause manageable. BMC Womens Health. 2008;8:22. doi: 10.1186/1472-6874-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleão JAS, Henriques HN, Carvalho ACB, Pollastri CE, Soares Filho PJ, Guzmán- Silva MA. Effect of tibolone on endometrium of castrated rats. Rev. Bras. Ginecol. Obstet. 2009;31:124–130. doi: 10.1590/s0100-72032009000300004. [DOI] [PubMed] [Google Scholar]
- Pessina MA, Hoyt RF, Jr, Goldstein I, Traish AM. Differential effects of estradiol, progesterone, and testosterone on vaginal structure integrity. Endocrinology. 2006;147:61–69. doi: 10.1210/en.2005-0870. [DOI] [PubMed] [Google Scholar]
- Piovesan AC, Soares Júnior JM, Mosquette R, Simões MJ, Simões RS, Baracat EC. Estudo morfológico e molecular da mama de ratas castradas tratadas com isoflavona ou estrogênios. Rev. Bras. Ginecol. Obstet. 2005;27:204–209. [Google Scholar]
- Rechberger T, Skorupski P. The controversies regarding the role of estrogens in urogynecology. Folia Histochem. Cytobiol. 2007;45:S17–S21. [PubMed] [Google Scholar]
- Rymer J, Chapman MG, Fogelman I, Wilson PO. A study of effect of tibolone on the vagina in postmenopausal women. Maturitas. 1994;18:127–133. doi: 10.1016/0378-5122(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Swanson SG, Drosman S, Helmond FA, Stathopoulos VM. Tibolone for the treatment of moderate to severe vasomotor symptoms and genital atrophy in postmenopausal women: multicenter, randomized, double-blind, placebo-controlled study. Menopause. 2006;13:917–925. doi: 10.1097/01.gme.0000247016.41007.c9. [DOI] [PubMed] [Google Scholar]
- Thayer SA, Doisy EA, Jr, Doisy EA. The bio-assay of β-estradiol. Yale J. Biol. Med. 1944;17:19–26. [PMC free article] [PubMed] [Google Scholar]
- Vasiadi M, Kempuraj D, Boucher W, Kalogeromitros D, Theoharides TC. Progesterone inhibits mast cell secretion. Int. J. Immunophatol. Pharmacol. 2006;19:787–794. doi: 10.1177/039463200601900408. [DOI] [PubMed] [Google Scholar]
- de Visser J, Coert A, Feenstra H, van de Vies J. Endocrinological studies with (7 alpha, 17 alpha)-17-hydroxy-7-methyl-19-norpregn-5(10)-en-20-yn-3-one (Org OD 14) Arzneimittelforschung. 1984;34:1010–1017. [PubMed] [Google Scholar]
- Wren BG. The benefits of oestrogen following menopause: why hormone replacement therapy should be offered to postmenopausal women. Med. J. Aust. 2009;190:321–325. doi: 10.5694/j.1326-5377.2009.tb02423.x. [DOI] [PubMed] [Google Scholar]
- Zárate A, Hernandéz M, Saucedo R. Lugar de la tibolona en la terapia de reemplazo hormonal en la postmenopausia. Acta Médica (Grupo Ángeles) 2004;2:193–195. [Google Scholar]


