Abstract
The pathogenesis of alcohol-induced osteonecrosis remains unclear. The purpose of the present study was to evaluate the morphological changes in bone marrow fat cells and the changes in the serum lipid levels in alcohol-treated rabbits. Fifteen rabbits were randomly assigned into three groups: Four rabbits intragastrically received low-dose alcohol (LDA) (15 ml/kg per day) containing 15% ethanol for 4 weeks, five rabbits received high-dose alcohol (HDA) (30 ml/kg per day) for 4 weeks and six rabbits received physiologic saline for 4 weeks as a control group. Six weeks after the initial alcohol administration, all rabbits were sacrificed. The mean size of the bone marrow fat cells in rabbits treated with HDA was significantly larger than that in the control group (P = 0.0001). Haematologically, the levels of triglycerides and free fatty acids in the rabbits treated with both low-dose and HDA were significantly higher than those in the control group (P = 0.001 for both comparisons). The results of this study are that there are lipid metabolism abnormalities, both morphologically and haematologically, after alcohol administration. Also these findings were more apparent in rabbits treated with HDA than those treated with LDA.
Keywords: alcohol, bone marrow fat cell, lipid metabolism abnormality, osteonecrosis, rabbits
Introduction
Osteonecrosis (ON) of the femoral head is a disabling condition of the hip joint (Assouline-Dayan et al. 2002). Non-traumatic ON has been proven to be associated with corticosteroid administration or alcohol abuse in many published reports (Merle d'Aubignéet al. 1965; Boettcher et al. 1970; Matsuo et al. 1988; Mont & Hungerford 1995; Assouline-Dayan et al. 2002).
Several possible factors in the pathogenesis of steroid-induced ON have been suggested based on human and animal studies, including hyperlipidaemia and coagulation abnormalities at the early phase of steroid administration (Yamamoto et al. 1997; Miyanishi et al. 2002; Motomura et al. 2004). Several experimental studies have demonstrated that abnormal lipid metabolism as judged by both morphological and haematological occurs in steroid-induced ON rabbits two weeks after the steroid injection (Miyanishi et al. 2002; Motomura et al. 2004; Nishida et al. 2008).
The pathogenesis of alcohol-induced ON remains unclear. Previous studies have demonstrated that long-term alcohol administration increases the volume of fatty marrow and intraosseous pressure in animals (Solomon 1985; Wang et al. 2003). Haematologically, previous papers have described the consumption of alcohol to elevate the serum lipid status including cholesterol, triglyceride and lipid peroxides (Shih et al. 1991; Wang et al. 2008). However, there have been no reports evaluating both morphological changes in bone marrow fat cells and sequential haematologic changes after different doses of alcohol administration in animals.
Thus the purpose of the present study was to evaluate changes in bone marrow fat cell morphologically as well as those in serum levels of lipid status after different doses of alcohol-treated rabbits.
Methods
All experiments were conducted in accordance with the Guidelines for Animal Experiments of Kyushu University, Japanese law (No. 105), and notification No. 6 of the government of Japan.
Animals
We studied 16 male Japanese white rabbits (Kyudo, Tosu, Japan), ranging in age from 30 to 32 weeks. Growth plate closure was confirmed histologically when they were sacrificed. Animals were housed at the Animal Center of Kyushu University and maintained on a standard diet and water. The body weights of the rabbits were measured prior to the experiment (3.3–4.1 kg) and every week (1–6 weeks). One of 16 rabbits initially used in this study died from aspiration because of tracheal tube insertion at 5 days after alcohol administration. Therefore, this rabbit was excluded from the analysis of the study.
Treatment
Fifteen rabbits were divided randomly into three groups. One group received low-dose alcohol (LDA group, n = 4, Japanese Sake; Gekkeikan Sake Company, Ltd., Kyoto, Japan: containing 15% ethanol, 15 ml/kg per day body weight), one received high-dose alcohol (HDA group; n = 5, 30 ml/kg per day), and one received physiologic saline (PS group; n = 6, 30 ml/kg per day) as a control. The rabbits were intragastrically administered alcohol or saline for 4 weeks, by passing the liquid through a rubber gastric tube into stomach. Two weeks after the non-treated periods (6 weeks after the initial alcohol administration), the rabbits were sacrificed and tissue specimens were prepared as described (Yamamoto et al. 1997).
Tissue preparation
For light microscopic examination, tissue samples were obtained from the femur, humerus and liver at the time of death and then were fixed for 1 week with 10% formalin-0.1 mol/l phosphate buffer, pH 7.4. The bone samples were decalcified with 25% formic acid for 3 days and then neutralized with 0.35 mol/l sodium sulphate for 3 days. The specimens were embedded in paraffin, cut into 4-μm sections and stained with haematoxylin and eosin.
Histopathological evaluation
Both femora and humeri were histopathologically examined for the incidence of ON (Yamamoto et al. 1997; Miyanishi et al. 2002). The liver was also histopathologically examined to identify any effects of alcohol administration (Yamamoto et al. 1997). We calculated the size of bone marrow fat cells as the mean of the maximal diameters of 25 fat cells in four randomly selected fields (one field = 25 × 10−8 m2), as well as the number of haematopoietic cells in four randomly selected field (one field = 4 × 10−8 m2), from the proximal one-third of the right femur using the NIH Image freeware (NIH, Bethesda, MD, USA), as previously described (Miyanishi et al. 2002; Motomura et al. 2004). In addition, we calculated the ratio of the bone volume to the total volume in the right femur as previously described (Parfitt et al. 1987). The evaluation of ON was also determined as previously described (Yamamoto et al. 1997). Briefly, the complete areas of the proximal one-third and distal condyle of both the femora and humeri (eight regions) were examined histologically for the presence of ON. A diagnosis of ON was made on the basis of the presence of diffuse empty lacunae or pyknotic nuclei of osteocytes within the bone trabeculae that were accompanied by surrounding bone marrow cell necrosis. Histopathological evaluations, including evaluations of the osteonecrotic lesion and liver degeneration, were made blindly by three authors (S.I, T.Y and G.M). If the evaluation differed between the three investigators, a consensus was reached by discussion of the histological findings without knowledge of the group from which the sample was obtained. Several calculations, including the sizes of bone marrow fat cells, the number of haematopoietic cells and the bone volume, were made blindly by one author (S.I).
Laboratory data examination
We collected blood samples from the auricular arteries while the animals were in a fasting state. Every week (0–6 week), samples were obtained in the early morning prior to alcohol administration. We examined the serum lipid levels including total cholesterol, triglycerides, the ratio of low-density lipoprotein (LDL) to high-density lipoprotein (HDL) cholesterol (LDL/HDL cholesterol ratio), which is considered a potential risk factor for corticosteroid-induced ON, and free fatty acid (Miyanishi et al. 2001). The hepatic enzymes, alanine aminotransferase and aspartate aminotransferase (AST), were also examined. Blood alcohol concentrations at 1 h after alcohol administrations were measured in rabbits of both the LDA and HDA groups. Measurements of blood samples were made by a company (CRC Inc., Fukuoka, Japan).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). The amounts of body weight, sizes of bone marrow fat cells, the numbers of bone marrow haematopoietic cells and the ratios of the bone volume to the total volume between three groups were compared using the one-way analysis of variance (anova) with Scheffe's post hoc test. Haematologic data were analysed for interaction with three groups by repeated-measures anova. Data from each group prior to alcohol administration (Week 0) and weekly thereafter were analysed by Dunnett-type multiple comparison. Data obtained at each time point were compared using the unpaired t-test. Statistical analyses were performed using Statistical Package for Social Sciences (spss Japan, Tokyo, Japan). P-values <0.05 were considered significant.
Results
The mean amount of body weight lost during the experimental period (between weeks 0 and 2) in the LDA group was 157 ± 139 g, the HDA group was 329 ± 90 g and the PS group was 32 ± 37 g. The mean body weight lost between weeks 0 and 2 in the HDA group was significantly higher than that lost by the PS group (P < 0.05).
Sizes of bone marrow fat cells
The mean size of bone marrow fat cells was significantly larger in the HDA group (53.5 ± 9.2 μm) than that in the PS group (44.0 ± 5.8 μm) (P = 0.0001) (Figure 1).
Figure 1.
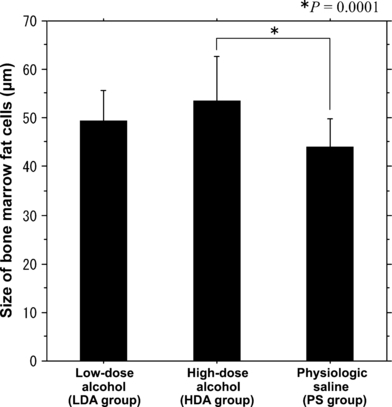
The sizes of the bone marrow fat cells between the three groups. The mean size of the bone marrow fat cells in the high-dose alcohol group was significantly larger (53.5 ± 9.2 μm) than that in the physiologic saline (PS) group (44.0 ± 5.8 μm) (P = 0.0001). The mean size of the bone marrow fat cells was larger in the low-dose alcohol (LDA) group (49.36 ± 6.4 μm) than in the PS group. However, there were no significant differences in the sizes of bone marrow fat cells between the LDA and PS groups (P = 0.0628).
Histopathological findings
Both the enlargement of adipocytes and diminished haematopoiesis were observed in the bone marrow of the LDA and HDA groups in comparison with the PS group. There were significant differences in the number of haematopoietic cells between the LDA group (107 ± 68) or the HDA group (63 ± 46) and the PS group (165 ± 46) (P < 0.01: LDA vs. PS, P = 0.0001: HDA vs. PS). The ratios of the bone volume to the total volume in the LDA (0.386 ± 0.011) and HDA groups (0.367 ± 0.087) were lower than those in the PS group (0.521 ± 0.088). There was a significant difference in the ratio of the bone volume to the total volume between the HDA and PS groups (P < 0.05). However, osteonecrotic lesions were not observed in either group histopathologically (Figure 2).
Figure 2.
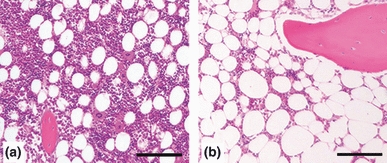
Histopathology of the metaphysis of the femur. (a) In the physiologic saline group, the marrow fat cells are almost homogeneous in size, and the space for the bone marrow haematopoietic cells seems to be preserved (haematoxylin and eosin (H and E), 40× magnification; scale bar = 100 μm). (b) In the high-dose alcohol group, marrow fat cells are increased in size. The space for the bone marrow haematopoietic cells was decreased. However, the osteonecrotic lesions were not observed (H and E, 40× magnification; scale bar = 100 μm).
Regarding liver degeneration, the HDA group revealed hepatocellular atrophy or periportal lymphocytic infiltration in all five rabbits (Figure 3). In contrast, there was no evidence of liver degeneration in any of the rabbits in the LDA group. Fatty livers were not apparent histologically. In HDA group, there was no relationship between the size of bone marrow fat cells and the degree of liver degeneration.
Figure 3.
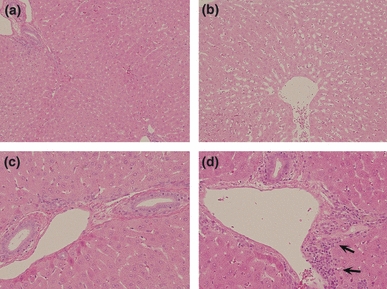
Histopathology in the liver. The diffuse hepatocellular atrophy [(b); H and E, 100×] as well as periportal lymphocytic infiltration [(d) 200× magnification; arrows] was observed in the high-dose alcohol group in comparison with a physiologic saline group [(a) 100×, (c) 200×].
Haematologic findings
The blood alcohol concentrations ranged from 100 to 150 and 290 to 330 mg/dl in the LDA group and the HDA group respectively. The levels of triglycerides and free fatty acid in both the LDA and HDA groups were significantly higher than those in the PS group (P = 0.001) (Figure 4a,b). The levels of total cholesterol in the HDA group at 3 and 4 weeks were significantly higher than those in the PS group (P < 0.05). The LDL/HDL cholesterol ratio observed in the HDA group at 3 and 4 weeks was also significantly higher than that in the PS group (P < 0.05) (Figure 4c). The levels of the hepatic enzyme AST in the HDA group at 1 week were significantly higher than those in the PS group (P < 0.05) (Figure 4d).
Figure 4.
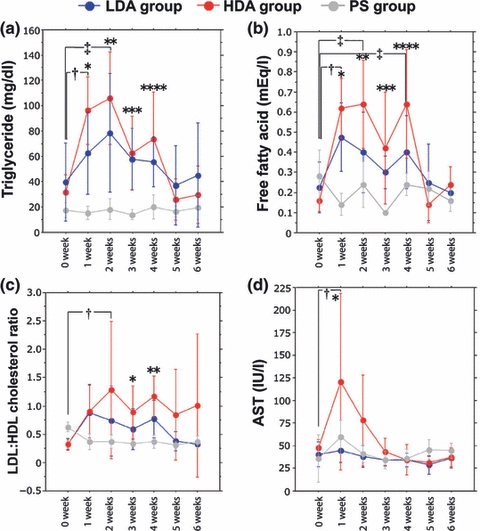
Sequential changes in the serum lipid status and hepatic enzyme. (a) The levels of triglyceride between three groups had a significant interaction (P = 0.001). In high-dose alcohol (HDA) group, the levels of triglyceride were significantly increased at 1 (†P = 0.005) and 2 weeks (‡P = 0.001) in comparison with those at week 0. At weeks 1, 2 and 3, the triglyceride levels in the low-dose alcohol (LDA) group were significantly higher than those in the physiologic saline (PS) group (* to ***P < 0.05). At weeks 1, 2, 3 and 4, the triglyceride levels in the HDA group were significantly higher than those in the PS group (*P = 0.001, **P = 0.001, ***P < 0.01 and ****P < 0.05 respectively). (b) The free fatty acid levels between three groups had a significant interaction (P = 0.001). In HDA group, the levels of free fatty acids were significantly increased at 1 (†P = 0.002), 2 (‡P = 0.001) and 3 weeks (‡P = 0.001) in comparison with those at week 0. At weeks 1 and 2, the levels of free fatty acids in the LDA group were significantly higher than those in the PS group (*P = 0.01 and **P < 0.05 respectively). At weeks 1, 2, 3 and 4, the levels of free fatty acids in the HDA group were significantly higher than those in the PS group (*P < 0.005, **P = 0.001, ***P < 0.05 and ****P < 0.05 respectively). (c) The low-density lipoprotein (LDL)/high-density lipoprotein (HDL) cholesterol ratio between three groups had no significant interaction (P = 0.406). In HDA group, the LDL/HDL cholesterol ratio was significantly increased at 2 weeks (†P < 0.05) in comparison with week 0. At weeks 3 and 4, levels of LDL/HDL cholesterol ratios in the HDA group were significantly higher than those in the PS group (*P < 0.05 and **P < 0.05 respectively). (d) The levels of aspartate aminotransferase (AST) between three groups had no significant interaction (P = 0.091). In HDA group, the levels of AST were significantly increased at 1 week (†P < 0.05) in comparison with those at week 0. At week 1, the AST level in the HDA group was significantly higher than that in the PS group (*P < 0.05).
Discussion
Previous studies (Solomon 1985; Wang et al. 2003) have described the morphological changes in fat cells in alcohol-treated animals. Wang et al. (2003) reported that fat cell hypertrophy in the subchondral area of the femoral head was present in rabbits administered alcohol for 6 months. Solomon (1985) reported a marked increase in the number of fat cells and a rise in intraosseous pressure in the femoral head in rats that received a supplementary diet of 12% ethanol for 40 weeks. Regarding the periods of alcohol administration, Wang et al. reported that there were no significant differences in fat cell diameter within the zone of the subchondral area, haematologic triglyceride and cholesterol levels between alcohol-treated animals and controls at 1 month. However, the current study indicated that there was enlargement of fat cells and an increase in lipid status after 4 weeks of alcohol administration. In addition, these findings were more apparent in rabbits treated with HDA than those treated with LDA.
Previous studies (Wang et al. 2003, 2008) have attempted to establish alcohol-induced ON models. In these reports, empty lacunae were observed in some parts of the subchondral bone trabeculae of the femoral head or metaphyseal region. However, in these studies, there were no obvious osteonecrotic lesions, which show diffuse empty lacunae within the bone trabeculae accompanied by surrounding bone marrow cell necrosis as an ON model in rabbits (Yamamoto et al. 1997; Miyanishi et al. 2002; Motomura et al. 2004). In this study, although the decreased bone volume was observed in rabbits treated with alcohol, the osteonecrotic lesion was not observed histopathologically.
The lipid metabolism abnormality was observed both morphologically and haematologically after alcohol administration. However, the mean size of bone marrow fat cells in the HDA group was 53.5 ± 9.2 μm, which is smaller than that in the steroid-induced ON rabbits (63.5 ± 5.8 μm) reported by Miyanishi et al. (2002). Haematologically, Motomura et al. (2008) reported the mean level of triglycerides 2 weeks after the injection of methylprednisolone (20 mg/kg) in rabbits was approximately 800 mg/dl, which is eight times higher than that in the HDA group. In addition, an experimental study (Yamamoto et al. 1997) observed that all rabbits with methylprednisolone injection had a fatty liver. In the current study, the fatty liver was not observed in any rabbits. We consider the abnormal lipid metabolism may be one of the factors considering the pathogenesis of alcohol-induced ON. However, further investigation is needed to clarify the pathogenesis of ON.
A human epidemiological study (Matsuo et al. 1988) reported that individuals with alcoholism have an elevated relationship: for <400, 400–1000 ml and more than 1000 ml of alcohol per week, the risk ratios were 3.3, 9.8 and 17.9 respectively. The amount of alcohol per week in the LDA group and the HDA group corresponds to approximately 800 and 1600 ml in humans respectively. In HDA group, the blood alcohol concentration was approximately 300 mg/dl. To assess the human relevance of alcohol-related studies, it may be noted that most non-alcoholic individuals become intoxicated at blood alcohol concentration between 100 and 200 mg/dl, whereas levels >400 mg/dl are considered lethal in individuals without a high tolerance to ethanol (Chakkalakal 2005).
The effects of different alcohol beverages on the blood biochemical findings have been shown to be based on the investigation of several types of alcohol intake in healthy young adults (Tousoulis et al. 2008). The ratio of plasminogen activator inhibitor (PAI-1) to tissue plasminogen activator (tPA) increased at 4 h after alcohol intake in the white wine, beer and whiskey groups. Plasminogen activator inhibitor-1/tPA ratio remained unchanged only in the red wine group. In addition, epidemiologic studies in Korea revealed that the rates of alcohol-induced ON are two to three times higher than those of steroid-induced ON, which are quite different from the rate reported for other countries (Kang et al. 2009). These results may indicate that there is a relationship between the types of alcohol and incidence of ON.
A recent study regarding alcohol-induced ON in human subjects revealed that expression of genes related to osteogenesis, including BMP-2, Runx2, osteocalcin, osteopoetin and osteonectin, was lower in the alcohol-induced ON group than in the non-ON group (Yeh et al. 2009). In contrast, the expression of adipogenic genes, including peroxisome proliferator-activated receptor γ (PPARγ) and adipsin, was higher in the alcohol-induced ON group than in the non-ON group (Yeh et al. 2009). An experimental study indicated that the activity of hepatic cytochrome P450 2E1 (CYP2E1) influences the expression of PPAR (Wan et al. 2001). Further investigations, regarding the correlation between the activity of CYP2E1 and changes in lipid status, might be necessary in alcohol-treated rabbits.
The current study has several limitations. The first limitation is that the osteonecrotic lesion was not observed histopathologically. The second limitation is that body weight loss was observed at the beginning of this experiment (between weeks 0 and 2). The third limitation is the small number of animals in each group. The final limitation is that only a few serum markers were investigated. Other risk factor markers for ON such as coagulation and apolipoprotein need to be monitored in future studies (Miyanishi et al. 1999; Motomura et al. 2004).
In summary, the results of the current study suggested the following conclusions: 1) Bone marrow fat cell enlargement, as well as diminished haematopoiesis, was observed in rabbits with alcohol administration for 4 weeks at the common site of ON in steroid-treated rabbits. 2) In addition, these findings were more apparent in rabbits treated with HDA than those treated with LDA.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Japan Society for the Promotion of Science (No. 21591948, 211160), Research Grant for Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan and a grant from Takeda Science Foundation.
References
- Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin. Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- Boettcher WG, Bonfiglio M, Hamilton HH, Sheets RF, Smith K. Nontraumatic necrosis of the femoral head. I. Relation of altered hemostasis to etiology. J. Bone Joint Surg. Am. 1970;52:312–321. [PubMed] [Google Scholar]
- Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol. Clin. Exp. Res. 2005;29:2077–2090. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- Kang JS, Park S, Song JH, Jung YY, Cho MR, Rhyu KH. Prevalence of osteonecrosis of the femoral head: a nationwide epidemiologic analysis in Korea. J. Arthroplasty. 2009;24:1178–1183. doi: 10.1016/j.arth.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Hirota T, Sugioka Y, Ikeda M, Fukuda A. Influence of alcohol intake, cigarette smoking and occupational status on idiopathic osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 1988;234:115–123. [PubMed] [Google Scholar]
- Merle d'Aubigné R, Postel M, Mazabraud A, Massias P, Gueguen J, France P. Idiopathic necrosis of the femoral head in adults. J. Bone Joint Surg. Br. 1965;47:612–633. [PubMed] [Google Scholar]
- Miyanishi K, Yamamoto T, Irisa T, et al. Increased level of apolipoprotein B/apolipoprotein A1 ratio as a potential risk for osteonecrosis. Ann. Rheum. Dis. 1999;58:514–516. doi: 10.1136/ard.58.8.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanishi K, Yamamoto T, Irisa T, et al. A high low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a potential risk factor for corticosteroid-induced osteonecrosis in rabbits. Rheumatology (Oxford) 2001;40:196–201. doi: 10.1093/rheumatology/40.2.196. [DOI] [PubMed] [Google Scholar]
- Miyanishi K, Yamamoto T, Irisa T, et al. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30:185–190. doi: 10.1016/s8756-3282(01)00663-9. [DOI] [PubMed] [Google Scholar]
- Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J. Bone Joint Surg. Am. 1995;77:459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- Motomura G, Yamamoto T, Miyanishi K, Jingushi S, Iwamoto Y. Combined effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Arthritis Rheum. 2004;50:3387–3391. doi: 10.1002/art.20517. [DOI] [PubMed] [Google Scholar]
- Motomura G, Yamamoto T, Irisa T, Miyanishi K, Nishida K, Iwamoto Y. Dose effects of corticosteroids on the development of osteonecrosis in rabbits. J. Rheumatol. 2008;35:2395–2399. doi: 10.3899/jrheum.080324. [DOI] [PubMed] [Google Scholar]
- Nishida K, Yamamoto T, Motomura G, Jingushi S, Iwamoto Y. Pitavastatin may reduce risk of steroid-induced osteonecrosis in rabbits: a preliminary histological study. Clin. Orthop. Relat. Res. 2008;466:1054–1058. doi: 10.1007/s11999-008-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Shih CH, Yang WE, Lee ZL, Kao YL, Hsueh S, Wei JS. Effect of long-term alcohol ingestion on the femoral head of rabbit. J. Formos. Med. Assoc. 1991;90:443–447. [PubMed] [Google Scholar]
- Solomon L. Mechanism of idiopathic osteonecrosis. Orthop. Clin. North Am. 1985;16:655–667. [PubMed] [Google Scholar]
- Tousoulis D, Ntarladimas I, Antoniades C, et al. Acute effects of different alcoholic beverages on vascular endothelium, inflammatory markers and thrombosis fibrinolysis system. Clin. Nutr. 2008;27:594–600. doi: 10.1016/j.clnu.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Wan YY, Cai Y, Li J, et al. Regulation of peroxisome proliferator-activated receptor alpha-mediated pathways in alcohol fed cytochrome P450 2E1 deficient mice. Hepatol. Res. 2001;19:117–130. doi: 10.1016/s1386-6346(00)00089-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin. Orthop. Relat. Res. 2003;410:213–224. doi: 10.1097/01.blo.0000063602.67412.83. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin L, Li Y, Liu P, Qui Q. Preventive effects of puerarin on alcohol-induced osteonecrosis. Clin. Orthop. Relat. Res. 2008;466:1059–1067. doi: 10.1007/s11999-008-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40:2055–2064. doi: 10.1002/art.1780401119. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Chang JK, Ho ML, Chen CH, Wang GJ. Different differentiation of stroma cells from patients with osteonecrosis: a pilot study. Clin. Orthop. Relat. Res. 2009;467:2159–2167. doi: 10.1007/s11999-009-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


