Abstract
Recent clinical studies have demonstrated that angiotensin II type 1 (AT1) receptor blockers (ARBs) reduce the onset of stroke, stroke severity and the incidence and progression of Alzheimer's disease and dementia. We can expect that ARBs exert these effects by both AT1 receptor blockade and angiotensin II type 2 (AT2) receptor stimulation. Moreover, recent experimental results support the notion that AT2 receptor stimulation with AT1 receptor blockade could contribute to protection against ischaemic brain damage at least partly due to an increase in cerebral blood flow and decrease in oxidative stress, and prevent cognitive decline. Cellular therapy has been focused on as a new therapeutic approach to restore injured neurons. In this context, it has been reported that AT2 receptor stimulation enhances neurite outgrowth and decreases neural damage, thereby enhancing neurogenesis. Moreover, additional beneficial effects of ARBs with an AT1 receptor blocking action with a partial peroxisome proliferator-activated receptor (PPAR)-γ agonistic effect have been reported, and interaction of AT2 receptor activation and PPAR-γ might be involved in these ARBs' effects. This article reviews the effects of regulation of activation of angiotensin II receptor subtypes on ischaemic brain damage and cognitive function, focusing on the effects of AT2 receptor stimulation.
LINKED ARTICLES
This article is one of a set of reviews submitted to BJP in connection with talks given at the September 2010 meeting of the International Society of Hypertension in Vancouver, Canada. To view the other articles in this collection visit http://dx.doi.org/10.1111/j.1476-5381.2011.01235.x, http://dx.doi.org/10.1111/j.1476-5381.2011.01260.x and http://dx.doi.org/10.1111/j.1476-5381.2011.01366.x
Keywords: angiotensin II, cognitive impairment, hypertension, receptor, stroke
Introduction
The renin-angiotensin system (RAS) in the brain is well known to be involved in systemic blood pressure control, including the regulation of cerebral blood flow (de Gasparo et al., 2000). Recent clinical trials have demonstrated that blockade of RAS could result in a reduction in the onset of stroke, probably independent of blood pressure lowering. Moreover, possible beneficial effects of RAS blockade on cognitive function are also becoming highlighted in the clinical field. These results led us to examine the roles of RAS in the brain, focusing on the pathogenesis of ischaemic stroke, cognitive impairment and neurological disorders. Consequently, it has been clarified that the local brain RAS plays an important role in a variety of neuronal functions. The major cardiovascular actions of angiotensin II have been reported to be mediated by the angiotensin II type 1 (AT1) receptor, whereas the role of a second receptor subtype known as the angiotensin II type 2 (AT2) receptor in brain ischaemic lesions is still an enigma. Accordingly, we here review the effects of activation of angiotensin II receptor subtypes on ischaemic brain damage and cognitive function, focusing on the function of AT2 receptor signalling.
Effects of angiotensin II type 1 receptor blocker treatment on ischaemic brain damage
Previous papers on the brain RAS mainly reported the regulatory mechanism of blood pressure (Jöhren et al., 1997; Davisson et al., 1998; 2000; Li et al., 2003). Recent clinical trials, such as LIFE (Dahlöf et al., 2002), MOSES (Schrader et al., 2005) and the JIKEI HEART STUDY (Mochizuki et al., 2007), demonstrated that administration of an AT1 receptor blocker (ARB) prevented the onset of stroke, independent of its blood pressure-lowering effect. This preventive effect of ARBs on the onset of stroke could be due to improvement of vascular remodelling, amelioration of the metabolic syndrome associated with anti-atherogenic effects, and reduction of atrial fibrillation (Figure 1). Moreover, a recent clinical study showed that prestroke treatment with an ARB reduced stroke severity, whereas this effect was not observed with an angiotensin converting enzyme (ACE) inhibitor (Fuentes et al., 2010). This finding is consistent with experimental data suggesting that ARBs could have cerebral protective effects.
Figure 1.
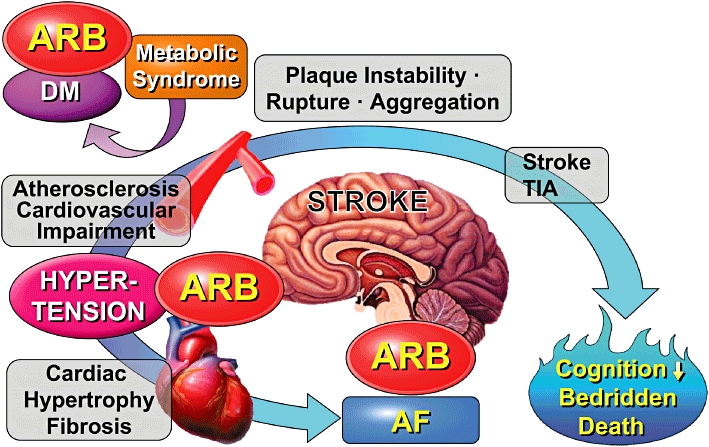
Angiotensin II type 1 receptor blockers (ARBs) improve vascular remodelling and metabolic syndrome associated with anti-atherogenic effects, and reduce atrial fibrillation, resulting in reduction of onset of stroke. AF, atrial fibrillation; DM, diabetes mellitus; TIA, transient cerebral ischaemic attack.
All components of the classical RAS exist in the brain (Jöhren et al., 1997; Saavedra, 2005). Mice with deletion of angiotensinogen (Maeda et al., 1999) or the AT1 receptor (Walther et al., 2002) show a reduction in the ischaemic area after middle cerebral artery (MCA) occlusion. Moreover, it has been reported that administration of an ARB decreases ischaemic brain damage (Iwai et al., 2004; Hamai et al., 2006; Saavedra et al., 2006; Zhou et al., 2006). Sustained blockade of AT1 receptors with an ARB could reverse pathological cerebrovascular change, oxidative stress and inflammation, thereby increasing cerebrovascular compliance and decreasing ischaemic brain damage (Chan, 2001; Ito et al., 2002; Unger, 2003). ARBs also could exert neuroprotective effects on ischaemic neuronal tissue and improve the neurological outcome of focal brain ischaemia (Engelhorn et al., 2004; Mogi et al., 2006).
Methods
We ensure that our target nomenclature conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2009).
Roles of AT1 and AT2 receptor stimulation in pathogenesis of ischaemic brain damage
Questions still remain as to whether activation of RAS could be really involved in exacerbation of ischaemic brain damage, and whether treatment with an ARB could prevent enhanced ischaemic brain damage induced by activation of the brain RAS. To examine these possibilities, we employed transgenic mice carrying both the human renin and angiotensinogen genes (hRN/hANG-Tg), which have been developed as a mouse model of human hypertension induced by activation of the human RAS (Fukamizu et al., 1993). Focal ischaemic brain damage was induced by permanent occlusion of the unilateral MCA by an intraluminal filament technique. The ischaemic brain area at 24 h after MCA occlusion was significantly enlarged in hRN/hANG-Tg mice, with a reduction of cerebral blood flow in the peripheral region of the MCA territory, increase in superoxide anion production in the brain and arteries, and increase in neurological deficit (Inaba et al., 2009b). Treatment with an ARB, valsartan, significantly reduced the ischaemic brain area and improved the neurological deficit after MCA occlusion in hRN/hANG-Tg mice, with restoration of cerebral blood flow in the peripheral region and decreases in superoxide anion production and blood pressure, whereas treatment with hydralazine, which decreased blood pressure to a level similar to that with ARB treatment in hRN/hANG-Tg mice, did not significantly reduce the brain ischaemic area. These results suggest that the preventive effects of ARBs on ischaemic brain damage are at least partly dependent on a decrease in oxidative stress and an increase in cerebral blood flow in the penumbra. We have also reported that an ARB increased capillary density, resulting in an increase in cerebral blood flow (Li et al., 2008). Therefore, a sustained reduction of activation of AT1 receptors by an ARB could be a therapeutic approach to prevent the progression of brain ischaemia, in addition to its hypotensive effect and preventive effect on the onset of stroke.
It has been reported that AT2 receptor stimulation antagonizes the effects of AT1 receptor stimulation in most tissues (Figure 2) (Horiuchi et al., 1999; de Gasparo et al., 2000). In the brain, AT2 receptors are expressed not only in the vascular wall, but also in the thalamus, hypothalamus and specific brain stem nuclei (Steckelings et al., 2005). Previous reports suggest that AT2 receptor stimulation is involved in axonal regeneration (Lucius et al., 1998), and memory and behaviour (Vervoort et al., 2002; Wright et al., 2002). These results point to the pathophysiological importance of the AT2 receptor in the clinical application of ARBs, which are widely used in patients of hypertension with the expectation of inhibition of the incidence and progression of cardiovascular disease (Figure 2). Indeed, we have previously reported that AT2 receptor stimulation could be involved in the beneficial effects of ARBs on vascular injury and cardiac remodelling (Wu et al., 2001; 2002;). However, the detailed function of AT2 receptor stimulation in the brain is not yet fully understood. We examined the roles of angiotensin II receptor subtypes in focal brain ischaemia and observed that the ischaemic area was significantly larger in AT2 receptor-deficient mice, with a decrease in surface cerebral blood flow and an increase in superoxide production (Iwai et al., 2004). ARB at a non-hypotensive dose significantly reduced the ischaemic area, neurological deficit and decrease of cerebral blood flow, as well as superoxide production and nicotinamide adenine dinucleotide phosphate oxidase activity in wild-type mice, whereas these inhibitory actions of ARB were weaker in AT2 receptor-deficient mice.
Figure 2.
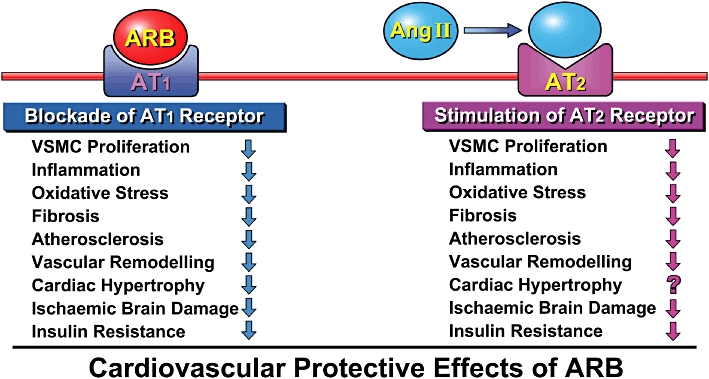
Angiotensin II type 1 (AT1) receptor blockers (ARBs) block AT1 receptor-mediated effects such as inflammation, oxidative stress and fibrosis, and unbound angiotensin II stimulates the angiotensin II type 2 (AT2) receptor. AT2 receptor stimulation is known to antagonize the effects of AT1 receptor stimulation in most tissues. VSMC, vascular smooth muscle cell.
Effects of angiotensin II type 1 receptor blocker treatment on cognitive function; roles of AT2 receptor stimulation
Dementia is a common serious health problem that impairs quality of life. Hypertension is a major risk factor for cerebrovascular disease including stroke, and contributes to the development of vascular dementia. Several clinical studies have shown that antihypertensive drug treatment is associated with reduced cognitive decline. However, it is still not clear which classes of antihypertensive drugs provide greater benefits than others. Recently, Li et al. reported that ARBs are associated with a significant reduction in the incidence and progression of Alzheimer's disease and dementia compared with ACE inhibitors and other cardiovascular drugs in a population of 819 491 predominantly male participants (98%) aged 65 years or more with cardiovascular disease (Li et al., 2010). We tested the hypothesis that continuous activation of the brain RAS is involved in impairment of cognitive function, using transgenic mice carrying both the human renin and angiotensinogen genes (Inaba et al., 2009a). Cognitive function evaluated by the shuttle avoidance test in wild-type mice gradually increased, whereas the avoidance rate in hRN/hANG-Tg mice did not increase from 14 weeks of age and it decreased thereafter with a decrease in cerebral surface blood flow (CBF) and increase in superoxide anion production. Administration of an ARB, olmesartan, attenuated the increase in blood pressure and ameliorated cognitive decline with enhancement of CBF and a reduction of oxidative stress in hRN/hANG-Tg mice. On the other hand, hydralazine did not improve the decrease in avoidance rate, in spite of a similar reduction of blood pressure to that by ARB. Angiotensin II induces cerebrovascular remodelling, promotes vascular inflammation and oxidative stress, and thereby impairs regulation of CBF (Kazama et al., 2004; Wei et al., 2007). Previous studies showed that endothelial function in cerebral vessels was impaired in a genetic model of angiotensin II-dependent hypertension (Didion et al., 2000; Franci et al., 2006). It is well known that CBF decreases with aging. These results suggest that continuous activation of the brain RAS impairs cognitive function via stimulation of the AT1 receptor with a decrease in CBF and increase in oxidative stress.
Oxidative stress has been implicated in age-related cognitive impairment (Liu et al., 2003; Dias-Santagata et al., 2007). In addition, there are reports indicating that oxidative stress is increased in the brain of Alzheimer's disease and other neurodegenerative disorders (Zhu et al., 2004). Taken together, these results suggest that the decline of cognitive function by continuous activation of the brain RAS in hRN/hANG-Tg mice is closely associated with enhanced oxidative stress due to excessive stimulation of the AT1 receptor. We propose that the sustained decrease in oxidative stress by blockade of the AT1 receptor by ARB could contribute to neural protection and an increase in CBF, resulting in the prevention of consequent cognitive decline (Figure 3).
Figure 3.
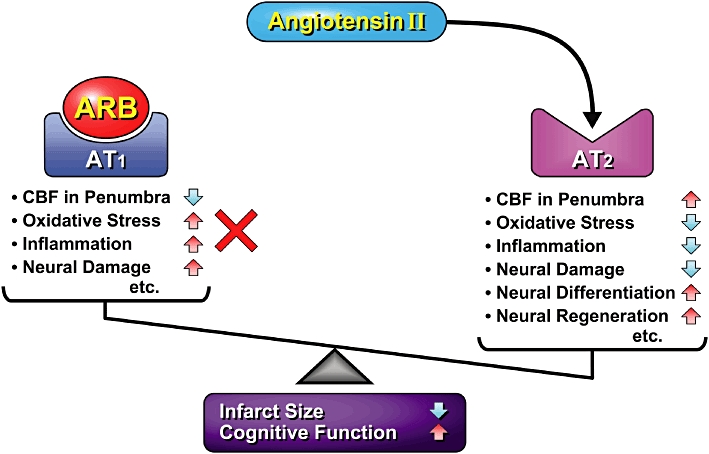
Blockade of angiotensin II type 1 (AT1) receptor by AT1 receptor blocker (ARB) results in increase in cerebral surface blood flow (CBF) in penumbra, and decreases in oxidative stress, inflammation and neural damage. Angiotensin II type 2 (AT2) receptor activation during ARB treatment could exert antagonistic effects on the AT1 receptor-mediated decrease in CBF, enhancement of oxidative stress, inflammation and neural damage, and increase neural differentiation and regeneration.
The importance of relative AT2 receptor stimulation during ARB treatment has been reported in terms of protection against brain damage (Iwai et al., 2004; Zhou et al., 2006). The AT2 receptor is reported to be expressed in areas related to learning and control of motor activity (Wright and Harding, 1995; Zhu et al., 2000), and is up-regulated after stroke (Lucius et al., 1998). Recent studies by Unger and colleagues demonstrated the possibility that stimulation of the AT2 receptor may promote cell differentiation and regeneration in neuronal tissue (Reinecke et al., 2003) and that AT2 receptor stimulation supported neuronal survival and neurite outgrowth in response to ischaemia-induced neuronal injury (Li et al., 2005). Moreover, Gallo-Payet et al. reported that angiotensin II induces neural differentiation and neurite outgrowth via mitogen-activated protein kinase (Gendron et al., 1999) or nitric oxide (Cote et al., 1998) through AT2 receptor activation, and is involved in cerebellar development (Cote et al., 1999). We also demonstrated that AT2 receptor mRNA expression was significantly increased in the ischaemic side of the brain after MCA occlusion, and that the passive avoidance rate to evaluate as an indicator of cognitive function was significantly impaired in AT2 receptor-null mice compared with wild-type mice (Mogi et al., 2006). Treatment with an ARB prevented the cognitive decline in wild-type mice, but this effect was weaker in AT2 receptor-null mice, suggesting that AT2 receptor stimulation during ARB treatment is important (Mogi et al., 2006). Moreover, compound 21, an orally active, non-peptidergic, highly selective AT2 receptor agonist, has been developed, which is reported to delay the occurrence of brain damage and prolong survival in spontaneously hypertensive stroke-prone rats by preventing renal damage (Gelosa et al., 2009).
We examined the possible signalling mechanism by which AT2 receptor stimulation could exert neuroprotective effects and enhance neural differentiation. We focused on MMS2 (methyl methanesulfonate sensitive 2), which belongs to a family of ubiquitin-conjugating enzyme variants (UEV) that are highly similar to ubiquitin-conjugating enzymes E2 (Ubc), and forms a complex with Ubc-13, as MMS2 was reported to be highly expressed in the rat brain in late embryonic development and then fall markedly during maturation of the central nervous system (Hofsaess and Kapfhammer, 2003), suggesting that it plays a pivotal role in neuronal development and differentiation. We demonstrated that neurons treated with small interfering (si) RNA of MMS2 failed to exhibit neurite outgrowth and synapse formation (Li et al., 2007). After AT2 receptor stimulation, ATIP (Nouet et al., 2004) (AT2 receptor interacting protein) (also known as AT2 receptor binding protein ATBP) (Wruck et al., 2005) and SHP-1 were translocated into the nucleus following formation of their complex and transactivated MMS2, resulting in neural differentiation and protection. Furthermore, we observed that in ischaemic brain regions, MMS2 was increased in wild-type mice but not in AT2 receptor-null mice, and that intracerebroventricular administration of MMS2-siRNA impaired the avoidance rate after MCA occlusion compared with that in control-siRNA-transfected mice (Mogi et al., 2006).
Cellular therapy has been focused on as a new therapeutic approach to restore injured neurons in the chronic stage (Shen et al., 2007) and to protect neurons from ischaemic-reperfusion damage in the acute phase of stroke (Zhao et al., 2006) using bone marrow stromal cells (BMSC), neural stem cells (Kelly et al., 2004), hematopoietic stem cells (Hayashi et al., 2006) and umbilical cord blood (Willing et al., 2003). We examined the possibility that deletion of the AT2 receptor in BMSC could attenuate the cerebroprotective effects of BMSC prepared from AT2 receptor-null mice (Iwanami et al., 2008). We reported that AT2 receptor-deficient BMSC-injected mice showed a marked decrease in survival rate after ischaemia followed by reperfusion, with increases in the ischaemic area and neurological deficit, brain oedema, and inflammatory response such as tumour necrosis factor-α. Mice with injection of wild-type BMSC treated with an ARB exhibited no operative death until 6 days after injury. These results suggest that AT1 receptor blockade and consequent AT2 receptor stimulation with unbound angiotensin II could contribute to the protective effects of BMSC. The more detailed mechanism, whether a direct or indirect mechanism by effect of BMSC such as involvement of neurohumoral factors, needs to be elucidated for future clinical application.
Metabosartans: AT1 receptor blocking action with partial peroxisome proliferator-activated receptor-γ agonistic effect
Recently, additional beneficial effects of ARBs have been highlighted (Kurtz and Klein, 2009). Some ARBs (so-called metabosartans) (Morishita et al., 2010) such as telmisartan and irebesartan have been reported to have an AT1 receptor-blocking action, with a partial peroxisome proliferator-activated receptor (PPAR)-γ agonistic effect (Benson et al., 2004; Schupp et al., 2004). PPAR-γ activation in the brain has been reported to prevent brain damage via anti-inflammatory effects in cells such as neurons (Luna-Medina et al., 2005), endothelial cells (Wang et al., 2002), astrocytes and microglia (Klotz et al., 2003), antioxidative actions and improvement of endothelial function (Camacho et al., 2004; Nakamura et al., 2007). Moreover, amyloid-beta (Aβ) clearance and neural stem cell proliferation are also reported to be enhanced by PPAR-γ activation (Camacho et al., 2004; Wada et al., 2006). Therefore, agents with a PPAR-γ agonistic effect are expected to be neuroprotective in ischaemic injury after stroke (Tureyen et al., 2007). We recently reported that telmisartan exerted protective effects against ischaemic brain damage through AT1 receptor blockade and PPAR-γ stimulation and had a preventive effect on cognitive impairment in a mouse model of Alzheimer's disease with intracerebroventricular injection of Aβ (Tsukuda et al., 2009; Iwanami et al., 2010). It has been also reported that the anti-inflammatory and antioxidative effects of telmisartan with PPAR-γ activation have protective roles against cognitive impairment and white matter damage after chronic cerebral hypoperfusion (Washida et al., 2010).
It has been reported that AT1 receptor blockade decreases NFκB activation, with PPAR-γ activation in the vasculature (Tham et al., 2002). AT1 receptor stimulation activates ERK (extracellular signal-regulated kinase), and PPAR-γ stimulation inhibits this ERK activation in vascular smooth muscle cells (VSMC) (Takeda et al., 2001). Moreover, PPAR-γ stimulation is known to suppress AT1 receptor expression in VSMC (Sugawara et al., 2001). Angiotensin II induces PPAR-γ activation in PC12W cells via AT2 receptor activation (Zhao et al., 2005), suggesting that metabosartans could further enhance PPAR-γ stimulation in the brain. Taken together, these results support the notion that metabosartans could exert protective effects against ischaemic brain damage via AT1 receptor blockade and PPAR-γ stimulation involving the AT2 receptor (Figure 4).
Figure 4.
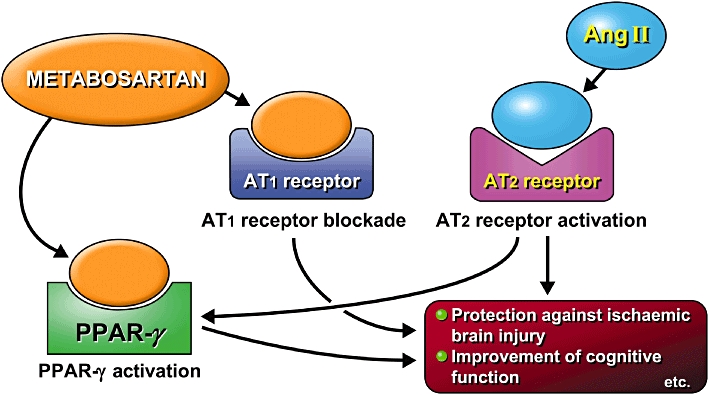
Metabosartans could exert protective effects on ischaemic brain damage, and increase cognitive function via angiotensin II type 1 (AT1) receptor blockade and partial peroxisome proliferator-activated receptor (PPAR)-γ stimulation with activation of angiotensin II type 2 (AT2) receptor.
Conclusion
The roles of AT2 receptor stimulation in the brain is still an enigma. Most studies addressing the roles of the AT2 receptor have been performed in genetically altered mice with or without ARB, or using the selective AT2 receptor antagonists such as PD123319 or PD123177 (Kaschina et al., 2008; Rompe et al., 2010). Therefore, elucidation of AT2 receptor-related effects has been difficult in the past because of the lack of a specific and selective AT2 receptor agonist. The most commonly used AT2 receptor agonist is the peptide CGP42112A having partly antagonistic properties, which rendered CGP42112A a problematic tool for research and prevented its development for clinical use (Rompe et al., 2010). In 2004, synthesis of the first selective, orally active AT2 receptor agonist, compound 21, has been published (Wan et al., 2004). Synthesis of this compound enables us to examine AT2 receptor actions in vitro and in vivo by direct receptor stimulation and also principally offers the possibility to use AT2 receptor stimulation as a therapeutic tool (Bosnyak et al., 2010; Unger and Dahlöf, 2010). It is reported that angiotensin II induces PPAR-γ activation in PC12W cells via AT2 receptor activation (Zhao et al., 2005), suggesting that possible crosstalk of AT2 receptor activation and PPAR-γ stimulation in the brain could contribute more protective effects against ischaemic brain damage and cognitive impairment. However, the possible beneficial roles of AT2 receptor activation with PPAR-γ stimulation have to be investigated and clarified in more detail. Moreover, addressing whether or not clinical efficacy correlates with degree of PPAR-γ stimulation could contribute to future development of more potent and therapeutically effective ARBs with the effects beyond simple AT1 receptor blockade and AT2 receptor stimulation. It is well known that diabetes is one of the major risk factors of the onset of stroke. People with diabetes mellitus are also at increased risk of cognitive dysfunction and dementia (Reijmer et al., 2010). Therefore, we could expect more potential beneficial effects of ARBs with PPAR-γ agonistic action in this population.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan.
Glossary
Abbreviations
- Aβ
amyloid-beta
- ACE
angiotensin converting enzyme
- ARB
angiotensin II type 1 receptor blocker
- AT1 receptor
angiotensin II type 1 receptor
- AT2 receptor
angiotensin II type 2 receptor
- BMSC
bone marrow stromal cell
- CBF
cerebral surface blood flow
- ERK
extracellular signal-regulated kinase
- hRN/hANG-Tg
human renin and angiotensinogen genes
- MCA
middle cerebral artery
- MMS2
methyl methanesulfonate sensitive 2
- PPAR
partial peroxisome proliferator-activated receptor
- RAS
renin-angiotensin system
- si
small interfering
- VSMC
vascular smooth muscle cell
Conflict of interest
None.
Supporting Information
Teaching Materials; Figs 1–4 as PowerPoint slide.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J Neurosci. 2004;24:10908–10917. doi: 10.1523/JNEUROSCI.3987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Cote F, Do TH, Laflamme L, Gallo JM, Gallo-Payet N. Activation of the AT(2) receptor of angiotensin II induces neurite outgrowth and cell migration in microexplant cultures of the cerebellum. J Biol Chem. 1999;274:31686–31692. doi: 10.1074/jbc.274.44.31686. [DOI] [PubMed] [Google Scholar]
- Cote F, Laflamme L, Payet MD, Gallo-Payet N. Nitric oxide, a new second messenger involved in the action of angiotensin II on neuronal differentiation of NG108-15 cells. Endocr Res. 1998;24:403–407. doi: 10.3109/07435809809032622. [DOI] [PubMed] [Google Scholar]
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. LIFE Study Group Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertensive study (LIFE); a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Yang G, Beltz TG, Cassell MD, Jhonson AK, Sigmund CD. The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res. 1998;83:1047–1058. doi: 10.1161/01.res.83.10.1047. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Santagata D, Fulga TA, Duttaroy A, Feany MB. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J Clin Invest. 2007;117:236–245. doi: 10.1172/JCI28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didion SP, Sigmund CD, Faraci FM. Impaired endothelial function in transgenic mice expressing both human renin and human angiotensinogen. Stroke. 2000;31:760–765. doi: 10.1161/01.str.31.3.760. [DOI] [PubMed] [Google Scholar]
- Engelhorn T, Goerike S, Doerfler A, Okorn C, Forsting M, Heusch G, et al. The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:467–474. doi: 10.1097/00004647-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Franci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: new insights from genetic models. J Cereb Blood Flow Metab. 2006;26:449–455. doi: 10.1038/sj.jcbfm.9600204. [DOI] [PubMed] [Google Scholar]
- Fuentes B, Fernández-Domínguez J, Ortega-Casarrubios MA, SanJosé B, Martínez-Sánchez P, Díez-Tejedor E. Treatment with angiotensin receptor blockers before stroke could exert a favourable effect in acute cerebral infarction. J Hypertens. 2010;28:575–581. doi: 10.1097/HJH.0b013e3283350f50. [DOI] [PubMed] [Google Scholar]
- Fukamizu A, Sugimura K, Takimoto E, Sugiyama F, Seo MS, Takahashi S, et al. Chimeric renin-angiotensin system demonstrates sustained increase in blood pressure of transgenic mice carrying both human renin and human angiotensinogen genes. J Biol Chem. 1993;268:11617–11621. [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Gelosa P, Pignieri A, Fandriks L, de Gasparo M, Hallberg A, Banfi C, et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009;27:2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- Gendron L, Laflamme L, Rivard N, Asselin C, Payet MD, Gallo-Payet N. Signals from the AT2 (angiotensin type 2) receptor of angiotensin II inhibit p21ras and activate MAPK (mitogen-activated protein kinase) to induce morphological neuronal differentiation in NG108-15 cells. Mol Endocrinol. 1999;13:1615–1626. doi: 10.1210/mend.13.9.0344. [DOI] [PubMed] [Google Scholar]
- Hamai M, Iwai M, Ide A, Tomochika H, Tomono Y, Mogi M, et al. Comparison of inhibitory action of candesartan and enalapril on brain ischemia through inhibition of oxidative stress. Neuropharmacology. 2006;51:822–828. doi: 10.1016/j.neuropharm.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, et al. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- Hofsaess U, Kapfhammer JP. Identification of numerous genes differentially expressed in rat brain during postnatal development by suppression subtractive hybridization and expression analysis of the novel rat gene rMMS2. Brain Res Mol Brain Res. 2003;113:13–27. doi: 10.1016/s0169-328x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- Inaba S, Iwai M, Furuno M, Tomono Y, Kanno H, Senba I, et al. Continuous activation of renin-angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension. 2009a;53:356–362. doi: 10.1161/HYPERTENSIONAHA.108.123612. [DOI] [PubMed] [Google Scholar]
- Inaba S, Iwai M, Tomono Y, Senba I, Furuno M, Kanno H, et al. Exaggeration of focal cerebral ischemia in transgenic mice carrying human renin and human angiotensinogen genes. Stroke. 2009b;40:597–603. doi: 10.1161/STROKEAHA.108.519801. [DOI] [PubMed] [Google Scholar]
- Ito T, Yamakawa H, Bregonzio C, Terrón JA, Falcón-Neri A, Saavedra JM. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002;33:2297–2303. doi: 10.1161/01.str.0000027274.03779.f3. [DOI] [PubMed] [Google Scholar]
- Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, et al. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation. 2004;110:843–848. doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]
- Iwanami J, Mogi M, Li JM, Tsukuda K, Min LJ, Sakata A, et al. Deletion of angiotensin II type 2 receptor attenuates protective effects of bone marrow stromal cell treatment on ischemia-reperfusion brain injury in mice. Stroke. 2008;39:2554–2559. doi: 10.1161/STROKEAHA.107.513275. [DOI] [PubMed] [Google Scholar]
- Iwanami J, Mogi M, Tsukuda K, Min LJ, Sakata A, Jing F, et al. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J Hypertens. 2010;28:1730–1737. doi: 10.1097/HJH.0b013e32833a551a. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Imboden H, Häuser W, Maye I, Sanvitto GL, Saavedra JM. Localization of angiotensin-covertingenzyme, angiotensin II, angiotensin II receptor subtypes, and vasopressin in the mouse hypothalamus. Brain Res. 1997;757:218–227. doi: 10.1016/s0006-8993(97)00220-5. [DOI] [PubMed] [Google Scholar]
- Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L, Sastre M, Kreutz A, Gavrilyuk V, Klockgether T, Feinstein DL, et al. Noradrenaline induces expression of peroxisome proliferator activated receptor gamma (PPARgamma) in murine primary astrocytes and neurons. J Neurochem. 2003;86:907–916. doi: 10.1046/j.1471-4159.2003.01909.x. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Klein U. Next generation multifunctional angiotensin receptor blockers. Hypertens Res. 2009;32:826–834. doi: 10.1038/hr.2009.135. [DOI] [PubMed] [Google Scholar]
- Li Z, Iwai M, Wu L, Shiuch T, Jinno T, Cui TX, et al. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Physiol Heart Circ Physiol. 2003;284:H116–H121. doi: 10.1152/ajpheart.00515.2002. [DOI] [PubMed] [Google Scholar]
- Li J, Culman J, Hortnagl H, Zhao Y, Gerova N, Timm M, et al. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 2005;19:617–619. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- Li JM, Mogi M, Tsukuda K, Tomochika H, Iwanami J, Min LJ, et al. Angiotensin II-induced neural differentiation via angiotensin II type 2 (AT2) receptor-MMS2 cascade involving interaction between AT2 receptor-interacting protein and Src homology 2 domain-containing protein-tyrosine phosphatase 1. Mol Endocrinol. 2007;21:499–511. doi: 10.1210/me.2006-0005. [DOI] [PubMed] [Google Scholar]
- Li JM, Mogi M, Iwanami J, Min LJ, Tsukuda K, Sakata A, et al. Temporary pretreatment with the angiotensin II type 1 receptor blocker, valsartan, prevents ischemic brain damage through an increase in capillary density. Stroke. 2008;39:2029–2036. doi: 10.1161/STROKEAHA.107.503458. [DOI] [PubMed] [Google Scholar]
- Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucius R, Gallinat S, Rosenstiel P, Herdegan T, Sievers J, Unger T. The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J Exp Med. 1998;188:661–670. doi: 10.1084/jem.188.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martinez A, Perez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor gamma activation. J Biol Chem. 2005;280:21453–21462. doi: 10.1074/jbc.M414390200. [DOI] [PubMed] [Google Scholar]
- Maeda K, Hata R, Bader M, Walther T, Hossmann KA. Larger anastomoses in angiotensinogen-knockout mice attenuate early metabolic disturbances after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1999;19:1092–1098. doi: 10.1097/00004647-199910000-00005. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Dahlöf B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, et al. Jikei Heart Study Group Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomized, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–1439. doi: 10.1016/S0140-6736(07)60669-2. [DOI] [PubMed] [Google Scholar]
- Mogi M, Li JM, Iwanami J, Min LJ, Tsukuda K, Iwai M, et al. Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension. 2006;48:141–148. doi: 10.1161/01.HYP.0000229648.67883.f9. [DOI] [PubMed] [Google Scholar]
- Morishita R, Kanda Y, Nakajima M. Irebesratn: second generation of ARB as Metabosartan. Curr Hypertens Rev. 2010;6:173–179. [Google Scholar]
- Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, et al. Pioglitazone exerts protective effects against stroke in stroke-prone spontaneously hypertensive rats, independently of blood pressure. Stroke. 2007;38:3016–3022. doi: 10.1161/STROKEAHA.107.486522. [DOI] [PubMed] [Google Scholar]
- Nouet S, Amzallag N, Li JM, Louis S, Seitz I, Cui TX, et al. Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor-interacting protein, ATIP. J Biol Chem. 2004;279:28989–28997. doi: 10.1074/jbc.M403880200. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26:507–519. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- Reinecke K, Lucius R, Reinecke A, Rickert U, Herdegen T, Unger T. Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kappaB. FASEB J. 2003;17:2094–2096. doi: 10.1096/fj.02-1193fje. [DOI] [PubMed] [Google Scholar]
- Rompe F, Artuc M, Hallberg A, Alterman M, Ströder K, Thöne-Reineke C, et al. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Benicky J, Zhou J. Mechanisms of the anti-ischemic effect of angiotensin II AT1 receptor antagonists in the brain. Cell Mol Neurobiol. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J, Lüders S, Kulschewski A, Hammersen F, Plate K, Berger J, et al. MOSES Study Group Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- Steckelings UM, Kaschina E, Unger T. The AT2 receptor-a matter of love and hate. Peptides. 2005;26:1401–1149. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sugawara A, Takeuchi K, Uruno A, Ikeda Y, Arima S, Kudo M, et al. Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology. 2001;142:3125–3134. doi: 10.1210/endo.142.7.8272. [DOI] [PubMed] [Google Scholar]
- Takeda K, Ichiki T, Tokunou T, Iino N, Takeshita A. 15-Deoxy-delta 12,14-prostaglandin J2 and thiazolidinediones activate the MEK/ERK pathway through phosphatidylinositol 3-kinase in vascular smooth muscle cells. J Biol Chem. 2001;276:48950–48955. doi: 10.1074/jbc.M108722200. [DOI] [PubMed] [Google Scholar]
- Tham DM, Martin-McNulty B, Wang YX, Wilson DW, Vergona R, Sullivan ME, et al. Angiotensin II is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiol Genomics. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- Tsukuda K, Mog M, Iwanami J, Min LJ, Sakata A, Jing F, et al. Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009;54:782–787. doi: 10.1161/HYPERTENSIONAHA.109.136879. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, et al. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Unger T. Inhibiting angiotensin receptors in the brain: possible therapeutic implications. Curr Med Res Opin. 2003;19:449–451. doi: 10.1185/030079903125001974. [DOI] [PubMed] [Google Scholar]
- Unger T, Dahlöf B. Compoun1, the first orally active, selective agonist of the angiotensin type 2 receptor (AT2): implications for AT2 receptor research and therapeutic potential. J Renin Angiotensin Aldosterone Syst. 2010;11:75–77. doi: 10.1177/1470320309347792. [DOI] [PubMed] [Google Scholar]
- Vervoort VS, Beachem MA, Edwards PS, Ladd S, Miller KE, de Mollerat X, et al. AGTR2 mutations in X-linked mental retardation. Science. 2002;296:2401–2403. doi: 10.1126/science.1072191. [DOI] [PubMed] [Google Scholar]
- Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, Kubota N, et al. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem. 2006;281:12673–12681. doi: 10.1074/jbc.M513786200. [DOI] [PubMed] [Google Scholar]
- Walther T, Olah L, Harms C, Maul B, Bader M, Hörtnagl H, et al. Ischemic injury in experimental stroke depends on angiotensin II. FASEB J. 2002;16:169–176. doi: 10.1096/fj.01-0601com. [DOI] [PubMed] [Google Scholar]
- Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, et al. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- Washida K, Ihara M, Nishio K, Fujita Y, Maki T, Yamada M, et al. Nonhypotensive dose of telmisartan attenuates cognitive impairment partially due to peroxisome proliferator-activated receptor-gamma activation in mice with chronic cerebral hypoperfusion. Stroke. 2010;41:1798–1806. doi: 10.1161/STROKEAHA.110.583948. [DOI] [PubMed] [Google Scholar]
- Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, et al. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension. 2007;50:384–391. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]
- Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. Brain angiotensin receptor subtypes AT1, AT2, and AT4 and their functions. Regul Pept. 1995;59:269–295. doi: 10.1016/0167-0115(95)00084-o. [DOI] [PubMed] [Google Scholar]
- Wright JW, Reichert JR, Davis CJ, Hatding JW. Neural plasticity and the brain renin-angiotensin system. Neurosci Biobehav Rev. 2002;26:529–552. doi: 10.1016/s0149-7634(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Wruck CJ, Funke-Kaiser H, Pufe T, Kusserow H, Menk M, Schefe JH, et al. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25:57–64. doi: 10.1161/01.ATV.0000150662.51436.14. [DOI] [PubMed] [Google Scholar]
- Wu L, Iwai M, Nakagami H, Chen R, Suzuki J, Akishita M, et al. Roles of angiotensin II type 2 receptor stimulation associated with selective angiotensin II type 1 receptor blockade with valsartan in the improvement of inflammation-induced vascular injury. Circulation. 2001;104:2716–2721. doi: 10.1161/hc4601.099404. [DOI] [PubMed] [Google Scholar]
- Wu L, Iwai M, Nakagami H, Chen R, Suzuki J, Akishita M, et al. Effect of angiotensin II type 1 receptor blockade on cardiac remodeling in angiotensin II type 2 receptor null mice. Arterioscler Thromb Vasc Biol. 2002;22:49–54. doi: 10.1161/hq0102.102277. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Foryst-Ludwig A, Bruemmer D, Culman J, Bader M, Unger T, et al. Angiotensin II induces peroxisome proliferator-activated receptor gamma in PC12W cells via angiotensin type 2 receptor activation. J Neurochem. 2005;94:1395–1401. doi: 10.1111/j.1471-4159.2005.03275.x. [DOI] [PubMed] [Google Scholar]
- Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, et al. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26:1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- Zhou J, Pavel J, Macova M, Yu ZX, Imboden H, Ge L, et al. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]
- Zhu YZ, Chimon GN, Zhu YC, Lu Q, Li B, Hu HZ, et al. Expression of angiotensin II AT2 receptor in the acute phase of stroke in rats. Neuroreport. 2000;11:1191–1194. doi: 10.1097/00001756-200004270-00009. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Lee HG, Casadesus G, Smith MA, Perry G. Oxidative stress signaling in Alzheimer's disease. Brain Res. 2004;1000:32–39. doi: 10.1016/j.brainres.2004.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


