Abstract
BACKGROUND AND PURPOSE
There is increasing evidence that potassium channels are involved in the cardiovascular dysfunction of sepsis. This evidence was obtained after the systemic inflammation, cardiovascular dysfunction and organ damage had developed. Here we have studied the consequences of early interference with potassium channels on development of sepsis.
EXPERIMENTAL APPROACH
Sepsis was induced by caecal ligation and puncture (CLP) or sham surgery in Wistar rats. Four hours after surgery, animals received tetraethylammonium (TEA; a non-selective potassium channel blocker) or glibenclamide (a selective ATP-sensitive potassium channel blocker). Twenty-four hours after surgery, inflammatory, biochemical, haemodynamic parameters and survival were evaluated.
KEY RESULTS
Sepsis significantly increased plasma NOx levels, expression of inducible nitric oxide synthase (NOS-2) protein in lung and thigh skeletal muscle, lung myeloperoxidase, urea, creatinine and lactate levels, TNF-α and IL-1β, hypotension and hyporesponsiveness to phenylephrine and hyperglycemia followed by hypoglycemia. TEA injected 4 h after surgery attenuated the increased NOS-2 expression, reduced plasma NOx, lung myeloperoxidase activity, levels of TNF-α and IL-1β, urea, creatinine and lactate levels, prevented development of hypotension and hyporesponsiveness to phenylephrine, the alterations in plasma glucose and reduced late mortality by 50%. Glibenclamide did not improve any of the measured parameters and increased mortality rate, probably due to worsening the hypoglycemic phase of sepsis.
CONCLUSIONS AND IMPLICATIONS
Early blockade of TEA-sensitive (but not the ATP-sensitive subtype) potassium channels reduced organ damage and mortality in experimental sepsis. This beneficial effect seems to be, at least in part, due to reduction in NOS-2 expression.
Keywords: potassium channels, septic shock, tetraethylammonium, glibenclamide, vascular hyporesponsiveness, sepsis, nitric oxide, NOS-2, peritonitis
Introduction
Sepsis is an infection-induced systemic inflammatory syndrome, accompanied by hypotension due to systemic vasodilatation, and affects every major organ within the body, potentially leading to their failure (see O'Brien et al., 2007).
Bacterial invasion releases inflammatory stimuli such as lipopolysaccharide (LPS), which evokes an overwhelming spectrum of biological activities and pro-inflammatory proteins, among them the inducible nitric oxide (NO) synthase (NOS-2) isoform (see Knowles and Moncada, 1994; Assreuy, 2006). Activation of macrophages and cytokines by inflammation and the subsequent formation of reactive nitrogen species have currently been regarded as central pathogenic importance in sepsis.
Plasma membrane potential has important consequences for cell signalling; for instance, macrophage TNF-α release is highly dependent on the cell membrane potential (Haslberger et al., 1992). Among other ion channels, potassium channels have an important role in macrophage activation being involved in IL-1β synthesis (Walev et al., 1995), NOS-2 expression and NO production (Lowry et al., 1998; Wu et al., 1998) and TNF-α release (Blunck et al., 2001; Seydel et al., 2001). Several groups including our own have been studying the role of potassium channels as effectors of the vascular effects of NO during sepsis (Hall et al., 1996; Taguchi et al., 1996; Chen et al., 1999; Silva-Santos et al., 2002; see Fernandes and Assreuy, 2008). It is well known that NO opens potassium channels (Williams et al., 1988; Fujino et al., 1991) either via cGMP (Robertson et al., 1993; Archer et al., 1994) or directly (Bolotina et al., 1994). There is increasing evidence from experimental studies that potassium channels are involved in the development of systemic vasodilatation and hyporesponsiveness to vasoconstrictors (Landry and Oliver, 1992; Vanelli et al., 1995; Gardiner et al., 1999; Lange et al., 2006). However, these studies and others have examined the role of potassium channels in mediating NO effects after sepsis has been established. For example, injection of potassium channel blockers acutely improves the responsiveness to phenylephrine if given 24 h after sepsis induction (Sordi et al., 2010). This beneficial effect however is evanescent and does not change the mortality or the general condition of the animals.
To better understand the role of potassium channels in sepsis, in this work, we employed two potassium channels blockers, tetraethylammonium (TEA) and glibenclamide, in the sepsis model of caecal ligation and puncture (CLP). The CLP model resembles ruptured appendicitis or perforated diverticulitis in humans, and it is pathophysiologically defined by two phases: a hyperdynamic phase (early) followed by a hypodynamic phase (late). The early phase is characterized by a hypermetabolic and hyperdynamic cardiovascular status, whereas the late phase sepsis is characterized by a hypometabolic and hypodynamic states (for review, see Buras et al., 2005). Therefore, the purpose of the present work was to evaluate the consequences of early blockade of potassium channels on sepsis development, using an experimental model of sepsis in rats.
Methods
Animals
All animal care and experimental procedures were approved by the University Institutional Ethics Committee and are in accordance with CONCEA (National Council for the Control of Animal Experimentation) Guidelines. Female Wistar rats (weighing 200–250 g) were housed in a temperature- and light-controlled room (23 ± 2°C; 12 h light/dark cycle), with free access to water and food.
CLP
Sepsis was induced with the CLP procedure, as previously described (Fernandes et al., 2009). Briefly, rats were anaesthetized with ketamine and xylazine (90 and 15 mg·kg−1 respectively, i.p.). Under aseptic conditions, a 2 cm midline laparotomy was performed to allow exposure of the caecum. The caecum was tightly ligated with a 3.0 silk suture at its base, below the ileocaecal valve, perforated 20 times with an 18 gauge needle, and a small amount of caecal content was squeezed through punctures. The caecum was replaced and the peritoneal wall and skin incisions were closed. Sham-operated rats underwent similar surgical procedures, but the caecum was not ligated or punctured. All animals received 10 mL·kg−1 of sterile warm Dulbecco's phosphate-buffered saline (PBS; in mM, 137 NaCl, 2.7 KCl, 1.5 KH2PO4 and 8.1 NaHPO4; pH 7.4) subcutaneously (s.c.).
Mean arterial pressure (MAP) measurement
Under anaesthesia with ketamine and xylazine (as above, supplemented at 45 to 60 min intervals), heparinized PE-20 and PE-50 polyethylene catheters were inserted into the left femoral vein for drug injections and into the right carotid artery for recording of MAP respectively. Heart rate was derived from the blood pressure signal. To prevent clotting, a bolus dose of heparin (300 IU) was injected immediately after vein cannulation. Animals were allowed to breathe spontaneously via a tracheal cannula. Body temperature was monitored by a rectal thermometer and maintained at 37 ± 1°C by means of a heating table. Blood pressure data were recorded with a catheter pressure transducer (Mikro-Tip®, Millar Instruments, Inc., Houston, TX, USA) coupled to a Powerlab 8/30 (AD Instruments Pty Ltd., Castle Hill, Australia). Results were expressed as mean ± SEM of the peak changes in MAP (as mmHg) relative to baseline and recorded following administration of a given compound. At the end of the experiment, animals were killed by an overdose of chloral hydrate.
Western blotting
Lung and thigh skeletal muscle tissues were homogenized in ice-cold buffer containing protease inhibitors (in mM: 50 HEPES, 1 MgCl2, 10 EDTA, and 1% Triton X-100, pH 6.4, containing 1 µg·mL−1 each of aprotinin, leupeptin, soy bean trypsin inhibitor and 1 mM phenylmethanesulphonyl fluoride). Protein samples (75 µg·lane−1) were subjected to denaturing gel electrophoresis (SDS-PAGE, 8% gel). After electrophoresis, proteins were electro-transferred to PVDF membranes (0.8 mA/cm2; 15 V in Tris-glycine buffer composed by 48 mM Tris–HCl/39 mM glycine/10% methanol). The membrane was incubated for 1 h at room temperature with T-PBS (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 10.8 mM Na2HPO4.2H2O and 0.05% Tween-20, pH 7.4) containing 5% skimmed milk. Membranes were then incubated with rabbit polyclonal anti-NOS-2 antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-actin antibody (1:1000; Santa Cruz Biotechnology) overnight at 4°C. Membranes were washed and incubated with biotinylated secondary antibodies (1:1000; Amersham Biosciences, Buckinghamshire, UK) and then with streptavidin horseradish peroxidase conjugate (1:1000; Amersham Biosciences). Immunocomplexes were visualized by chemiluminescence, and band intensity was quantified by densitometry using Scion Image software (Scion Co., Frederick, MD, USA).
NOx (nitrite + nitrate) assay
Briefly, zinc sulphate–deproteinized plasma samples were subjected to nitrate conversion. Nitrate was converted to nitrite using Escherichia coli nitrate reductase for 3 h at 37°C. Samples were centrifuged to remove bacteria, and 100 µL of each sample was mixed with Griess reagent (1% sulphanilamide in 10% phosphoric acid/0.1% naphthylethylenediamine in Milli-Q water) in a 96-well plate and read at 540 nm in a plate reader. Standard curves of nitrite and nitrate (0–150 µM) were run simultaneously. As under these conditions nitrate conversion was always greater than 90%, no corrections were made. Values are expressed as µM NOx (nitrate + nitrite).
Myeloperoxidase (MPO) assay
Lungs were homogenized in hexadecyltrimetylammonium bromide buffer (HTAB; 0.5% in 80 mM sodium phosphate buffer, pH 5.4). Supernatant was assayed for MPO activity using a published method (Bradley et al., 1982). Total protein was determined according the Bradford method. Myeloperoxidase activity was expressed as optical density at 450 nm per mg of protein.
Glucose, urea, creatinine and lactate levels
Tail blood glucose was measured electrochemically by using Trackease Smart System (Home Diagnostics, Inc., Fort Lauderdale, FL, USA). Plasma levels of urea nitrogen, creatinine and lactate were measured by using commercially available clinical assay kits (Gold Analisa Diagnostica and BioClin, Belo Horizonte, MG, Brazil).
Leukocyte and blood bacteria counts
Total leukocyte numbers were determined by haemocytometer cell counter and was reported as 103cells mm−3. Blood was collected 24 h after CLP or sham surgery under sterile conditions. Serial 10-fold dilutions of blood were prepared in sterile water, and 10 µL of each dilution was plated on Mueller-Hinton agar dishes and incubated at 37°C; colony-forming units (CFU) were analysed after 24 h, and the results were expressed as CFU mL−1 of blood.
Determination of cytokine levels
TNF-α and IL-1β in serum were measured by commercially available enzyme-linked immunosorbent assay kits (PeproTech Inc., Rocky Hill, NJ, USA).
Experimental design
Survival
Rats were sham-operated or submitted to CLP as described above and randomized to receive TEA (8 or 16 mg·kg−1; s.c.), glibenclamide (1 or 20 mg·kg−1; s.c.) or vehicle [PBS or dimethyl sulphoxide (DMSO) respectively] 4 h after CLP procedure. Groups (TEA or glibenclamide) were studied on different days, and each group was compared with its control group, because they had received different vehicles (PBS or DMSO). The injected volume of DMSO was never higher than 0.5 mL·kg−1. In order to evaluate the effect of a partial or a total inducible NOS-2 inhibition on survival rate, 12 h after CLP surgery, a group of animals received aminoguanidine (10 or 200 mg·kg−1; s.c.) or 1400W [N-(3-(aminomethyl)-benzyl)acetamidine; 0.3 mg·kg−1; s.c.] or vehicle (PBS). Survival was recorded every 12 h over a 3 day period. Doses of potassium channel blockers and NOS-2 inhibitors were chosen based on our own experience (Silva-Santos and Assreuy, 1999; Silva-Santos et al., 2002; Sordi et al., 2010) and from others (Sorrentino et al., 1999; Benjamim et al., 2000).
Vascular reactivity and tissue collection
Rats were sham-operated or submitted to CLP as described above and randomized to receive TEA (8 mg·kg−1; s.c.), glibenclamide (1 mg·kg−1; s.c.) or vehicle (PBS or DMSO) 4 h after CLP surgery. Twenty hours later, animals were prepared for MAP recording, and a randomized dose–response curve to phenylephrine (3, 10 and 30 nmol·kg−1) was obtained. Each dose of the vasoconstrictor was given only when the pressor effect of the preceding one had fully subsided. Basal MAP and heart rate (HR) were also recorded. Immediately after the experiment, blood samples were obtained for measurement of plasma urea, creatinine and NOx levels and lung and thigh skeletal muscle tissues for NOS-2 immunoelectrophoresis. For cytokine measurement, blood samples were collected, and serum was obtained for the assays. An additional group of animals was included where animals received NOS-2 inhibitors (1400W 0.3 mg·kg−1 or aminoguanidine 10 or 200 mg·kg−1) 12 h after surgery, and blood samples were obtained for plasma NOx levels measurement.
Blood glucose measurement
Rats were sham- or CLP-operated and randomized to receive TEA (8 mg·kg−1; s.c.), glibenclamide (1 mg·kg−1; s.c.) or vehicle (PBS or DMSO) 4 h after the surgery. Six, 12 and 24 h after CLP procedure, 1 µL of blood was obtained from the tail tip, and blood glucose levels were measured as described above.
Statistical analysis
Data are expressed as mean ± SEM of n animals. Statistical significance was analysed by two-way analysis of variance (anova) followed by the appropriate post hoc test as indicated in the figure legends. Differences in the survival were determined with log rank test. A P-value of less than 0.05 was considered significant. Two-way anova analyses were performed using the Statistica® software package (StatSoft Inc., Tulsa, OK, USA), and the other tests were performed using GraphPad Prism software (San Diego, CA, USA).
Materials
TEA chloride, aminoguanidine, 1400W, glibenclamide and phenylephrine chloride were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Heparin was a kind gift of Cristália Laboratories (São Paulo, SP, Brazil). All drug and receptor nomenclature follows Alexander et al., 2009.
Results
Effects of potassium channel blockers on survival rate
Sepsis induced a progressive mortality in animals submitted to CLP surgery (Figure 1). TEA treatment (8 mg·kg−1) given 4 h after CLP surgery protected half of animals from death. Curiously, a higher dose of TEA (16 mg·kg−1) did not change the mortality when compared with CLP control group (Figure 1A). On the other hand, glibenclamide treatment in both doses increased CLP mortality (Figure 1B). No mortality was observed in sham, sham + TEA or sham + glibenclamide groups (Figure 1 A and B). Based on this result, the subsequent experiments were performed with TEA 8 mg·kg−1 and glibenclamide 1 mg·kg−1.
Figure 1.
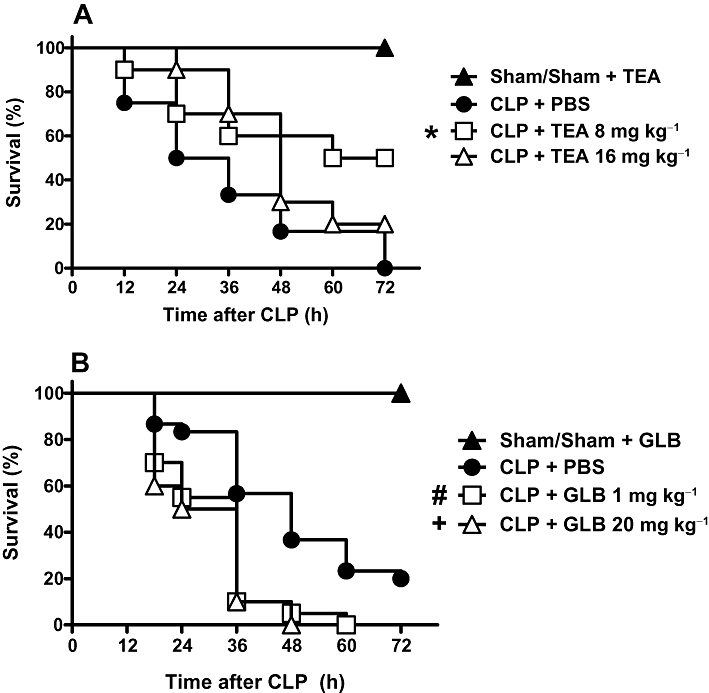
Effect of potassium channels blockers on survival of septic rats. A. Effect of tetraethylammonium (TEA). Rats were sham-operated or submitted to CLP procedure. CLP animals were treated subcutaneously with PBS, TEA 8 mg·kg−1 or TEA 16 mg·kg−1 4 h after the surgery. Sham animals received PBS or TEA and, because there was no difference between these groups, their values are presented as one group. B. Effect of glibenclamide (GLB). CLP animals were treated subcutaneously with DMSO or GLB 1 mg·kg−1 or 20 mg·kg−1 4 h after surgery. Sham-operated animals that received DMSO or glibenclamide were grouped together because there was no difference between them. Statistical analysis was performed using log-rank test. *P = 0.01, #P = 0.0004, +P = 0.001 significantly different from CLP+PBS rats, n = 20 per group. Results were expressed as percent survival and are representative of two different experiments. The significance symbols beside the survival curve means that the curve was statistically different from the reference survival (CLP+PBS).
Effects of potassium channel blockers on MAP, HR and vasoconstrictor response
Sham animals exhibited a MAP of 105 ± 6 mmHg and a HR of 232 ± 6 bpm. Twenty-four hours after CLP procedure, MAP had decreased (Figure 2A), and heart rate was increased (Figure 2B), both by 30%. In addition, there was a substantial reduction (about 50%) in phenylephrine response (Figure 2C). The early injection (4 h after CLP surgery) of only one dose of TEA was enough to completely restore the HR and phenylephrine response to normal values. Early TEA injection, however, only partially blocked the development of hypotension. TEA did not affect MAP, HR or phenylephrine response in sham-operated animals (Figure 2). On the other hand, the early injection of glibenclamide did not change the sepsis-induced cardiovascular dysfunction (Figure 3, Panel A, B and C).
Figure 2.
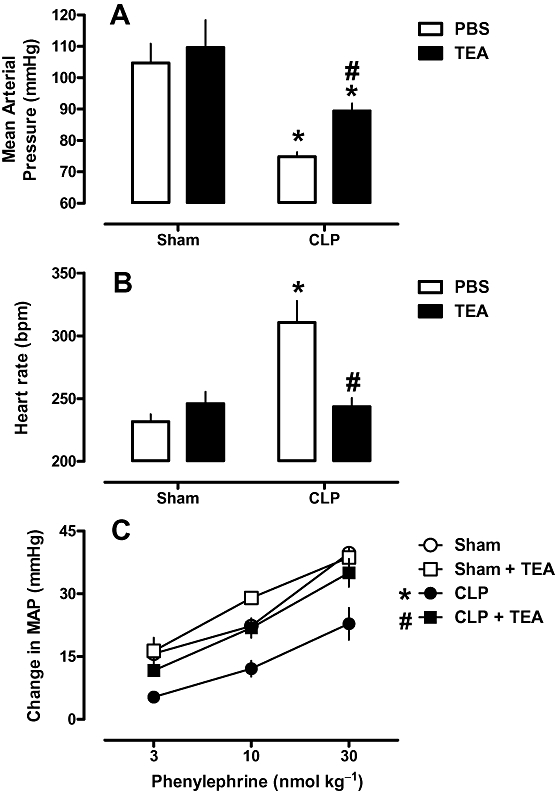
Effects of tetraethylammonium (TEA) on haemodynamic parameters of rats after CLP. Rats were injected with TEA (8 mg·kg−1, s.c.) 4 h after CLP or sham surgery. Twenty-four hours after surgery, mean arterial pressure (A), heart rate (B) and phenylephrine dose–response curves (C) were evaluated. Each bar represents the mean of six to eight animals, and vertical lines are the SEM. Statistical analyses were performed using two-way anova followed by Newman–Keuls post hoc test. *P < 0.05 significantly different from sham-operated group and #P < 0.05 significantly different from CLP group.
Figure 3.
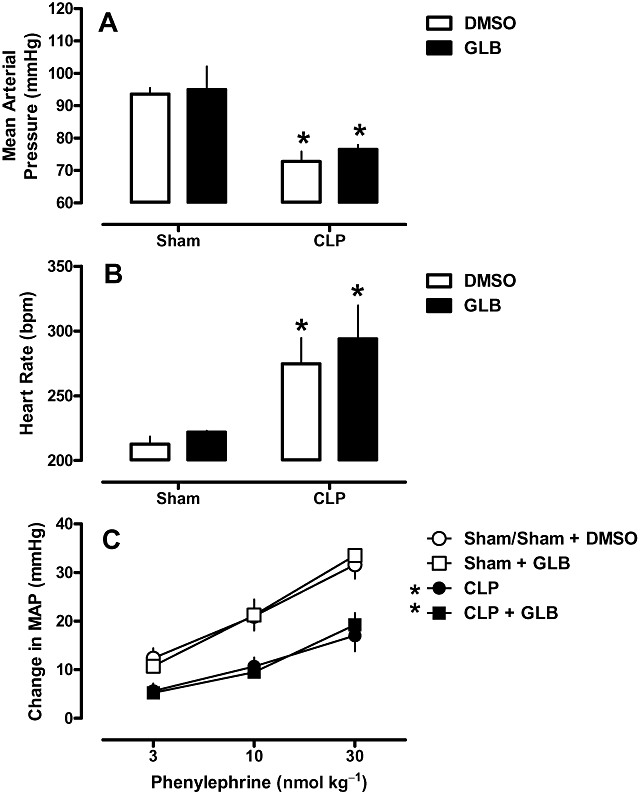
Effects of glibenclamide (GLB) on haemodynamic parameters of rats after CLP. Rats were injected with glibenclamide (1 mg·kg−1, s.c.) or vehicle (DMSO; 100 µL) 4 h after CLP or sham surgery. Twenty-four hours after surgery, mean arterial pressure (A), heart rate (B) and phenylephrine dose–response curves (C) were evaluated. Each bar represents the mean of six to eight animals, and vertical lines are the SEM. Statistical analysis was performed using two-way anova followed by Newman–Keuls post hoc test. *P < 0.05 significantly different from corresponding sham group.
Effects of potassium channel blockers on organ damage and inflammatory parameters
Sepsis induced renal damage and tissue hypoperfusion as demonstrated by increased plasma levels of urea, creatinine and lactate. CLP animals developed leucopenia and increased myeloperoxidase activity in the lung (Tables 1 and 2). Levels of the cytokines TNF-α and IL-1β were increased in serum from rats after CLP surgery (Figure 4). Early TEA treatment reduced the magnitude of all these changes after CLP (Table 1 and Figure 4, Panels A and C), except the leucopenia. On the other hand, glibenclamide treatment (Table 2) either failed to improve or exacerbated these changes. Neither the potassium channels blockers nor their vehicles changed these variables in sham-operated animals.
Table 1.
Effects of the early injection of tetraethylammonium (TEA) on blood biochemistry and inflammatory markers in rats after CLP or sham surgery
| Experimental groups | ||||
|---|---|---|---|---|
| Sham | CLP | |||
| PBS | TEA | PBS | TEA | |
| Urea | 217 ± 15 | 222 ± 27 | 390 ± 51* | 247 ± 23# |
| Creatinine | 2.0 ± 0.1 | 2.4 ± 0.4 | 4.4 ± 0.4* | 3.1 ± 0.2*# |
| Lactate | 91 ± 32 | 75 ± 14 | 221 ± 26* | 129 ± 16# |
| Leukocytes | 7.7 ± 0.68 | 8.0 ± 0.45 | 4.6 ± 0.49* | 4.9 ± 0.65* |
| MPO activity | 63 ± 11.6 | 93 ± 4.7 | 202 ± 24.6* | 143 ± 12.1*# |
Rats were injected with TEA (8 mg·kg−1, s.c.) or vehicle (PBS) 4 h after CLP or sham surgery and the variables measured 20 h later. Urea, creatinine and lactate values are expressed as mg L–1; lung myeloperoxidase (MPO) activity is expressed as optical density units (mg protein)−1; total leukocyte numbers are expressed as 103cells mm–3. Data are expressed as mean ± SEM. Statistical analyses were performed using two-way anova followed by Newman–Keuls post hoc test.
P < 0.05 significantly different from sham animals
P < 0.05 significantly different from CLP animals.
Table 2.
Effects of the early injection of glibenclamide (GLB) on blood biochemistry and inflammatory markers in rats after CLP or sham surgery
| Experimental Group | ||||
|---|---|---|---|---|
| Sham | CLP | |||
| DMSO | GLB | DMSO | GLB | |
| Urea | 284 ± 17 | 312 ± 31 | 615 ± 151* | 429 ± 66* |
| Creatinine | 1.7 ± 0.5 | 1.9 ± 0.4 | 3.9 ± 0.5* | 3.4 ± 0.4* |
| Lactate | 245 ± 17 | 226 ± 24 | 357 ± 28* | 340 ± 11* |
| Leukocytes | 7.7 ± 0.66 | 7.1 ± 0.24 | 4.7 ± 0.11* | 5.1 ± 0.47* |
| MPO activity | 118 ± 23.1 | 90 ± 10.1 | 294 ± 63.8* | 306 ± 74.5* |
Rats were injected with glibenclamide (1 mg·kg−1, s.c.) or vehicle (DMSO) 4 h after CLP or sham surgery and the variable measured 20 h later. Other details are as in Table 1.
Figure 4.
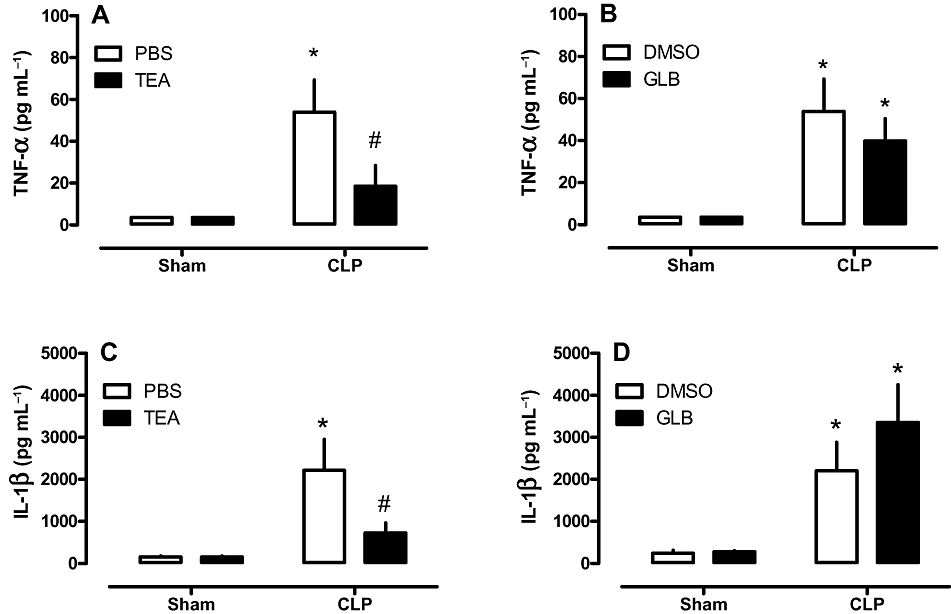
Effects of potassium channel blockers on serum cytokine levels. Rats were injected with vehicle (PBS or DMSO), tetraethylammonium (TEA; A and C; 8 mg·kg−1; s.c.) or glibenclamide (GLB; B and D; 1 mg·kg−1; s.c.) 4 h after CLP or sham surgery. Twenty hours later, blood samples were collected and assayed for cytokine levels; TNF-α (A and B); IL-1 β (C and D). Each bar represents the mean of 8–10 animals, and vertical lines are SEM. Statistical analyses were performed using two-way anova followed by Newman–Keuls post hoc test. *P < 0.05 significantly different from corresponding sham group and #P < 0.05 significantly different from corresponding CLP rats.
Effects of potassium channel blockers on plasma NOx levels
NOx levels in plasma were increased in animals after CLP (Figure 5). Treatment of CLP rats with TEA significantly reduced plasma NOx levels (Figure 5A), whereas GLB was without effect (Figure 5B). None of the blockers or their vehicles altered NOx levels in sham-operated animals (Figure 5 A and B).
Figure 5.
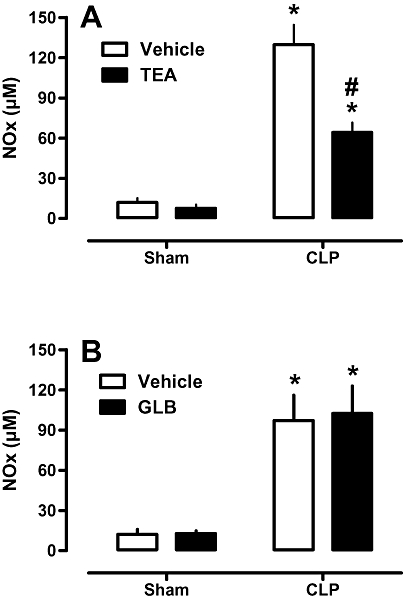
Effects of potassium channel blockers on NOx plasma levels. Rats were injected with vehicle (PBS or DMSO), tetraethylammonium (TEA: A; 8 mg·kg−1; s.c.) or glibenclamide (GLB; B; 1 mg·kg−1; s.c.) 4 h after CLP or sham surgery. Twenty hours later, blood samples were collected and assayed for NOx levels. Each bar represents the mean of 8–10 animals, and vertical lines are SEM. Statistical analyses were performed using two-way anova followed by Newman–Keuls post hoc test. *P < 0.05 significantly different from corresponding sham group and #P < 0.05 significantly different from corresponding CLP rats.
Effects of TEA on lung and thigh skeletal muscle NOS-2 expression
NOS-2 protein in extracts of lung or thigh skeletal muscle was increased by CLP, when measured 24 h later. TEA injected 4 h after CLP surgery reduced these levels in lung (Figure 6 A and B) and thigh skeletal muscle (Figure 6 A and C). Levels of β-actin in these tissues were not altered by CLP surgery or TEA treatment (Figure 6 A). Because glibenclamide did not alter NOx levels (Figure 5 B), NOS-2 expression was not studied in this group.
Figure 6.
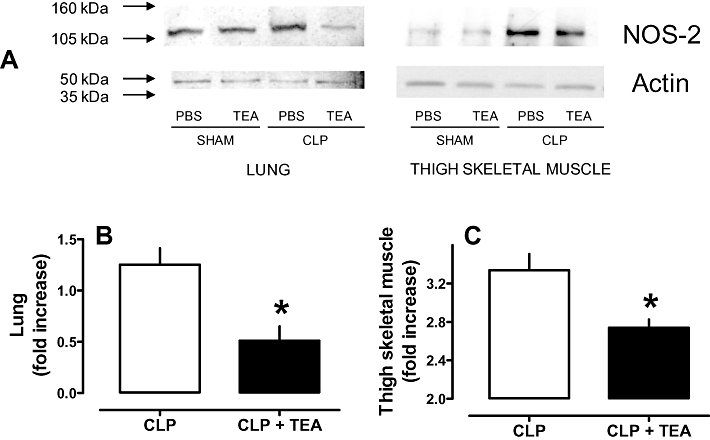
Effect of tetraethylammonium (TEA) on inducible nitric oxide synthase (NOS-2) expression. A. Representative immunoelectrophoresis of NOS-2 and β-actin in extracts of lung and thigh skeletal muscle from rats after CLP and treated with TEA (8 mg·kg−1; s.c.) 4 h after surgery. Tissues were harvested 24 h after CLP, homogenized and centrifuged. The same amount of protein (75 µg) was loaded in each well, and a representative experiment is shown. Band intensity of lung (B) and thigh skeletal muscle (C) NOS-2 were quantified by densitometry, and all data have been normalized to the levels of sham-operated animals. Each bar represents the mean of three to four independent experiments, and vertical lines are SEM. Statistical analysis was performed using two-way anova followed by Newman–Keuls post hoc test. *P < 0.05 significantly different from corresponding CLP group.
Effects of potassium channel blockers on blood bacterial counts in sepsis
The bacterial load in blood samples obtained from CLP rats was, as expected, significantly higher than in the sham group (Figure 7). Early treatment with potassium channel blockers did not change bacterial counts in the blood of sham or CLP animals.
Figure 7.
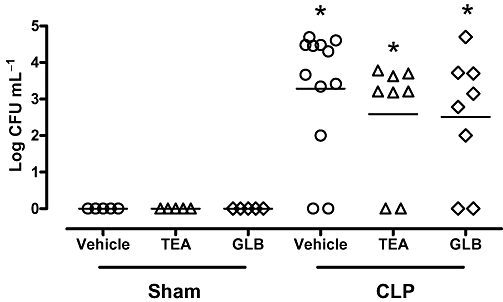
Effects of potassium channel blockers on blood bacterial load. Animals were treated 4 h after sham or CLP surgery with vehicle (PBS or DMSO), tetraethylammonium (TEA; 8 mg·kg−1; s.c.) or glibenclamide (GLB; 1 mg·kg−1; s.c.). Peripheral blood was collected 24 h after CLP, and serial dilutions were placed on Mueller-Hinton agar plates. Colony-forming units (CFU) in blood were determined 24 h after plating and were expressed as log CFU mL−1 blood. Individual animals are represented by individual symbols. Sham and CLP control animals received PBS or DMSO and, because there was no difference between these groups, their values were presented as one group (vehicle). Statistical analyses were performed using two-way anova followed by Newman–Keuls post hoc test. *P < 0.05 significantly different from corresponding sham group.
Effects of potassium channel blockers on blood glucose levels
CLP surgery induced biphasic changes in blood glucose. Six hours after CLP, animals presented with hyperglycemia, and, from 12 h onwards, a progressive hypoglycemia developed (Figure 8 A). Treatment of CLP rats with TEA prevented both sepsis-induced hypoglycemia and hyperglycemia (Figure 8 C). In both sham and sham + TEA groups, blood glucose levels were not significantly changed during the experimental period (Figure 8 B). On the other hand, glibenclamide reduced blood glucose levels in sham-operated animals (Figure 8 D) and worsened the hypoglycemic phase in CLP animals (Figure 8 E).
Figure 8.
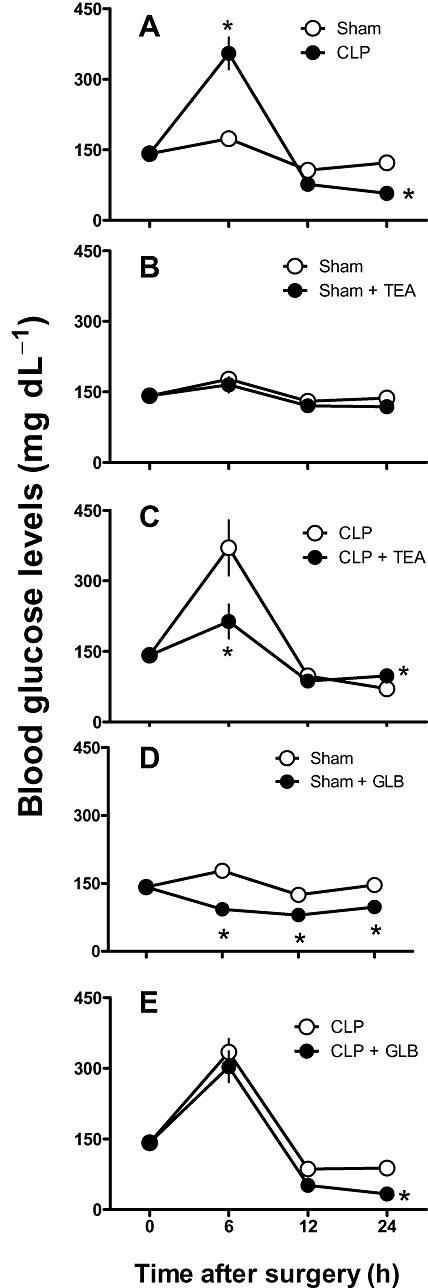
Effect of potassium channel blockade on blood glucose level changes induced by CLP. Six, 12 and 24 h after CLP, blood glucose levels were measured electrochemically in blood withdrawn from the tail tip. A. Blood glucose level in sham and CLP animals. B and D. Sham animals were randomized and 4 h after surgery treated with tetraethylammonium (TEA) 8 mg·kg−1 (B), glibenclamide (GLB) 1 mg·kg−1 (D;) or vehicle. C and E. CLP animals were randomized and 4 h after surgery were treated with TEA 8 mg·kg−1 (C), GLB 1 mg·kg−1 (E) or vehicle. Data were expressed as mean ± SEM from four to six animals. Statistical analysis was performed using two-way anova followed by Newman–Keuls post hoc test. *P < 0.05 significantly different from corresponding sham-operated group and #P < 0.05 significantly different from corresponding CLP control group.
Effects of inducible NOS-2 inhibition on NOx levels and survival
Taking into account that the partial reduction in NOS-2 levels had several beneficial effects, we wondered if a partial reduction in NOS-2 activity (assessed by plasma NOx) would have a similar outcome. The effects of NOS-2 inhibition on survival rate were assessed using a lower and a higher dose of aminoguanidine (10 and 200 mg·kg−1; i.p.) or a low dose of 1400W (0.3 mg·kg−1; i.p.), given to the animals 12 h after surgery. The lower dose of aminoguanidine improved the survival, whereas the high dose did not (Figure 9A). The low dose of 1400W, considered a selective NOS-2 inhibitor, also improved survival (Figure 9B). NOx levels in animals with sepsis and treated with the lower dose of both inhibitors were partially reduced (Figure 10 A and B, respectively), whereas those after treatment with the higher dose of aminoguanidine were similar to levels found in control animals (Figure 10 A).
Figure 9.
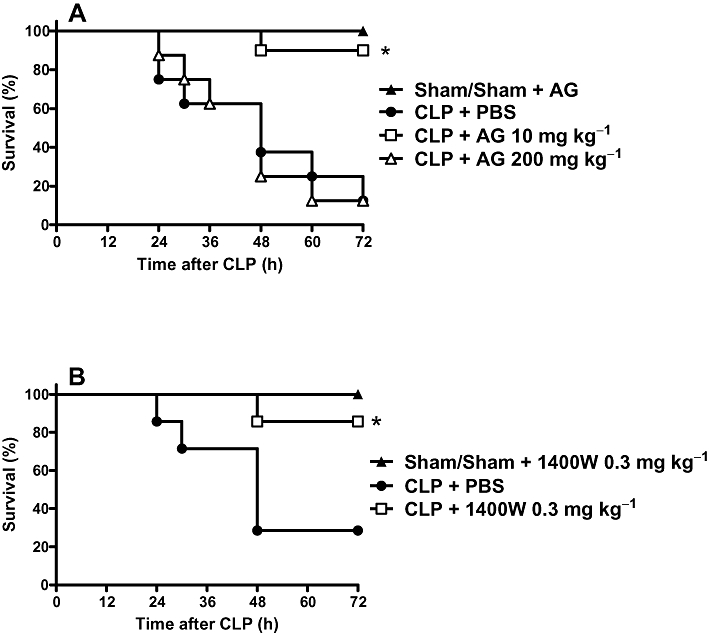
Effect of NOS-2 inhibitors on the survival of rats with sepsis. A. Aminoguanidine (AG). Rats were sham-operated or submitted to CLP procedure. CLP animals were treated subcutaneously with PBS, AG 10 mg·kg−1 or 200 mg·kg−1, 12 h after the surgery. Sham animals received PBS or AG, and because there was no difference between these groups, their values are presented as one group. Statistical analysis was performed using log-rank test. *P < 0.0013 significantly different from CLP+PBS rats, n = 10 per group. B. Effects of 1400W. CLP animals were treated subcutaneously with PBS or 1400W 0.3 mg·kg−1 12 h after surgery. Sham-operated animals that received PBS or 1400W were grouped together because there was no difference between them. Statistical analysis was performed using log-rank test. *P < 0.007 significantly different from CLP+PBS rats, n = 10 per group. Results were expressed as percent survival and are representative of two different experiments.
Figure 10.
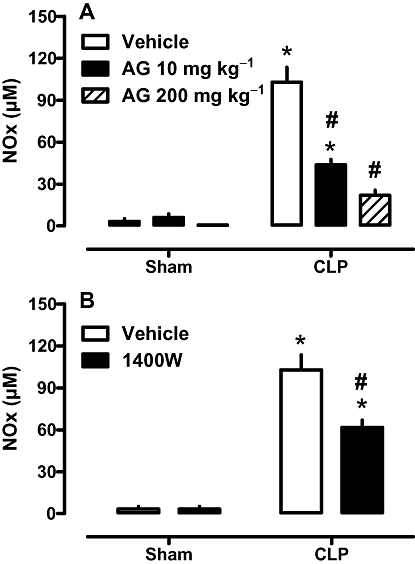
Effect of NOS-2 inhibitors on NOx plasma levels. A. Effects of aminoguanidine (AG). B. Effect of 1400W. Rats were treated subcutaneously 12 h after CLP surgery with vehicle or inhibitors as in Figure 9. Twelve hours later, blood samples were collected and assayed for NOx levels. Each bar represents the mean of 8–10 animals, and vertical lines are SEM. Statistical analyses were performed using two-way anova followed by Newman–Keuls post hoc test. *P < 0.05 significantly different from corresponding sham group and #P < 0.05 significantly different from corresponding CLP rats.
Discussion and conclusions
The main finding of the present report is that the early blockade of potassium channels with TEA dramatically improves cardiovascular and inflammatory parameters and decreases the mortality rate of septic rats. On the other hand, the selective blockade of ATP-sensitive potassium (KATP) channel by glibenclamide not only did not provide any improvement but actually increased mortality.
This latter finding was somewhat surprising because there are several reports showing beneficial effects of KATP channel inhibitors in experimental sepsis and endotoxemia. For instance, intravenous infusion of glibenclamide reversed endotoxin-induced systemic hypotension in dogs (Landry and Oliver, 1992), pigs (Vanelli et al., 1995) and rats (Gardiner et al., 1999). Two recent studies investigated the effect of KATP channel blockade in patients with septic shock (Warrilow et al., 2006; Morelli et al., 2007) and, in contrast to the animal studies, glibenclamide failed to improve haemodynamics or to reduce noradrenaline requirements in both studies. Although these studies have been questioned (Oliver and Landry, 2006), they would be compatible with our results. Moreover, recent work shows that improvement in sepsis survival by hydrogen sulphide was mediated by KATP channel activation and glibenclamide treatment prevented these beneficial effects (Spiller et al., 2010). Our results point to the activation, and not the blockade, of KATP channels as being beneficial in sepsis.
In striking contrast, the early treatment with only one injection of TEA improved the inflammatory and dysfunctional haemodynamic parameters of septic animals. TEA treatment early in sepsis reduced the late consequences of the systemic inflammation in septic rats, as shown by the reduced myeloperoxidase activity and decrease in NOx, TNF-α and IL-1β levels. These data corroborate previous work showing that potassium channels are involved in transmembrane signal transduction in macrophages and that blockers of these channels reduced cytokine production in these cells (Blunck et al., 2001; Papavlassopoulos et al., 2006). Animals with sepsis also exhibited a decrease in blood leukocytes. The leukocyte number drops during sepsis because these cells, mainly neutrophils, transmigrate to the infectious focus and to others organs such as the lung (Alves-Filho et al., 2008), which in turn contributes to the multiple organ dysfunction of sepsis (Souto et al., 2011). This is confirmed by the increase in lung MPO activity in rats after CLP, reflecting a systemic inflammation that the treatment with TEA was able to reduce. The reduced systemic inflammatory response, in turn, diminished the development and/or maintenance of organ dysfunction and hence would contribute to the increased survival.
It is important to note that the reduction in NOx levels was partial and probably due to a similarly partial reduction in NOS-2 expression. Although certainly not the only relevant mechanism, NO/NOS-2 reduction can be regarded as an important contribution to the beneficial effects of TEA. Non-selective NOS inhibition has harmful consequences to the host (Lopez et al., 2004). However, our results suggest that a partial reduction in NOS-2 expression may be advantageous in sepsis because it seemed to reduce the unwanted cardiovascular and perfusional consequences of increased NO synthesis, while preserving other important NO actions, such as host defense. Interestingly enough, the partial reduction of NOS-2 expression, although not affecting the bacteremia, had important consequences for the cardiovascular system. TEA was able to prevent hypotension and vascular hyporesponsiveness to phenylephrine and reduced hypoxia and tissue damage as suggested by the decrease in lactate, urea and creatinine levels. Following the same line of reasoning, if TEA had totally inhibited NOS-2 expression, we would expect that the ability of the host to deal with the infection would be compromised. However, our results show that the bacterial burden was not changed by the early injection of TEA and the reduced NOS-2 expression, suggesting the ability to deal with the infection was unaffected, even with reduced cytokine levels. Although NO and cytokines are important to host defense, others mechanisms such as the complement system and reactive oxygen species are also important and may compensate for the reduction in NO and cytokine production.
The consequences of the partial reduction in NO production induced by TEA suggested that this would be advantageous to animals with sepsis. As we found, the increase in survival after low doses of aminoguanidine and 1400W was so clear that this strategy is worth exploring in clinical studies. Another interesting aspect of our results is that the higher dose of aminoguanidine probably lost selectivity for the NOS-2 isoform, corroborating the results of a study in humans, indicating that non-selective NOS inhibition was harmful to the host (Lopez et al., 2004).
In contrast to our data, previous studies of TEA in sepsis have shown no improvement in blood pressure or mortality (Clayton et al., 2005; Cauwels and Brouckaert, 2008). The most likely explanation for the discrepancy between our data and these two studies lies with the animal model used and the dose of TEA. TEA is regarded as a non-selective inhibitor of potassium channels; at low doses, TEA inhibits preferentially calcium-activated potassium channels, whereas at higher doses, it inhibits most, if not all, potassium channel subtypes (Nelson and Quayle, 1995). In our study, only the lower dose of TEA was effective in reducing the mortality, whereas the higher dose had no effect on survival. Maybe the higher dose of TEA inhibited other potassium channel subtypes (including the ATP-sensitive subtype) that somehow counteracted the beneficial effects of the lower dose. TEA reversed the attenuated vasoconstrictor response to noradrenaline during experimental human endotoxemia mediated by NO-induced opening of calcium-activated potassium channels (Pickkers et al., 2006), suggesting that the beneficial effects of a lower dose of TEA shown here might be due to inhibition of calcium-activated potassium channels. This suggestion was strengthened by unpublished experiments from our group with the KV channel blocker 4-aminopyridine (10 and 100 µg·kg−1; s.c.; 4 h after surgery). Blockade of this potassium channel subtype did not induce any effects, either protective or harmful, in animals with sepsis.
As in human sepsis, CLP in rodents results in an early hyperdynamic and hypermetabolic phase followed by a later hypodynamic and hypometabolic phase (Siegel, 1981; Maitra et al., 2000). As can be seen from our results, experimental sepsis in rats reproduced the metabolic biphasic pattern. Hyperglycemia is a complication that contributes to morbidity and mortality of septic shock (Buras et al., 2005). It is well established that hyperglycemia has toxic effects besides increasing susceptibility to infection and the severity of hyperglycemia has been repeatedly associated with adverse outcomes of sepsis (Van den Berghe, 2003; Vanhorebbek et al., 2007). In our hands, TEA treatment prevented the biphasic changes in blood glucose levels observed in animals with sepsis. The lack of effect on sham-operated rats of glycaemia is further evidence that the protective effect of TEA was not due to KATP channel blockade. As expected, glibenclamide decreased blood glucose levels of sham animals, but it did not prevent sepsis-induced hyperglycemia and in fact worsened the hypoglycemic phase of septic shock. This fact probably contributed to the increased mortality in rats treated with glibenclamide. To assess whether glibenclamide-induced hypoglycemia could be masking a putative protective effect on mortality, glucose was supplemented to septic animals treated with glibenclamide. Although infusion of glucose O'Brien et al. (2009) was not feasible in our protocols, injection of glucose every 8 h in rats after CLP and treated with glibenclamide provided blood glucose levels similar to those in CLP+PBS animals. Survival of the glucose supplemented group was identical to that of the CLP group (data not shown), indicating that, although glibenclamide-induced hypoglycemia was contributing to the higher mortality, blockade of KATP channels did not provide any benefit in sepsis. Our data are in line with the observation that selective KATP blockade with sulphonylurea derivatives may not be helpful in the management of septic shock (Singer et al., 2005). Furthermore, there is an apparent dissociation between the sulphonylurea receptor and the channel pore in prolonged sepsis in vitro (O'Brien et al., 2005). In this way, the use of specific pore blockers of KATP channels may be more interesting than sulphonylurea derivatives.
Our study has two main limitations. The first is that our pharmacological approach relied on several compounds with limited selectivity (except 1400W), because highly selective blockers for some targets discussed here are still lacking. The second limitation is a general one concerning the translation of animal studies to human conditions. Sepsis is no exception. For example, animal sepsis models develop a rapid disease as compared with the human condition and do not fully resemble the human disease or involve care identical to that delivered to patients with sepsis (see Buras et al., 2005).
We have not yet established the mechanism by which the early blockade of potassium channel reduces NOS-2 expression; this is currently being investigated in our laboratory. However, in spite of this caveat, the data presented here point out to a hitherto unexamined possibility of interfering with NO pathway in sepsis, namely the beneficial effect of a partial reduction in NOS-2 expression, as distinct from total inhibition. The results presented here suggest that blocking potassium channels with TEA early after the onset of sepsis has several beneficial consequences, such as reduction of inflammatory response and improvement in cardiac and vascular conditions, leading to better organ perfusion. Probably due to the overall improvement in the condition of the animals, survival substantially increased. The beneficial haemodynamic effects caused by TEA in rats with septic shock seem to be, at least in part, due to a partial reduction in NOS-2 expression. Because selective blockade of voltage-gated and ATP-sensitive potassium channels have not reproduced the beneficial effects of TEA, the calcium-activated potassium channels are the more likely sites of action of TEA.
Acknowledgments
The skillful technical assistance of Mrs Adriane Madeira is gratefully acknowledged. Cristália Pharmaceutical Industries (São Paulo, SP, Brazil) is gratefully acknowledged for the gift of heparin. This study was supported by CNPq, CAPES and FAPESC (Brazil).
Glossary
Abbreviations
- 1400W
N-(3-(aminomethyl)-benzyl)acetamidine
- AG
aminoguanidine
- CLP
caecal ligation and puncture
- HR
heart rate
- KATP
channel, ATP-sensitive potassium channel
- KV
voltage-gated potassium channels
- MAP
mean arterial pressure
- NOS-2
inducible nitric oxide synthase
- NOx
nitrite/nitrate
- TEA
tetraethylammonium
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–10 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl. 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- Archer SL, Huang JM, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of charybdotoxin-sensitive K channels by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assreuy J. Nitric oxide and cardiovascular dysfunction in sepsis. Endocr Metab Immune Disord Drug Targets. 2006;6:165–173. doi: 10.2174/187153006777442314. [DOI] [PubMed] [Google Scholar]
- Benjamim CF, Ferreira SH, Cunha FQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis. 2000;182:214–223. doi: 10.1086/315682. [DOI] [PubMed] [Google Scholar]
- Blunck R, Scheel O, Müller M, Brandenburg K, Seitzer U, Seydel U. New insights into endotoxin-induced activation of macrophages: involvement of a K+ channel in transmembrane signaling. J Immunol. 2001;166:1009–1015. doi: 10.4049/jimmunol.166.2.1009. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibe S, Palacino JJ, Pagano JP, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Cauwels A, Brouckaert P. Critical role for small and large conductance calcium-dependent potassium channels in endotoxemia and TNF toxicity. Shock. 2008;29:577–582. doi: 10.1097/shk.0b013e31815071e9. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Wu CC, Yen MH. Role of nitric oxide and K+-channels in vascular hyporeactivity induced by endotoxin. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:493–499. doi: 10.1007/pl00005381. [DOI] [PubMed] [Google Scholar]
- Clayton NP, LeDuc BW, Kelly LJ. Effect of potassium channel and cytochrome P450 inhibition on transient hypotension and survival during lipopolysaccharide-induced endotoxic shock in the rat. Pharmacology. 2005;73:113–120. doi: 10.1159/000081631. [DOI] [PubMed] [Google Scholar]
- Fernandes D, Assreuy J. Nitric oxide and vascular reactivity in sepsis. Shock. 2008;30:10–13. doi: 10.1097/SHK.0b013e3181818518. [DOI] [PubMed] [Google Scholar]
- Fernandes D, Sordi R, Pacheco LK, Nardi GM, Heckert BT, Villela CG, et al. Late, but not early, inhibition of soluble guanylate cyclase decreases mortality in a rat sepsis model. J Pharmacol Exp Ther. 2009;328:991–999. doi: 10.1124/jpet.108.142034. [DOI] [PubMed] [Google Scholar]
- Fujino K, Nakaya S, Wakatsuki T, Miyoshi Y, Nakaya Y, Mori H, et al. Effects of nitroglycerin on ATP-induced Ca(++)-mobilization, Ca(++)-activated K channels and contraction of cultured smooth muscle cells of porcine coronary artery. J Pharmacol Exp Ther. 1991;256:371–377. [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, March JE, Bennett T. Regional haemodynamic responses to infusion of lipopolysaccharide in conscious rats: effects of pre- or post-treatment with glibenclamide. Br J Pharmacol. 1999;128:1772–1778. doi: 10.1038/sj.bjp.0702985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Turcato S, Clapp L. Abnormal activation of K+ channels underlies relaxation to bacterial lipopolysaccharide in rat aorta. Biochem Biophys Res Commun. 1996;224:184–190. doi: 10.1006/bbrc.1996.1005. [DOI] [PubMed] [Google Scholar]
- Haslberger A, Romanin C, Koerber R. Membrane potential modulates release of tumor necrosis factor in lipopolysaccharide-stimulated mouse macrophages. Mol Biol Cell. 1992;4:451–460. doi: 10.1091/mbc.3.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry DW, Oliver JA. The ATP-sensitive K-channel mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J Clin Invest. 1992;89:2071–2074. doi: 10.1172/JCI115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Szabo C, Van Aken H, Williams W, Traber DL, Daudel F, et al. Short-term effects of glipizide (an adenosine triphosphate-sensitive potassium channel inhibitor) on cardiopulmonary hemodynamics and global oxygen transport in healthy and endotoxemic sheep. Shock. 2006;26:516–521. doi: 10.1097/01.shk.0000228795.33421.45. [DOI] [PubMed] [Google Scholar]
- Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- Lowry MA, Goldberg JI, Belosevic M. Induction of nitric oxide (NO) synthesis in murine macrophages requires potassium channel activity. Clin Exp Immunol. 1998;111:597–603. doi: 10.1046/j.1365-2249.1998.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra SR, Wojnar MM, Lang CH. Alterations in tissue glucose uptake during the hyperglycemic and hypoglycaemic phases of sepsis. Shock. 2000;13:379–385. doi: 10.1097/00024382-200005000-00006. [DOI] [PubMed] [Google Scholar]
- Morelli A, Lange M, Ertmer C, Broeking K, Van Aken H, Orecchioni A, et al. Glibenclamide dose-response in patients with septic shock: effects on norepinephrine requirements, cardiopulmonary performance, and global oxygen transport. Shock. 2007;28:530–535. doi: 10.1097/shk.0b013e3180556a3c. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- O'Brien AJ, Thakur G, Buckley JF, Singer M, Clapp LH. The pore-forming subunit of the K(ATP) channel is an important molecular target for LPS-induced vascular hyporeactivity in vitro. Br J Pharmacol. 2005;144:367–375. doi: 10.1038/sj.bjp.0706065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JM, Jr, Ali NA, Aberegg SK, Abraham E. Sepsis. Am J Med. 2007;120:1012–1022. doi: 10.1016/j.amjmed.2007.01.035. [DOI] [PubMed] [Google Scholar]
- O'Brien A, Stidwill RP, Clapp LH, Singer M. Variable effects of inhibiting iNOS and closing the vascular ATP-sensitive potassium channel (via its pore-forming and sulfonylurea receptor subunits) in endotoxic shock. Shock. 2009;31:535–541. doi: 10.1097/SHK.0b013e31818b99c2. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Landry DW. Potassium channels and septic shock. Crit Care Med. 2006;34:1255–1257. doi: 10.1097/01.CCM.0000208107.11781.B5. [DOI] [PubMed] [Google Scholar]
- Papavlassopoulos M, Stamme C, Thon L, Adam D, Hillemann D, Seydel U, et al. MaxiK blockade selectively inhibits the lipopolysaccharide-induced I kappa B-alpha/NF-kappa B signaling pathway in macrophages. J Immunol. 2006;177:4086–4093. doi: 10.4049/jimmunol.177.6.4086. [DOI] [PubMed] [Google Scholar]
- Pickkers P, Dorresteijn MJ, Bouw MP, van der Hoeven JG, Smits P. In vivo evidence for nitric oxide-mediated calcium-activated potassium-channel activation during human endotoxemia. Circulation. 2006;114:414–421. doi: 10.1161/CIRCULATIONAHA.105.590232. [DOI] [PubMed] [Google Scholar]
- Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265:299–303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Seydel U, Scheel O, Müller M, Brandenburg K, Blunck R. A K+ channel is involved in LPS signaling. J Endotoxin Res. 2001;7:243–247. [PubMed] [Google Scholar]
- Siegel JH. Relations between circulatory and metabolic changes in sepsis. Annu Rev Med. 1981;32:175–194. doi: 10.1146/annurev.me.32.020181.001135. [DOI] [PubMed] [Google Scholar]
- Silva-Santos JE, Assreuy J. Long-lasting changes of rat blood pressure to vasoconstrictors and vasodilators induced by nitric oxide donor infusion: involvement of potassium channels. J Pharmacol Exp Ther. 1999;290:380–387. [PubMed] [Google Scholar]
- Silva-Santos JE, Terluk MR, Assreuy J. Differential involvement of guanylate cyclase and potassium channels in nitric oxide-induced hyporesponsiveness to phenylephrine in endotoxemic rats. Shock. 2002;17:70–76. doi: 10.1097/00024382-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Singer M, Coluzzi F, O'Brien A, Clapp LH. Reversal of life-threatening, drug-related potassium-channel syndrome by glibenclamide. Lancet. 2005;365:1873–1875. doi: 10.1016/S0140-6736(05)66619-6. [DOI] [PubMed] [Google Scholar]
- Sordi R, Fernandes D, Assreuy J. Differential involvement of potassium channel subtypes in early and late sepsis-induced hyporesponsiveness to vasoconstrictors. J Cardiovasc Pharmacol. 2010;56:184–189. doi: 10.1097/FJC.0b013e3181e74d6a. [DOI] [PubMed] [Google Scholar]
- Sorrentino R, d'Emmanuele di Villa Bianca R, Lippolis L, Sorrentino L, Autore G, Pinto A. Involvement of ATP-sensitive potassium channels in a model of a delayed vascular hyporeactivity induced by lipopolysaccharide in rats. Br J Pharmacol. 1999;127:1447–1453. doi: 10.1038/sj.bjp.0702666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am J Respir Crit Care Med. 2011;183:234–242. doi: 10.1164/rccm.201003-0416OC. [DOI] [PubMed] [Google Scholar]
- Spiller F, Orrico MI, Nascimento DC, Czaikoski PG, Souto FO, Alves-Filho JC, et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am J Respir Crit Care Med. 2010;182:360–368. doi: 10.1164/rccm.200907-1145OC. [DOI] [PubMed] [Google Scholar]
- Taguchi H, Heistad DD, Chu Y, Rios CD, Ooboshi H, Faraci FM. Vascular expression of inducible nitric oxide synthase is associated with activation of Ca(++)-dependent K+ channels. J Pharmacol Exp Ther. 1996;279:1514–1519. [PubMed] [Google Scholar]
- Van den Berghe G. Insulin therapy for the critically ill patient. Clin Cornerstone. 2003;5:56–63. doi: 10.1016/s1098-3597(03)90018-4. [DOI] [PubMed] [Google Scholar]
- Vanelli G, Hussain SN, Aguggini G. Glibenclamide, a blocker of ATP-sensitive potassium channels, reverses endotoxin-induced hypotension in pig. Exp Physiol. 1995;80:167–170. doi: 10.1113/expphysiol.1995.sp003832. [DOI] [PubMed] [Google Scholar]
- Vanhorebbek I, Langouche L, Van den Berghe G. Modulating the endocrine response in sepsis: insulin and blood glucose control. Novartis Found Symp. 2007;280:204–215. [PubMed] [Google Scholar]
- Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrilow S, Egi M, Bellomo R. Randomized, double-blind, placebo-controlled crossover pilot study of a potassium channel blocker in patients with septic shock. Crit Care Med. 2006;34:980–985. doi: 10.1097/01.CCM.0000206114.19707.7C. [DOI] [PubMed] [Google Scholar]
- Williams DL, Katz GM, Roy-Contancin L, Reuben JP. Guanosine 5′-monophosphate modulates gating of high-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Proc Natl Acad Sci. 1988;85:9360–9364. doi: 10.1073/pnas.85.23.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Chen SJ, Yen MH. Nitric oxide-independent activation of soluble guanylyl cyclase contributes to endotoxin shock in rats. Am J Physiol. 1998;275:H1148–H1157. doi: 10.1152/ajpheart.1998.275.4.H1148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


