Abstract
BACKGROUND AND PURPOSE
Peptide YY (PYY) and neuropeptide Y (NPY) are involved in regulating gut and brain function. Because gastrointestinal inflammation is known to enhance anxiety, we explored whether experimental colitis interacts with genetic deletion (knockout) of PYY and NPY to alter emotional-affective behaviour.
EXPERIMENTAL APPROACH
Male and female wild-type, NPY (NPY−/−), PYY (PYY−/−) and NPY−/−; PYY−/− double knockout mice were studied in the absence and presence of mild colitis induced by ingestion of dextran sulphate sodium (2%) in drinking water. Anxiety-like behaviour was tested on the elevated plus maze and open field, and depression-like behaviour assessed by the forced swim test.
KEY RESULTS
In the absence of colitis, anxiety-like behaviour was increased by deletion of NPY but not PYY in a test- and sex-dependent manner, while depression-like behaviour was enhanced in NPY−/− and PYY−/− mice of either sex. The severity of DSS-induced colitis, assessed by colonic myeloperoxidase content, was attenuated in NPY−/− but not PYY−/− mice. Colitis modified anxiety- and depression-related behaviour in a sex-, genotype- and test-related manner, and knockout experiments indicated that NPY and PYY were involved in some of these behavioural effects of colitis.
CONCLUSIONS AND IMPLICATIONS
These data demonstrate sex-dependent roles of NPY and PYY in regulation of anxiety- and depression-like behaviour in the absence and presence of colitis. Like NPY, the gut hormone PYY has the potential to attenuate depression-like behaviour but does not share the ability of NPY to reduce anxiety-like behaviour.
Keywords: anxiety-like behaviour, colitis, corticosterone, depression-like behaviour, dextran sulphate sodium, elevated plus maze, forced swim test, myeloperoxidase, neuropeptide Y, peptide YY
Introduction
Peptide YY (PYY) and neuropeptide Y (NPY) belong to the pancreatic polypeptide-fold family of biologically active peptides, which share a number of structural and functional similarities. Their biological actions are mediated by five types of receptors, termed Y1, Y2, Y4, Y5 and y6, which are coupled to Gi/o signalling pathways (Redrobe et al., 2004; receptor nomenclature follows Alexander et al., 2009). PYY is secreted post-prandially from endocrine L cells of the hindgut, and the major circulating form of this peptide, PYY(3–36), is thought to be a satiety signal (McGowan and Bloom, 2004; Ueno et al., 2008). PYY(3–36) is able to reduce food intake in rodents and humans primarily via binding to autoinhibitory Y2 receptors in the arcuate nucleus of the hypothalamus (Batterham et al., 2002; McGowan and Bloom, 2004; Ueno et al., 2008), although corticolimbic areas are also involved (Batterham et al., 2007).
In contrast, NPY is a messenger widely distributed in the central and peripheral nervous system. Its many functional implications include the central regulation of energy balance, cognition, mood, anxiety and stress sensitivity (Heilig, 2004; Lin et al., 2004; Eva et al., 2006; Morales-Medina et al., 2010). Haplotype-driven expression of NPY in humans predicts brain responses to emotional and stress challenges and inversely correlates with trait anxiety (Zhou et al., 2008). The concentration of NPY in the cerebrospinal fluid is reduced in patients with post-traumatic stress disorder (Sah et al., 2009), and NPY gene polymorphisms modify the antidepressant treatment response in depression (Domschke et al., 2010), while NPY gene copy variations are relevant to attention-deficit hyperactivity disorder (Lesch et al., 2010).
The functional implications of NPY and PYY as signalling molecules have been explored by gene knockout approaches and, where available, pharmacological antagonism of Y receptors. The phenotype of NPY knockout (NPY−/−) and PYY knockout (PYY−/−) mice is characterized by alterations in feeding behaviour and energy homeostasis (Bannon et al., 2000; Lin et al., 2004; Batterham et al., 2006; Boey et al., 2006; Karl et al., 2008; Edelsbrunner et al., 2009). In addition, there are sex-dependent changes in anxiety- and depression-related behaviour of NPY−/− mice (Bannon et al., 2000; Lin et al., 2004; Karl et al., 2008), while the emotional-affective behaviour of PYY−/− mice has not yet been addressed. The observations in NPY−/− and PYY−/− mice are complemented by distinct alterations of anxiety- and depression-related behaviour in Y1, Y2 and Y4 knockout mice (Redrobe et al., 2003; Tschenett et al., 2003; Eva et al., 2006; Karl et al., 2006; Painsipp et al., 2008; Tasan et al., 2009).
NPY and PYY also play a role in the gut–brain axis beyond the regulation of appetite and energy homeostasis. For instance, endogenous NPY acting via Y2 and Y4 receptors seems to depress the afferent input from the acid-challenged stomach to the nucleus tractus solitarii by a presumably central site of action (Wultsch et al., 2005). Based on this finding, we reasoned that PYY and NPY could also contribute to the ability of gastrointestinal inflammation to alter emotional-affective behaviour. This argument is supported by the ability of experimental gastritis (Painsipp et al., 2007) and experimental infection with Campylobacter jejuni (Goehler et al., 2008) to enhance anxiety-related behaviour in mice. In addition, NPY itself has an impact on the severity of experimental inflammation, given that NPY−/− mice are less susceptible to dextran sulphate sodium (DSS)–induced colitis than wild-type (WT) mice (Chandrasekharan et al., 2008), an outcome that is reproduced by knockout or antagonism of Y1 receptors (Hassani et al., 2005) and treatment with a NPY antisense oligodeoxynucleotide (Pang et al., 2010).
Against this background, the present study was set out to study four specific questions. The first aim was to compare the phenotype of NPY−/−, PYY−/− and NPY−/−; PYY−/− double knockout mice as regards their anxiety-and depression-like behaviour as assessed in the elevated plus maze (EPM) test, open field (OF) test and forced swim test (FST). The second goal was to evaluate whether DSS-induced colitis is associated with changes in anxiety-and depression-related behaviour. The third aim was to assess whether NPY and PYY play a role in the effect of DSS-induced colitis to modify anxiety-and depression-related behaviour. The fourth goal was to examine whether changes in circulating corticosterone, the final output of the hypothalamic–pituitary–adrenal axis, could explain some of the behavioural changes evoked by knockout of NPY and/or PYY. The study was carried out with female and male mice, given that affective disorders are more prevalent in women than in men (Gorman, 2006), and there is a need to overcome the sex bias in experimental neuroscience (Beery and Zucker, 2011).
Methods
Experimental animals
All animal care and experimental procedures were approved by an ethical committee at the Federal Ministry of Science and Research of the Republic of Austria and conducted according to the Directive of the European Communities Council of 24 November 1986 (86/609/EEC). The experiments were designed in such a way that the number of animals used and their suffering was minimized.
The study was conducted with adult mice of four different genotypes: WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− double knockout mice of either sex, all on a mixed C57BL/6:129/SvJ (1:1) background. Germ-line PYY−/− mice (Boey et al., 2006) and germ-line NPY−/− mice (Karl et al., 2008), in which the entire coding sequence including the initiation start codon was removed, were generated as reported. Double knockout (NPY−/−; PYY−/−) mice were generated by crossing the single gene knockout strains and in a second step by crossing the double heterozygous mice. The presence or deletion of PYY and/or NPY was verified by polymerase chain reaction (Boey et al., 2006; Karl et al., 2008). Homozygous male and female WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice were obtained from the Neurobiology Research Program of the Garvan Institute of Medical Research (Sydney, Australia) via the Institute of Pharmacology of the Medical University of Innsbruck (Innsbruck, Austria). No more than three generations of these homozygous animals were bred at the Institute of Experimental and Clinical Pharmacology of the Medical University of Graz (Graz, Austria).
The animals were housed in groups of three to five per cage in cages of size IIL (length × width × height = 26 cm × 20.5 cm × 14 cm) under controlled temperature (set point 22°C), relative air humidity (set point 50%) and light conditions (lights on at 6:00 h, lights off at 18:00 h, maximal intensity 150 lux). Tap water and standard laboratory chow were provided ad libitum throughout the study.
Induction of experimental colitis
Colitis was induced by adding DSS (2%) to the drinking water (tap water) for 11 days (Mitrovic et al., 2010). The control animals received normal tap water. The DSS-containing drinking water was made up freshly every day to avoid bacterial contamination.
Experimental protocols
Prior to the behavioural tests, the mice were allowed to adapt to the test room (set points 22°C and 50% relative air humidity, lights on at 6:00 h, lights off at 18:00 h, maximal light intensity 100 lux) for at least 2 days. Two different protocols were used.
Protocol 1 was used to study the effect of DSS-induced colitis on anxiety-related behaviour in the EPM and OF tests, depression-related behaviour in the FST, body temperature and circulating corticosterone in male and female WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice (weights shown in the Results section). The animals were treated with DSS (2%), added to the drinking water, for 11 days (days 1–11) or drank normal tap water (control mice). The behavioural tests were performed on day 8 (EPM test), day 9 (OF test), day 10 (measurement of body temperature) and day 11 (FST). Twenty-eight minutes after the start of the FST, the animals were killed with an overdose of pentobarbital (150 mg·kg−1 injected intraperitoneally), which permitted the collection of trunk blood for determination of the circulating levels of corticosterone and of the colon for determination of the myeloperoxidase content 30 min after the start of the FST.
Protocol 2 involved male and female WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice in the naïve state, which were used for the determination of basal plasma levels of corticosterone. In these experiments, blood was sampled by tail incision (Fluttert et al., 2000).
EPM test
The animals were placed in the centre of a maze with four arms arranged in the shape of a plus (Belzung and Griebel, 2001). The maze consisted of a central quadrangle (5 × 5 cm), two opposing open arms (30 cm long, 5 cm wide) and two opposing closed arms of the same size but equipped with 15 cm high walls at their sides and the far end. The device was made of opaque grey plastic and elevated 70 cm above the floor. The light intensity at the central quadrangle was 70 lux, on the open arms 80 lux and in the closed arms 40 lux.
At the beginning of each trial, the animals were placed on the central quadrangle facing an open arm. The movements of the animals during a 5 min test period were tracked by a video camera above the centre of the maze and recorded with the software VideoMot2 (TSE Systems, Bad Homburg, Germany). This software was used to evaluate the animal tracks and to determine the number of their entries into the open arms, the time spent on the open arms, the total distance travelled on the EPM and the total number of entries into any arm. Entry into an arm was defined as the instance when the mouse placed its four paws on that arm. A reduction of the open arm time and/or the open arm entries was interpreted as an increase in anxiety-like behaviour.
OF test
The OF consisted of a box (50 × 50 × 30 cm) that was made of opaque grey plastic and illuminated by 80 lux at floor level. The ground area of the box was divided into a 36 × 36 cm central area and the surrounding border zone. The mice were placed individually in a corner of the OF, and their behaviour during a 5 min test period was tracked by a video camera positioned above the centre of the OF and recorded with the software VideoMot2 (TSE Systems). This software was used to evaluate the time spent in the central area, the number of entries into the central area and the total distance travelled in the OF. A reduction of the central area time and/or the central area entries was interpreted as an increase in anxiety-like behaviour.
Body temperature
The rectal temperature of the mice was measured with a digital thermometer (BAT-12, Physitemp Instruments, Clifton, NJ) equipped with a rectal probe for mice. The temperature recordings were taken between 13:00 and 13:30 h.
FST
The mice were placed individually in a glass cylinder (diameter: 16 cm, height: 23 cm) containing tap water of 25°C at a depth of 16 cm, which prevented the mice from touching the bottom with their paws or the tail. The mice were tested for 6 min, and their behavioural activity was scored by a trained observer blind to genotype and treatment. The time each mouse spent climbing, swimming and floating (immobile) was expressed as a percentage of the test duration. Mice were considered immobile when they floated passively in the water, performing only movements that enabled them to keep their heads above the water level (Cryan and Mombereau, 2004). An increase in the immobility time (at the expense of swimming and/or climbing time) was interpreted as an increase in depression-like behaviour. After the FST, the mice were placed for 6 min under a warming lamp and then returned to their home cage.
Myeloperoxidase (MPO) levels in the colon
The tissue levels of MPO were used to quantify inflammation-associated infiltration of neutrophils and monocytes into the tissue (Krawisz et al., 1984). After killing, full-thickness pieces of the distal colon were excised, shock-frozen in liquid nitrogen and stored at −70°C until assay. After weighing, the frozen tissues were placed, at a ratio of 1 mg : 0.02 mL, in MPO lysis buffer (Hycult Biotechnology, Uden, The Netherlands). The composition of this buffer was as follows: 200 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycine, 0.1 mM phenylmethylsulphonyl fluoride, 1 µg·ml−1 leupeptide, 28 µg·ml−1 aprotinin, pH 7.4. The samples were homogenized on ice with an Ultraturrax (IKA, Staufen, Germany) and then subjected to two centrifugation steps at 6000×g and 4°C for 15 min. The MPO (donor: H2O2 oxidoreductase, EC 1.11.1.7) content of the supernatant was measured with an enzyme-linked immunosorbent assay kit specific for the rat and mouse protein (Hycult Biotechnology). The sensitivity of this assay is 1 ng·mL−1 at an intra- and inter-assay variation of around 10%.
Circulating corticosterone levels
The plasma levels of corticosterone were determined between 11:00 h and 13:00 h. Blood was collected into vials coated with EDTA (Greiner, Kremsmünster, Austria) kept on ice. Following centrifugation for 20 min at 4°C and 1200×g, blood plasma was collected and stored at −70°C until assay. The plasma levels of corticosterone were determined with an enzyme immunoassay kit (Assay Designs, Ann Arbor, MI). According to the manufacturer's specifications, the sensitivity of the assay is 27 pg·mL−1, and the intra- and inter-assay coefficient of variation amounts to 7.7% and 9.7% respectively.
Statistics
Statistical evaluation of the results was made with SPSS 16.0 (SPSS Inc., Chicago, IL). The data were analysed with Student's t test, one-way or two-way analysis of variance (anova), as appropriate. The homogeneity of variances was assessed with the Levene test. In case of sphericity violations, the Greenhouse-Geisser correction was applied. Post-anova analysis of group differences was performed with the Tukey HSD (honestly significant difference) test, when the variances were homogeneous, and with the Games–Howell test, when the variances were unequal. In select cases, planned comparisons with one-way anova were performed (Kirk, 1995). Probability values P≤ 0.05 were regarded as statistically significant. All data are presented as means ± SEM, n referring to the number of mice in each group.
Materials
DSS (molecular weight: 36 000–50 000; MP Biochemicals, Illkirch, France) was added to the drinking water at a concentration of 2%. Pentobarbital (Sigma-Aldrich, Vienna, Austria) was dissolved in an aqueous solution containing 20% ethylene glycol and 10% ethanol at a concentration of 120 mg·mL−1.
Results
Effect of DSS-induced colitis on rectal temperature, body weight and colonic MPO content in female WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice
Rectal temperature was determined on day 10, while body weight and colonic MPO content were measured on day 11 of the treatment protocol. Two-way anova revealed that the rectal temperature differed with genotype (F(3,57) = 15.08, P < 0.001) but not with treatment, with a significant interaction between these factors (F(3,57) = 4.37, P = 0.008). Post hoc analysis showed that the rectal temperature of female PYY−/− and NPY−/−; PYY−/− mice was higher than that of WT mice (Table 1). PYY−/− mice presented the highest rectal temperature, exceeding that of NPY−/− and NPY−/−; PYY−/− mice (Table 1). DSS treatment reduced the rectal temperature in female PYY−/− mice but not in the other female genotypes under study (Table 1).
Table 1.
Rectal temperature (°C) measured in female and male mice of the wild-type (WT), NPY−/−, PYY−/− and NPY−/−; PYY−/− genotype
| Female Mice | Male mice | |||
|---|---|---|---|---|
| Genotype | Control | DSS | Control | DSS |
| WT mice | 36.36 ± 0.19 | 36.69 ± 0.15 | 37.05 ± 0.21 | 35.88 ± 0.10++ |
| NPY−/− mice | 36.60 ± 0.15 | 36.63 ± 0.13 | 35.91 ± 0.05** | 35.65 ± 0.09+ |
| PYY−/− mice | 37.69 ± 0.07** | 37.00 ± 0.14+ | 37.11 ± 0.30 | 36.30 ± 0.10+ |
| NPY−/−; PYY−/− mice | 37.01 ± 0.12* | 36.88 ± 0.12 | 36.20 ± 0.21* | 36.38 ± 0.21 |
The animals were treated with dextran sulphate sodium (DSS, 2%) added to the drinking water for 9 days before the recording, while control mice drank normal tap water. The values represent means ± SEM, n = 8–9.
P < 0.05
P < 0.01 versus values in WT control mice of the same sex
P < 0.05
P < 0.01 versus values in control mice of the same genotype and sex.
The weight of the animals was measured immediately before the treatment with DSS and on day 11 of the DSS treatment, 30 min after the start of the FST. Two-way anova with repeated measurements demonstrated that the weight of the female animals depended on genotype (F(3,56) = 14.49, P < 0.001) but not on treatment, without a significant interaction between these factors. Table 2 shows that the body weight did not change after DSS treatment.
Table 2.
Body weight (g) of female and male mice of the wild-type (WT), NPY−/−, PYY−/− and NPY−/−; PYY−/− genotype
| Female mice | Male mice | |||
|---|---|---|---|---|
| Genotype | Control | DSS | Control | DSS |
| WT mice | 22.09 ± 0.23 | 21.96 ± 0.22 | 28.86 ± 0.60 | 22.99 ± 0.79++ |
| NPY−/− mice | 24.92 ± 0.53 | 25.55 ± 0.53 | 31.88 ± 0.67* | 30.71 ± 0.80 |
| PYY−/− mice | 22.28 ± 0.66 | 23.53 ± 0.83 | 31.63 ± 0.40* | 29.96 ± 0.76 |
| NPY−/−; PYY−/− mice | 25.29 ± 0.86 | 24.82 ± 0.52 | 29.02 ± 0.96 | 26.12 ± 0.77+ |
The animals were treated with dextran sulphate sodium (DSS, 2%) added to the drinking water for 10 days before the recording, while control mice drank normal tap water. The values represent means ± SEM, n = 8.
P < 0.05 versus values in WT control mice of the same sex
P < 0.05
P < 0.01 versus values in control mice of the same genotype and sex.
Treatment with DSS induced colitis as shown by an increase in the tissue levels of MPO measured on day 11 of the DSS treatment, 30 min after the start of the FST (Figure 1A). Specifically, the colonic MPO content differed with genotype (F(3,56) = 13.67, P < 0.001) and treatment (F(1,56) = 77.88, P < 0.001), with a significant interaction between these factors (F(3,56) = 9.62, P < 0.001). Post hoc analysis disclosed that the pre-treatment levels of MPO in female NPY−/− and NPY−/−; PYY−/− mice were lower than those in female WT mice (Figure 1A). DSS treatment increased the levels of MPO in all female genotypes, except in NPY−/− mice (Figure 1A). The post-treatment content of MPO in PYY−/− and NPY−/−; PYY−/− mice was significantly higher (P < 0.01) than that in WT and NPY−/− mice (Figure 1A).
Figure 1.

Colonic myeloperoxidase (MPO) content of female (A) and male (B) mice of the wild-type (WT), NPY−/−, PYY−/− and NPY−/−; PYY−/− genotype. The animals were treated with dextran sulphate sodium (DSS, 2%) added to the drinking water for 10 days before the recording, while control (CO) mice drank normal tap water. The values represent means ± SEM, n = 7–8. *P < 0.05, **P < 0.01 versus values in WT control mice of the same sex, +P < 0.05, ++P < 0.01 versus values in control mice of the same genotype and sex.
Effect of DSS-induced colitis on rectal temperature, body weight and colonic MPO content in male WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice
Two-way anova showed that the rectal temperature differed with genotype (F(3,56) = 9.73, P < 0.001) and treatment (F(1,56) = 17.07, P < 0.001), with a significant interaction between these factors (F(3,56) = 5.62, P = 0.002). Post hoc analysis showed that the rectal temperature of male NPY−/− and male NPY−/−; PYY−/−, but not male PYY−/−, mice was lower than that of male WT mice (Table 1). DSS treatment led to a significant drop of body temperature in WT, NPY−/− and PYY−/−, but not NPY−/−; PYY−/−, mice (Table 1).
The weight of male mice varied with genotype (F(3,56) = 24.67, P < 0.001) and treatment (F(1,56) = 31.21, P < 0.001), with a significant interaction between these factors (F(3,56) = 4.11, P = 0.01). Specifically, the pre-treatment weight of male NPY−/− and PYY−/−, but not NPY−/−; PYY−/−, mice was higher than that of male WT mice (Table 2). The body weight of WT and NPY−/−; PYY−/− mice, but not of NPY−/− and PYY−/− mice, decreased significantly after DSS treatment (Table 2).
The induction of DSS-evoked colitis in male mice was shown by an increase in the tissue level of MPO (Figure 1B). The colonic MPO content differed with genotype (F(3,55) = 19.00, P < 0.001) and treatment (F(1,55) = 149.70, P < 0.001), with a significant interaction between these factors (F(3,55) = 13.26, P < 0.001). The pre-treatment levels of MPO did not significantly differ between the male genotypes. DSS treatment increased the level of MPO in all genotypes, but to a different extent (Figure 1B). Thus, the post-treatment content of MPO in WT, PYY−/− and NPY−/−; PYY−/− mice was significantly higher (P < 0.05) than that in WT and NPY−/− mice (Figure 1B).
Effect of DSS-induced colitis on anxiety- and depression-related behaviour and circulating corticosterone in female WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice
The behaviour of female WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice in the EPM test differed with genotype but not with treatment (Figure 2, left panel). Two-way anova showed that the genotype influenced the time spent on the open arms (F(3,56) = 4.77, P = 0.005), the number of open arm entries (F(3,56) = 7.93, P < 0.001), the total travelling distance (F(3,56) = 3.34, P = 0.03) and the number of total entries into any arm (F(3,56) = 9.35, P < 0.001). However, none of the parameters of the EPM test was affected by the factor ‘treatment’, nor was there any interaction between genotype and treatment.
Figure 2.

Behaviour of female (A–D) and male (E–H) mice of the wild-type (WT), NPY−/−, PYY−/− and NPY−/−; PYY−/− genotype in the elevated plus maze (EPM) test. The animals were treated with dextran sulphate sodium (DSS, 2%) added to the drinking water for 7 days before the EPM test, while control (CO) mice drank normal tap water. The graphs show the time spent on the open arms (A, E), the number of entries into the open arms (B, F), the total distance travelled in the open and closed arms (C, G) and the total number of entries into any arm (D, H) during the 5 min test session. The time spent on the open arms is expressed as a percentage of the test duration. The values represent means ± SEM, n = 8. **P < 0.01 versus WT control mice of the same sex, ++P < 0.01 versus control mice of the same genotype and sex.
Analysis of the behaviour in the OF test revealed both genotype- and treatment-related effects (Figure 3, left panel). Specifically, the time spent in the central area of the OF differed with genotype (F(3,56) = 52.28, P < 0.001) but not with treatment, with a significant interaction between these factors (F(3,56) = 5.20, P = 0.003). The number of central area entries depended on genotype (F(3,56) = 44.11, P < 0.001) and treatment (F(1,56) = 22.25, P < 0.001), with a significant interaction between these factors (F(3,56) = 9.24, P < 0.001). In contrast, the total travelling distance was affected by the genotype only (F(3,56) = 25.31, P < 0.001), while treatment had no effect, and there was no interaction between genotype and treatment. Post hoc analysis disclosed that female NPY−/− control mice spent less time in the central area (Figure 3A) and entered the central area less often (Figure 3B) than female WT control mice. NPY−/−; PYY−/− control mice likewise entered the central area less often than WT control mice (Figure 3B), while PYY−/− and WT control mice did not grossly differ in their OF behaviour (Figure 3A,B,C). DSS treatment prolonged the time spent in the central area and the number of central area entries in PYY−/− mice and lengthened the central area time in NPY−/− mice but had no significant effect in WT and NPY−/−; PYY−/− mice (Figure 3A, B).
Figure 3.
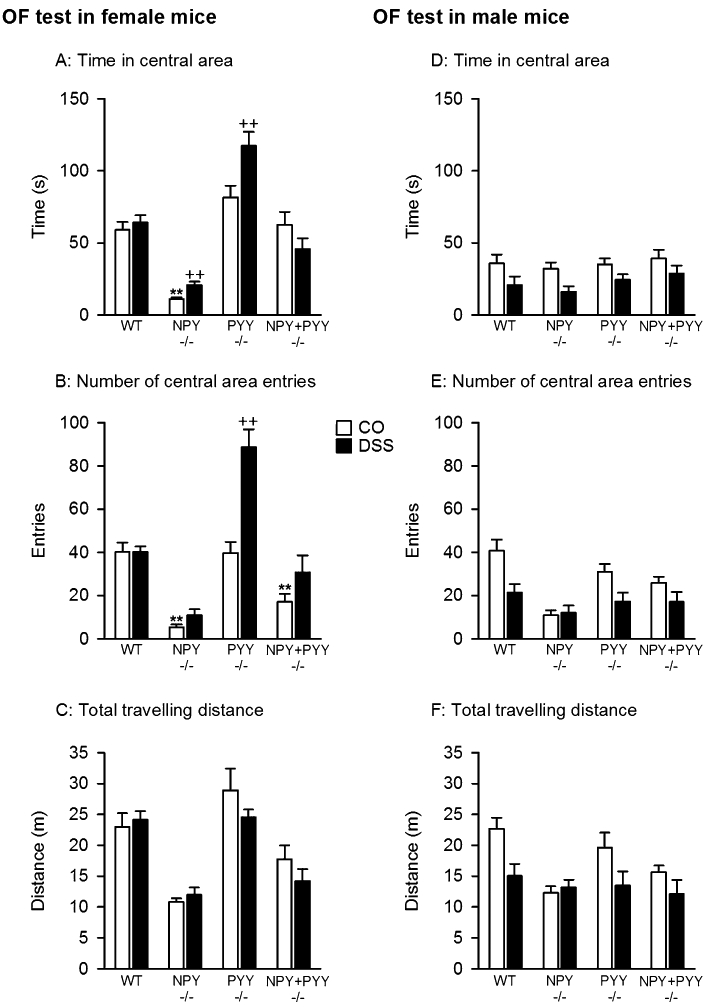
Behaviour of female (A–C) and male (D–F) mice of the wild-type (WT), NPY−/−, PYY−/− and NPY−/−; PYY−/− genotype in the open field (OF) test. The animals were treated with dextran sulphate sodium (DSS, 2%) added to the drinking water for 8 days before the OF test, while control (CO) mice drank normal tap water. The graphs show the time spent in the central area (A, D), the number of entries into the central area (B, E) and the total distance travelled (C, F) during the 5 min test session. The time spent in the central area is expressed as a percentage of the test duration. The values represent means ± SEM, n = 8. **P < 0.01 versus WT control mice of the same sex, ++P < 0.01 versus control mice of the same genotype and sex.
Depression-like behaviour was assessed with the FST, and two-way anova revealed that the time of immobility was related to genotype (F(3,56) = 48.73, P < 0.001) and treatment (F(1,56) = 17.77, P < 0.001), with a significant interaction between these factors (F(3,56) = 2.67, P = 0.05). The time of swimming likewise depended on genotype (F(3,56) = 4.90, P = 0.004) and treatment (F(1,56) = 22.21, P < 0.001), with a significant interaction between these factors (F(3,56) = 4.92, P = 0.004). In contrast, the time of climbing differed only with genotype (F(3,56) = 44.31, P < 0.001) but not with treatment, without a significant interaction between these factors. Post hoc analysis disclosed that the time of immobility in female PYY−/−, NPY−/− and NPY−/−; PYY−/− control mice was significantly longer than in female WT control mice (Figure 4A). The prolongation of the immobility time was associated with a shortening of the swimming time in NPY−/− control mice (Figure 4B). Treatment with DSS prolonged the duration of immobility in female WT and NPY−/−; PYY−/− mice, an effect that occurred at the expense of swimming time, while in female PYY−/− and NPY−/− mice, no significant effect of DSS treatment was noted (Figure 4A,B).
Figure 4.

Behaviour of female (A–C) and male (D–F) mice of the wild-type (WT), NPY−/−, PYY−/− and NPY−/−; PYY−/− genotype in the forced swim test (FST). The animals were treated with dextran sulphate sodium (DSS, 2%) added to the drinking water for 10 days before the FST, while control (CO) mice drank normal tap water. The graphs show (A, D) the duration of immobility, (B, E) the duration of swimming and (C, F) the duration of climbing, these parameters being expressed as a percentage of the test duration. The values represent means ± SEM, n = 8. *P < 0.05, **P < 0.01 versus WT control mice of the same sex, ++P < 0.01 versus control mice of the same genotype and sex.
Thirty minutes after the FST was begun, the levels of circulating corticosterone were determined. Given that the stress associated with the FST causes a rise of circulating corticosterone, planned comparisons were used to compare the post-FST plasma levels of corticosterone in control and DSS-treated mice with basal plasma levels of corticosterone from a separate group of female animals. The basal levels of corticosterone in female NPY−/− mice were higher than in female WT mice but did not significantly differ in female PYY−/− and NPY−/−; PYY−/− mice (Figure 5A). Exposure to the FST enhanced circulating corticosterone in both control and DSS-treated female mice of the WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− genotype. The post-FST levels of corticosterone did not significantly differ between control and DSS-treated WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice (Figure 5A).
Figure 5.

Plasma levels of corticosterone in female (A) and male (B) mice of the wild-type (WT), NPY−/−, PYY−/− and NPY−/−; PYY−/− genotype. The animals were treated with dextran sulphate sodium (DSS, 2%) added to the drinking water for 10 days before the FST, while control (CO) mice and mice used for measuring basal corticosterone levels drank normal tap water. Circulating corticosterone was determined at baseline and 30 min after the forced swim test had begun. The values represent means ± SEM, n = 8. *P < 0.05, **P < 0.01 versus basal values in WT mice of the same sex, +P < 0.05, ++P < 0.01 versus basal values in mice of the same genotype and sex, P < 0.05, P < 0.01 versus CO+FST values in mice of the same genotype and sex.
Effect of DSS-induced colitis on anxiety- and depression-related behaviour and circulating corticosterone in male WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice
The behaviour of male WT, NPY−/−, PYY−/− and NPY−/−; PYY−/− mice in the EPM test was subject to genotype and treatment effects (Figure 2, right panel). Two-way anova showed that the genotype influenced the time spent on the open arms (F(3,56) = 13.83, P < 0.001), the total travelling distance (F(3,56) = 9.09, P < 0.001) and the number of total entries into any arm (F(3,56) = 5.74, P = 0.002). The time spent on the open arms (F(1,56) = 12.73, P = 0.001) and the number of open arm entries (F(1,56) = 10.92, P = 0.002) were affected by DSS treatment, with a significant interaction between genotype and treatment for both parameters (time spent on the open arms: F(3,56) = 6.12, P = 0.001; number of open arm entries: F(3,56) = 4.74, P = 0.005). Figure 2E illustrates that male NPY−/− control mice spent less time on the open arms than male WT control mice. DSS treatment shortened the time spent on the open arms in male WT mice and reduced the number of open arm entries in male NPY−/− mice (Figure 2E, F).
Assessment of the behaviour in the OF test revealed both genotype- and treatment-related effects (Figure 3, right panel). While the time spent in the central area of the OF differed with treatment (F(1,56) = 13.00, P = 0.001) but not genotype, without a significant interaction between these factors, the number of central area entries depended on genotype (F(3,56) = 8.96, P < 0.001) and treatment (F(1,56) = 14.07, P < 0.001), without a significant interaction between these factors. Likewise, the total travelling distance was affected by genotype (F(3,56) = 4.44, P = 0.007) and treatment (F(1,56) = 9.89, P = 0.003), without a significant interaction between these factors.
Depression-like behaviour as assessed with the FST was subject to genotype and DSS treatment effects. Thus, the time of immobility was affected by genotype (F(3,56) = 20.64, P < 0.001) but not by treatment, with a significant interaction between these factors (F(3,56) = 3.93, P = 0.01). Similarly, the time of swimming depended on genotype (F(3,56) = 10.43, P < 0.001) but not on treatment, with a significant interaction between genotype and treatment (F(3,56) = 4.92, P = 0.004). In contrast, there was no significant interaction between these factors as regards the time of climbing which differed only with genotype (F(3,56) = 18.66, P < 0.001) but not treatment. Post hoc analysis indicated that the time of immobility in male NPY−/−, PYY−/− and NPY−/−; PYY−/− control mice was significantly longer than in male WT control mice (Figure 4D), which was associated with a shortening of the swimming time in male PYY−/− and NPY−/−; PYY−/− control mice (Figure 4E). Treatment with DSS shortened the time of immobility in male PYY−/− mice, an effect that was associated with an increase in the swimming time (Figure 4D,E).
The levels of circulating corticosterone, determined at basal conditions and 30 min after the FST had begun, differed with genotype and treatment (Figure 5B). The basal levels of corticosterone in male PYY−/− animals were higher, and those in male NPY−/−; PYY−/− mice lower, than the basal corticosterone levels in male WT mice. Exposure to the FST enhanced circulating corticosterone in both control and DSS-treated male mice of all genotypes under study, but to a significantly different degree (Figure 5B). Following DSS treatment, the post-FST concentration of corticosterone in male WT, PYY−/− and NPY−/−; PYY−/− mice was significantly higher than that under control conditions, whereas in male NPY−/− control mice the FST-evoked increase in circulating corticosterone was as large as that evoked in male DSS-treated NPY−/− mice (Figure 5B).
Discussion
The major aim of this study was to evaluate whether knockout of PYY and/or NPY and mild experimental colitis interacted in modifying emotional-affective behaviour in a sex-related manner. This goal was addressed by examining (i) sex-related effects of NPY and/or PYY knockout on emotional-affective behaviour in the absence of colitis and (ii) sex- and genotype-related effects of DSS-induced colitis on emotional-affective behaviour. The major findings of the study are summarized in Table 3.
Table 3.
Summary of findings in female and male wild-type (WT), NPY−/−, PYY−/− and NPY−/−; PYY−/− mice
| A. Genotype-related changes of parameters in control mice, relative to WT mice | ||||||
|---|---|---|---|---|---|---|
| NPY−/− | PYY−/− | NPY−/−; PYY−/− | ||||
| Test parameter | Female | Male | Female | Male | Female | Male |
| Body weight |  |
 |
||||
| Rectal temperature |  |
 |
 |
 |
||
| Colonic myeloperoxidase |  |
 |
||||
| Anxiety-related behaviour (EPM) |  |
|||||
| Anxiety-related behaviour (OF) |  |
|||||
| Depression-related behaviour |  |
 |
 |
 |
 |
 |
| Basal corticosterone |  |
 |
 |
|||
| B.DSS-induced changes of parameters, relative to control mice | ||||||||
|---|---|---|---|---|---|---|---|---|
| WT | NPY−/− | PYY−/− | NPY−/−; PYY−/−- | |||||
| Test parameter | Female | Male | Female | Male | Female | Male | Female | Male |
| Body weight |  |
 |
||||||
| Rectal temperature |  |
 |
 |
 |
||||
| Colonic myeloperoxidase |  |
 |
 |
 |
 |
 |
 |
|
| Anxiety-related behaviour (EPM) |  |
|||||||
| Anxiety-related behaviour (OF) |  |
 |
||||||
| Depression-related behaviour |  |
 |
 |
|||||
| Corticosterone post-FST |  |
 |
 |
|||||
 Increase,
Increase,  decrease. These symbols denote statistically significant changes. The changes in anxiety-related behaviour refer to the time spent on the open arms (EPM) and in the central area (OF), while the changes in depression-related behaviour refer to the time of immobility in the FST.
decrease. These symbols denote statistically significant changes. The changes in anxiety-related behaviour refer to the time spent on the open arms (EPM) and in the central area (OF), while the changes in depression-related behaviour refer to the time of immobility in the FST.
Effects of PYY and/or NPY knockout on body weight, rectal temperature and emotional-affective behaviour in the absence of colitis
The findings that male NPY−/− and male PYY−/− mice were heavier than WT mice of the same sex agree in part with other observations (Batterham et al., 2006; Boey et al., 2006; Edelsbrunner et al., 2009), which are not entirely consistent with each other. In addition, the rectal temperature in female PYY−/− and NPY−/−; PYY−/− animals was higher, and that in male NPY−/− and NPY−/−; PYY−/− mice lower, than the temperature in the respective WT animals. It is likely that these differences in temperature are due to differences in locomotion and bodily activity. Indeed, the EPM and OF test in the current experiments and a previous study of the circadian activity (Edelsbrunner et al., 2009) disclosed genotype-related differences in locomotion (total travelling distance). However, these data have a limited bearing on the present temperature recordings under group housing conditions, given that circadian activity was recorded under single housing conditions (Edelsbrunner et al., 2009) and locomotor behaviour of individual animals on the EPM and OF is determined by their reaction to novelty.
When studying anxiety-and depression-like behaviour, both sex- and test-related differences were observed as, for instance, anxiety-like behaviour on the OF but not EPM was enhanced by knockout of NPY in female mice whereas, in male mice, deletion of NPY caused an increase in anxiety-like behaviour on the EPM but not OF (Table 3). In contrast, knockout of PYY in addition to NPY cancelled the anxiogenic effect of NPY deletion alone in female mice (OF), as well as male mice (EPM). Deletion of PYY alone did not have any significant influence on anxiety-like behaviour in the EPM and OF test, either in female or in male mice.
Depression-like behaviour in the FST was enhanced by knockout of both NPY and PYY, alone or in combination, a change that was seen in both female and male mice and characterized by a prolongation of the immobility time at the expense of climbing time. In analogy with the behavioural effects of antidepressant drugs, this pattern of effect may provide a clue to the underlying neurochemical mechanisms. Antidepressants that act primarily by reinforcing 5-hydroxytryptaminergic transmission increase predominantly swimming behaviour, whereas antidepressants that act primarily by reinforcing noradrenergic transmission increase predominantly climbing behaviour (Detke et al., 1995; Cryan et al., 2005). It would seem therefore that deletion of NPY and/or PYY enhances depression-like behaviour by an action on noradrenergic mechanisms, a suggestion that provides a lead for further investigations.
The current findings reveal that deletion of PYY, a gut hormone, enhances depression-like behaviour in the absence of a change in anxiety-related behaviour or, in other terms, that endogenous PYY has an antidepressant action. This discovery adds support to the notion that gut hormones can influence emotional-affective behaviour as has been noted, for example, for ghrelin, glucagon-like peptide-1 and glucagon-like peptide-2 (Möller et al., 2002; Kinzig et al., 2003; Lutter et al., 2008; Iwai et al., 2009). PYY released from endocrine cells of the hindgut into the bloodstream circulates primarily as PYY(3–36), which can gain access to hypothalamic and extrahypothalamic sites in the brain (McGowan and Bloom, 2004; Batterham et al., 2007; Ueno et al., 2008). Although PYY(3–36) has some selectivity for Y2 receptors (Alexander et al., 2009), it would be premature to surmise that the antidepressant action of PYY, envisaged from the current data, is mediated by Y2 receptors. If so, deletion of Y2 receptors should, like deletion of PYY, enhance depression-like behaviour, whereas the opposite is the case: Y2 receptor deletion attenuated anxiety- and depression-like behaviour, an effect in which the amygdala plays a major role (Redrobe et al., 2003; Tschenett et al., 2003; Painsipp et al., 2008; Tasan et al., 2010).
The finding that knockout of NPY causes a sex- and test-related increase in anxiety and decrease in locomotion is in overall agreement with previous reports on the behavioural phenotype of NPY−/− mice (Bannon et al., 2000; Lin et al., 2004; Karl et al., 2008). The current study adds that knockout of NPY enhances depression-like behaviour in a sex-independent fashion. The levels of circulating corticosterone measured at baseline and post-FST do not support the conclusion that the pro-depressant effect of NPY and PYY deletion is related to altered activity of the hypothalamic-pituitary-adrenal axis.
Effect of DSS-induced colitis on body weight, rectal temperature and colonic MPO
DSS (2% in the drinking water) increased the colonic MPO content by a factor of 1.8–7.9, depending on the genotype. We consider this effect of DSS to be indicative of a mild non-necrotic form of colitis because even a 17-fold increase of the colonic MPO content, as seen with DSS (2%) in the outbred OF1 mouse strain, was not associated with a change in the architecture of the colonic mucosa and in the circadian pattern of activity and ingestion (Mitrovic et al., 2010). In the present study, DSS failed to significantly alter the body weight of female mice of any genotype, whereas male WT and NPY−/−; PYY−/− mice lost some weight during the 11 day course of DSS treatment (Table 3). In addition, colitis lowered rectal temperature in female PYY−/− and in male WT, NPY−/− and PYY−/− mice. This sex difference needs be taken into account when the sex-related impact of DSS-induced colitis on emotional-affective behaviour is considered. The oestrous cycle was not monitored in the present experiments in order to avoid additional stress, but we consider it unlikely that our data were significantly biased by this potentially confounding factor, given that the oestrous cycle is largely synchronized across the cages under study (Painsipp et al., 2007).
The magnitude of the effect of DSS to enhance the colonic MPO content as an index of inflammation depended on both genotype and sex, given that the DSS-evoked increase in the colonic MPO content of male WT, PYY−/− and NPY−/−; PYY−/− mice was larger than that in the respective female mice. This particular susceptibility of male mice to DSS-induced inflammation does not apply to NPY−/− mice because deletion of NPY had a sex-independent anti-inflammatory effect. Thus, the colonic levels of MPO in female NPY−/− and NPY−/−; PYY−/− mice in the absence of DSS treatment were lower than in the respective WT mice, and the DSS-induced increase in the colonic MPO content in female and male NPY−/− mice was much lower than in WT, PYY−/− and even NPY−/−; PYY−/− mice (Table 3). We can infer therefore that the anti-inflammatory action of NPY knockout was opposed by the additional knockout of PYY or, in other terms, that the pro-inflammatory influence of NPY is antagonized by PYY.
The ability of NPY knockout to attenuate the DSS-evoked accumulation of MPO in the colon confirms other reports that deletion of NPY blunts DSS-induced colitis (Chandrasekharan et al., 2008), an outcome that is reproduced by knockout or antagonism of Y1 receptors (Hassani et al., 2005) and treatment with a NPY antisense oligodeoxynucleotide (Pang et al., 2010). An involvement of NPY and PYY in colonic inflammation is suggested by several other findings. For instance, DSS-induced colitis was associated with an increase in the expression of NPY in enteric neurons (Chandrasekharan et al., 2008), a reduction of Y1 receptor expression and a loss of the anti-secretory action of NPY in the colon (Klompus et al., 2010). In contrast, the colonic levels of PYY were decreased in rats with DSS-induced colitis (Hirotani et al., 2008). These experimental data are in line with the observed decrease of colonic PYY levels in patients with inflammatory bowel disease (Tari et al., 1988; El-Salhy et al., 1997; Schmidt et al., 2005), while circulating levels of PYY and NPY are enhanced (Adrian et al., 1986; Straub et al., 2002).
Interaction of DSS-induced colitis with NPY and/or PYY knockout in modifying emotional-affective behaviour
Gastrointestinal inflammation is increasingly recognized to contribute to alterations in emotional-affective behaviour (Painsipp et al., 2007; Goehler et al., 2008). While female WT mice did not change in their anxiety-like behaviour on the EPM and OF in response to colitis, male WT mice placed on the EPM were more anxious after DSS treatment. In contrast, when female NPY−/− and PYY−/− mice were tested on the OF, colitis had an anxiolytic effect, whereas combined deletion of NPY and PYY prevented the ability of colitis to alter anxiety-like behaviour. It would seem therefore that NPY and PYY have the potential to modify the effect of colitis on anxiety in a sex-dependent manner. An aspect worth considering is that, in NPY−/− mice, colitis had a distinct effect on anxiety-like behaviour although the severity of colitis in these mice was significantly attenuated. It remains to be explored whether DSS- or colitis-induced factors that are of minor relevance to the severity of inflammation determine the impact of DSS treatment on brain circuits governing behaviour. The paradoxical observation that DSS-induced colitis attenuated anxiety-like behaviour in female NPY−/− and PYY−/− mice on the OF but not EPM remains to be confirmed and further analysed.
Depression-like behaviour was enhanced by DSS-induced colitis in female, but not male, WT mice. Knockout of NPY and PYY prevented the pro-depressant effect of colitis seen in female WT mice, which supports the conclusion that NPY and PYY play a role in the ability of colitis to increase depression-like behaviour, this role being lost when both NPY and PYY were knocked out together. In female WT and NPY−/−; PYY−/− mice, DSS-induced colitis prolonged the immobility time in the FST at the expense of swimming time, whereas in male PYY−/− mice DSS-induced colitis shortened the immobility time in the FST with a concomitant gain in swimming time. Although the influence of colitis on depression-like behaviour differed with sex and genotype, the alteration of immobility time in exchange with swimming time suggests that the impact of colitis on depression-like behaviour involves primarily 5-hydroxytryptaminergic mechanisms. The effect of colitis to amplify the post-FST levels of circulating corticosterone did not provide any useful clues to a specific involvement of the hypothalamic–pituitary–adrenal axis in the colitis-induced changes of depression-like behaviour.
Concluding considerations
Five major conclusions are drawn from this study. (i) Like deletion of the neuropeptide NPY, deletion of the gut hormone PYY enhances depression-like behaviour, whereas anxiety-like behaviour is enhanced by deletion of NPY but not PYY. (ii) The severity of DSS-induced colitis is attenuated by knockout of NPY but not PYY. (iii) DSS-induced colitis modifies anxiety- and depression-related behaviour, effects in which NPY and PYY appear to be involved. (iv) Sex and behavioural tests are important factors that determine the effect of experimental interventions such as colitis and genetic modifications on emotional-affective behaviour. (v) While these data demonstrate distinct roles of PYY and NPY in the regulation of anxiety- and depression-like behaviour in the absence and presence of colitis, the possibility that developmental compensations may mask the full implication of PYY and NPY in these functions cannot be discounted.
Experiments involving the combined knockout of NPY and PYY were carried out to explore whether these two members of the pancreatic polypeptide-fold peptide family serve additive, synergistic or antagonistic functions. In several instances, double knockout of NPY plus PYY cancelled the effects of knocking out NPY or PYY alone. It would thus seem that NPY and PYY are functional antagonists, the actions of which are brought about by different receptors at different cellular locations. In the brain, postsynaptic Y1 and presynaptic Y2 receptors mediate antagonistic effects of Y1 and Y2 receptor agonists, for example anxiolytic and antidepressant responses to Y1 receptor stimulation, and opposite effects due to stimulation of presynaptic autoinhibitory Y2 receptors (Heilig, 2004; Morales-Medina et al., 2010; Tasan et al., 2010). It remains to be examined whether the gut hormone PYY, degraded to PYY(3–36), exerts its effects on the gut–brain axis predominantly via activation of Y2 receptors. Because brain Y1 and Y2 receptor binding is increased in NPY−/− mice (Gehlert and Shaw, 2007), it is also possible that genetic deletion of NPY plus PYY leads to compensatory changes in other systems in such a way that the phenotype of NPY−/− and PYY−/− mice is lost.
Acknowledgments
This study was supported by the Zukunftsfonds Steiermark (grant 262) and the Austrian Science Funds (FWF grant L25-B05). The authors thank Margit Eichholzer for the assays of myeloperoxidase and corticosterone, Dr Birgit Ebner, Karin Meister and Dr Christian Gülly for genotyping the animals, and Professor Andreas Tiran for his support to conduct the study at the Centre for Medical Research of the Medical University of Graz.
Glossary
Abbreviations
- DSS
dextran sulphate sodium
- EPM
elevated plus maze
- FST
forced swim test
- MPO
myeloperoxidase
- NPY
neuropeptide Y
- NPY−/− mice
homozygous NPY knockout mice
- OF
open field
- PYY
peptide YY
- PYY−/− mice
homozygous PYY knockout mice
- WT
wild type
Conflict of interest
None.
References
- Adrian TE, Savage AP, Bacarese-Hamilton AJ, Wolfe K, Besterman HS, Bloom SR. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology. 1986;90:379–384. doi: 10.1016/0016-5085(86)90936-4. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. (4th edn.) 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, et al. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, et al. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia. 2006;49:1360–1370. doi: 10.1007/s00125-006-0237-0. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, et al. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One. 2008;3:e3304. doi: 10.1371/journal.pone.0003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berlin) 1995;21:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, et al. Neuropeptide Y (NPY) gene: impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol. 2010;20:301–309. doi: 10.1016/j.euroneuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Edelsbrunner ME, Herzog H, Holzer P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian cycle- and gender-dependent manner. Behav Brain Res. 2009;203:97–107. doi: 10.1016/j.bbr.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front Neuroendocrinol. 2006;27:308–339. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim. 2000;34:372–378. doi: 10.1258/002367700780387714. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Shaw JL. Increased brain neuropeptide Y1 and Y2 receptor binding in NPY knock out mice does not result in increased receptor function. Peptides. 2007;28:241–249. doi: 10.1016/j.peptides.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RPA. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun. 2008;22:354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM. Gender differences in depression and response to psychotropic medication. Gender Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol. 2005;288:G550–G556. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hirotani Y, Mikajiri K, Ikeda K, Myotoku M, Kurokawa N. Changes of the peptide YY levels in the intestinal tissue of rats with experimental colitis following oral administration of mesalazine and prednisolone. Yakugaku Zasshi. 2008;128:1347–1353. doi: 10.1248/yakushi.128.1347. [DOI] [PubMed] [Google Scholar]
- Iwai T, Hayashi Y, Narita S, Kasuya Y, Jin K, Tsugane M, et al. Antidepressant-like effects of glucagon-like peptide-2 in mice occur via monoamine pathways. Behav Brain Res. 2009;204:235–240. doi: 10.1016/j.bbr.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Karl T, Burne TH, Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav Brain Res. 2006;167:87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur J Neurosci. 2008;28:173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design. Procedures for the Behavioral Sciences. 3rd edn. Belmont, CA: Wadsworth; 1995. [Google Scholar]
- Klompus M, Ho W, Sharkey KA, McKay DM. Antisecretory effects of neuropeptide Y in the mouse colon are region-specific and are lost in DSS-induced colitis. Regul Pept. 2010;165:138–145. doi: 10.1016/j.regpep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Lesch KP, Selch S, Renner TJ, Jacob C, Nguyen TT, Hahn T, et al. Genome-wide copy number variation analysis in attention-deficit/hyperactivity disorder: association with neuropeptide Y gene dosage in an extended pedigree. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.29. doi: 10.1038/mp.2010.29. [DOI] [PubMed] [Google Scholar]
- Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan BMC, Bloom SR. Peptide YY and appetite control. Curr Opin Pharmacol. 2004;4:583–588. doi: 10.1016/j.coph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Mitrovic M, Shahbazian A, Bock E, Pabst MA, Holzer P. Chemo-nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin 1 channels. Br J Pharmacol. 2010;160:1430–1442. doi: 10.1111/j.1476-5381.2010.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller C, Sommer W, Thorsell A, Rimondini R, Heilig M. Anxiogenic-like action of centrally administered glucagon-like peptide-1 in a punished drinking test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:119–122. doi: 10.1016/s0278-5846(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Shahbazian A, Edelsbrunner M, Kreissl MC, Schirbel A, et al. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience. 2007;150:522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Edelsbrunner ME, Tasan RO, Singewald N, Herzog H, et al. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav. 2008;7:532–542. doi: 10.1111/j.1601-183X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang XH, Li TK, Xie Q, He FQ, Cui DJ, Chen YQ, et al. Amelioration of dextran sulfate sodium-induced colitis by neuropeptide Y antisense oligodeoxynucleotide. Int J Colorectal Dis. 2010;25:1047–1053. doi: 10.1007/s00384-010-0964-z. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav Brain Res. 2003;141:251–255. doi: 10.1016/s0166-4328(02)00374-1. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Carvajal C, Kask A, Dumont Y, Quirion R. Neuropeptide Y and its receptor subtypes in the central nervous system: emphasis on their role in animal models of psychiatric disorders. Handb Exp Pharmacol. 2004;162:101–136. [Google Scholar]
- Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, et al. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66:705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PT, Ljung T, Hartmann B, Hare KJ, Holst JJ, Hellström PM. Tissue levels and post-prandial secretion of the intestinal growth factor, glucagon-like peptide-2, in controls and inflammatory bowel disease: comparison with peptide YY. Eur J Gastroenterol Hepatol. 2005;17:207–712. doi: 10.1097/00042737-200502000-00012. [DOI] [PubMed] [Google Scholar]
- Straub RH, Herfarth H, Falk W, Andus T, Schölmerich J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J Neuroimmunol. 2002;126:116–125. doi: 10.1016/s0165-5728(02)00047-4. [DOI] [PubMed] [Google Scholar]
- Tari A, Teshima H, Sumii K, Haruma K, Ohgoshi H, Yoshihara M, et al. Peptide YY abnormalities in patients with ulcerative colitis. Jpn J Med. 1988;27:49–55. doi: 10.2169/internalmedicine1962.27.49. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Lin S, Hetzenauer A, Singewald N, Herzog H, Sperk G. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009;158:1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, et al. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, et al. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul Pept. 2008;145:12–16. doi: 10.1016/j.regpep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Wultsch T, Painsipp E, Thoeringer CK, Herzog H, Sperk G, Holzer P. Endogenous neuropeptide Y depresses the afferent signaling of gastric acid challenge to the mouse brainstem via neuropeptide Y type Y2 and Y4 receptors. Neuroscience. 2005;136:1097–1107. doi: 10.1016/j.neuroscience.2005.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch M, Scott D, Sinha R, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]


