Abstract
To elucidate the cognitive structures of animals, neuroscientists use several behavioral tasks. Therefore, it is imperative to have a firm understanding of each task’s behavioral parameters in order to parse out possible task effects. We compare two operant discrimination tasks (Go/No-Go: GNG; Two-Alternative Choice: TAC) that are commonly used in olfactory research. Past research has suggested that solving the two tasks requires divergent cognitive strategies. One hypothesis is that the two tasks differ in how an animal optimizes reward rate by means of a speed-accuracy trade-off (SAT). If this is true, then changing tasks could give researchers an additional tool to understand animal cognition. However, no study has systematically analyzed the two tasks in parallel using odor stimuli.
Using standardized training protocols, we test GNG and TAC in parallel. Our protocols allow us to isolate the stimulus sampling period from a general reaction time period. We find that the two tasks do not differ with regard to the stimulus sampling period and conclude that the two tasks do not differ in the amount of time it takes an animal to perform a discrimination. Instead, tasks differ in the time it takes to make an overt behavioral response, with GNG showing shorter periods than TAC. We also find no evidence of rats using either task specific or intertrial interval dependent SAT schema in order to optimize reward rate. We show that similarities between dependent variables, with the possible exception of response delay, appear to be under experimenter control.
Keywords: Olfactory behavior, go/no-go, two-alternative choice, rat operant behavior, odor discrimination, speed-accuracy trade-off
Olfactory research has used Go/No-Go (GNG) and Two-Alternative Choice (TAC) discrimination tasks to characterize rodent (rats and mice) psychophysics (Frederick, Barlas, Ievins, & Kay, 2009; Kay, Krysiak, Barlas, et al., 2006; B. M. Slotnick & Nigrosh, 1974; B. Slotnick, 2007) as well as the physiological correlates of odorant discrimination (Beshel, Kopell, & Kay, 2007; Carey, Verhagen, Wesson, Pírez, & Wachowiak, 2009; Friedrich, 2006; Gervais, Buonviso, Martin, & Ravel, 2007; Rajan, Clement, & Bhalla, 2006; Uchida & Mainen, 2003; Verhagen, Wesson, Netoff, White, & Wachowiak, 2007; Wesson, Carey, et al., 2008; Wesson, Donahou, et al., 2008; Wesson, Verhagen, & Wachowiak, 2009). Results suggest that in some circumstances rats need only a minimal sampling duration (∼1–2 sniffs) to discriminate accurately. However, results also suggest that rodents may behave differently in the two tasks, using longer stimulus sampling durations and discrimination times in GNG than in TAC tasks (Abraham et al., 2004; Gervais, Buonviso, Martin, & Ravel, 2007; Rinberg, Koulakov, & Gelperin, 2006; Uchida & Mainen, 2003). Mice engaged in a GNG task have been reported to increase their discrimination times to maintain a high level of accuracy for harder discriminations, while rats engaged in a TAC task have been reported not to engage in this behavior (Abraham et al., 2004; Uchida & Mainen, 2003). It is also generally assumed that TAC is more difficult for rodents to learn (B. M. Slotnick & Nigrosh, 1974; B. Slotnick, 2007).
These reported differences have been hypothesized to be due to either a speed-accuracy trade-off (SAT) (Rinberg, Koulakov, & Gelperin, 2006) and/or fundamentally different cognitive structures or strategies used in the two tasks (Kay, Beshel, & Martin, 2006). Rodents engaged in a TAC task may prefer a strategy that biases speed over accuracy in order to maximize reward rate, even when longer sampling durations may improve per trial performance (Friedrich, 2006; Rinberg, Koulakov, & Gelperin, 2006). This may be because in a TAC task a subject can usually receive rewards for correct responses to all stimuli whereas in GNG only one of the stimuli is paired with a reward. However, the fundamental difference between the two tasks is the stimulus-response pairing. In GNG, a subject associates a response to one stimulus but refrains from responding to a different stimulus. In TAC, a subject associates distinct responses to distinct stimuli. Differences in reward schedule are not therefore a fundamental difference between the tasks.
Inferences drawn from previous studies are potentially confounded by the fact that laboratories used different training and testing protocols, odorant types, exposure lengths, concentrations and delivery methods, animal restraining conditions, as well as different model species (mouse and rat). To address this, we tested two independent groups of rats, each engaged in either a GNG or TAC task. The groups were trained using the same protocol up until a final branching point where the task specific stimulus-response pairings were learned. We analyzed the behavioral distance between the two tasks along five dimensions: learning rate, performance, response delay, odorant sampling duration, and delay to initiate the start of a trial (nose-poke delay).
The reported differences between the two tasks could be due to each task having a different psychophysical curve dictated by the task’s unique stimulus-response pairing. One task may just require a rat to sample the stimulus longer, and we would therefore expect a difference in sampling duration and not in performance or other behavioral variables. A second possibility is that rats employ a different strategy in the two tasks that seeks to maximize reward rate for each task, possibly employing a SAT. To test this, we also varied the intertrial interval (ITI) in both tasks. If rats engage in a SAT meant to optimize reward rates, then a perturbation of the ITI should cause a shift in behavioral variables to compensate for the change in reward probability.
Methods
Subjects
Twenty-five adult male Sprague-Dawley rats (Harlan HSD, Dublin, VA and Indianapolis, IN) were used, but only twenty-four are included in the current report because one rat failed to learn the task (Phase 2) and was removed from the study. Of the twenty-four rats used for the main experiment, sixteen were randomly selected for use in the Phase 3 Variable ITI experiment (See Methods and Materials: Phase 3 Variable ITI). Seven of the twenty-four rats were randomly selected for use in control experiments (See Methods and Materials: Control Tests). All rats were individually housed in standard clear polycarbonate home cages with filter tops and maintained on a 14/10 hour light/dark cycle (lights on at 8:00 A.M. central standard time). All experiments were performed during the light phase, between 9:00 A.M. and 5:00 P.M. Rats were dieted to 85% of their ad libitum weights prior to Phase 0 (See Methods and Materials: Tasks Paradigms) and were maintained within 10% of their target weight for the duration of the experiment by food restriction. Rats were fed daily, at the same time, following their individual experimental session. Rats were run daily, at the same time, in groups of four without interruption from the start of training until a rat finished its experimental protocol. All experimental procedures were done with approval and oversight by the University of Chicago Institutional Animal Care and Use Committee, according to Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Odorant Delivery System
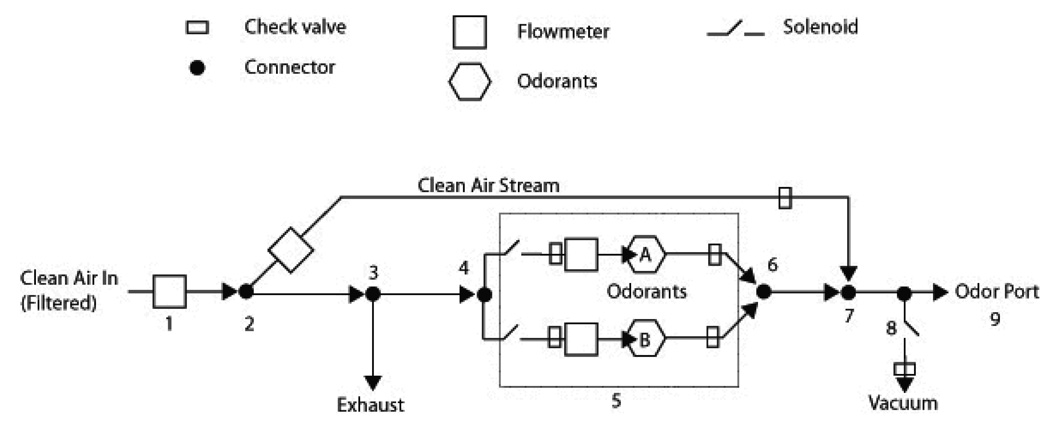
The odorant delivery system and behavioral apparatuses were constructed in-house using parts from Med Associates (St Alban, VT). All tubing connections were located outside of the operant chamber. The odorant delivery was a positive-pressure, air-dilution system constructed with C-flex tubing, glass test tubes for odorants, acrylic flowmeters, and solenoid-operated valves controlled by a computer running Med Associates MedPC IV software. The final odorant concentration was approximately 16% of the saturated vapor, based upon relative flow rates of clean air and odorized air. A schematic of the odorant delivery system is presented in Figure 2.
Figure 2. Odorant Delivery System Schematic.
Clean air enters the system after being passed through a carbon filter (1). The clean air is then split into a clean air stream (2) and two odor streams (4). An exhaust vent (3) prevents pressure problems. The odor streams pass through the odorants (5), which are housed in test tubes (A, B). The odorant streams then meet (6) and rejoin the clean air stream (7) at which point the odorized air has approximately 16% saturated vapor. If the vacuum line is open (8), the odorized air is diverted to an exhaust. If the vacuum line is closed, then the odorized air is able to flow into the odor port (9). Arrows indicate the direction of flow. Solenoids are controled by a computer. All connectors, except the one for the vacuum, which used a T-connector, were Y-connectors. Check valves ensured flow direction throughout the system. Not drawn to scale (for actual dimensions, see Materials and Methods: Odorant Delivery System).
The odorant delivery system had one input (clean air) and three outputs: an exhaust, a vacuum line, and the odor-port in the operant chamber (Figure 2: item 3, 8, and 9). Air was obtained from the building’s central line and passed through a carbon filter (Whatman InLine Carbon Filter, Kent, UK). C-flex tubing (I.D. 1/8", 6424–67, Cole-Parmer, Vernon Hills, IL) carried the clean air, unless otherwise specified. Downstream to the filter, air passed through 2 m of tubing and then through an acrylic flowmeter (5 LPM, Cole-Parmer; Figure 2, item 1). Then, 0.5 m after leaving the flowmeter, air was separated into two parallel streams (Figure 2, item 2) labeled as 1) the clean air stream and 2) the odor input stream. The odor input stream was separated into three parallel odor channels: two streams delivered air into the odorant test tubes (odor streams A and B; Figure 2, item 4), and a third, exhaust stream (0.6 m of tubing with open ending; Figure 2, item 3), was used to regulate excess pressure in the system. Then, 0.3 m prior to the odor test tubes, separate acrylic flowmeters (0.5 LPM, Cole-Parmer) were used to monitor flow in each odorant stream. Solenoid-operated 2-way pinch valves (R-98302–16, Cole-Parmer) were positioned 0.15 m upstream of the flowmeters, one per line, to control airflow (Figure 2, item 5). Odor tubes were located 0.3 m downstream of flowmeters, and their outputs channeled through odorized air streams, 0.6 m of parallel tubing paths (silicone tubing; one per odorant line). Then, 7 cm upstream of the odor port, the odorized air streams combined at a Y-connector into a single tubing segment (Figure 2, item 6), the final stream. The clean air stream mixed with the final stream 0.9 m from its initial diversion (Figure 2, item 2) and 4 cm upstream of the odor port (about halfway through the final stream; Figure 2; item 7). This resulted in dilution of the odorized air prior to reaching the odor port. Finally, 2 cm before reaching the odor port, a T-connector was inserted and tubing was diverted to a vacuum line (Figure 2, item 8) and to the odor port (Figure 2, item 9).
Solenoid valves for the odorants and the vacuum line, as well as session events, were controlled via custom-written code within Med Associates Med-PC IV software. Airflow through the clean air stream was never interrupted during the session. Two seconds prior to trial start, a solenoid valve opened to allow airflow to one odorant tube, in order to charge the tubing with odorized air. During this period, the vacuum was on, which prevented the odorized air from reaching the odor port. At the end of the 2 seconds, a house-light turned on, which signaled to the rat that the trial could begin. Then, a nose-poke by the rat would trigger the closing of the vacuum valve, which would allow the odorized stream to flow into the odor port. While the rat kept its nose in the odor port (i.e., while the photobeam was interrupted) the vacuum valve remained closed. Withdrawal of the nose from the odor port turned off the odorant solenoid and opened the vacuum solenoid thereby stopping any additional odorant from entering the odor port. Additionally, because the vacuum flow was greater than the odor stream flow, there was a slight negative flow from the odor port into the vacuum. This helped to clear the port of residual odorant between trials.
When an odorant’s solenoid valve was open, airflow to the odorant tube was 0.2 L/min and clean airflow was 1 L/min. Assuming that the odorized air was close to saturation, the final odorant concentration was approximately 16% of the saturated vapor. We estimate, based upon flow rates, length of tubing from the vacuum branch point and the solenoid manufacturer’s response time specifications , that there is approximately a 55 ms delay from the time the vacuum solenoid closes until odorant reaches the odor port. We present all sampling durations without subtracting this estimated delay.
Anisole (99+%, Fluka, Sigma Aldrich, St Louis, MO) and amyl acetate (99%, Acros, New Jersey) were used as odorants. Based on odorant assignment, we distinguished two groups of rats that are named Odor Set 1 and Odor Set 2. For Odor Set 1, amyl acetate was designated as odorant A (rewarded for response in GNG task, rewarded for left-side response in TAC task), and anisole was designated odorant B (refrain from response in GNG, rewarded for right-side response in TAC). For Odor Set 2, the roles were reversed; anisole was odorant A and amyl acetate was odorant B (See Table 1).
Table 1.
Odorants and Treatment Groups
| Phase 1 | Phase 2 | Phase 3 | ||
|---|---|---|---|---|
|
Group 1 (n=12 rats) |
Odor A GNG: Response TAC: Response left |
Amyl Acetate | Amyl Acetate | Amyl Acetate |
|
Odor B GNG: No response TAC: Response right |
– | – | Anisole | |
|
Group 2 (n=12 rats) |
Odor A GNG: Response TAC: Response left |
Anisole | Anisole | Anisole |
|
Odor B GNG: No response TAC: Response right |
– | – | Amyl Acetate | |
Task Paradigms
We used a 2×2 factorial design with the following treatments: odor set (Odor Set 1, 2) and task (GNG, TAC) with 6 rats per cell for a total of 24 rats. Rats were randomly assigned to one of four possible daily time slots and the assignments were balanced across the 2×2 design. A schematic outline of an individual trial is presented, along with descriptive statistics, in Figure 3E.
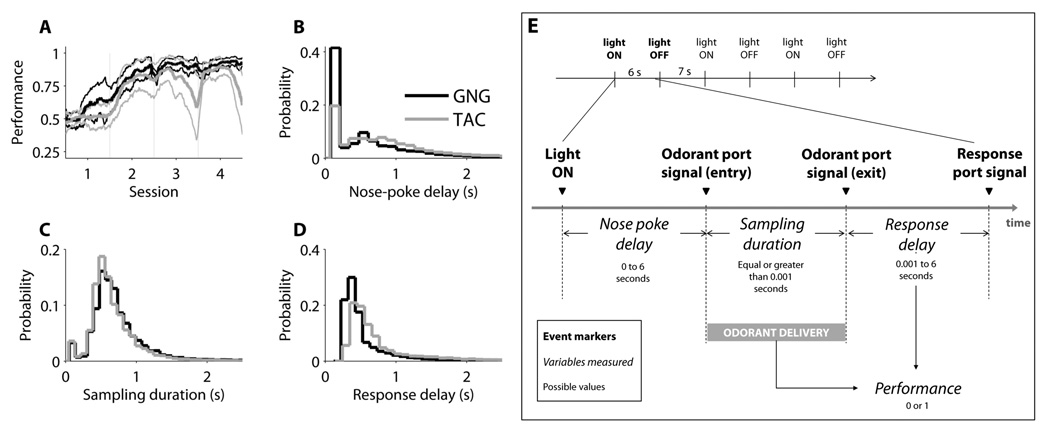
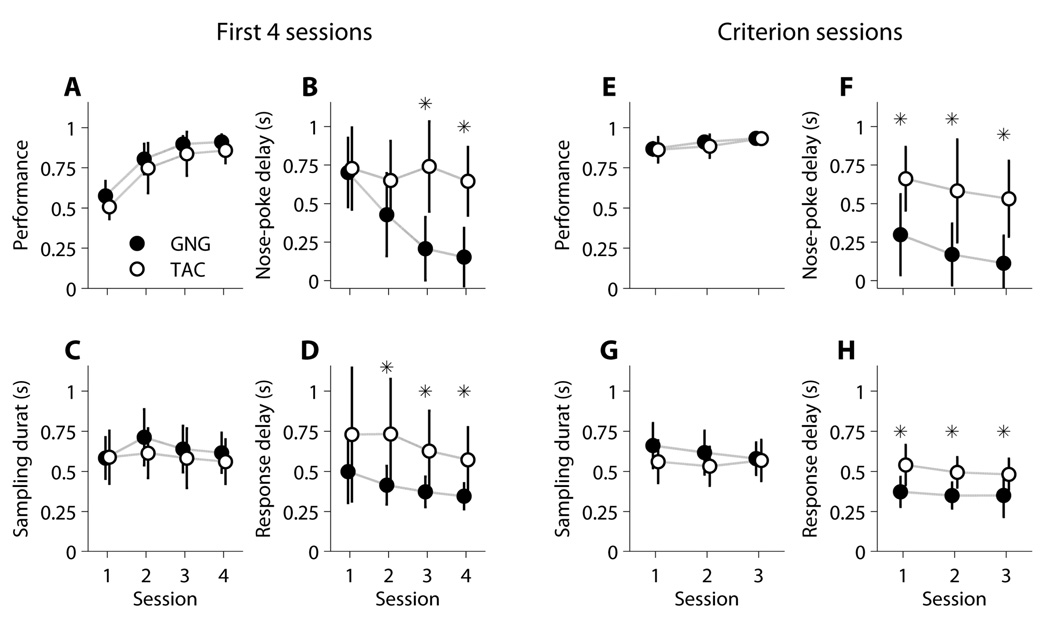
Figure 3. Descriptive Plots of the Measured Dependent Variables: Performance, Nose-poke Delay, Sampling Duration, and Response Delay.
3A: Performance. Moving mean performance (window = 40 trials, step size = 1 trial) across the first four sessions of Phase 3 Fixed ITI. Performance is expressed as the cumulative fraction of correct trials. Mean is shown as the bold line and one standard deviation as the pale lines. 3B: Nose-Poke Delay. This corresponds to the time between house-light on and initial odor nose-poke. 3C: Sampling Duration. This is the time spent in the odor port on each trial. 3D: Response Delay. This is the time between withdrawal of nose from the odor port to delivery of a response in response port. For GNG, response delay distribution includes only odorant A (‘Go’) trials. All distribution plots (B–D) were constructed using all sessions from Phase 3 Fixed ITI and all attempted trials (n = 24 rats, 106 sessions). 3E: temporal structure of an attempted trial. The horizontal line at the top represents the time and the two events that occur in the chamber if the rat does nothing (6-second light-on period followed by a 7-second light-off period). These two events mark the session trials. If the rat nose pokes at the odor port during the light-on period, the trial is considered attempted and the behavioral variables are measured (shown in the diagram at the bottom horizontal line). The time elapsed between light-on and odor nose-poke is the nose poke delay, which can go up to six seconds (duration of light-on period). The time spent by the rat with the nose in the odor port is the sampling duration, which corresponds to the period of odor delivery and it can be as long as the rat decides. After withdrawing the nose from the odor port, the rat has 6 seconds to deliver a response in the response port, and the period between nose withdrawal from odor port and execution of the response is call the response delay. Based on the type of odorant delivered and the response of the rat, the performance is calculated as a binary variable, 1 if correct and 0 if incorrect.
Training involved progression through four consecutive Phases, which we designate as Phase 0, 1, 2, and 3. Depending on the Phase, an experimental session (hereafter, session) consisted of between 100 and 400 total trials. Any single rat was run only one session per day, in consecutive days. Therefore, a rat never progressed through Phases within the same day. Performance criteria to move from one Phase to the next are described below.
We defined a trial as the period between the house-light on (start of trial) and the house-light off (end of trial), regardless of whether the rat engaged in expected task behavior. A rat had 6 seconds after the light on in which to initiate a trial. During this period, a nose-poke in the odor port caused the vacuum’s solenoid to close, which then allowed the free flow of an odorant stream into the odor-port only while the rat kept its nose in the odor port. The house-light remained on until the end of the trial. A trial’s duration depended upon Phase and performance.
Within a session, we consider a trial to be a binary event that could either be attempted (i.e., the rat nose-poked into the odor port), or not. The ITI was defined as the minimal time between the house-light off and the next house-light on. All Phases, with the exception of Phase 3 Variable ITI, had a 7-second ITI. The number of trials per session within a Phase was as follows: Phase 1, 100 total trials; Phase 2, 200 total trials; Phase 3, either 400 total trials or 300 attempted trials. Sessions ended when these trial criteria were met.
All training and testing, with the exception of Phase 0, occurred in the same operant chamber constructed from Med Associates components. Two opposed walls of the chamber were made of clear polycarbonate (chamber was the extra tall rat operant test chamber from Med Associates, ENV-007). The other two walls were made of aluminum and contained three panels where the different devices (i.e., odor and response ports, house light, and reward dish) could be located. One aluminum wall (back) had the house-light at the top and center. The other, opposing, wall (front) contained at the center the odor port, the reward dish below it, and response ports on one or both sides, depending on the task and Phase (See Figure 1). The floor of the chamber consisted of a stainless steel grid floor attached to the walls and a piece of Plexiglas on top of it. The chamber was mounted on a base of white polypropylene. The odor-port consisted of two normal odor-ports glued together to extend the inner distance. The first odor port (closest to the operant chamber) housed the photobeam detector. The second odor port had a hole drilled in the bottom in which the odorized stream was delivered. In the second odor port, the holes that would have normally housed a photobeam detector were left open to aid in clearing the port between trials.
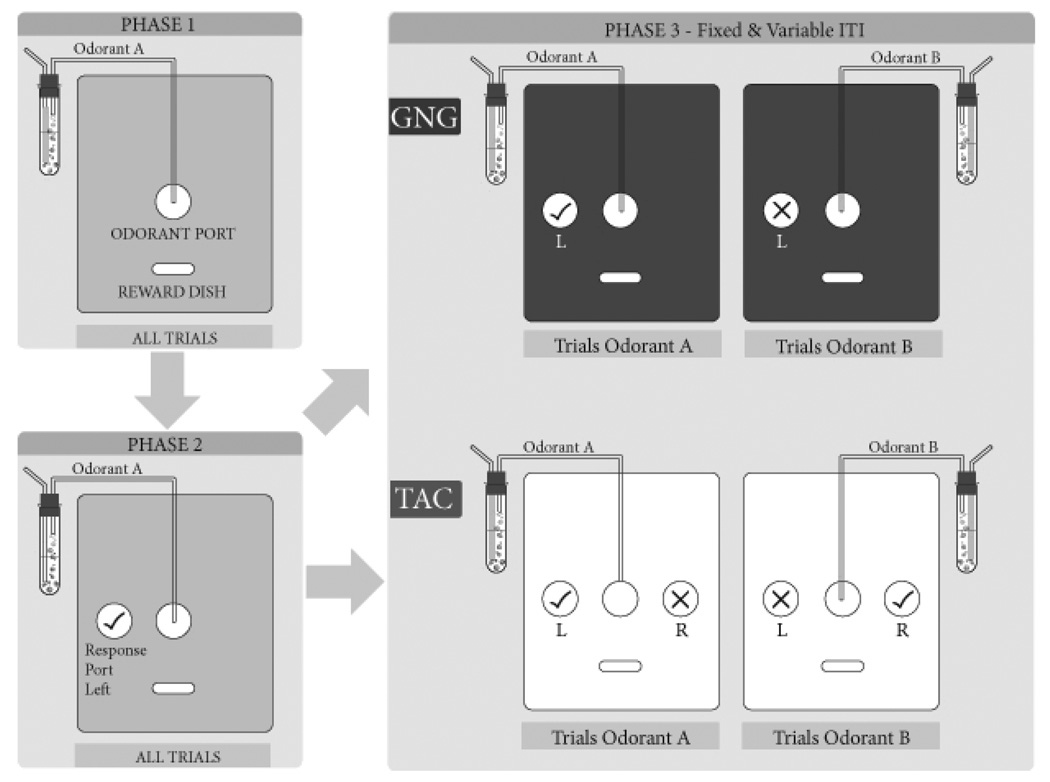
Figure 1. Overview of Experimental Design and Methods.
Rats were trained in three sequential Phases, the first two being common to all subjects, using only one odorant. The last Phase introduced the specifics of each task, Go/No-Go (GNG) or Two-Alternative Choice (TAC), and the odorant discrimination proper. The panels in the diagram represent the wall of the operant chamber containing the operandi. In Phase 1 (top left), rats were trained to associate a nose-poke in the odor port during trial time with a sugar pellet delivered in the reward dish. In Phase 2 (lower left), a response port was introduced to the left of the odor port (L) and rats were rewarded for nose-poking in it after sampling the odorant. In Phases 1 and 2 the same odorant (A) was delivered for each trial. In Phase 3 (right column), each rat was randomly assigned to either GNG or TAC. For any given trial, one of two odorants was delivered. The two tasks differ in their stimulus-response pairings. For GNG, odorant A required a nose-poke in the left response port (GNG left panel) and odorant B required refraining from the same response (GNG right panel). For TAC, odorant A was paired with a response in the left response port (TAC left panel) and odorant B was paired with a response in the right response port (TAC right panel). GNG rats could only receive a reward for correct odorant A responses, while TAC rats could receive rewards for correct responses to odorant A or B.
All task parameters were controlled using Med Associates’ hardware and their MEDPC IV software interface. Individual trial types (odorant A or odorant B presentations) were pseudo-randomly selected with replacement using MEDPC IV’s RANDI method. Within the text we label these trials as being randomly selected to conform to conventions of the literature. The trials were not presented in blocks, as is done in other experimental preparations.
Phase 0
Phase 0 (one day prior to Phase 1), rats were given 45-mg sugar pellets (Research Diets, Inc., New Brunswick, NJ) in a Petri dish in their home cages. This same type of sugar pellet was used as rewards for the rest of the Phases. Our earlier experience in training rats indicated that rats without previous exposure to sugar pellets had to learn to eat the pellets. Therefore, to reduce the burden in Phase 1, we habituated rats to the sugar pellets prior to the first day of training.
Phase 1
In Phase 1, rats learned to associate a nose-poke into the odor port during the trial (6 seconds after house light on) period with a reward given in the reward dish that was delivered as soon as the nose was withdrawn from the odor port. Rats were given an extra 5 seconds of house light on to eat the reward. There was no odorant in the response port. After the first nose-poke, repeated attempts (i.e., multiple nose-pokes at odor port) within the same trial did not result in additional odorant delivery. To encourage learning, a rat received two ‘free’ sugar pellets once every 20 trials, but only if the rat had not correctly performed 15 attempts. Rats were run for 100 total trials per session. In order for a rat to complete Phase 1, it had to complete at least 80 attempts in two consecutive days, with the second day having higher performance than the first (See Table 2).
Table 2.
Criterion levels for each phase.
| Phase | Criteria | ||
|---|---|---|---|
| 1 | >80 attempts over 2 days | 2nd day > 1st day | |
| 2 | >50% for 2 consecutive days | 2nd day > 1st day | |
| 3 | 3 days > 80% | 2 of 3 days, >90% | OR 7 total days |
Phase 2
In Phase 2, rats learned to pair the initial nose-poke in the odor port and stimulus sampling with a second nose-poke into a response port. We label this positive operant behavior a response. As in Phase 1, rats had 6 seconds to initiate a trial by nose-poking in the central odor port. The response port was introduced on the first day of Phase 2 and was located to the left of the odor port (See Figure 1). If the rat nose-poked in the odor port during a trial, it had 5 seconds after retracting its nose from the odor port to nose-poke at the response port (termed a response), which resulted in receiving a reward. A nose-poke at the response port did not result in odorant delivery. To keep rats motivated during learning (i.e., while they discovered there was a response port and learned the correct response), ‘free’ rewards were randomly (1/3 probability) delivered for Phase 1 type behavior (nose poke without response). Also, during the first 5 attempts, if a rat did not make a response after 4 seconds, it received a ‘free’ reward.
As in Phase 1, only a single odorant (odorant A) was delivered. Rats were run for 200 total trials per session. In order for a rat to complete Phase 2, it had to achieve session performance (correct attempts / total attempts) greater than 50% for two consecutive days with the performance of the second day greater than the first (See Table 2). If, after 7 sessions, a rat had not yet reached the criterion level, the rat progressed to a variation of Phase 2 that decreased the free reward probability from 1/3 to 1/20. Three of the original twenty-five rats had to do this variation. Of the three, only one did not reach criterion performance and was removed from the study and replaced.
Phase 3 - Fixed ITI
In Phase 3, rats learned to perform an olfactory discrimination. This was the first Phase in which two different odorants were used in the same session. Phase 3 is also the first time that there was a task difference between GNG and TAC groups. Rats were required to achieve three days above 80% with two of those days above 90%. If a rat did not achieve criterion performance after seven days, it was stopped and the final performance levels were recorded (See Table 2, criterion levels). Four rats did not achieve our specified criterion level and were stopped after 7 days. However, all rats had an average performance of at least 75% for the three criterion sessions and, therefore, we did not remove any rats due to Phase 3 performance. On each trial, one of two odorants (Odorant A, used in Phases 1 and 2; Odorant B, new) was randomly selected (uniform probability). Just as in Phases 1 and 2, rats (GNG and TAC) had 6 seconds in which to attempt a trial (i.e., to nose-poke in the odor port) after house-light on.
Go/No-Go (GNG)
For the GNG task no new ports were added to the operant chamber. A correctly attempted trial was one in which rats delivered the operant response (within 5 seconds) if Odorant A was presented and refrained from doing so (See Figure 1) for 5 seconds if Odorant B was presented. Rats could only receive a reward for correct responses. When a rat correctly performed for Odorant A, it received a pellet and the house-light remained on for a 5-second reward period. Responses could be made until the house-light turned off, which was 5 seconds after the rat withdrew its nose from the odor port. If a rat delivered the response for Odorant B, the house-light was immediately extinguished and an extra 7 seconds (penalty delay) was added to the normal ITI.
Two-Alternative Choice (TAC)
For the TAC task an additional response port was introduced and placed to the right side of the odor port (See Figure 1). As in GNG, one of two odorants (Odorant A, used in Phases 1 and 2; Odorant B, novel) was randomly (uniform probability) selected on each trial. A correct trial was one in which rats delivered the response in the left response port if Odorant A was presented, and delivered the response in the right response port if Odorant B was presented (See Figure 1). Rats received a reward for all correct responses, which could be made until the house-light turned off 5 seconds after nose withdrawal from the odor port. If a response was delivered in the wrong response port, the house-light was immediately extinguished and the same penalty delay used in GNG (7 seconds) was added to the normal ITI. If the rat refrained from delivering a response during the 5 seconds following nose withdrawal, the house-light was turned off and a new trial initiated following the regular ITI.
Phase 3 - Variable ITI
After completion of Phase 3 Fixed ITI, sixteen of the original twenty-four rats (randomly selected; eight each of GNG and TAC) were given three days of rest prior to the start of the Variable ITI experiment. With the exception of the ITI manipulation, the protocols were the same as those used in Phase 3 Fixed ITI. We used four ITI levels: 3, 5, 9, and 11 seconds. ITI test order was randomly assigned. The penalty delay of 7 seconds was maintained across training and ITI manipulation. There was no performance criterion, as rats were only tested one day per ITI level.
Control Tests
Following completion of testing, a subset of rats (n=7) completed one of two, but not both, control tests. These controls were designed to test the integrity of the odor delivery apparatus and behavior. The first control (auditory cues) was designed to ensure that rats were not discriminating between the presented stimuli based upon the sound of the odorant solenoids opening. The second control (vacuum leakage) was designed to ensure that the vacuum setup prevented any, or enough, odorant leakage into the odor port that could be used by the rats for early detection.
For the first control (auditory cues), all odorant tubing was replaced and a single test tube, instead of the normal two test tubes, filled with distilled water was used as the stimulus. Except for this change in stimulus, all other task parameters were the same as in a typical Phase 3 Fixed ITI session. The crucial factor is that both odor streams went through this single test tube. This meant that both stimuli types (odorant A, B) were the same distilled water, the only difference between the two presentations would be the auditory sound of the respective solenoid opening when the odorant was turned on to charge the tubing. We reasoned that if rats were using auditory cues, either in addition to or in place of olfactory cues, then they should still be able to discriminate the stimuli above chance. For this control, three rats were used (GNG: n=2; TAC: n=1), and as expected, performance was at chance levels (M=0.5008, SD=0.0282). We, therefore, conclude that the rats were not making any, or substantial, use of auditory cues for discrimination.
The second control (vacuum leakage) again consisted of all but one task parameter being the same as a normal Phase 3 Fixed ITI session. The one change was that when a rat nose-poked into the central odor port, the vacuum was not turned off. By not turning off the vacuum, we reasoned that if rats were still able to discriminate, then there was enough odor leaking into the odor port past the vacuum. If rats could discriminate the odors with the vacuum on, they could have been sampling the odorant stimulus prior to entering the odor port, which would confound analysis of sampling durations. However, if rats were not able to significantly discriminate, then we could be confident that rats did not receive enough odor information during the charging period in order to form a discrimination. For this control, four rats were used (GNG: n=2; TAC: n=2), and as expected rats performance was at chance levels (M=0.53, SD=0.0033). Therefore, we conclude that rats could not receive any significant quantity of odorant during the charging period and that the sampling duration reflects the period in which rats actually sampled the odor stimulus.
Statistical Analysis & Protocols
Analysis was guided by two goals: 1) comparison of GNG and TAC tasks, 2) investigation of the effect of ITI on dependent variables for possible speed-accuracy trade-off effects. We defined three independent variables: task type (GNG, TAC), odor set (Set 1: amyl acetate – odorant A, anisole – odorant B; Set 2: anisole – odorant A, amyl acetate – odorant B), and odorant. We defined four dependent variables (also called session variables): performance, nose-poke delay, sampling duration, and response delay. For each trial, the session variable was recorded. Then, for each session variable, the session statistic, which is the session level estimate of the variable, was calculated as detailed below.
Performance: For GNG, a trial was considered correct if the rat responded to odorant A and refrained from responding for odorant B. For TAC, a trial was considered correct only if the rat responded with the correct behavior for the presented stimulus (e.g., response in the left response port for odorant A, response in the right response port for odorant B).
Nose-Poke Delay: the time between house-light on and the first nose-poke in the odor port that signaled the start of an attempted trial. For nose-poke delay, we subtract the time of light on from the time of nose-poke on to determine the nose-poke delay. If a nose-poke was initiated prior to house-light on and the rat remained within the odor port as the house-light turned on, then the associated nose-poke delay was set to 0 seconds. Analysis conducted without zeroing this variable revealed the same results. Therefore, we only present the results of zeroing the variable.
Sampling Duration: the duration between the first entry into the odor port and first nose-poke withdrawal. Although rats were free to nose-poke again, the odorant was on only for the duration of the first nose-poke. Therefore, we use only the time for which the animal could receive odorant streams.
Response Delay: the duration from the first nose-poke withdrawal from the odor port until an entry, if there was one, into a response port. Only trials in which rats attempted a response were used for analysis of response delay.
The session performance was calculated as the total number of correct attempts over the total number of attempts (for Phase 2 and 3) or as the total number of attempts out of 100 (for Phase 1). Unless otherwise indicated within the text, the remaining session statistics were calculated as the median of a session variable (e.g., the session statistic nose-poke delay is the median of all nose-poke delays within the session). We selected the median because it is more robust to outliers and non-normal skewed distributions.
For Phases 1 and 2, to determine if there was an effect of odor set, we compared the final session results using independent samples t-test (factor, odor set). We also constructed a difference measure (difference of final and first session statistics paired by rat) to see if a variable changed between the first and last session of a phase.
For Phase 3 Fixed ITI analysis, the sessions were divided into two non-disjoint sets. The first set (First 4 Sessions) include sessions 1 to 4. We selected four days because the fourth day is the last day in which all rats were still in the experiment (i.e., some rats completed Phase 3 Fixed ITI in 4 days). The second set (Criterion Sessions) included the three criterion sessions, which were the final three sessions of Phase 3 Fixed ITI for each rat. This division into two sets allowed us to investigate differences of the session statistics during the initial (learning) stages of the Phase and criterion performance. Conditional probability plots, P(Performance | Sampling Duration), were computed using all trials from Phase 3 Fixed and Variable ITI sessions. Plots are shown for descriptive purposes.
For Phase 3 Variable ITI, data were analyzed along two different dimensions: 1) within ITI, 2) between ITI. A priori hypotheses dictated that if a main effect of ITI treatment levels were to occur, the effects would be most obvious in the extremes. Finally, we also report the inter-attempt interval (IAI), which is the time between successive nose-poke attempts. To access if rats changed their IAI dependent upon ITI, we subtracted the ITI from each rat’s IAI. If rats did change their IAI in an ITI-dependent manner, then the remainder (after subtracting the ITI) should change.
Unless otherwise stated within the text, all analysis was conducted with an alpha value of 0.05. All analyses were done in MATLAB (vR2010a; Natick, MA). Statistical results are reported in the text except when such reporting would be cumbersome, then the statistics are reported in tables. Mean and standard deviation values are presented paired with statistical tests when appropriate and are indicated as M (mean) and SD (standard deviation). P-values are reported exactly unless the associated F-statistic is less than 1. Cohen’s d and eta-square statistics were used to evaluate effect sizes and are presented for any significant effect. For paired t-tests, Cohen’s d is calculated using the standard deviation of the first group, instead of using a pooled deviation. The first group is indicated in the text.
Results
Phase 1 & Phase 2
Phase 1 and 2 were the initial training phases that all rats went through regardless of whether they would eventually be used in GNG or TAC tasks. Training the rats in the same setup allowed us to guard against any possible differences between GNG and TAC tasks that would be a result of random assignment. We observed no differences between GNG and TAC rats in either Phase 1 or Phase 2. First and final session variable values are shown in Figure 4.
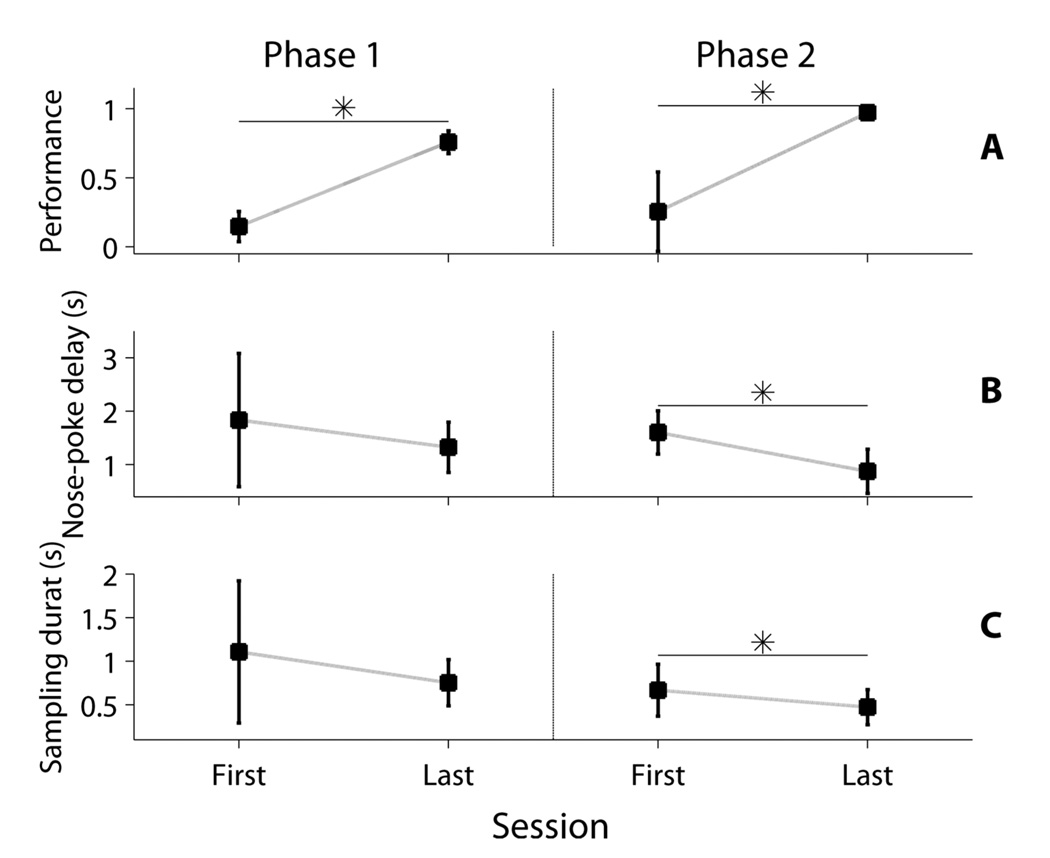
Figure 4. First and Final Sessions for Phases 1 and 2.
For each session we calculated the median value for each rat using all attempted trials, and then computed the mean and SD of these medians (n=24 rats). Left section on each plot corresponds to Phase 1, right section to Phase 2. 4A: Performance. Performance was calculated as the fraction of correct trials. As required by our criteria, performance increased from first to last day in both Phases. 4B: Nose-poke delay. The nose-poke delay decreased from first to last day of Phase 1, and then decreased again during Phase 2. 4C: odorant sampling duration. The sampling duration, like nose-poke delay, decreased throughout Phases 1 and 2. Asterisks indicate significant differences between first and last sessions as determined by paired t-test at the alpha=0.05 level.
Phase 1 (P1)
Rats completed P1 in a median of 3 sessions (M=2.7917, SD=0.5882) and there was no effect of odor set (t(22)=1.0430, p=0.3083). There was also no effect of odor set on the final day’s performance (t(22)=−1.0963, p=0.2848, M=0.7583, SD=0.0823), nose-poke delay (t(22)=−0.8134, p=0.4247, M=1.3260, SD=0.4695), or sample duration (t(22)=−1.6687, p=0.1093, M=0.7540, SD=0.2648) using independent samples t-test. As expected, there was a significant increase in performance from the first (M=0.1475, SD=0.1090) to the last (M=0.7583, SD=0.0823) session (t(23)=20.3410, p=3.44E-16, M=0.6108, SD=0.1471, d=5.6062). Cohen’s d was calculated using the standard deviation of the first day, instead of the pooled standard deviation. There were no significant changes between the first and last sessions for either nose poke delay (first session: M=1.8348, SD=1.2467; final session: M=1.3260, SD=0.4695; t(23)=−1.1933, p=0.0657) or sampling duration (first session: M=1.1081, SD=0.8152; final session: M=0.7540, SD=0.2648; t(23)=−1.8921, p=0.0711).
Phase 2 (P2)
Rats completed P2 in a median of 3 days (M=4, SD=2.6043) and there was no effect of odor set (t(22)=2.0000, p=0.0580). There was also no effect of odor set on the final day’s performance (t(22)=0.2590, p=0.7980), nose-poke delay (t(22)=−1.4016, p=0.1750), sampling duration (t(22)=−1.9694, p=0.0616), or response delay (t(22)=−0.9786, p=0.3384) using independent samples t-test. As expected, there was a significant increase in performance from the first to the last session (first session: M=0.2555, SD=0.2878; final session: M=0.9716, SD=0.0366; t(23)=12.5495, p=9E-12, M=0.7161, SD=0.2796, d=2.4885) as indicated by paired t-test. There were significant decreases between the first and last sessions for nose-poke delay (first session: M=1.6146, SD=0.4026; final session: M=0.8846, SD=0.4147; t(23)=−8.5197, p=1.43E-8, M:-0.73, SD=0.4198, d=−1.8130), sampling duration (first session: M=0.6679, SD=0.2978; final session: M=0.4746, SD=0.2012; t(23)=−3.8318, p=8.53E-4, M=−0.1933, SD=0.2472, d=−0.6491), and response delay (first session: M=3.1271, SD=1.1366; final session: M=0.6565, SD=0.2824; t(23)=−10.1474, p=5.80E-10, M: −2.4706, SD: 1.1928, d=−2.1737) as indicated by paired t-tests. Cohen’s d was calculated using the standard deviation of the first sessions.
Phase 3 Fixed ITI
Rats completed Phase 3 in a median of 5 days (min=4 days, max=7 days; M=5.4583, SD=1.1788) and there was a main effect of task (F(1,23)=10.9223, p=0.0035, η2=0.2934) with TAC rats needing more days to reach criterion performance (M=6.0833, SD=1.0836) than GNG rats (M=4.8333, SD=0.9374). There was also a significant interaction (task x odor set; F(1,23)=5.8738, p=0.0250, η2=0.1578), but no main effect of odor set (F(1,23)=0.4369, ns).
Rats reached performance greater than 80% in a median of 2 days (M: 2.5, SD: 0.7802). In contrast to the total number of days to complete Phase 3, there was neither a main effect of task (F(1,23)=1.0256,p=0.3233) nor odor set (F(1,23)=0.2564, ns) or an interaction effect (F(1,23)=0.2564, ns) for the number of sessions to reach performance greater than 80%.
While running the experiments, we noticed that not all rats would complete the prescribed 300 attempted trials. To investigate the possible difference between the tasks, we looked at the average number of attempts each rat performed as well as each rat’s average attempt ratio (attempted trials / total trials). For average number of attempts (GNG: M=299.2000, SD=2.7713; TAC: M=287.6383, SD=17.8800), there was a main effect of task (F(1,23)=4.5253, p=0.046, η2=0.1822), but neither a main effect of odor set (F(1,23)=0.0500, ns) nor an interaction effect (F(1,23)=0.2683, ns). For attempt ratio (GNG: M=0.9455, SD=0.030; TAC: M=0.8422, SD=0.1103), there was also a main effect of task (F(1,23)=8.9278, p=0.0073; η2=0.3083), but neither a main effect of odor set (F(1,23)=0.0068, ns) nor an interaction (F(1,23)=0.0211, ns). This difference, TAC rats attempting fewer trials than GNG rats, may be due to the difference in reward rate between the two tasks (See Materials and Methods: Task Paradigms; also Discussion).
Because there was variability in the number of sessions rats took to complete Phase 3, we constructed two non-disjoint sets for analysis to capture Phase 3 dynamics (See Materials & Methods: Statistical Analysis and Protocols). The first set consists of the first four sessions. The second set consists of the final three sessions of Phase 3, called the Criterion Sessions.
First 4 Sessions (4S)
Statistical results (F, p-values, and η2 values for significant p-values) are presented in Table 3 and graphically in Figure 5A–D. There were neither significant main effects nor interaction effects of task and odor set on performance for any of the first four sessions. A main effect of task on nose-poke delay became statistically significant in Session 3 and continued to be significant for Session 4. There were neither significant main effects of odor set nor interactions for any session for nose-poke delay. For sampling duration, there were neither significant main effects nor interaction effects of task and odor set. For response delay in session 1, there were neither significant main effects nor interaction effects of task and odor set. However, for sessions 2 to 4, there was a main effect of task, but still neither a main effect of odor set nor an interaction effect.
Table 3. Phase 3 Fixed ITI First 4 Sessions Statistics.
Results from two-way factorial ANOVAs are presented. Each analysis had 1 and 23 degrees of freedom.
| Session Variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Session | Effect | Performance | Nose-poke delay | Sample Duration | Response Delay | ||||
| F | P (η2) | F | P (η2) | F | P (η2) | F | P (η2) | ||
| 1 | Task | 3.7082 | 0.0685 | 0.0589 | ns | 0.0073 | ns | 2.7478 | 0.1130 |
| Odor Set | 0.2791 | ns | 0.0099 | ns | 3.2014 | 0.0887 | 0.0881 | ns | |
| Interaction | 0.8377 | ns | 0.2717 | ns | 0.1696 | ns | 0.0026 | ns | |
| 2 | Task | 1.1505 | 0.2962 | 4.0331 | 0.0583 | 2.0920 | 0.1636 | 8.4805 | 0.0086 (0.2961) |
| Odor Set | 0.0627 | ns | 0.6562 | ns | 1.2747 | 0.2722 | 0.0575 | ns | |
| Interaction | 2.0008 | 0.1726 | 0.0216 | ns | 0.0164 | ns | 0.0998 | ns | |
| 3 | Task | 1.9171 | 0.1814 | 24.4268 | 7.85E-5 (0.5490) | 0.6491 | ns | 10.0412 | 0.0048 (0.3298) |
| Odor Set | 0.3267 | ns | 0.0418 | ns | 3.11E-04 | ns | 0.3385 | ns | |
| Interaction | 0.0125 | ns | 0.0250 | ns | 0.2269 | ns | 0.0622 | ns | |
| 4 | Task | 4.1341 | 0.0555 | 31.8161 | 1.61E-5 (0.6083) | 1.0816 | 0.3107 | 12.0898 | 0.0024 (0.3721) |
| Odor Set | 2.0256 | 0.1701 | 0.0039 | ns | 2.0478 | 0.1679 | 0.0147 | ns | |
| Interaction | 1.5629 | 0.2257 | 0.4796 | ns | 0.4012 | ns | 0.3830 | ns | |
Figure 5. Phase 3 - Fixed ITI Sessions.
For each session we calculated the median value for each rat using all attempted trials, and then computed the mean and SD of these medians (GNG: n=12 rats; TAC: n=12 rats). Phase 3 Fixed ITI sessions were analyzed using two non-disjoint sets: the first four sessions and the final three (criterion) sessions (See Methods: Statistical analysis and protocols). A-D: First 4 Sessions. 5A: Performance. We found no difference between the tasks in performance, indicating that in average they are equally difficult for the rats. 5B: Nose-Poke Delay. The mean nose-poke delay decreased across the first four days for GNG rats, but this did not happen for TAC rats, which resulted in a difference between tasks in sessions 3 and 4. (See Results: Nose-poke delay analysis). 5C: Sampling Duration. We found no difference between tasks or between sessions in sampling duration. 5D: Response Delay. For GNG, only odorant A attempted trials are used. In sessions 2 to 4, it took TAC rats longer to respond, compared to GNG rats. E-H: Criterion Sessions. We observed the same differences and similarities that we obtained when looking at the first four sessions: no differences between tasks in performance or sampling duration, and differences between tasks in nose-poke delay and response delay, with the differences in the same direction as before. Asterisks indicate main effects of the task (GNG, TAC) determined by 2-way ANOVAs at the alpha=0.05 level. Full statistical results are presented in Table 3 and Table 4.
Criterion Sessions (3C)
Statistical results (F, p-values, and η2 values for significant p-values) are presented in Table 4 and graphically in Figure 5E–H. There were neither main effects nor interaction effects of task and odor set on performance for criterion sessions 1 and 2. For session 3, there was a significant interaction of task and odor set on performance, but neither a main effect of task nor odor set. For nose-poke delay, there were significant main effects of task (all three sessions) and interaction effects between task and odor set (sessions 1 and 2, but not 3). There were no significant main effects of odor set on nose-poke delay for any session. Sampling duration had neither significant main effects nor interaction effects of task and odor set for any criterion session. Response delay had main effects of task for all three sessions and a significant interaction effect between task and odor set for the third session; however, there were no significant effects of odor set.
Table 4. Phase 3 Fixed ITI Criterion Sessions Statistics.
Results from two-way factorial ANOVAs are presented. Each analysis had 1 and 23 degrees of freedom.
| Session Variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Session | Effect | Performance | Nose-poke delay | Sample Duration | Response Delay | ||||
| F | P (η2) | F | P (η2) | F | P (η2) | F | P (η2) | ||
| 1 | Task | 0.0365 | ns | 15.8483 | 7.35E-4 (0.3929) | 3.1373 | 0.0918 | 14.5794 | 0.0011 (0.3860) |
| Odor Set | 0.3329 | ns | 0.0368 | ns | 1.1931 | 0.2877 | 0.0058 | ns | |
| Interaction | 0.0545 | ns | 4.4487 | 0.0477 (0.1103) | 0.2983 | ns | 3.1886 | 0.0893 | |
| 2 | Task | 1.4374 | 0.2446 | 17.7576 | 4.27E-4 (0.3800) | 2.6810 | 0.1172 | 17.2233 | 4.95E-4 (0.4224) |
| Odor Set | 1.5978 | 0.2207 | 1.0787 | 0.3114 | 2.6286 | 0.1206 | 0.0466 | ns | |
| Interaction | 3.6077 | 0.0720 | 7.8923 | 0.0108 (0.1689) | 0.0126 | ns | 3.5012 | 0.0760 | |
| 3 | Task | 0.0197 | ns | 21.4202 | 1.62E-4 (0.5075) | 0.0651 | ns | 8.4621 | 0.0087 (0.2566) |
| Odor Set | 0.0257 | ns | 0.0260 | ns | 3.1920 | 0.0892 | 0.0451 | ns | |
| Interaction | 5.9947 | 0.0237 (0.2301) | 0.7576 | ns | 0.1609 | ns | 4.4723 | 0.0472 (0.1356) | |
Phase 3 - Variable ITI
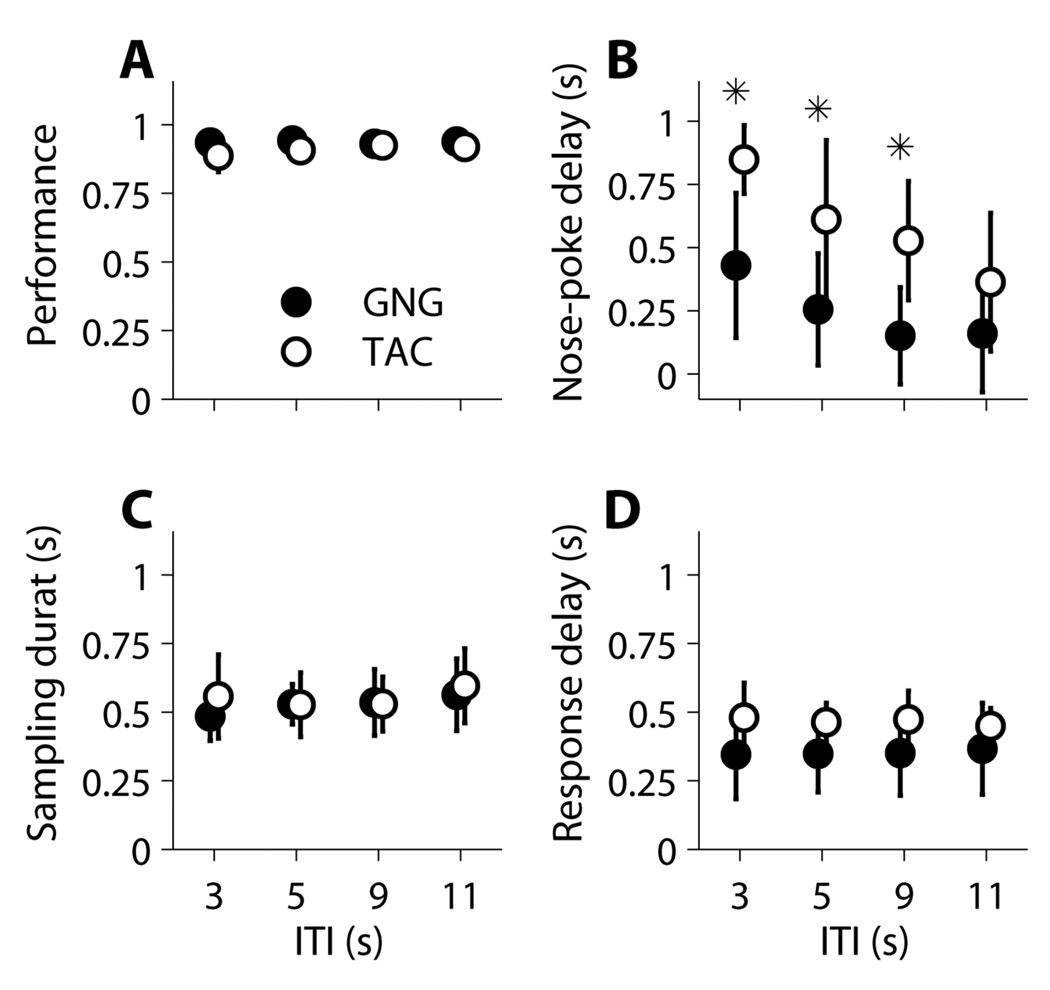
The ITI manipulation was designed to test one aspect of the speed-accuracy trade-off (SAT) hypothesis. If rats engage in SAT in order to maximize reward rate, then we expected that for increased inter-trial intervals rats would spend more time sampling the odorant to increase their accuracy. This was deduced under the assumption that increased time between trials would make rewards more temporally scarce and therefore rats would optimize performance by sampling longer thereby increasing reward frequency. If intertrial intervals were decreased, then we expected rats to spend a shorter amount of time sampling the odorant, resulting in increased errors but still allowing the rats to maintain a high reward rate as rewards could be more frequent. We performed analysis along two dimensions. In the first dimension, within ITI, we examined differences between GNG and TAC. In the second dimension, between ITI, we examined comparisons within task (i.e., GNG or TAC). Results are shown in Figure 6.
Figure 6. Phase 3 - Variable ITI Sessions.
For each session we calculated the median value for each rat using all attempted trials, and then computed the mean and SD of these medians (GNG: n=8 rats; TAC: n=8 rats). Plots are in the same order and have the same specifications as in Figure 5 (A: Performance, B: Nose-Poke Delay, C: Sampling Duration, D: Response Delay). Each session corresponds to a different ITI, ranging from 3 to 11 seconds. Most differences and similarities between tasks found in Phase 3 with fixed ITI were conserved in the case of variable ITI (no difference in performance and sampling duration, difference in nose-poke delay with TAC displaying longer times). There was no difference in response delay between tasks. For TAC rats, there was an effect of ITI duration on nose-poke delay (top right), with ITI 3 and ITI 11 sessions being significantly different from each other (See Results: ITI). Asterisks indicate main effects of the task (GNG, TAC) determined by 2-way ANOVAs at the alpha=0.05 level. Full statistical results are presented in Table 5.
Within ITI
Statistical results (F, p-values, and η2 values for significant p-values) are presented in Table 5. The within ITI contrasts largely replicated the results from Phase 3 Criterion Sessions. For performance, the only significant effect was a main effect of odor set for ITI 3. For nose-poke delay, there were significant main effects of task for ITI durations 3, 5, and 9, but no significant main effect for ITI 11. There were neither main effects of odor set nor interaction effects on nose-poke delay for any ITI duration. For sampling duration, there was never a main effect of task or an interaction. However, there were main effects of odor set for ITI 3, 5, and 9, but no such main effect for ITI 11.
Table 5. Phase 3 Variable ITI Within Session Statistics.
Results from two-way factorial ANOVAs are presented. Each analysis had 1 and 15 degrees of freedom.
| Session Variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ITI | Effect | Performance | Nose-poke delay | Sample Duration | Response Delay | ||||
| F | P (η2) | F | P (η2) | F | P (η2) | F | P (η2) | ||
| 3 | Task | 5.9995 | 0.0306 (0.2429) | 12.2821 | 0.0043 (0.5002) | 1.7662 | 0.2086 | 3.2646 | 0.0959 |
| Odor Set | 3.6666 | 0.0797 | 0.0655 | ns | 5.4624 | 0.0376 (0.2733) | 0.9415 | 0.3510 | |
| Interaction | 2.8939 | 0.1147 | 0.2065 | ns | 0.7581 | ns | 0.2690 | ns | |
| 5 | Task | 3.2932 | 0.0946 | 7.7180 | 0.0167 (0.3302) | 0.0020 | ns | 4.1736 | 0.0637 |
| Odor Set | 0.2212 | ns | 0.8757 | ns | 5.8951 | 0.0319 (0.3130) | 0.7120 | ns | |
| Interaction | 0.3691 | ns | 2.7785 | 0.1214 | 0.9374 | 0.3520 | 0.7890 | ns | |
| 9 | Task | 0.0897 | ns | 14.6520 | 0.0024 (0.4687) | 0.0157 | ns | 3.3720 | 0.0912 |
| Odor Set | 0.0418 | ns | 1.6934 | 0.2176 | 8.7771 | 0.0119 (0.4143) | 0.4301 | ns | |
| Interaction | 0.5120 | ns | 2.9159 | 0.1134 | 0.3918 | ns | 1.2640 | 0.2829 | |
| 11 | Task | 1.9767 | 0.1851 | 2.2216 | 0.1619 | 0.2597 | ns | 1.9323 | 0.1898 |
| Odor Set | 2.9494 | 0.1116 | 0.1449 | ns | 2.9754 | 0.1102 | 1.9329 | 0.1898 | |
| Interaction | 2.0244 | 0.1803 | 0.0017 | ns | 0.3458 | ns | 1.7068 | 0.2159 | |
For all ITI’s, there was a significant effect of task type on inter-attempt intervals (difference of the means and pooled standard deviations are: ITI 3: M=−3.4671, SD=1.5322; ITI 5: M=−4.3078, SD=3.0617; ITI 9: M=−5.4146, SD=3.6726; ITI 11: M=−5.1822, SD=3.8838, see Table 8). This reflected that GNG rats (ITI 3: M=10.113, SD=0.3214; ITI 5: M=12.247, SD=0.5479; ITI 9: M=15.989, SD=0.4392; ITI 11: M=17.966, SD=0.5445) had shorter IAIs than TAC rats (ITI 3: M=13.58,SD=2.0013; ITI 5: M=16.555,SD=4.013; ITI 9: M=21.404, SD=4.8385; ITI 11: 23.148, SD=5.1089).
Table 8.
Inter -attempt intervals (IAI) and attempt ratio (AR) statistical results. Two sample t-tests with task as the factor
| IAI | AR | |||||
|---|---|---|---|---|---|---|
| ITI | T | P | d | T | P | d |
| 3 | −4.526 | 0.0005 | −2.419 | 3.7941 | 0.002 | 2.028 |
| 5 | −2.814 | 0.0138 | −1.504 | 2.8826 | 0.0121 | 1.5408 |
| 9 | −2.949 | 0.0106 | −1.576 | 3.1254 | 0.0074 | 1.6706 |
| 11 | −2.669 | 0.0183 | −1.426 | 2.7367 | 0.0161 | 1.4628 |
There was also a significant effect of task on attempt ratio for each ITI (difference of the means and pooled standard deviations are: ITI 3: M=0.1814, SD=0.0956; ITI 5: M=0.1844, SD=0.1280; ITI 9: M=0.1925, SD=0.1232; ITI 11: M=0.1625, SD=0.1187, see Table 8). GNG rats (ITI 3: M=0.9613, SD=0.0231; ITI 5: M=0.9548, SD=0.0390; ITI 9: M=0.9740, SD=0.0186; ITI 11: M=0.9723, SD=0.0241) had a consistently higher attempt ratio than TAC rats (ITI 3: M=0.7800, SD=0.1243; ITI 5: M=0.7704, SD=0.1647; ITI 9: M=0.7815, SD=0.1620; ITI 11: M=0.8098, SD=0.1553).
Based upon these two analyses, we conclude that the task effect on IAI could most likely be explained by the difference in attempt ratio.
Between ITI
For GNG and TAC, separately, we used one-way ANOVAs to determine if ITI had a significant effect on a dependent variable. For GNG, there was no effect of ITI on any of the dependent variables (Performance: F(3,31)=0.2237, ns; Nose-poke delay: F(3,31)=2.4109, p=0.0879; Sampling duration: F(3,31)=0.7557, ns; Response delay: F(3,31)=0.0290, ns). For TAC, there was no effect of ITI on performance (F(3,31)=1.3689, p=0.2726), sampling duration (F(3,31)=0.5039, ns), or response delay (F(3,31)=0.1612, ns). However, there was a significant effect of ITI on nose-poke delay (F(3,31)=5.3338, p=0.0041, η2=0.3722). Given that we found a significant effect of ITI on nose-poke delay, we then used multiple comparisons with Bonferroni correction (corrected alpha=0.0083) to assess pairwise significance. There were two significant comparisons: ITI 3,9 (t(14)=3.4056, p=0.0043, d=1.7785) and ITI 3,11 (t(14)=4.4810, p=5.18E-4, d=2.3401). All other comparisons were not significant (ITI 3,5: t(14)=1.9137, p=0.0763; ITI 5,9: t(14)=0.7274, p=0.4789; ITI 5,11: t(14)=1.8196, p=0.0903; ITI 9,11: t(14)=1.2991, p=0.2149). This effect led us to conduct additional analyses (See Results: Nose-poke Delay Analysis).
Finally, we tested if changing ITI affected the IAI. To do this we calculated the IAI, then, for each ITI duration/session, subtracted off the fixed (or minimal) ITI. The remainder is the time that is under the rat’s control. If rats were selecting different IAIs based upon the ITI, then one would expect differences across ITIs. Instead, rats did not change their IAIs based upon ITIs (GNG: F(3,31)=0.5237, ns; TAC: F(3,31)=0.2639, ns). Also, there was no effect of ITI on attempt ratio (GNG: F(3,31)=0.7825, ns; TAC: F(3,31)=0.0869, ns).
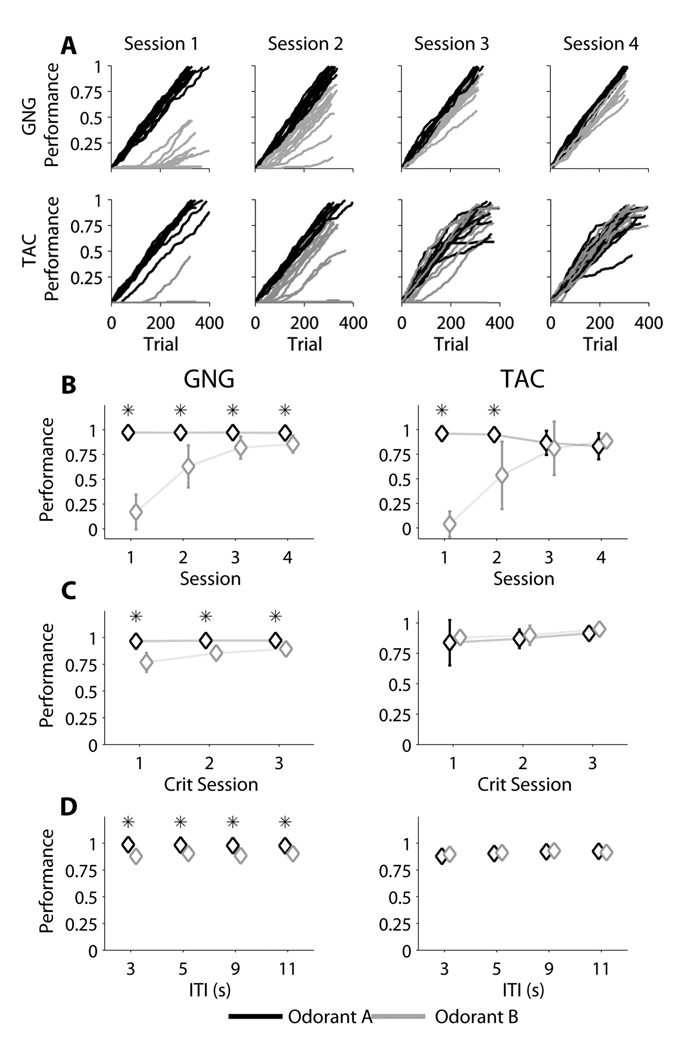
Performance by Odorant
Although group analysis showed equal distributions of performance between GNG and TAC, we observed a possible difference in performance between odorants A and B. To investigate this, we analyzed the difference in performance for GNG and TAC for each odorant (See Figure 7).
Figure 7. Session Performance by Odorant Type.
For GNG, odorant A was the Go-stimulus and odorant B was the No-Go stimulus. For TAC, odorant A was the Go-Left stimulus and odorant B was the Go-Right stimulus. 7A: Within-session performance: All trials from the first four sessions were used (GNG: n=12 rats, TAC: n=12 rats). For a given trial, performance is calculated as the total number of correct trials up to and including that trial, divided by the total number of trials for the session. On each plot, each rat is represented as two separate traces, one per odorant. Top Row: GNG, Bottom Row: TAC. Notice the difference in performance by odorant that can be seen in GNG but not in TAC (Sessions 3 and 4). 7B: Phase 3 Fixed ITI First 4 Sessions. Notice that while for TAC odorant A performance falls in session 3 and meets the performance of odorant B, for GNG odorant A performance remains high, and there is a statistical difference between the two odorants. For TAC there is no statistical difference after Day 3. 7C: Phase 3 Fixed ITI Criterion Sessions. 7D: Phase 3 Variable ITI.
The statistical results for GNG and TAC are presented in Table 6. For GNG, there were significant differences in performance for the two odorants for Phase 3 Fixed ITI First 4 Sessions (Figure 7B left), Phase 3 Fixed ITI 3 Criterion Sessions (Figure 7C left), and Phase 3 Variable ITI (Figure 7D left). For TAC, only the first two sessions of Phase 3 Fixed ITI showed significant differences between the two odorants (Figure 7B, right). There were no significant differences for Phase 3 Fixed ITI 3 Criterion Sessions (Figure 7C right) or Phase 3 Variable ITI (Figure 7D right).
Table 6. Performance by Odorant Statistical Results.
Phase 3 Fixed had 11 degrees of freedom, while Phase 3 Variable ITI had 7.
| Phase 3 Fixed ITI First 4 Sessions | |||||||
|---|---|---|---|---|---|---|---|
| GNG | TAC | ||||||
| Session | T | P | d | Session | T | P | d |
| 1 | 14.3634 | 1.80E-08 | 33.8933 | 1 | 22.8661 | 1.26E-10 | 24.4479 |
| 2 | 5.1444 | 3.21E-04 | 9.7993 | 2 | 4.0206 | 2.00E-03 | 13.3130 |
| 3 | 4.1731 | 0.0016 | 5.8365 | 3 | 0.5805 | 0.5733 | --- |
| 4 | 4.0764 | 0.0018 | 3.8710 | 4 | -1.4916 | 0.1639 | --- |
| Phase 3 Fixed ITI Criterion Sessions | |||||||
| GNG | TAC | ||||||
| Session | T | P | d | Session | T | P | d |
| 1 | 6.7104 | 3.33E-05 | 5.6771 | 1 | −0.7057 | 0.4951 | --- |
| 2 | 4.7200 | 6.29E-04 | 4.2478 | 2 | −1.5830 | 0.1417 | --- |
| 3 | 3.9112 | 0.0024 | 2.8436 | 3 | −2.0333 | 0.0669 | --- |
| Phase 3 Variable ITI Sessions | |||||||
| GNG | TAC | ||||||
| ITI | T | P | d | ITI | T | P | d |
| 3 | 5.2220 | 0.0012 | 8.8725 | 3 | −1.4473 | 0.1911 | --- |
| 5 | 5.2986 | 0.0011 | 3.3170 | 5 | −0.3299 | 0.7511 | --- |
| 9 | 3.7350 | 0.0073 | 5.1628 | 9 | −0.3320 | 0.7496 | --- |
| 11 | 3.7942 | 0.0068 | 3.5517 | 11 | 0.3755 | 0.7184 | |
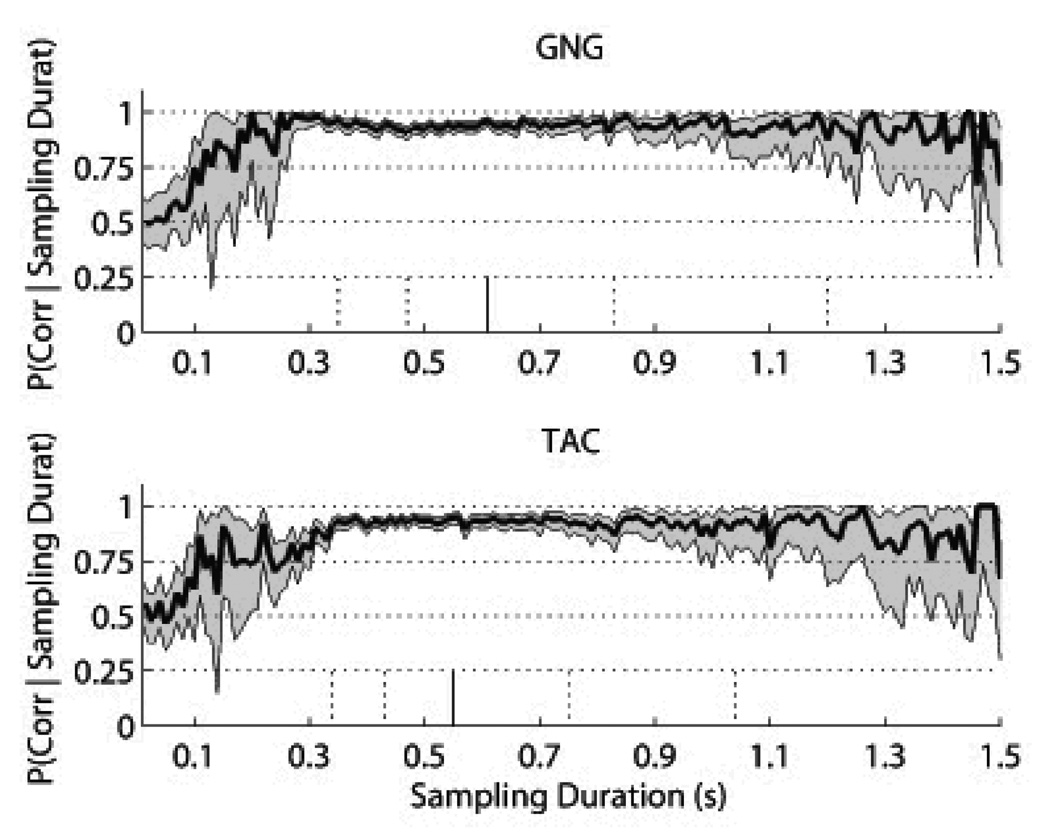
Conditional probability plots were constructed as Pr(Correct | Sampling Duration) with 10 ms sampling duration bins and are presented in Figure 8. Binomial 95-percent confidence intervals were calculated for each vertical slice (i.e., for each conditional probability). For GNG, the sampling durations for the percentiles are: 0.35, 0.47, 0.61, 0.83, 1.2s. For TAC, the sampling durations for the percentiles are: 0.34, 0.43, 0.55, 0.75, 1.04s.
Figure 8. Performance Conditioned on Sampling Duration.
For the Phase 3 Fixed ITI Criterion Sessions (3 sessions per rat; GNG: n=12 rats, TAC: n=12 rats) and the Phase 3 Variable ITI sessions (4 sessions per rat; GNG: n=8 rats, TAC: n=8 rats), we concatenated all trials and calculated the conditional probability of performance for each given sampling duration in 10ms bins. The short stubs at the bottom are the 10th, 25th, 50th, 75th, and 90th percentiles of sampling duration calculated on the entire set (i.e., both odorants) for GNG and TAC. The shaded regions represent the 95-percent binomial confidence intervals for each vertical section. The x-axis is limited to 1.5 seconds, which contains the 80-percent central distribution of the sampling duration. Above 1.5 seconds, there were too few values per bin to give an accurate graph.
Although the conditional performance does show what appears to be a monotonically increasing function between 0 and ∼300ms, which could be construed as an example of a speed-accuracy trade-off, we instead suggest that this is merely a psychometric function indicating the fact that performance below 300ms has not yet saturated. For this to be a speed-accuracy trade-off meant to optimize reward rate, then rats should skew their distribution downwards. Instead, the times between 0 and ∼300 ms correspond to less than 10% of the total trials.
Stimulus Sampling Duration by Odorant
The statistical results for sampling duration for each odorant in GNG and TAC are presented in Table 7. Similar to performance by odorant, we analyzed sampling durations as a function of the individual odorants. Contrary to previous research (Slotnick, 2007) we were unable to find a difference between odorants. For GNG and TAC, there was never a significant difference between odorant sampling duration for any session considered.
Table 7.
Sampling Duration by Odorant Statistical Results
| Phase 3 Fixed ITI First 4 Sessions | |||||
|---|---|---|---|---|---|
| GNG | TAC | ||||
| Session | T | P | Session | T | P |
| 1 | 0.8917 | 0.3916 | 1 | 1.4990 | 0.1620 |
| 2 | −0.3889 | 0.7048 | 2 | 1.7133 | 0.1147 |
| 3 | 0.4094 | 0.8576 | 3 | 1.2408 | 0.2408 |
| 4 | 0.6941 | 0.5020 | 4 | 1.4393 | 0.1779 |
| Phase 3 Fixed ITI Criterion Sessions | |||||
| GNG | TAC | ||||
| Session | T | P | Session | T | P |
| 1 | 0.1376 | 0.8930 | 1 | 0.4882 | 0.6350 |
| 2 | 0.9067 | 0.3840 | 2 | 0.1215 | 0.9055 |
| 3 | 0.7555 | 0.4658 | 3 | 0.1343 | 0.8956 |
| Phase 3 Variable ITI Sessions | |||||
| GNG | TAC | ||||
| ITI | T | P | ITI | T | P |
| 3 | −0.9153 | 0.3905 | 3 | 0.4077 | 0.6957 |
| 5 | 0.2457 | 0.8129 | 5 | −1.1667 | 0.2815 |
| 9 | 0.1297 | 0.9004 | 9 | 0.2013 | 0.8462 |
| 11 | −0.1559 | 0.8805 | 11 | −0.0870 | 0.9331 |
Nose-Poke Delay Analysis
Throughout the sessions, we observed that rats quite often would engage in grooming behavior after eating the pellet, which led us to think that the observed decrease in nose-poke delay with increasing ITI could depend on whether or not the rat received a pellet in the previous trial. To test this hypothesis, we divided each of a rat’s Phase 3 Variable ITI sessions into two disjoint sets conditioned upon either 1) the odorant (A or B) used in the previous trial, or 2) whether or not the rat received a pellet in the previous trial. We chose to analyze the ITI sessions because they would give us the additional information regarding whether or not ITI duration interacted with nose-poke delay. If the delay was driven, in part, by behavior during the ITI period, then with increasing ITI the behavior should have had time to be completed and the rat to reset for the next trial. If however, the difference is due, at least in part, to some behavioral or cognitive difference between the tasks, then regardless of the ITI duration the differences should persist.
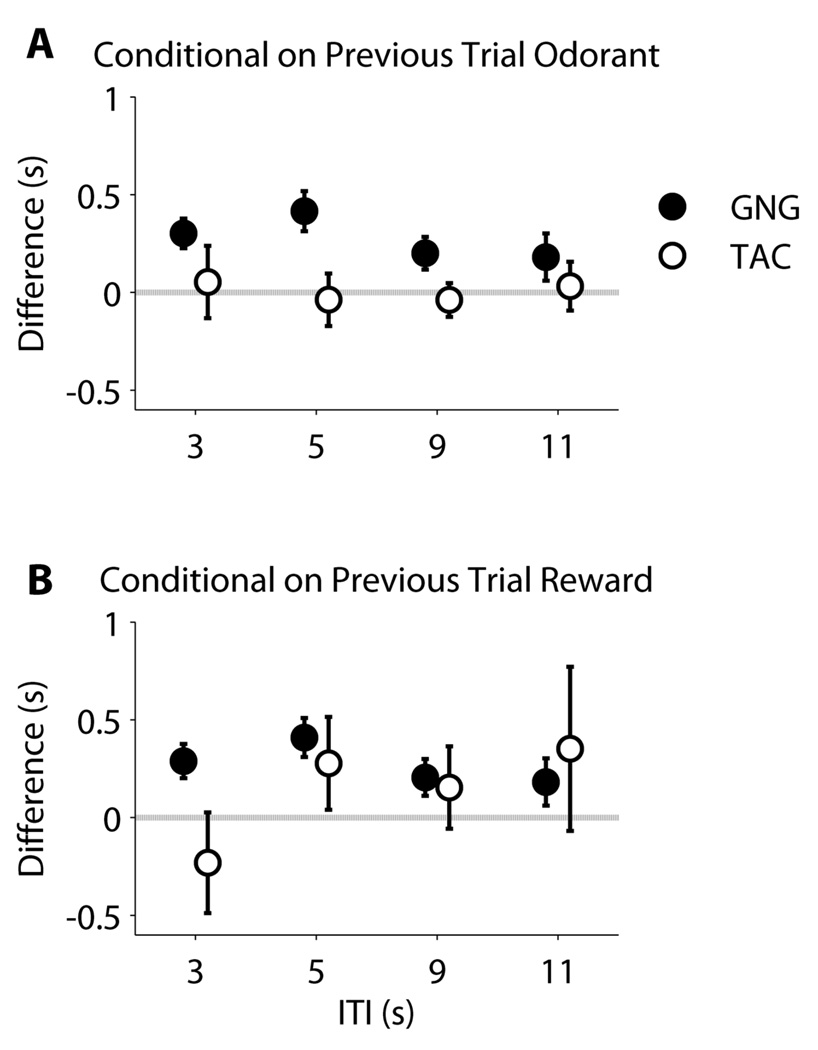
Results are presented in Figure 9. For GNG, regardless of how we constructed the difference set, there were statistically significant differences in nose-poke delay times for all ITIs (Figure 9 A, B; all green boxes are above the zero line, showing longer delays if the previous trial was odor B or was not rewarded). For TAC, the only significant difference in nose-poke delay was for ITI 5 conditioned upon previous trial being rewarded (Figure 9B). In this instance, TAC rats took longer to initiate a trial if the previous trial was not rewarded.
Figure 9. Phase 3 - Variable ITI Conditional Nose-poke delay differences.
To assess whether or not the previous trial type had a conditional influence on nose-poke delay, we calculated differences between mean delays in two ways. 9A: Difference conditional on previous trial odorant type. This corresponds to the difference between mean delay when the previous trial was odorant B minus when it was odor A, across rats. 9B: Difference conditional on receiving a reward in previous trial. This corresponds to differences between mean nose-poke delay for trials preceded by an unrewarded trial minus trials preceded by a rewarded trial, across rats. (Differences of the means and 95% confidence intervals are shown. Zero indicates no difference.)
Discussion
Past research in olfactory psychophysics indicated that mice and rats are able to perform odor discriminations in a relatively short period (< 300 ms) that may correspond to one or two sniffs (Rinberg, Koulakov, & Gelperin, 2006; Uchida & Mainen, 2003). The literature also suggested that rodents (mice and rats) may differently engage GNG and TAC operant tasks, exhibiting shorter sampling durations and discrimination times when engaged in a TAC task than when engaged in a GNG task (Friedrich, 2006). Furthermore, mice have been shown to increase their discrimination times in order to maintain accuracy in a GNG task (Abraham et al., 2004), while mice and rats in TAC tasks have not (Rinberg, Koulakov, & Gelperin, 2006; Uchida & Mainen, 2003). It is also commonly suggested that a GNG task is easier for rats to learn than a TAC task (B. M. Slotnick & Nigrosh, 1974; B. Slotnick, 2007). Surprisingly, no research has systematically explored the possible similarities and differences between the two tasks in rodent olfaction, although a recent study has modeled the two tasks in humans, in the context of lexical decision and word recognition, and has suggested that the differences are not in cognitive demands (Gomez, Ratcliff, & Perea, 2007).
We hypothesized that these reported differences could be explained by the two tasks engaging different cognitive processes and that these differences would be, at least in part, intrinsic to the tasks (Kay, 2009; Kay, Beshel, & Martin, 2006). Given the differences in reward schedule typically used for the tasks, we thought that rats would engage in a speed-accuracy trade-off behavior meant to optimize reward rate. In GNG, only half of the trials could be rewarded, whereas in TAC all trials have the potential to be rewarded. Past literature has shown that there is a positive correlation between forced sampling duration and performance (Rinberg, Koulakov, & Gelperin, 2006). Therefore, rats engaged in a GNG task should spend more time sampling the odorants in order to gain additional olfactory information that would correlate with better performance. In contrast, TAC rats would be more likely to have shorter sampling durations, indicating a preference for a fast decision even if longer sampling time improved individual trial performance. It was also expected that this behavioral difference would lead to GNG rats learning faster than TAC rats. To address our hypotheses, we constructed a standardized training protocol and testing procedure that allowed us to compare the two tasks in parallel and to manipulate the ITI. Our protocols also allowed us to isolate the stimulus sampling period from a general reaction time period.
We find that the two tasks do differ, but that the difference is not absolute and depends upon which dependent variable one examines and how one selects the values of task parameters. This is crucial, because for all but two dependent variables (individual odorant performance and perhaps response delay) we were able to make the tasks arbitrarily similar with modification to the basic task parameters (e.g., ITI duration). Furthermore, we find no evidence of a speed-accuracy trade-off meant to optimize reward rates, instead rats appear to focus on total performance that would optimize total rewards.
We found that the average amount of time to initiate a trial (nose-poke delay) was shorter for GNG rats than TAC rats. However, this difference disappeared for long ITI durations (See Figure 6). By constructing conditional difference sets we see that TAC rats generally have the same delay regardless of whether the previous trial was rewarded or whether it was odorant A or B. However, GNG rats show significantly shorter nose-poke delays when the previous trial was either odorant A or was rewarded (See Figure 9). Anecdotally, during GNG sessions, we observed that rats would often turn away from the front panel, which contained the odor and response ports, after refraining from responding. This behavior may have resulted in the rats being farther from the odor port at the start (house light on) of the next trial and consequently would mean that they would need more time to get to the odor port to initiate the trial. If rats were attempting to optimize reward rates, then one would expect that this is one variable that they could easily decrease to increase their rates. This does not appear to happen.
For one variable that may not be under experimenter control – response delay – we found that the time to perform a response following odorant sampling was shorter for GNG odorant A than for either odorant in TAC. However, this difference was only significant for Phase 3 Fixed ITI sessions and was not significant for any ITI duration in Phase 3 Variable ITI sessions. The lack of an effect might be explained by reduced power, if the difference represents an intrinsic difference between the tasks. Or it could be that TAC simply requires more time to achieve proficiency such that the motor response would be executed as fast as the GNG ‘go’ response.
Contrary to our expectations, the odorant sampling duration differed neither between the tasks nor between the odorants (See Figure 5, Figure 7 and Table 7). Additionally, the sampling durations were not affected by ITI duration (See Figure 6). Initially, we had expected that GNG rats would spend more time sampling the odorants in order to ensure correct identification of the stimulus. We imagined that this would be the case because, on average, rats engaged in GNG tasks typically are only able to receive rewards for correctly performing the ‘go-behavior’ and would therefore select a sampling duration that would help them to maximize their rewards. Instead, we found that GNG rats had the same sampling duration as TAC rats (for both odorants) and that both GNG and TAC rats could perform to a high degree of accuracy for short sampling duration (∼300ms), although they typically took longer (See Figure 8). Taken together, these results suggest that GNG and TAC tasks may not differ in stimulus sampling durations, which is in agreement with research suggesting that any difference between the tasks is at the level of post-sensory experience (Gomez, Ratcliff, & Perea, 2007).
Average session performance was the same for GNG and TAC and not affected by ITI duration (See Figure 5 and Figure 6). However, there were differences in individual odorant performance within a task. In general, GNG rats performed much better for odorant A (the positively re-enforced stimulus) than odorant B, whereas TAC rats showed no preference. This difference in odorant performance, but not session performance, might account for the observation that GNG rats reached criterion performance sooner than TAC rats, given our initial criterion level (See Results: Phase 3 Fixed ITI). However, when examined with a more lenient criterion level (number of days to reach 80% session performance) the two tasks did not differ. We hypothesize that the difference in odorant performance leads to the difference in number of days to reach our criterion performance level and that the differences are driven by animal motivation, which is in turn driven by the reward schedule. Therefore, if one were to reward the no-go behavior in the GNG task, then the animal would have a positive incentive to increase performance for odorant B, which might then translate into parity performance between the odorants, similar to the TAC task. If this is true, then this task difference would also be under experimenter control.
Another example of how experimenter selection of task parameters may influence conclusions is as follows. If one were to run the experiments only using a 3-second ITI, then one may conclude that the tasks are essentially different because there are main effects of task on session performance, nose-poke delay (See Table 5), and individual odorant performance (See Table 7). However, if one were to run the same experiment using an 11-second ITI, then one may conclude that the tasks are essentially the same (with the exception of individual odorant performance).
Although we limited our experiments to a single odor set whose odorants were easily discriminated, we reasoned that if the two tasks are fundamentally different, then that difference should emerge regardless of the odor set used. In fact, we found distinct differences between the tasks for several dependent variables: nose-poke delay, individual odorant performance, and response delay. Furthermore, we found no evidence that rats engage in systematic self-directed speed-accuracy tradeoff meant to optimize reward rates. Perhaps the strongest evidence against them doing so in our experiment is the fact that although rats could perform with a very high level of accuracy (See Figure 8) for very short sampling durations, they chose these sampling durations for less than 10% of the trials. This goes against the view that rats may use SAT to maximize reward rate. Instead, our evidence points to the more likely inference that rats maximize total reward, in which case SAT is not needed, since rats are engaged in experimental protocols with fixed numbers of total trials and/or total attempts. It is of course still possible that some SAT-based differences between the tasks would manifest themselves in more difficult discriminations, and this remains to be tested in the future, using multiple odor sets and odor mixtures.
Except for individual odorant performance, which we hypothesize to be due to reward structure, all differences between dependent variables appear to be under the experimenter’s control. These factors are important for sensory physiology and studies involving odor coding. Researchers should take this ability to make GNG and TAC behavior arbitrarily similar into account when designing learning and performance studies. Future research should be able to take advantage of this ability to explore whether or not these behavioral modulations correlate with neuronal activity patterns, to elucidate the neurobiological basis underlying the observed tasks’ differences and/or similarities.
Acknowledgments
We thank Stephanie Tacopina and Hannah Lee for help in animal handling and data collection. We also thank Romina Kuppe for design help on Figure 1. This research was supported by funding from NIDCD CRCNS R01-DC007995 and an Institute for Mind and Biology Seed Grant to LM Kay.
Footnotes
The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Contributor Information
Donald E. Frederick, Department of Psychology and Institute for Mind & Biology, The University of Chicago.
Daniel Rojas-Líbano, Committee on Neurobiology and Institute for Mind & Biology, The University of Chicago.
Meagen Scott, Institute for Mind & Biology, The University of Chicago.
Leslie M. Kay, Department of Psychology, Committee on Neurobiology, and Institute for Mind & Biology, The University of Chicago
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. Journal of Neuroscience. 2007;27:8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pírez N, Wachowiak M. Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. Journal of Neurophysiology. 2009;101:1073–1088. doi: 10.1152/jn.90902.2008. doi: 10.1152/jn.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DE, Barlas L, Ievins A, Kay LM. A critical test of the overlap hypothesis for odor mixture perception. Behavioral Neuroscience. 2009;123:430–437. doi: 10.1037/a0014729. doi: 10.1037/a0014729. [DOI] [PubMed] [Google Scholar]
- Friedrich RW. Mechanisms of odor discrimination: neurophysiological and behavioral approaches. Trends in Neurosciences. 2006;29:40–47. doi: 10.1016/j.tins.2005.10.004. doi: 10.1016/j.tins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Gervais R, Buonviso N, Martin C, Ravel N. What do electrophysiological studies tell us about processing at the olfactory bulb level? Journal of Physiology, Paris. 2007;101:40–45. doi: 10.1016/j.jphysparis.2007.10.006. doi: 10.1016/j.jphysparis.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Gomez P, Ratcliff R, Perea M. A model of the go/no-go task. Journal of Experimental Psychology. General. 2007;136:389–413. doi: 10.1037/0096-3445.136.3.389. doi: 10.1037/0096-3445.136.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM. Oscillatory modes and the role of task structure in early olfactory processing. Chemical Senses. 2009;34:A5–A6. [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Líbano D, Kopell N. Olfactory oscillations: the what, how and what for. Trends in Neurosciences. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Martin C. When good enough is best. Neuron. 2006;51(3):277–278. doi: 10.1016/j.neuron.2006.07.015. doi: 10.1016/j.neuron.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Kay LM, Krysiak M, Barlas L, Edgerton GB. Grading odor similarities in a Go/No-Go task. Physiology & behavior. 2006;88:339–346. doi: 10.1016/j.physbeh.2006.04.007. doi: 10.1016/j.physbeh.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311:666–670. doi: 10.1126/science.1122096. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006;51:351–358. doi: 10.1016/j.neuron.2006.07.013. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Olfactory stimulus control evaluated in a small animal olfactometer. Perceptual and Motor Skills. 1974;39(1 Pt 2):583–597. doi: 10.2466/pms.1974.39.1.583. [DOI] [PubMed] [Google Scholar]
- Slotnick B. Odor-sampling time of mice under different conditions. Chemical Senses. 2007;32:445–454. doi: 10.1093/chemse/bjm013. doi: 10.1093/chemse/bjm013. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nature Neuroscience. 2003;6:1224–1229. doi: 10.1038/nn1142. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White Ja, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nature Neuroscience. 2007;10:631–639. doi: 10.1038/nn1892. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PLoS Biology. 2008;6:e82. doi: 10.1371/journal.pbio.0060082. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing behavior of mice during performance in odor-guided tasks. Chemical Senses. 2008;33:581–596. doi: 10.1093/chemse/bjn029. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Verhagen JV, Wachowiak M. Why sniff fast? The relationship between sniff frequency, odor discrimination, and receptor neuron activation in the rat. Journal of Neurophysiology. 2009;101:1089–1102. doi: 10.1152/jn.90981.2008. doi: 10.1152/jn.90981.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]