Abstract
Background
Very low levels of cardiac troponin T associate with increased risk of cardiovascular death in patients with stable chronic coronary disease. Whether high-sensitivity cardiac troponin T (hsTnT) levels associate with adverse cardiovascular outcomes in individuals without cardiovascular disease (CVD) has not been well studied.
Methods and Results
Utilizing two complementary study designs, we evaluated the relationship between baseline cardiac troponin and incident CVD events among diabetic and non-diabetic participants in the Women’s Health Study (median follow-up 12.3 years). All diabetic women with blood specimens were included in a cohort study (n=512 diabetic women, n=65 events) and non-diabetic women were sampled for inclusion in a case-cohort analysis (n=564 comprising the subcohort, n=479 events). HsTnT was detectable (≥0.003 μg/L) in 45.5% of diabetic women and 30.3% of non-diabetic women (P<0.0001). In models adjusted for traditional risk factors and hemoglobin A1c, detectable hsTnT was associated with subsequent CVD (myocardial infarction, stroke, cardiovascular death) in diabetic women (adjusted HR 1.79, 95% CI 1.04-3.07, P=0.036), but not non-diabetic women (adjusted HR 1.13, 95% CI 0.82-1.55, P=0.46). Further adjustment for NT-proBNP and estimated renal function did not substantially alter this relationship among diabetic women (HR 1.76, 95% CI 1.00-3.08, P=0.0499), which appeared to be driven by a threefold increase in CVD death that was not observed in non-diabetic women.
Conclusions
Very low but detectable levels of cardiac troponin T associate with total CVD and CVD death in women with diabetes. Among healthy non-diabetic women, detectable compared to undetectable troponin was not associated with CVD events.
Keywords: Primary prevention, cardiovascular disease risk factors, troponin, diabetes mellitus type 2, women
Introduction
The presence of circulating cardiac troponin, when measured with conventional troponin assays, is a strong predictor of adverse outcome in patients with acute coronary syndromes, heart failure, and chronic coronary disease.1-8 However, these conventional assays are of limited use in healthier patients without preexisting heart disease, because most healthy patients do not have detectable levels of cardiac troponin using these standard assays.9
Recently, newer assays for circulating cardiac troponin with nearly 10 times the detection limit of conventional assays have become available. When compared to the conventional assays, newer assays improve the speed and accuracy of the diagnosis of acute coronary syndromes.10, 11 In high-risk patients with established coronary disease or heart failure, troponin levels that are below the limit of detection of conventional assays and within the normal range of the newer assays predict adverse cardiovascular outcomes, including cardiovascular death.12, 13Recently published results from the Cardiovascular Health Study and the Dallas Heart Study extend those observations to a primary prevention population of elderly men and women and to a general population inclusive of subjects with prior overt or subclinical heart disease.14, 15 However, data in lower risk populations, such as middle-aged women, are lacking, and many of the published studies of patients with chest pain, heart failure, or coronary disease have included few women.12, 13, 16 Finally, data are scarce among patients with diabetes, a condition known to associate with elevated baseline conventional troponin levels.9
In order to further evaluate these issues, we measured cardiac troponin T levels using a novel high-sensitivity assay in baseline blood samples among two groups of women: diabetic and non-diabetic participants in the Women’s Health Study. Women were followed prospectively for incident myocardial infarction, stroke, and cardiovascular death. We hypothesized that circulating cardiac troponin levels would be higher among diabetic than non-diabetics subjects, and that troponin levels that were detectable but nonetheless in the normal range would associate with future cardiovascular events in both groups of women.
Methods
Study Participants and Endpoint Ascertainment
Study participants were enrolled in the Women’s Health Study (WHS), a completed randomized, double-blind, placebo-controlled 2×2 factorial trial of aspirin and vitamin E in the prevention of cardiovascular disease and cancer among 39,876 female health professionals aged 45 years and older. Enrollment began in 1992, and a total of 28,345 women provided a blood sample at baseline. Among these, 19,871 (70.1%) were fasting at time of sample collection. The randomized portion of the study was completed in March 2004, and women were invited to participate in continued observational follow up. Subjects have been followed continuously for incident cardiovascular disease (CVD), a composite of non-fatal myocardial infarction, non-fatal ischemic stroke, or cardiovascular death. The methods of endpoint validation have been described elsewhere in detail.17
Laboratory Analysis
Plasma high-sensitivity troponin T was measured using an electrochemiluminescent immunoassay assay provided by Roche Diagnostics (Indianapolis, Indiana). According to the manufacturer, the lower limit of detection of the high-sensitivity assay is 0.003 μg/L. The lower limit of detection with the conventional Roche assay is 0.01 μg/L. The value at the 99th percentile in a population of healthy blood donors is 0.0135 μg/L, and the 10% coefficient of variation concentration has been reported to be less than this value.18 Recent work has defined an abnormal level of hsTnT to be ≥ 0.014 μg/L, so we have adopted that convention for this study.19
Study Subjects
All subjects were selected from among women with adequate fasting baseline blood specimens. Two complimentary study designs were implemented based upon baseline diabetes status.
Diabetic Women
All women who reported a diagnosis of diabetes on the enrollment questionnaire and had blood samples available for analysis were included in the diabetes cohort (n=512).
Non-diabetic Women
We designed a case-cohort study of non-diabetic women by first selecting a random sample of CVD cases (n=464) from among the entire WHS cohort. A randomly selected subcohort of non-diabetic women (n=564) was then chosen as a reference risk set. Selection of this reference subcohort of non-diabetic women was independent of CVD case status. Fifteen participants who were randomly selected for the reference subcohort of non-diabetic women developed incident CVD, increasing the total number of CVD events included in the case-cohort analysis to 479. The selection of the reference subcohort was stratified by race/ethnicity (White vs. non-White) as well as age (in 5-year strata) to approximately match the distribution among CVD cases. This non-diabetic reference subcohort, which comprised a total of 564 women, was used for the analysis of the distribution of hsTnT levels and associated covariates.
Statistical Analysis
HsTnT Levels and Associated Covariates in Diabetic and Non-Diabetic Women
Baseline clinical covariates and the distribution of hsTnT in diabetic women (n=512) and the reference subcohort of non-diabetic women (n=564) were compared using chi-square and Wilcoxon rank-sum tests for categorical and continuous variables, respectively. Non-diabetic women selected solely on the basis of CVD case status (n=464) were not included in this analysis. On an a priori basis we defined 3 ways to compare hsTnT levels in diabetic women with those in the reference subcohort of non-diabetic women: as a continuous variable, as the proportion of women with detectable hsTnT levels (≥ 0.003 μg/L), and as the proportion of women with abnormal hsTnT levels (≥0.014 μg/L).19
We assessed clinical and biochemical correlates of baseline detectable (≥0.003 μg/L) as compared to undetectable (<0.003 μg/L) troponin by calculating odds ratios (OR), 95% confidence intervals (CI) and P-values separately for diabetic and non-diabetic women from the reference subcohort. Beta coefficients for each univariable correlate of detectable hsTnT in diabetic women were compared to those in non-diabetic women with a two-sided Z-test. Two independent multivariable adjusted regression models for diabetic and non-diabetic women were constructed using backwards selection, with an inclusion threshold for the final model of P<0.15. Candidate variables included those analyzed in the previous univariable logistic regression analysis. When current smoking was selected for multivariable models, past smoking was forced into the model so that the referent group remained never smokers. During the model selection process, in order to prevent the addition of two variables that were likely to be collinear, we included systolic blood pressure rather than a history of hypertension and total cholesterol rather than low-density lipoprotein cholesterol (LDL-C) or history of elevated cholesterol. Models including history of hypertension in place of systolic blood pressure or LDL-C or history of elevated cholesterol in place of total cholesterol yielded similar results (data not shown). As a secondary analysis, independent stepwise logistic regression procedures in diabetic and non-diabetic women were used to confirm that no important associations between baseline clinical covariates and a detectable hsTnT were missed. The significance thresholds for covariable entry and retention were set at P<0.15.
HsTnT and Incident Cardiovascular Disease in Diabetic and Non-Diabetic Women
Cox proportional hazards models were used to calculate adjusted hazard ratios (HR) and 95% CIs for incident cardiovascular disease (non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death) according to the presence of detectable troponin (≥0.003 μg/L). The risk of incident CVD associated with a detectable troponin was calculated separately for diabetic women (analyzed as a cohort) and non-diabetic women (analyzed as a case-cohort). Similar Cox proportional hazards modeling techniques were used in diabetic and non-diabetic women, except that in non-diabetic women, adjustments were made to account for the weighted sampling technique and the case-cohort study design.20 Evidence for statistically significant differences in beta coefficients for the association between a detectable troponin (≥0.003 μg/L) and incident cardiovascular disease in the diabetic as compared to the non-diabetic women were assessed using a two-sided Z-test. Women without complete information for all covariables included in multivariable models were excluded from those analyses. A maximum of 40 of the 512 diabetic women (7.8%) and 32 of the 1028 non-diabetic women (3.1%) were excluded for missing covariate information. The proportional hazards assumption was examined in diabetic and non-diabetic women by including a time by detectable hsTnT interaction term in the model. No violation of this assumption was detected. All analyses were performed using SAS 9.1 for UNIX.
Results
Baseline Characteristics and Distribution of hsTnT
Baseline characteristics of the diabetic women and the non-diabetic women from the reference subcohort are displayed in Table 1. Women with diabetes were similar in age to non-diabetic women, but were more likely to have a history of high cholesterol and elevated blood pressure. Diabetic women had higher median body mass index, high-sensitivity C-reactive protein, estimated glomerular filtration rate and HbA1c levels and lower high-density lipoprotein cholesterol levels.
Table 1.
Baseline characteristics of diabetic women and non-diabetic women selected for inclusion in the reference subcohort. Continuous variables are presented as medians with interquartile ranges in parentheses. P-values are from Wilcoxon rank-sum or Chi-square tests for continuous and categorical characteristics, respectively.
| Characteristic | Diabetic Women (N=512) | Reference Subcohort of Non-Diabetic Women (N=564) | P-value |
|---|---|---|---|
| Age (years) | 56.2 (50.6, 63.3) | 56.7 (50.9, 64.3) | 0.30 |
| White race, N (%) | 453 (88.5) | 519 (92.0) | 0.05 |
| History of cholesterol > 240 mg/dL, N (%) | 253 (49.4) | 191 (33.9) | <0.0001 |
| History of hypertension, N (%) | 330 (64.5) | 180 (31.9) | <0.0001 |
| Systolic blood pressure, mmHg | 135 (125, 145) | 125 (115, 135) | <0.0001 |
| Parental history of myocardial infarction at age < 60 years, N (%) | 65 (14.2) | 68 (13.2) | 0.66 |
| Current Smoking, N (%) | 59 (11.5) | 56 (9.9) | 0.40 |
| Body mass index, kg/m2 | 30.1 (26.4, 34.4) | 25.6 (23.0, 28.3) | <0.0001 |
| Alcohol, at least 1 serving/week, N (%) | 96 (18.8) | 225 (39.9) | <0.0001 |
| Exercise at least 1 time/week, N (%) | 164 (32.0) | 260 (46.1) | <0.0001 |
| Aspirin use, N (%) | 263 (51.4) | 277 (49.1) | 0.46 |
| Treatment for high cholesterol, N (%) | 59 (11.6) | 24 (4.3) | <0.0001 |
| Treatment for hypertension, N (%) | 235 (46.0) | 95 (16.8) | <0.0001 |
| Hormone therapy use, N (%) | 141 (27.6) | 243 (43.2) | <0.0001 |
| Cholesterol, mg/dL | |||
| Total | 215.0 (193.0, 245.0) | 214 (188, 240) | 0.12 |
| Low density lipoprotein | 130.3 (108.0, 152.8) | 125.4 (107.0, 148.8) | 0.18 |
| High density lipoprotein | 41.4 (35.0, 50.0) | 51.6 (43.0, 62.1) | <0.0001 |
| High-sensitivity C-reactive protein, mg/L | 4.79 (2.57, 8.34) | 2.16 (0.94, 4.61) | <0.0001 |
| HbA1c, % | 7.00 (5.97, 8.38) | 5.02 (4.86, 5.20) | <0.0001 |
| Estimated glomerular filtration rate, mL/min | 53.7 (44.8, 66.2) | 46.9 (39.4, 57.2) | <0.0001 |
Abbreviations: HbA1c, hemoglobin A1c.
HsTnT levels in the women with diabetes were higher than among non-diabetic women (P<0.0001). While 30.3% of non-diabetic women had detectable levels of hsTnT, this proportion was much higher among diabetic women (45.5%, P<0.0001; Table 2). Only 2% of healthy non-diabetic women included in the cohort had elevated (≥0.014 μg/L) hsTnT levels. Among diabetic women, that proportion was nearly twice as high (3.9%), although the difference was not statistically significant (P=0.055).
Table 2.
Proportion of diabetic and non-diabetic women with detectable (≥0.003 μg/L) and elevated levels (≥0.014 μg/L) of high sensitivity cardiac troponin T (hsTnT).
| Characteristic | Diabetic Women (N=512) | Reference Subcohort of Non-Diabetic Women (N=564) | P-value |
|---|---|---|---|
| Detectable hsTnT (≥ 0.003 μg/L), N (%) | 233 (45.5) | 171 (30.3) | <0.0001 |
| Elevated hsTnT (≥ 0.014 μg/L), N (%) | 20 (3.9) | 11 (2.0) | 0.055 |
Abbreviations: hsTnT, high sensitivity cardiac troponin T; IQR, interquartile range.
The distribution of detectable hsTnT levels in diabetic and non-diabetic women is displayed in the Figure. Among those with a detectable hsTnT, the median (IQR) hsTnT was higher in diabetic women [0.0060 μg/L (0.0044, 0.0084)] than in non-diabetic women [0.0049 μg/L (0.0039, 0.0071); P=0.0002). Only 2 out of 512 (0.4%) diabetic women and 2 out of 564 (0.4%) non-diabetic women had troponin levels that would have been detectable with previous generation assays (hsTnT ≥ 0.030 μg/L).
Figure.
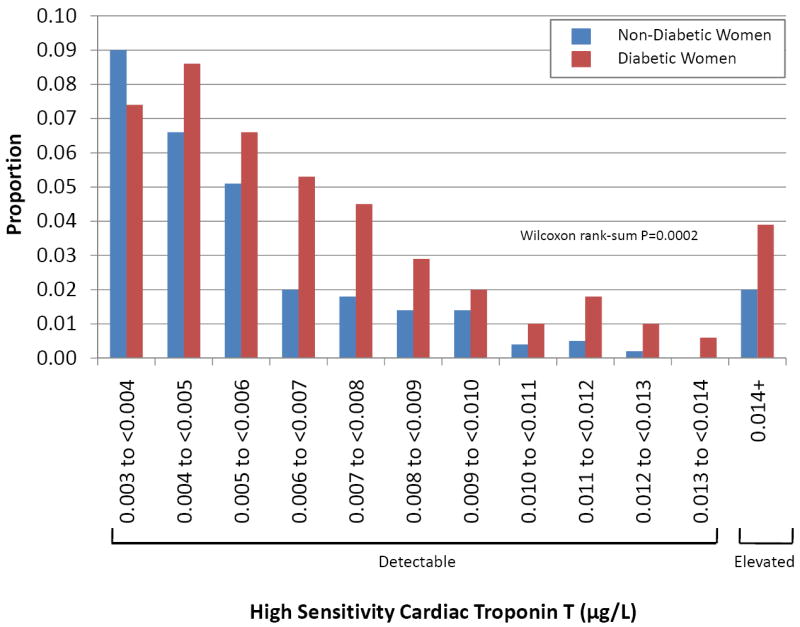
Distribution of detectable hsTnT levels (≥ 0.003 μg/L) in diabetic women and in the reference subcohort of non-diabetic women.
Baseline Characteristics Associated with a Detectable hsTnT
The univariable association of clinical and biochemical covariates with a detectable hsTnT among diabetic women and the non-diabetic reference subcohort are displayed in Table 3. The strength and direction of association for most correlates was similar in women with and without diabetes. However, increasing levels of total and LDL cholesterol were associated with increased odds of a detectable hsTnT in diabetic women, and decreased odds of detectable hsTnT in non-diabetic women, a difference that was statistically significant (Table 3). HbA1c correlated with hsTnT in diabetic and non-diabetic women, but the relationship was significantly stronger among non-diabetic women. Variables retained in the multivariable models are shown in Table 4. Similar results were observed for all covariates when using a stepwise regression procedure, when LDL-C was included in models instead of total cholesterol, and when history of hypertension was used instead of systolic blood pressure.
Table 3.
Univariable association between baseline characteristics of diabetic and non-diabetic women and a detectable high-sensitivity cardiac troponin T (≥0.003 μg/L) at baseline in the Women’s Health Study.
| Diabetic Women* | Reference Subcohort of Non-Diabetic Women† | Test for Difference in Association | |||
|---|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | P-value |
| Age, per 5 year increase | 1.18 (1.05-1.32) | 0.006 | 1.35 (1.21-1.51) | <0.0001 | 0.09 |
| White race (as compared to non-White race) | 0.85 (0.49-1.46) | 0.55 | 1.38 (0.68-2.79) | 0.37 | 0.29 |
| History of hypertension | 1.80 (1.24-2.61) | 0.002 | 1.78 (1.22-2.60) | 0.0026 | 0.97 |
| Systolic Blood Pressure, per 10 mmHg increase | 1.28 (1.12-1.45) | 0.0002 | 1.22 (1.08-1.37) | 0.0009 | 0.60 |
| Treatment for hypertension | 1.33 (0.93-1.88) | 0.12 | 1.60 (1.01-2.52) | 0.05 | 0.53 |
| Current smoking‡ | 0.93 (0.53-1.65) | 0.81 | 0.39 (0.18-0.83) | 0.015 | 0.07 |
| Past smoking‡ | 1.20 (0.82-1.75) | 0.35 | 0.90 (0.61-1.31) | 0.57 | 0.29 |
| History of cholesterol > 240 mg/dL | 0.94 (0.66-1.32) | 0.70 | 1.30 (0.89-1.89) | 0.17 | 0.21 |
| Treatment for high cholesterol | 1.38 (0.80-2.38) | 0.24 | 0.76 (30-1.94) | 0.56 | 0.28 |
| Parental history of myocardial infarction at age <60y | 0.76 (0.44-1.29) | 0.31 | 0.98 (0.56-1.72) | 0.95 | 0.51 |
| Body mass index (per 1 kg/m2 increase) | 1.02 (0.99-1.05) | 0.32 | 1.03 (1.00-1.07) | 0.07 | 0.47 |
| Total cholesterol (per 1 SD increase) | 1.24 (1.06-1.44) | 0.008 | 0.84 (0.70-1.01) | 0.06 | 0.002 |
| LDL cholesterol (per 1 SD increase) | 1.21 (1.03-1.42) | 0.02 | 0.92 (0.76-1.10) | 0.35 | 0.03 |
| HDL cholesterol (per 1 SD increase) | 1.01 (0.84-1.21) | 0.92 | 0.87 (0.73-1.05) | 0.15 | 0.28 |
| Natural logarithm of hsCRP (per 1 SD increase) | 1.01 (0.84-1.21) | 0.93 | 1.09 (0.91-1.31) | 0.35 | 0.55 |
| Fibrinogen (per 1 SD increase) | 1.14 (0.99-1.32) | 0.07 | 1.22 (1.02-1.46) | 0.03 | 0.57 |
| Hemoglobin A1c (per 1 SD increase) | 1.05 (1.02-1.08) | 0.003 | 1.28 (1.07-1.53) | 0.007 | 0.03 |
| Natural logarithm of the EGFR (per 1 SD increase) | 1.12 (0.96-1.32) | 0.16 | 0.91 (0.76-1.09) | 0.32 | 0.09 |
| Natural logarithm of NT-proBNP (per 1 SD increase) | 1.28 (1.08-1.52) | 0.004 | 1.17 (0.98-1.40) | 0.09 | 0.46 |
| Natural logarithm of Lp(a) (per 1 SD increase) | 1.07 (0.92-1.25) | 0.39 | 1.01 (0.84-1.21) | 0.94 | 0.61 |
Of the 512 diabetic women, 233 (45.5%) had a detectable hsTnT.
Of the 564 non-diabetic women in the reference subcohort, 171 (30.3%) had a detectable hsTnT.
Current and past smokers are included in the same logistic regression model, such that never smokers are the referent group.
Abbreviations: EGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; LDL, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); SD, standard deviation
Table 4.
Multivariable adjusted association between baseline characteristics of diabetic and non-diabetic women and a detectable high sensitivity cardiac troponin (≥0.003 μg/L) at baseline in the Women’s Health Study. Backward selection (p-value threshold of <0.15) was used to choose covariates in separate analyses for diabetic and non-diabetic women. Odds ratios (ORs) and P-values are presented only for those correlates included in the final adjusted model. Models including low density lipoprotein cholesterol (instead of total cholesterol) or history of hypertension (instead of systolic blood pressure) yielded similar results.
| Diabetic Women* | Reference Subcohort of Non-Diabetic Women† | |||
|---|---|---|---|---|
| Characteristic | Adjusted Odds Ratio (95% CI) | P-value | Adjusted Odds Ratio (95% CI) | P-value |
| Age, per 5 year increase | 1.21 (1.05-1.41) | 0.01 | 1.30 (1.15-1.47) | <0.0001 |
| Systolic blood pressure, per 10 mmHg increase | 1.20 (1.04-1.38) | 0.01 | 1.12 (0.98-1.27) | 0.09 |
| Current smoking‡ | - | 0.43 (0.20-0.94) | 0.04 | |
| Past smoking‡ | - | 0.91 (0.61-1.36) | 0.20 | |
| Total cholesterol (per 1 SD increase) | 1.18 (0.99-1.40) | 0.07 | 0.74 (0.60-0.91) | 0.005 |
| Hemoglobin A1c (per 1 SD increase) | 1.04 (1.01-1.08) | 0.01 | 1.19 (0.98-1.44) | 0.07 |
| Natural logarithm of the EGFR (per 1 SD increase) | 1.22 (1.01-1.48) | 0.04 | - | |
| Natural logarithm of NT-proBNP (per 1 SD increase) | 1.18 (0.97-1.43) | 0.10 | - | |
Abbreviations: EGFR, estimated glomerular filtration rate; NT-proBNP, amino-terminal pro-B-type natriuretic peptide.
Of the 512 diabetic women, 233 (45.5%) had a detectable hsTnT.
Of the 564 non-diabetic women in the reference subcohort, 171 (30.3%) had a detectable hsTnT.
Past smoking was included in combination with current smoking, such that never smokers were the referent group.
HsTnT and Incident Cardiovascular Events
Among diabetic women, a detectable level of hsTnT at baseline was associated with nearly double the risk of the composite cardiovascular endpoint (HR 1.95, 95% CI 1.17-3.23, P=0.01) after adjustment for age and race (Table 5). This risk estimate was modestly attenuated but remained statistically significant after the addition of traditional cardiovascular risk factors and hsCRP (Model 2, Table 5) and the addition of HbA1c (Model 3, Table 5). After adjustment for the complete set of covariables, including NT-proBNP and estimated glomerular filtration rate, we observed a 76% increase in risk of the composite CVD endpoint (HR 1.76, 95% CI 1.00-3.08, P=0.0499; Model 4, Table 5). By contrast, among non-diabetic women, a detectable hsTnT was not associated with incident CVD (Table 5). As shown, the calculated hazard ratios (HR) and 95% CI ranged from 1.07 (0.81-1.40) in models adjusted for age and race to 1.15 (0.82-1.59) in models adjusted for traditional cardiovascular risk factors, hsCRP, HbA1c, NT-proBNP, and estimated glomerular filtration rate (Table 5). This difference in association between hsTnT and CVD in diabetic as compared to non-diabetic women was statistically significant in the age-and race-adjusted Model 1 (P=0.04), but not after further adjustment (P-values for Models 2 through 4 ranged from 0.12 to 0.20).
Table 5.
The adjusted risk of the composite cardiovascular endpoint of myocardial infarction, stroke, or cardiovascular death according to the presence of a detectable hsTnT in women with and without diabetes in the Women’s Health Study.
| Diabetic Women* | Non-Diabetic Women† | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Model 1 | 1.95 (1.17-3.23) | 0.010 | 1.07 (0.81-1.40) | 0.63 |
| Model 2 | 1.85 (1.09-3.17) | 0.024 | 1.13 (0.82-1.54) | 0.45 |
| Model 3 | 1.79 (1.04-3.07) | 0.036 | 1.13 (0.82-1.55) | 0.46 |
| Model 4 | 1.76 (1.00-3.08) | 0.0499 | 1.15 (0.82-1.59) | 0.42 |
65 out of 512 diabetic women developed the composite cardiovascular endpoint during follow up.
The case-cohort study of non-diabetic women included 479 cases of the composite cardiovascular endpoint and a reference subcohort of 564 women.
Model 1: Age, race adjusted
Model 2: Age, race, body mass index (kg/m2), history of hypertension, current smoking, parental history of myocardial infarction, total cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), natural logarithm high-sensitivity C-reactive protein (mg/L).
Model 3: Model 2 plus hemoglobin A1c (%)
Model 4: Model 2 plus hemoglobin A1c (%) and the natural logarithms of NT-proBNP and estimated glomerular filtration rate.
The association between a detectable hsTnT and incident CVD among women with diabetes appeared to be driven by a more than 3-fold increase in the risk of cardiovascular death in age-and race-adjusted models (HR 3.25, 95% CI 1.17-9.01, P=0.02; Table 6). This risk was only modestly attenuated after adjustment for the full set of covariables (HR 3.13, 95% CI 0.99-9.95, P=0.053). Apart from this finding in women with diabetes, a detectable hsTnT was not associated with an increased risk of myocardial infarction, stroke, or coronary revascularization in either group of women (Table 6). Excluding women with elevated levels of troponin (hsTnT ≥ 0.014 μg/L) did not substantially alter these results. In diabetic women, the addition of hsTnT to fully adjusted models for either the composite cardiovascular endpoint or cardiovascular death significantly improved model fit (P-values 0.046 and 0.037, respectively). The association between detectable troponin levels and incident CVD in women with diabetes was not modified by HbA1c levels above or below the median of 7% (P-interaction=0.10), or by a history of hypertension, a history of high cholesterol, age ≥ 60 years, or BMI ≥ 30 kg/m2 (each P-interaction >0.40). The lack of association between a detectable hsTnT and incident CVD amongst non-diabetic women was also consistent across these subgroups.
Table 6.
The adjusted risk of the composite and individual cardiovascular endpoints according to the presence of a detectable hsTnT in women with and without diabetes in the Women’s Health Study.
| Diabetic Women* | Non-Diabetic Women† | |||||
|---|---|---|---|---|---|---|
| Endpoint | Events (N) | Hazard Ratio (95% CI) | P-value | Events (N) | Hazard Ratio (95% CI) | P-value |
| Age and race adjusted | ||||||
| Total Cardiovascular Disease | 65 | 1.95 (1.17-3.23) | 0.01 | 479 | 1.07 (0.81-1.40) | 0.63 |
| Cardiovascular Death | 20 | 3.25 (1.17-9.01) | 0.02 | 108 | 1.13 (0.73-1.73) | 0.59 |
| Total Myocardial Infarction | 27 | 1.41 (0.66-3.04) | 0.38 | 167 | 0.80 (0.54-1.17) | 0.25 |
| Total Stroke | 28 | 1.95 (0.89-4.26) | 0.09 | 254 | 1.14 (0.83-1.58) | 0.42 |
| Multivariable adjusted‡ | ||||||
| Total Cardiovascular Disease | 60 | 1.76 (1.00-3.08) | 0.0499 | 456 | 1.15 (0.82-1.59) | 0.42 |
| Cardiovascular Death | 18 | 3.13 (0.99-9.95) | 0.053 | 101 | 1.24 (0.74-2.09) | 0.42 |
| Total Myocardial Infarction | 24 | 1.25 (0.53-2.95) | 0.61 | 155 | 0.76 (0.46-1.26) | 0.29 |
| Total Stroke | 27 | 1.92 (0.81-4.52) | 0.14 | 245 | 1.26 (0.87-1.83) | 0.22 |
65 out of 512 diabetic women developed the composite cardiovascular endpoint during follow up.
The case-cohort study of non-diabetic women included 479 cases of the composite cardiovascular endpoint and a reference subcohort of 564 women.
Multivariable adjusted hazard ratios are adjusted for covariables included in Model 4 from Table 5, including age, race, body mass index (kg/m2), history of hypertension, current smoking, parental history of myocardial infarction, total cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), natural logarithm high-sensitivity C-reactive protein, hemoglobin A1c (%), and the natural logarithms of NT-proBNP and estimated glomerular filtration rate. Women without complete information for all covariables included in the multivariable model were excluded from the analysis.
Because prior studies in secondary prevention13, elderly14and general15populations have reported an increased risk of CVD even among those with normal hsTnT levels (<0.014 μg/L), we conducted a secondary analysis in the non-diabetic women to determine whether slightly higher hsTnT levels associated with increased CVD risk. We identified non-diabetic women with hsTnT levels equal to or greater than the median detectable hsTnT level in the reference subcohort (0.00521 μg/L). Compared to women with undetectable hsTnT levels, these women were at modestly, non-significantly increased risk of all CVD (HR 1.33, 95% CI 0.86-2.06, P=0.30) and CVD death (HR 1.46, 95% CI 0.76-2.81, P=0.34).
Discussion
In these prospective analyses, we report that nearly half of women with diabetes and a third of women without diabetes had levels of troponin that were detectable with a new generation troponin assay, and that all but 4 of those women had troponin levels that were below the limit of detection of the previous generation of assays. Furthermore, in women with diabetes, these very low but detectable troponin levels were associated with a more than 3-fold increase in the adjusted risk of cardiovascular death, even though the vast majority of women had troponin levels within the range considered normal. By contrast, among women without diabetes, these very low but detectable levels of troponin were not significantly associated with the composite cardiovascular endpoint or any of its components, including cardiovascular death.
We believe these data are of clinical importance for several reasons. First, very low levels of troponin were detectable in a significant minority (30%) of the women without diabetes, and a larger proportion (45%) of women with diabetes. The prevalence of an elevated hsTnT (≥ 0.014 μg/L) in the reference subcohort of non-diabetic women was 2%, a number consistent with data from other studies of healthy men and women.13, 15, 18 These data suggest that while the prevalence of an elevated hsTnT in our non-diabetic cohort is similar to other healthy populations, there is evidence of chronic, ongoing release of small amounts of myocardial troponin into the circulation in both diabetic and non-diabetic women. Not surprisingly, the prevalence of a detectable troponin in both of these groups of women, who do not have clinically evident CVD, is lower than what has been observed in higher risk populations, such as those with prevalent coronary disease, heart failure, or advanced age.13, 14, 21 Our observation that increasing age, blood pressure, HbA1c, and NT-proBNP are associated with increased odds of a detectable hsTnT is consistent with prior data,9, 13, 15, 16 while the lack of association between other risk markers, such as low-density lipoprotein cholesterol, hsCRP, and body mass index, is consistent with some7, 9, 12, 16 but not other13 published reports. Recent findings from the Dallas Heart Study support an independent association between detectable hsTnT and cardiac structural abnormalities that relate to heart failure risk, such as increased left ventricular mass, wall thickness, or chamber size. By contrast, markers of myocardial infarction risk, such as angina, prior myocardial infarction, or coronary artery calcium, were not independent predictors of detectable hsTnT.15Other studies have also reported correlations between structural heart disease and detectable levels of hsTnT.14-16 While we were unable to assess these associations in our study, we did observe a strong univariable correlation between the metabolic marker HbA1c and detectable hsTnT, even among non-diabetic women. These results raise the possibility that alterations in glucose metabolism that precede the onset of clinical diabetes may be an important mechanism of myocardial cell injury.
Second, these very low but detectable levels of troponin were associated with a significantly increased risk of incident cardiovascular disease in women with diabetes but not in women without diabetes. The increased risk of the combined cardiovascular endpoint in women with diabetes appeared to be largely due to increased risk of cardiovascular death, rather than non-fatal myocardial infarction or stroke. These data are consistent with two prior studies of the novel assay conducted in higher risk patients, including those with established but stable coronary disease and the elderly men and women enrolled in the Cardiovascular Health Study.13, 14 Both studies observed an increased risk of cardiovascular death even for subjects with hsTnT levels that were well below the limit of detection of conventional assays, and within the normal range of the novel assay used in this study. While the pathobiologic mechanisms leading to this increased risk of cardiovascular death among those with a detectable hsTnT have yet to be clearly defined, they are likely to be related to myocardial structural abnormalities that have been previously reported to associate with circulating cardiac troponin.14-16
There are a number of possible explanations for our findings in non-diabetic women. First, while the prevalence of detectable troponin in otherwise healthy women was high, it was lower than other populations of more advanced age. Thus, some level of circulating troponin may be normal, at least in women without diabetes who are at low absolute CVD risk.13, 14We did note a subtle, non-significant increase in CVD risk with slightly higher levels of hsTnT in the non-diabetic women. This finding is consistent with previously published observations in other populations, and suggests that a value closer to the 99th percentile in healthy populations might be a better threshold to use to determine cardiovascular risk among healthy, non-diabetic subjects.18 However, recently published results from the Dallas Heart Study suggest an elevated risk of both all-cause and cardiovascular mortality across a range of hsTnT levels that are still within what is defined as normal (<0.014 μg/L). This risk was consistent across sex, diabetes status, and the presence of preexisting cardiovascular disease.15 One possible explanation for the different results in our study is that the predominantly white female health professionals enrolled in the WHS had better access to routine preventive medical care, which may have attenuated mortality risk associated with an elevated hsTnT.
Alternatively, detectable troponin in women without diabetes may reflect a normal biologic process of myocyte turnover that at very low levels is not associated with increased risk of adverse cardiovascular outcome, while the observation of relatively higher prevalence of detectable troponin in women with diabetes may reflect a more malignant process of accelerated cell turnover, either due to subclinical epicardial coronary disease, chronic microvascular ischemia, or cardiomyopathy with attendant risk of adverse cardiovascular outcomes. One mechanism specific to the diabetic heart is a shift to fatty acid metabolism, which may lead to increased oxidative stress, which has previously shown in animal models to precipitate troponin release.22, 23In multivariable models, blood pressure and NT-proBNP, both measures of cardiac wall stress, were associated with detectable hsTnT among diabetic women but not among non-diabetic women. This observation raises the possibility that the diabetic myocardium may be more vulnerable to the deleterious effects of elevated blood pressure. Alternatively, the observed discrepancy between women with and without diabetes in this study may be due to absence of congestive heart failure in our CVD definition. This endpoint has been associated with detectable troponin levels in patients with chronic heart failure,12 chronic stable coronary disease,13and the elderly.14 Thus, if non-diabetic women with detectable hsTnT were more likely to develop heart failure than die of cardiovascular causes, those events would not have been captured by our study design.
Finally, the fact that our data suggest that very low but detectable levels of troponin provide independent prognostic information for women with diabetes, even after controlling for traditional and novel cardiovascular risk markers, raises the possibility that high sensitivity troponin could serve as a complimentary marker of cardiovascular risk and a means of further risk stratification in patients with diabetes. This possibility is intriguing, because the rates of macrovascular complications of diabetes remain high, even with treatment strategies designed for aggressive control of glucose,24-26 blood pressure,27 and lipids.28
Strengths and Limitations
Our study has a number of strengths and limitations which merit consideration. Our study was performed in middle-aged women, who have been underrepresented in previous studies of new high-sensitivity troponin assays,12, 13, 16 and who were healthy at the time of enrollment and blood sampling. Our prospective study design included a large number of incident cardiovascular events, including many cardiovascular deaths. Baseline diabetes was assessed by self-report, thus, the possibility of misclassification exists. However, WHS participants were all female health professionals, among whom self-report of various chronic health conditions has proven accurate in prior validation studies.29 Nonetheless, misclassificaiton by diabetes status would tend to bias our results toward the null. Although our sample may not be representative of the overall U.S. population, the prevalence of an elevated troponin in our cohort is consistent with that observed in other healthy populations (i.e., approximately 1-2%). As such, we were unable to assess whether an elevated hsTnT level (≥ 0.014 μg/L), instead of a detectable hsTnT level (≥ 0.003 μg/L), is a marker of future cardiovascular risk. Finally, validated heart failure events were not available, limiting our ability to evaluate the reported association between a detectable hsTnT and incident heart failure in a healthy population.
Conclusions
In conclusion, very low but detectable levels of cardiac troponin T are common and are associated with incident cardiovascular disease, and cardiovascular death in particular, in women with diabetes. Among initially healthy women without diabetes, detectable compared to undetectable troponin was not associated with incident cardiovascular events, including cardiovascular death.
Acknowledgments
Funding Sources The Women’s Health Study was supported by grants HL-043851, HL-080467 and HL-099355 from the National Heart, Lung and Blood Institute and CA-047988 from the National Cancer Institute. Support for the hsTnT and NT-proBNP assays was provided through an investigator-initiated grant to Dr. Everett from Roche Diagnostics and by a Discovery Award from the Brigham and Women’s Hospital Cardiovascular Leadership Council. Support for the construction of the case-cohort study was provided through an investigator-initiated grant to Dr. Ridker from Roche Diagnostics. The funders had no role in the design and conduct of the study, the collection, management, or analysis of the data.
Footnotes
Conflict of Interest Disclosures Drs. Everett and Ridker are the recipients of investigator-initiated research awards from Roche Diagnostics. Drs. Magnone and Bobadilla are employees of Hoffmann-La Roche Ltd.
References
- 1.Hamm CW, Ravkilde J, Gerhardt W, Jorgensen P, Peheim E, Ljungdahl L, Goldmann B, Katus HA. The prognostic value of serum troponin T in unstable angina. N Engl J Med. 1992;327:146–150. doi: 10.1056/NEJM199207163270302. [DOI] [PubMed] [Google Scholar]
- 2.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH, Apple FS, Francis G, Tang W. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552–574. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 4.Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, Fischer GA, Fung AY, Thompson C, Wybenga D, Braunwald E. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 5.Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003;108:833–838. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 6.Perna ER, Macin SM, Canella JP, Augier N, Stival JL, Cialzeta JR, Pitzus AE, Garcia EH, Obregon R, Brizuela M, Barbagelata A. Ongoing myocardial injury in stable severe heart failure: value of cardiac troponin T monitoring for high-risk patient identification. Circulation. 2004;110:2376–2382. doi: 10.1161/01.CIR.0000145158.33801.F3. [DOI] [PubMed] [Google Scholar]
- 7.Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 2006;113:1071–1078. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]
- 8.Sundström J, Ingelsson E, Berglund L, Zethelius B, Lind L, Venge P, Arnlöv J. Cardiac troponin-I and risk of heart failure: a community-based cohort study. Eur Heart J. 2009;30:773–781. doi: 10.1093/eurheartj/ehp047. [DOI] [PubMed] [Google Scholar]
- 9.Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, Wians F, Sabatine MS, Morrow DA, de Lemos JA. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 10.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth-Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–877. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 11.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 12.Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN, Investigators V-H. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 13.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. New Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of Serial Measures of Cardiac Troponin T Using a Sensitive Assay With Incident Heart Failure and Cardiovascular Mortality in Older Adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Januzzi JL, Bamberg F, Lee H, Truong QA, Nichols JH, Karakas M, Mohammed AA, Schlett CL, Nagurney JT, Hoffmann U, Koenig W. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation. 2010;121:1227–1234. doi: 10.1161/CIRCULATIONAHA.109.893826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 18.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 19.Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High-sensitivity cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem. 2010;56:642–650. doi: 10.1373/clinchem.2009.134460. [DOI] [PubMed] [Google Scholar]
- 20.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 21.Hallen J, Johansen OE, Birkeland KI, Gullestad L, Aakhus S, Endresen K, Tjora S, Jaffe AS, Atar D. Determinants and prognostic implications of Cardiac Troponin T measured by a sensitive assay in Type 2 Diabetes Mellitus. Cardiovasc Diabetol. 2010;9:52. doi: 10.1186/1475-2840-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart Fail Rev. 2002;7:149–159. doi: 10.1023/a:1015328625394. [DOI] [PubMed] [Google Scholar]
- 23.Nie J, Close G, George KP, Tong TK, Shi Q. Temporal association of elevations in serum cardiac troponin T and myocardial oxidative stress after prolonged exercise in rats. Eur J Appl Physiol. 2010;110:1299–1303. doi: 10.1007/s00421-010-1604-6. [DOI] [PubMed] [Google Scholar]
- 24.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 25.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 27.Blood pressure control in type 2 diabetes. N Engl J Med. 2010;363:695–697. doi: 10.1056/NEJMc1006411. [DOI] [PubMed] [Google Scholar]
- 28.Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010;363:692–695. doi: 10.1056/NEJMc1006407. [DOI] [PubMed] [Google Scholar]
- 29.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]


